Abstract
Aims
We previously reported that preconditioning of stem cells with insulin-like growth factor-1 (IGF-1) translocated connexin-43 (Cx-43) into mitochondria, causing cytoprotection. We posit that these preconditioning effects could be simulated by mitochondria-specific overexpression of Cx-43.
Methods and results
During IGF-1-induced preconditioning of C57black/6 mouse bone marrow stem cell antigen-1+ (Sca-1+) cells, Cx-43 was mainly translocated onto the mitochondrial inner membrane, which was abrogated by an extracellular signal-regulated kinases 1 and 2 (ERK1/2) blocker PD98059. To investigate the role of mitochondrial Cx-43, we successfully designed a vector coding for full-length mouse Cx-43 with a mitochondria-targeting sequence (mito-Cx-43) and cloned into a shuttle vector (pShuttle-IRES-hrGFP-1) for mitochondria-specific overexpression of Cx-43 (mito-Cx-43). Sca-1+ cells with mito-Cx-43 reduced cytosolic accumulation of cytochrome c, lowered caspase-3 activity, and improved survival during index oxygen–glucose deprivation as determined by terminal deoxynucleotidyl transferase dUTP nick-end labelling and lactate dehydrogenase assays. Computational analysis revealed a B-cell lymphoma-2 (Bcl-2) homology domain-3 (BH3) motif in Cx-43 with a conserved pattern of amino acids consistent with the Bcl-2 family that regulated cytochrome c release. Moreover, computational secondary structure prediction indicated an extended α-helix in this region, a known condition for BH3-driven protein–protein interactions.
Conclusion
Cx-43 translocation into mitochondria during preconditioning was ERK1/2-dependent. Expression of mito-Cx-43 simulated the cytoprotective effects of preconditioning in stem cells. Structural features of Cx-43 were shared with the Bcl-2 family as determined by computational analysis.
Keywords: Apoptosis, Connexin-43, Heart, IGF-1, Mitochondria, Sca-1+
1. Introduction
Connexin-43 (Cx-43) is a predominant junctional protein in the intercellular communication channels that critically regulates translocation of ions and small molecules and is responsible for electrical communication between varieties of cell types, especially the cardiomyocytes.1 Additionally, Cx-43 also participates in other cellular functions such as cell growth, differentiation, and death/survival signalling.2–4 Nevertheless, the involvement of Cx-43 in these functions is independent of its role in intercellular communication. Therefore, intracellular translocation of Cx-43 between the cell organelles occurs under the influence of various physiological or pathophysiological stimuli to achieve the required effects.5–7 We have already reported a dual role for Cx-43 in preconditioned stem cells wherein it helped the preconditioned cells to integrate in the host myocardium and promoted their post-engraftment survival.8 We observed that insulin-like growth factor-1 (IGF-1)-induced preconditioning promoted Cx-43 translocation into mitochondria which was responsible for its pro-survival activity. These novel findings underscored the need to elucidate the mechanism by which IGF-1-induced preconditioning produced Cx-43 translocation from the cytosol into mitochondria and its exact site of localization. It also remains to be seen whether the cytoprotective effects of mitochondrial Cx-43 during IGF-1-induced preconditioning could be replicated by mitochondria-specific transgenic overexpression of Cx-43. We therefore hypothesized that mitochondria-specific Cx-43 is an effective alternative strategy to promote stem cell survival by duplicating preconditioning effects of IGF-1. Moreover, Cx-43 in the mitochondria would prevent the release of cytochrome c into the cytosol, thus reducing its availability to activate caspase-3 cascade. On the basis of the protein structural analysis using computational methods, we found that Cx-43 contained a B-cell lymphoma-2 (Bcl-2) homology domain-3 (BH3) domain, thus suggesting its anti-apoptotic role via a mechanism similar to other members of the Bcl-2 family.
2. Methods
All experiments described herein were performed at least three times (n = 3) to ascertain reproducibility of the data.
2.1. Isolation of bone marrow stem cell antigen-1+ cells
Bone marrow was harvested from 6- to 8-week male C57Black6 mice and stem cell antigen-1+ (Sca-1+) cells were purified and propagated as described previously.8 The cells were rich in Sca-1 expression (93%) and low in ckit and CD45 antigens.8 The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1985) and a protocol approved by Institutional Animal Care and Use Committee, University of Cincinnati.
2.2. Mitochondrial fractionation and subfractionation
Mitochondrial fractionation was performed with ProteoExtract Cytosol/Mitochondria Fractionation Kit (EMD Bioscience, CA, USA) as per the manufacturer's instructions. A mitochondrial subfractionation method was adopted from a previously published report except that 0.4% digitonin concentration was used7 and given in Supplementary material online.
2.3. Western blot analysis
Protein concentration of the lysate samples from cytosolic and mitochondrial fractions was determined with Lowry's method. Western blot analysis for cell lysate samples was performed as described previously.8 The antibodies and their concentrations used have been given in Supplementary material online, Table S1.
2.4. RNA isolation and RT–PCR
RNA was harvested with Trizol Reagent (Invitrogen, CA, USA). Samples were subjected to chloroform extraction and two rounds of acid phenol:chloroform extractions. The supernatant was collected and equal volume of isopropanol was added for precipitation. Samples were then subjected to centrifugation at 23 000 g for 30 min. The RNA pellets were washed with 70% ethanol and dried. Pellets were dissolved with DNAse and RNAse-free water, and concentration was determined with a spectrometer. RNA reverse transcription was performed with Cloned AMV First-Strand cDNA Synthesis Kit as per the manufacturer's instructions (Invitrogen).
2.5. Cloning of mitochondria-targeted GFP and Cx-43 vectors
Mitochondria-targeted GFP expression vector pCMV/myc/mito/GFP was used as the starting backbone vector. The vector was double digested with PstI and NotI to release GFP fragment. Mice Cx-43 full-length cDNA was amplified from Sca-1+ cells using HotStar HiFidelity Taq Polymerase (Qiagen, CA, USA) with the following primers: Fwd5′-AACTGCAGATGGTGACTGGAGCGCCTT-3′ and Rev5′-AAGCGGCCGCTTAAATCTCCAGGTCATCAG-3′. The RT–PCR product was double digested with PstI and NotI followed by ligation with a previously double-digested vector. Mito-Cx-43 fragment was amplified from pCMV/myc/mito/Cx-43 using the following primers: Fwd5′-AAAGATCTATGTCCGTCCTGACGCCG-3′ and Rev5′-AACTCGAGAATCTCCAGGTCATCAGGC-3′. The PCR product was then subcloned into pShuttle-IRES-hrGFP-1 using XhoI and BglII recognition sites. Mito-GFP fragment was amplified from pCMV/myc/mito/GFP using the following primers: Fwd5′-AAAGATCTATGTCCG TCCTGACGCCG-3′ and Rev5′-AACGATCGGTTTGTAGAGCTCATCCATG-3′. The PCR product was then subcloned into pShuttle-IRES-hrGFP-1 using PvuI and BglII recognition sites. Sequencing was performed (Genewiz Inc., NJ, USA) and verified with BLAST from NCBI (NIH, MD, USA). Restriction enzymes were either from Promega (WI, USA) or New England Biolabs (MA, USA).
2.6. Transfection of Sca-1+ cells for mitochondrial overexpression of GFP or Cx-43
Sca-1+ cells were transfected with respective plasmids for overexpression of mito-GFP-FLAG and mito-Cx-43-FLAG. Transfection was carried out using Attractene Reagent (Qiagen) following the customized protocol for bone marrow stem cells. Briefly, 3 × 105 cells were seeded into 35 mm dish in 2 mL high-glucose DMEM with 10% FBS. Plasmid DNA (1.2 μg) was diluted in 100 μL culture medium without serum and mixed with 4.5 μL Attractene transfection reagent by vortexing. The complex was incubated for 10–15 min at room temperature. The cells were incubated with the transfection complex under their normal growth conditions for 48 h. Transfection efficiency was determined by performing GFP immunostaining and visualization of cell nuclei with DAPI. The cells showing co-localization of GFP expression with DAPI were counted at low-power magnification (×20) in multiple microscopic fields. Transfection efficiency was calculated by dividing the GFP-positive cells with total number of cells (determined by DAPI-positive nuclei). The cells were subsequently used in different experiments. For exposure to oxygen–glucose deprivation (OGD), the cells were cultured in glucose-free medium and exposure to anoxia for 8 h as described in Supplementary material online, Methods. For mitochondrial fractionation, transfections were performed in 100 mm dish.
2.7. Lactate dehydrogenase assay
Intracellular lactate dehydrogenase (LDH) release was measured using Homogeneous Membrane Integrity Assay (Promega) as described earlier and in Supplementary material online, Methods.9
2.8. Terminal deoxynucleotidyl transferase dUTP nick-end labelling assay
Terminal deoxynucleotidyl transferase dUTP nick-end labelling (TUNEL) assay was performed on paraformaldehyde-fixed cells with in situ cell death detection kit (TMR Red) (Roche, Germany). The results were analysed and recorded with a fluorescence microscope.
2.9. Caspase-3 activity assay
Sca-1+ cells were exposed to OGD, cell lysates were collected, and caspase-3 activity was measured with caspase-3 immunoassay kit as per instructions of the manufacturer (EMD Bioscience).
2.10. Immunostaining
Cells were fixed with 4% paraformaldehyde at room temperature for 5 min followed by permeabilization with 0.2% Triton X-100 for another 5 min. Blocking was performed at room temperature for 30 min with 10% animal serum from the same animal species as secondary antibodies. A primary mouse anti-FLAG antibody (Sigma; 1:500) and a goat anti-mouse Alexa Fluor-546-conjugated secondary antibody (Invitrogen; 1:400) were diluted with blocking buffer and were incubated at room temperature for 45 and 30 min, respectively. After each incubation, slides were subjected to wash three times with PBS. Cell nuclei were stained with DAPI and slides were mounted with Fluoromount G (SouthernBiotech, AL, USA). Pictures were taken with a fluorescence microscope (Olympus, Japan).
2.11. Computational analysis
Protein sequences were retrieved from the UniProt database. Bcl-2 family members were found in the Pfam10 and Bcl-2 family11 databases. Sequence similarity search and multiple sequence alignment were performed using BLAST12 and Clustal W,13 respectively. The SABLE method14 was used for secondary structure prediction. The location of transmembrane (TM) domains was predicted using the MINNOU method.15
2.12. Statistical analysis
All the data were expressed as mean ± SEM. The comparisons among groups were made using Student's t-test or ANOVA with a mixed-effect model to account for the random effect from different repetition and unequal variance. A value of P ≤ 0.05 was considered statistically significant.
3. Results
3.1. Role of mitogen-activated protein kinase in mitochondrial translocation of Cx-43 and cytoprotection
Our previous data showed that Cx-43 played an essential role in IGF-1-induced preconditioning-mediated cytoprotection, whereas knockdown of Cx-43 abolished its cytoprotective effects. Our current data revealed submitochondrial translocation of Cx-43 in the preconditioned cells occurred in a p42/44 mitogen-activated protein kinase (MAPK)-dependent manner.
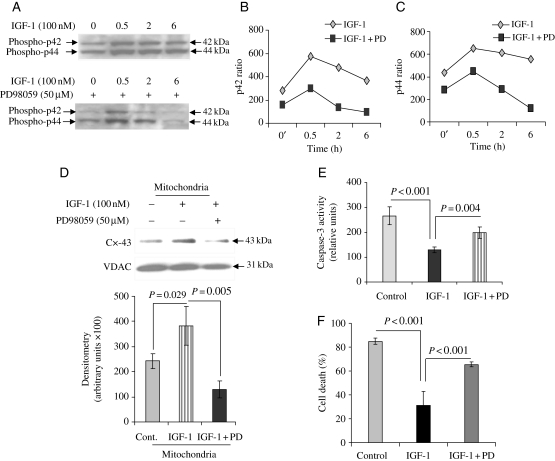
The role of IGF-1 in activation of MAPK is reported previously.16 Sca-1+ cells treated with 100 nM IGF-1 for various time durations showed significant activation of MAPK which was abrogated by pre-treatment with 50 µM MAPK blocker PD98059 (Figure 1A). Densitometry for phospho-p42 MAPK and phospho-p44 MAPK showed that preconditioning dramatically up-regulated activities for both p42 and p44 MAPKs (Figure 1B and C). MAPK activity peaked at 30 min after IGF-1 treatment which coincided with a simultaneous increase in mitochondrial Cx-43. Interestingly, pre-treatment of the IGF-1-treated cells with an MAPK inhibitor PD9805943 significantly reduced Cx-43 translocation from the cytosol into mitochondria (Figure 1D; purity of mitochondrial fraction was determined by western blotting using Cox-IV as a marker of purity of the mitochondrial fraction as shown in Supplementary material online, Figure S1). These data show the p42/44 MAPK dependence of Cx-43 for mitochondrial translocation during IGF-1-induced preconditioning. We also observed that the MAPK blocker abolished IGF-1-induced cytoprotection by preventing caspase-3 activation under OGD (Figure 1E and F).
Figure 1.
(A) Western immunoblot showing phospho-MAPK in Sca-1+ cells preconditioned with 100 nM IGF-1 for different time durations. Parallel experiment was performed with pre-treatment of Sca-1+ cells with 50 μM of MAPK blocker PD98059 for 30 min followed by 100 nM IGF-1 for the stipulated time durations. Western blot for p42/44 MAPK with/without PD98059 was performed on the same membrane and subjected to the same antibody incubation, detection, and exposure. The blots were later edited to align for comparison. (B and C) Densitometry for p42 MAPK and p44 MAPK for different durations of treatment showed the kinetics of MAPK phosphorylation over time and abrogation after PD98059 blocker treatment. (D) Representative western immunoblot from subfractionated cell lysate samples from preconditioning of Sca-1+ cells showing enhanced mitochondrial translocation of Cx-43 which was blocked by 50 μM PD98059 for 30 min (densitometric results are an average of three separate experiments). (E) Parallel experiments were performed to show that caspase-3 activity was significantly increased in the preconditioned Sca-1+ cells pre-treated with PD98059 with concomitant reduction in cell survival as determined by LDH release assay (F) (the number of experiments performed, n = 3).
3.2. Mitochondrial subfractionation and distribution of translocated Cx-43 between inner and outer mitochondrial membranes in preconditioned Sca-1+ cells
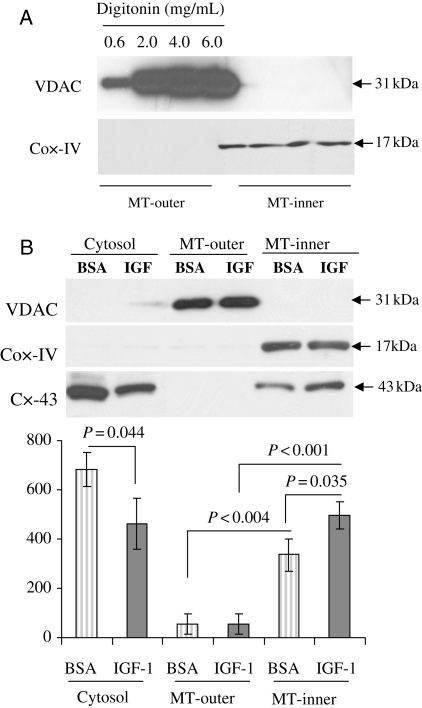
Mitochondria from Sca-1+ cells were successfully isolated and subfractionationated using different concentrations of digitonin (0.6–6 mg/mL). Equal amounts of lysate samples from the cytosol, mitochondrial outer membrane, and mitochondrial inner membrane thus obtained were analysed by western blot for VDAC and Cox-IV as markers of outer and inner membranes (Figure 2A). The band intensity for VDAC from mitochondrial outer membrane fraction increased in parallel with the concentration of digitonin from 0.6 to 6 mg/mL and reached the maximum at 4–6 mg/mL. Cox-IV was only detected in mitochondrial inner membrane fraction, whereas it was not detected in mitochondrial outer membrane, thus indicating that there was no cross-contamination between inner and outer mitochondrial membrane fractions (Figure 2A). Moreover, we did not use the matrix-containing pelleted fraction in the last step of the mitochondrial fractionation procedure (refer to Supplementary material online, Methods). Thus, 4 mg/mL digitonin was applied into the mitochondrial isolation buffer throughout the study.
Figure 2.
Mitochondrial translocation of Cx-43 in preconditioned stem cells. (A) Western immunoblot showing purity of mitochondrial subfractionations in Sca-1+ cells using different concentrations of digitonin (ranging from 0.6 to 6 mg/mL) in the mitochondria lysis buffer. VDAC and Cox-IV were used as the mitochondrial outer and inner membrane markers, respectively. MT-outer, mitochondria outer membrane; MT-inner, mitochondria inner membrane. (B) Western immunoblot showing translocation of Cx-43 subsequent to preconditioning of Sca-1+ cells using 100 μM IGF-1 (the number of experiments performed, n = 3). BSA, bovine serum albumin.
Sca-1+ cells were preconditioned with 100 nM IGF-1 or BSA as a control. Cells were collected and subjected to mitochondrial subfractionation. In untreated control cells, Cx-43 was detected in both cytosolic fraction and mitochondrial inner membrane fraction (Figure 2B). Upon IGF-1 treatment, a reduction of Cx-43 in cytosolic fraction was observed with a concomitant Cx-43 increase in mitochondrial inner membrane fraction, thus suggesting that translocation of Cx-43 occurred from the cytosol to mitochondria inner membrane during IGF-1-induced preconditioning (Figure 2B).
3.3. Construction of Cx-43 plasmid for mitochondria-specific overexpression of Cx-43
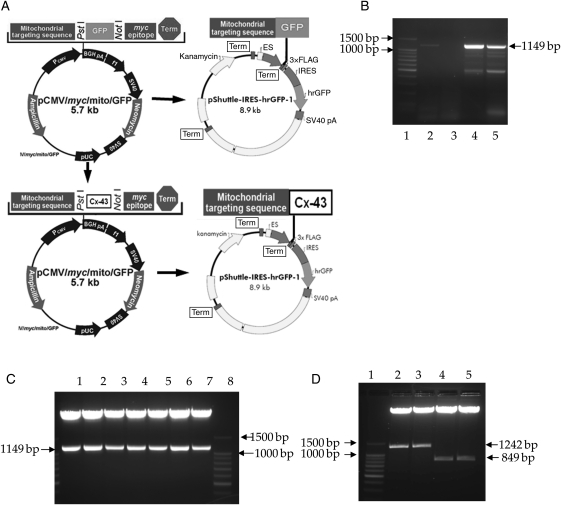
Figure 3A illustrates the schematic description of Cx-43 plasmid construct for mitochondria-specific Cx-43 transgenic overexpression. Detailed methodology is available in Section 2. Briefly, mouse Cx-43 full-length cDNA, an 1149 bp fragment (Figure 3B), was amplified by RT–PCR from bone marrow-derived Sca-1+ cells and cloned into pCMV/myc/mito/GFP in-frame, downstream of mitochondria-targeting sequence. Positive bacterial colonies that released the insertion fragments after double digestion of minipreped plasmids with PstI and NotI could be seen from Figure 3C. The fragment that contained mitochondria-targeting sequence and Cx-43 cDNA (mito-Cx-43 fragment) was amplified from positive colonies by PCR. This fragment was then subcloned into pShuttle-IRES-hrGFP-1, and positive colonies were double digested with XhoI and BglII. A 1242 bp fragment which represented mito-Cx-43 was released (Figure 3D). Mito-GFP fragment was also subcloned separately into pShuttle-IRES-hrGFP-1 and an 849 bp mito-GFP fragment was released (Figure 3D). There was a 3×FLAG tag that fused in-frame downstream of both mito-Cx-43 and mito-GFP which served as a detection and purification marker for potential protein–protein interaction studies. The vector contained a CMV promoter and a bicistronic expression cassette in which the multiple cloning sites was followed by the EMCV-IRES for directed translation of a humanized recombinant green fluorescent protein (hrGFP) as a second opening frame. This allowed expression of mito-Cx-43-FLAG to be monitored at the single-cell level due to the expression of the hrGFP on the same transcript. The sequence of mito-Cx-43-FLAG and its in-frame fusion with mitochondria-targeting sequence and FLAG tag were confirmed by sequencing with Cx-43 forward and reverse primers (see Supplementary material online, Figure S2).
Figure 3.
Development of vector for mitochondria-specific overexpression of Cx-43. (A) Schematic diagram of full-length Cx-43 gene cloning into pCMV/Myc/mito-GFP and subcloning of mito-GFP and mito-Cx-43 into pShuttle-IRES-hrGFP-1. (B) RT–PCR of mice Cx-43 from Sca-1+ cells with or without buffer Q (Qiagen). Mice Cx-43 whole cDNA was 1149 bp (lane 1, ladder; lanes 2 and 3, RT–PCR without buffer Q; lanes 4 and 5, RT–PCR with buffer Q). (C) Restriction enzyme digestion of miniprep plasmid from colonies 1–7. Positive colonies expressed mice Cx-43 whole cDNA fragment (lanes 1–7; lane 8, ladder). (D) Lane 1, ladder; lanes 2 and 3, restriction enzyme digestion of miniprep plasmid from colonies obtained after subcloning of mito-Cx-43 into pShuttle-IRES-hrGFP-1. Released fragment is mitochondria-targeting sequence plus Cx-43 whole cDNA. Lanes 4 and 5, restriction enzyme digestion of miniprep plasmid from colonies obtained from subcloning of mito-GFP into pShuttle-IRES-hrGFP-1. Released fragment is mitochondria-targeting sequence plus GFP whole cDNA.
3.4. Mitochondria-specific transgenic overexpression of Cx-43 in Sca-1+ cells
Successful transfection of mito-Cx-43-FLAG and mito-GFP-FLAG into Sca-1+ cells was observed by hrGFP expression in the transfected cells (Figure 4A and B). The overall transfection efficiency was estimated at 30%. Western blot studies using cell lysate fractions of mitochondria and sarcolemma showed high specificity of transgenic Cx-43 overexpression in mitochondria, whereas no transgenic overexpression of Cx-43 was observed in other compartments of the cells, i.e. sarcolemma (Figure 4C). Transfected cells were also subjected to mitochondrial fractionation and the lysate samples thus obtained from mitochondrial subfractionation were electrophoresed on SDS–PAGE, transferred onto the blot, and immuno-probed with an anti-FLAG-specific primary antibody. A single band was detected at ∼43 kDa in the cells transfected with mito-Cx-43-FLAG vector, whereas a single band around ∼27 kDa was detected in the cells transfected with mito-GFP-FLAG (Figure 4D). Since mitochondria-targeting sequence and 3×FLAG tag only contributed little to molecular weights to both constructs, it minimally altered the molecular weights of Cx-43 and GFP alone. Mock-transfected cells did not show the signal for FLAG tag (Figure 4D). The lysates were further probed with a Cx-43-specific antibody to confirm its expression, using Cox-IV as a marker to confirm the mitochondrial fraction (Figure 4E). Densitometry showed higher expression of Cx-43 in mito-Cx-43-FLAG vector-transfected mitochondrial fractions when compared with the mito-GFP-FLAG vector-transfected cells. Immunostaining performed with an anti-FLAG antibody on cells transfected with mito-Cx-43-FLAG showed a punctuate distribution pattern of the Cx-43-FLAG fusion protein in the mito-Cx-43-FLAG vector-transfected cells (Figure 4F–I).
Figure 4.
Mitochondria-specific transgenic overexpression of Cx-43. Fluorescence images of Sca-1+ cells overexpressing transgenes; (A) mito-GFP-FLAG and (B) mito-Cx-43-FLAG after successful transfection with their respective vectors (original magnification = ×200). (C) Western blot showing a highly specific mitochondrial transgenic overexpression of Cx-43 in Sca-1+ cells. No transgenic overexpression of Cx-43 was observed in sarcolemmal fraction. Cox-IV and Na+/K+ ATPase were used as markers of mitochondrial and sarcolemmal fractions, respectively. (D) Western immunoblots showing expression of fusion proteins mito-Cx-43-FLAG and mito-GFP-FLAG. Cell lysate samples were obtained from mitochondrial fraction of Sca-1+ cells after transfection and presence of respective antigen was detected with an anti-FLAG antibody (lane 1, mito-Cx-43 transfected; lane 2, mito-GFP transfected; lane 3, mock transfected). (E) Western immunoblot showing Cx-43 expression in Sca-1+ cells after transfection with mito-Cx-43-FLAG. Cell lysate samples were obtained from mitochondrial inner membrane fraction. Densitometry for Cx-43 and Cox-IV was performed and relative Cx-43 expression was determined as a ratio with Cox-IV expression (lane 1, mito-Cx-43 transfected; lane 2, mito-GFP transfected). (F–H) Fluorescence immunostaining of Sca-1+ cells with an anti-FLAG antibody after transfection with mito-Cx-43-FLAG. The expression of fusion protein was detected using an anti-FLAG antibody and the primary antigen–antibody reaction was detected with an Alexa Fluor-546 secondary antibody (red fluorescence; white arrows). Nuclei were stained with DAPI (blue fluorescence; original magnification = oil). (I) Magnified image of a cell from (H) (white box) to show punctuate distribution of transfected Cx-43-FLAG fusion protein (the number of experiments performed, n = 3, except experiments involving mitochondrial fractionation and subfractionation, n = 6).
3.5. Mitochondrial Cx-43 overexpression is cytoprotective
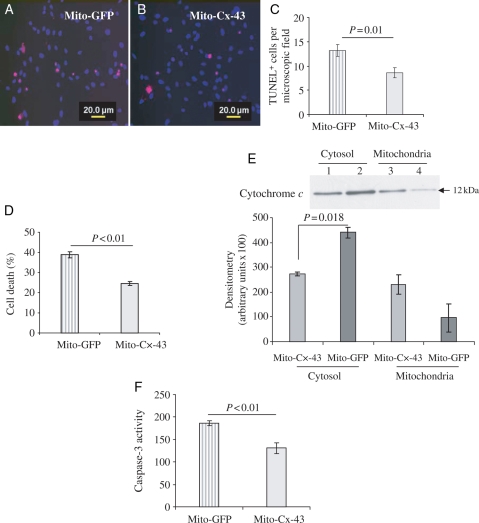
The role of mitochondria-specific transgenic overexpression of Cx-43 in cytoprotection was assessed by subjecting mito-Cx-43-FLAG-transfected Sca-1+ cells to OGD, using mito-GFP-FLAG-transfected cells under the same set of culture conditions as controls. TUNEL assay showed significantly lower TUNEL-positive cells, counted from random-selected fields, in mito-Cx-43-FLAG-transfected cells (P< 0.01 vs. mito-GFP-FLAG-transfected cells; Figure 5A–C). These results were confirmed by LDH release assay which showed significantly higher cell viability in mito-Cx-43-FLAG-transfected cells (Figure 5D).
Figure 5.
Pro-survival activity of mitochondrial Cx-43 in stem cells. Sca-1+ cells were transfected with either mito-GFP-FLAG (control) or mito-Cx-43-FLAG and later were subjected to 8 h OGD. (A–C) The number of TUNEL+ cells per microscopic field (×400) was significantly reduced in Sca-1+ cells transfected with mito-Cx-43-FLAG when compared with the control (P < 0.01 vs. control cells). (D) LDH release assay showed significantly higher cell death in mito-GFP-FLAG-transfected control cells when compared with mito-Cx-43-FLAG cells (P < 0.01 vs. control). (E) Western immunoblots for cytochrome c using cell lysate samples from cytosolic fraction and mitochondrial inner membrane fraction. The cells after their transfection with the respective vectors were subjected to 8 h OGD for collection of lysate samples. The cells transfected with mito-Cx-43 showed reduced cytochrome c release from mitochondria into the cytosol (P = 0.018 vs. control; lanes 1 and 3, mito-Cx-43 transfected; lanes 2 and 4, mito-GFP transfected). Concomitantly, caspase-3 activity (F) was significantly reduced in mito-Cx-43-transfected Sca-1+ cells after 8 h OGD when compared with the control cells (P < 0.01 vs. control) (the number of experiments performed, n = 3).
We next elucidated the mechanism through which mitochondrial Cx-43 protected Sca-1+ cells from OGD-induced injury. Subcellular fractions of Sca-1+ cells transfected with mito-GFP-FLAG followed by exposure to OGD showed significantly higher presence of cytochrome c in cytosolic fraction when compared with mito-Cx-43-FLAG-transfected cells (Figure 5E). On the contrary, more cytochrome c was retained in the mitochondrial fraction of mito-Cx-43-FLAG-transfected cells when compared with cells transfected with mito-GFP-FLAG (Figure 5E). Parallel with the release of cytochrome c, we observed higher caspase-3 activation in cells transfected with mito-GFP-FLAG when compared with mito-Cx-43-FLAG. These data implied the importance of mitochondrial overexpression of Cx-43 in Sca-1+ cells under OGD to prevent cytochrome c release into the cytosol and the subsequent activation of caspase-3 under OGD. Taken together, our results showed that mitochondrial Cx-43 was critical in Sca-1+ cells for their cytoprotection against OGD-induced injury.
3.6. Cx-43 shares a common BH3 motif with Bcl-2 family members
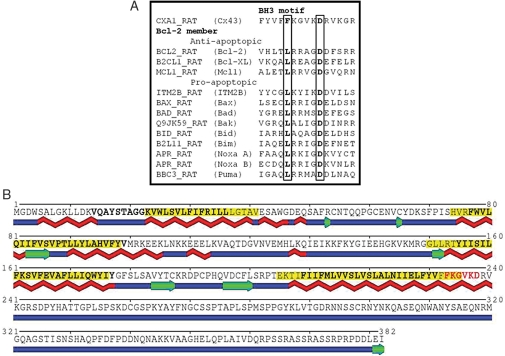
Western blot studies showed up-regulation of Cx-43 in IGF-1 preconditioned cells which was associated with a simultaneous increase in Bcl-2 in mitochondria (our unpublished data). Sequence homology search revealed a BH3 domain in Cx-43 when aligned to the integral membrane protein 2B (ITM2B), a BH3-only member of the Bcl-2 family.17,18 SwissProt IDs of the corresponding sequences used for alignment included CXA1_RAT and ITM2B_RAT. Subsequent multiple sequence alignment of this region of Cx-43 with the other members of the Bcl-2 family revealed consistency in the physico-chemical profile of amino acids within the BH3 domain across the selected proteins (Figure 6A). Using the state-of-the-art SABLE method14 for prediction of the secondary structure, we found an extended α-helix in this region (Figure 6B, amino acids 207–240). Additionally, to localize TM domain in Cx-43 and compare its topology with other Bcl-2 family members, large-scale prediction of TM domains was conducted using MINNOU.15 The prediction showed four TM regions in Cx-43 (Figure 6B, amino acids highlighted yellow). These findings were consistent with the UniProt19 annotation (SwissProt ID: CXA1_RAT), although borders of putative membrane-spanning regions slightly differed (Figure 6B, amino acids with bold face). Among 357 proteins available from Pfam (Bcl-2 family ID: PF00452; using full sequences)10 as of March 2010, 76 members were predicted with no TM domains, 266 were predicted with a single membrane-spanning region, 12 with two, one with three, and two with four regions. These results indicated that proteins with the four-TM topology were rare but present in the Bcl-2 family.
Figure 6.
Identification of the BH3 domain in Cx-43. (A) Multiple sequence alignment of Cx-43 with BH3 domain of representative Bcl-2 family members. Highlighted positions are the most conserved amino acids. (B) Secondary structure prediction using SABLE.14 Blue elements indicate unstructured loops (coil state), green are β-strands, and red are α-helices. Putative membrane spanning regions as predicted by MINNOU15 are yellow, as annotated in UniProt19 are with bold face. Residues highlighted in red represent a core of the BH3 domain.
4. Discussion
We have previously reported that IGF-1-induced preconditioning of Sca-1+ cells promoted their survival and differentiation to adopt cardiac phenotype with involvement of Cx-43 which was translocated into mitochondria during preconditioning.8 The present study determined the exact submitochondrial localization site of the translocated Cx-43 in preconditioned Sca-1+ cells and demonstrated that its pro-survival effects might be simulated by mitochondria-specific transgenic overexpression of Cx-43. To achieve this study endpoint, we successfully designed and developed a plasmid coding for the full length of the mouse Cx-43 transgene with a mitochondria-specific localization signal. The addition of a FLAG tag helped us to identify transfection efficiency of the cells, in addition to serving as a localization site marker of the transgenic Cx-43-FLAG fusion protein in the mitochondria. Mitochondria specificity of transgenic overexpression was confirmed by western blotting. The mitochondrial subfractionation protocol was optimized and submitochondrial markers such as VDAC and Cox-IV were used to show that no significant subfraction cross-contamination occurred during the course of the present study. Our western blot data, supported by fluorescence immunocytochemistry, showed that Cx-43 was translocated onto the inner membrane of the mitochondria following preconditioning of Sca-1+ cells. The initial evidence for a link between Cx-43 and cytoprotection came from the study of ischaemic preconditioning (IPC)-induced cardioprotection in mice hearts.20 Compared with the wild-type mice, preconditioning-induced cardioprotection was lost in heterozygous Cx-43-deficient mice (Cx-43+/−). The role of preconditioning-induced Cx-43 was more as a regulatory protein in survival signalling, which was independent of its presence as a gap junction protein for intercellular communication.21,22 While advancing these data, Rodriguez-Sinovas et al. have elegantly shown that homozygous replacement of Cx-43 with Cx-32 led to reduced infarct size subsequent to ischaemia–reperfusion injury. However, there was concomitant abrogation of cytoprotective response to both IPC and pharmacological preconditioning unlike heterozygous replacement of Cx-43 wherein cytoprotective response to IPC still persisted.23 These data, besides signifying the isoform-dependent nature of preconditioning responses, clearly supported our findings that Cx-43 had a dual role of helping the cell to integrate besides participation in cytoprotective response during IGF-1-induced preconditioning.8 Further studies reported mitochondrial localization of Cx-43 in the cardiomyocytes during IPC of animal hearts which resulted in cardiomyocyte protection.5 Similar observations have also been made during pharmacological preconditioning of rat heart with diazoxide where Cx-43 was translocated onto the mitochondrial inner membrane in the cardiomyocytes with possible involvement of heat shock protein-90-dependent Tom-20 (translocase of the outer membrane-20) complex pathway.7
Despite numerous reports on the presence of mitochondrial Cx-43 in cardiomyocytes, its existence and biological importance in stem cells, especially during preconditioning, remains less well studied. We have therefore adopted a molecular approach to achieve mitochondria-specific overexpression of transgenic Cx-43 to study a cause–effect relationship between its translocation and cytoprotection in preconditioned stem cells. Similar approach was previously employed to express Kir6.2 in mitochondria of HEK293 and HL-1 cells to show that an increased influx K+ into mitochondria during hypoxic stress prevented the detrimental effects of Ca2+ overload.24 We improvised Ljubkivic's approach however, and instead of inserting Cx-43 downstream of mitochondria-targeting sequence and GFP, we removed GFP and inserted Cx-43 gene directly downstream of mitochondria-targeting sequence. This insertional modification in the plasmid alleviated the possibility that a 27 kDa GFP might change the topology, and hence change the native function, of 43 kDa Cx-43 upon mitochondrial targeting. Secondly, we subcloned mito-Cx-43 into a bicistronic vector with a second open-reading frame for GFP downstream of mito-Cx-43, thus allowing cellular level GFP reporter gene expression on the same transcript.
We observed that mitochondria-specific transgenic overexpression of Cx-43 in stem cells was indeed protective for the cells under OGD. The cytoprotective effects of mitochondria-specific transgenic overexpression of Cx-43 in stem cells were mediated via abrogation of cytochrome c release from mitochondria into the cytosol during OGD. While simulating the effect of preconditioning, we observed that transgenic overexpression of Cx-43 in the mitochondria helped the cells to avert leakage of cytochrome c from mitochondria into the cytosol. This prevented caspase-3 activation by cytochrome c, one of the major events in apoptosis. We observed 1.61-fold higher cytochrome c levels in the cytosol of mito-GFP-FLAG-transfected cells under OGD when compared with the cytosol of mito-Cx-43-FLAG-transfected cells. On the other hand, retention of cytochrome c was significantly higher in the mitochondrial fraction of mito-Cx-43-FLAG-transfected cells (2.4-fold vs. mito-GFP-FLAG-transfected cells) under OGD. These observations were supported by reduced activation of caspase-3 and decreased cell death in mito-Cx-43-FLAG-transfected cells, thus suggesting a link between mitochondrial Cx-43 and cytochrome c release. We showed that caspase-3 activation was p42/44 MAPK-dependent during preconditioning of Sca-1+ cells with IGF-1 which was similar to the previously published studies involving IPC.25 Phosphorylated MAPK was co-immunoprecipitated with Cx-43 in the diazoxide-treated myocardium. An MAPK inhibitor PD98059 abolished diazoxide-induced cardioprotection.26 Similar to our observations, these data strongly suggested a connection between MAPK and Cx-43. We have also shown that induction of Cx-43 during preconditioning of stem cells with IGF-1 and its cytoprotective effects were Akt-dependent and abrogation of Akt activation with a specific blocker resulted in simultaneous loss of cytoprotection.8
Although the role of mitochondrial Cx-43 in cytoprotection of stem cells under OGD has been highlighted by these data which also showed the significance of mitochondria-specific transgenic Cx-43 overexpression as an alternative strategy to simulate the effects of preconditioning, further studies are required to elucidate the actual mechanism by which Cx-43 in the mitochondria prevented the release of cytochrome c into the cytosol. Whether Cx-43 becomes integrated into the mitochondrial transition pores or makes a complex by interacting with cytochrome c through possible hydrophobic interaction remains to be determined. A recent study has shown that mito-Cx-43 formed hemichannels at the inner mitochondrial membrane as a possible mechanism by which Cx-43 prevented the release of cytochrome c into the cytosol.27 Bcl-2, which resides in the mitochondria membrane, plays an essential role in repressing apoptosis by preventing cytochrome c release from mitochondria into the cytosol.28,29 Sequence homology search revealed the presence of BH3 in Cx-43. Mutation of leucine (L) and aspartic acid (D) in this domain would abolish apoptosis regulatory function of Bcl-2 proteins.30,31 Although Cx-43 has a phenylalanine (F) instead of a leucine (L), they are both hydrophobic. Cx-43 has a similar domain organization with a Bcl-2 protein (ITM2B), except that Cx-43 has four TM regions as opposed to one in ITM2B. The latter is an integral membrane protein which has a pro-apoptotic role by interacting with Bcl-2.18 Whether Cx-43 is indeed an apoptotic regulatory protein together with a deeper insight into the mechanism behind its anti-apoptotic role awaits further research. Our data from IGF-1-induced preconditioning and mitochondria-specific overexpression of Cx-43 combined with computational analysis establish a link between mitochondria Cx-43 and cytochrome c release and suggest Cx-43 to be a candidate member of the Bcl-2 family.
Besides these significant findings, the study has some limitations. We managed only 30% transfection efficiency of Sca-1+ stem cells using non-viral vector to achieve mitochondria-specific overexpression of Cx-43 transgene. Improvement of transfection efficiency with viral vector will help in proof-of-the-principle studies to show the significance of mitochondria-specific overexpression of Cx-43 to improve stem cell survival, besides determining its role in stem cell proliferation and differentiation. Secondly, the detailed mechanism behind the new function of Cx-43 awaits further research. To this end, our current efforts are focused to study the functional alternations of the Cx-43 mutants which would change its binding affinity with Bcl-2 family members and therefore alter cytoprotective effects of preconditioning in stem cells.
In conclusion, we report that targeting Cx-43 overexpression in mitochondria in Sca-1+ cells can simulate the cytoprotective effects of IGF-1-induced preconditioning and Cx-43, per se, may possess apoptosis regulatory function. Our current experimental evidence establishes a link between mitochondrial Cx-43 and cytochrome c release, and in conjunction with structural considerations, Cx-43 is a likely candidate to belong to the Bcl-2 family.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by NIH Grants # R37-HL074272, HL-080686, and HL-087246 (M.A.); HL-087288 and HL-089535 (H.K.H.); and P30-ES006096 (A.P.)
Supplementary Material
Acknowledgements
We gratefully acknowledge Dr Martin Bienengraeber (Medical College of Wisconsin, WI, USA) for his help with mitochondria-targeting vector and Prof. Muhammad Matlib (University of Cincinnati, OH, USA) for his technical help in subfractionation of mitochondria.
Conflict of interest: none declared.
References
- 1.Duffy HS, Fort AG, Spray DC. Cardiac connexins: genes to nexus. Adv Cardiol. 2006;42:1–17. doi: 10.1159/000092550. doi:10.1159/000092550. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Sinovas A, Cabestrero A, Lopez D, Torre I, Morente M, Abellan A, et al. The modulatory effects of connexin-43 on cell death/survival beyond cell coupling. Prog Biophys Mol Biol. 2007;94:219–232. doi: 10.1016/j.pbiomolbio.2007.03.003. doi:10.1016/j.pbiomolbio.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Mesnil M, Krutovskikh V, Piccoli C, Elfgang C, Traub O, Willecke K, et al. Negative growth control of HeLa cells by connexin genes: connexin species specificity. Cancer Res. 1995;55:629–639. [PubMed] [Google Scholar]
- 4.Doble BW, Dang X, Ping P, Fandrich RR, Nickel BE, Jin Y, et al. Phosphorylation of serine 262 in the gap junction protein connexin-43 regulates DNA synthesis in cell–cell contact forming cardiomyocytes. J Cell Sci. 2004;117:507–514. doi: 10.1242/jcs.00889. doi:10.1242/jcs.00889. [DOI] [PubMed] [Google Scholar]
- 5.Boengler K, Dodoni G, Rodriguez-Sinovas A, Cabestrero A, Ruiz-Meana M, Gres P, et al. Connexin-43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc Res. 2005;67:234–244. doi: 10.1016/j.cardiores.2005.04.014. doi:10.1016/j.cardiores.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Heinzel FR, Luo Y, Li X, Boengler K, Buechert A, Garcia-Dorado D, et al. Impairment of diazoxide-induced formation of reactive oxygen species and loss of cardioprotection in connexin-43 deficient mice. Circ Res. 2005;97:583–586. doi: 10.1161/01.RES.0000181171.65293.65. doi:10.1161/01.RES.0000181171.65293.65. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Sinovas A, Boengler K, Cabestrero A, Gres P, Morente M, Ruiz-Meana M, et al. Translocation of connexin-43 to the inner mitochondrial membrane of cardiomyocytes through the heat shock protein 90-dependent TOM pathway and its importance for cardioprotection. Circ Res. 2006;99:93–101. doi: 10.1161/01.RES.0000230315.56904.de. doi:10.1161/01.RES.0000230315.56904.de. [DOI] [PubMed] [Google Scholar]
- 8.Lu G, Haider HK, Jiang S, Ashraf M. Sca-1+ stem cell survival and engraftment in the infarcted heart: dual role for preconditioning-induced connexin-43. Circulation. 2009;119:2587–2596. doi: 10.1161/CIRCULATIONAHA.108.827691. doi:10.1161/CIRCULATIONAHA.108.827691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Won Kim H, Haider HK, Jiang S, Ashraf M. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein-2. J Biol Chem. 2009;284:33161–33168. doi: 10.1074/jbc.M109.020925. doi:10.1074/jbc.M109.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finn RD, Tate J, Mistry J, Coggill PC, Sammut SJ, Hotz HR, et al. The Pfam protein families database. Nucleic Acids Res. 2008;36:D281–D288. doi: 10.1093/nar/gkm960. doi:10.1093/nar/gkm960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blaineau SV, Aouacheria A. BCL2DB: moving ‘helix-bundled' BCL-2 family members to their database. Apoptosis. 2009;14:923–925. doi: 10.1007/s10495-009-0376-0. doi:10.1007/s10495-009-0376-0. [DOI] [PubMed] [Google Scholar]
- 12.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 13.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. doi:10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 14.Adamczak R, Porollo A, Meller J. Accurate prediction of solvent accessibility using neural networks-based regression. Proteins. 2004;56:753–767. doi: 10.1002/prot.20176. doi:10.1002/prot.20176. [DOI] [PubMed] [Google Scholar]
- 15.Cao B, Porollo A, Adamczak R, Jarrell M, Meller J. Enhanced recognition of protein transmembrane domains with prediction-based structural profiles. Bioinformatics. 2006;22:303–309. doi: 10.1093/bioinformatics/bti784. doi:10.1093/bioinformatics/bti784. [DOI] [PubMed] [Google Scholar]
- 16.Webster J, Prager D, Melmed S. Insulin-like growth factor-1 activation of extracellular signal-related kinase-1 and -2 in growth hormone-secreting cells. Mol Endocrinol. 1994;8:539–544. doi: 10.1210/mend.8.5.8058064. doi:10.1210/me.8.5.539. [DOI] [PubMed] [Google Scholar]
- 17.Petros AM, Olejniczak ET, Fesik SW. Structural biology of the Bcl-2 family of proteins. Biochim Biophys Acta. 2004;1644:83–94. doi: 10.1016/j.bbamcr.2003.08.012. doi:10.1016/j.bbamcr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Fleischer A, Ayllon V, Dumoutier L, Renauld JC, Rebollo A. Proapoptotic activity of ITM2B(s), a BH3-only protein induced upon IL-2-deprivation which interacts with Bcl-2. Oncogene. 2002;21:3181–3189. doi: 10.1038/sj.onc.1205464. doi:10.1038/sj.onc.1205464. [DOI] [PubMed] [Google Scholar]
- 19.(UniProt) TUPR. The anti-apoptotic molecules Bcl-xL and Bcl-w target protein phosphatase 1alpha to Bad. Nucleic Acids Res. 2009;37:D169–174. doi: 10.1093/nar/gkn664. doi:10.1093/nar/gkn664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwanke U, Konietzka I, Duschin A, Li X, Schulz R, Heusch G. No ischemic preconditioning in heterozygous connexin-43-deficient mice. Am J Physiol Heart Circ Physiol. 2002;283:H1740–H1742. doi: 10.1152/ajpheart.00442.2002. [DOI] [PubMed] [Google Scholar]
- 21.Padilla F, Garcia-Dorado D, Rodriguez-Sinovas A, Ruiz-Meana M, Inserte J, Soler-Soler J. Protection afforded by ischemic preconditioning is not mediated by effects on cell-to-cell electrical coupling during myocardial ischemia–reperfusion. Am J Physiol Heart Circ Physiol. 2003;285:H1909–H1916. doi: 10.1152/ajpheart.00438.2003. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Heinzel FR, Boengler K, Schulz R, Heusch G. Role of connexin-43 in ischemic preconditioning does not involve intercellular communication through gap junctions. J Mol Cell Cardiol. 2004;36:161–163. doi: 10.1016/j.yjmcc.2003.10.019. doi:10.1016/j.yjmcc.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Sinovas A, Sanchez JA, Gonzalez-Loyola A, Barba I, Morente M, Aguilar R, et al. Effects of substitution of Cx-43 by Cx-32 on myocardial energy metabolism, tolerance to ischaemia and preconditioning protection. J Physiol. 2010;588:1139–1151. doi: 10.1113/jphysiol.2009.186577. doi:10.1113/jphysiol.2009.186577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ljubkovic M, Marinovic J, Fuchs A, Bosnjak ZJ, Bienengraeber M. Targeted expression of Kir6.2 in mitochondria confers protection against hypoxic stress. J Physiol. 2006;577:17–29. doi: 10.1113/jphysiol.2006.118299. doi:10.1113/jphysiol.2006.118299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–H976. doi: 10.1152/ajpheart.00374.2004. doi:10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 26.Naitoh K, Ichikawa Y, Miura T, Nakamura Y, Miki T, Ikeda Y, et al. MitoKATP channel activation suppresses gap junction permeability in the ischemic myocardium by an ERK-dependent mechanism. Cardiovasc Res. 2006;70:374–383. doi: 10.1016/j.cardiores.2006.01.023. doi:10.1016/j.cardiores.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 27.Miro-Casas E, Ruiz-Meana M, Agullo E, Stahlhofen S, Rodriguez-Sinovas A, Cabestrero A, et al. Connexin-43 in cardiomyocyte mitochondria contributes to mitochondrial potassium uptake. Cardiovasc Res. 2009;83:747–756. doi: 10.1093/cvr/cvp157. doi:10.1093/cvr/cvp157. [DOI] [PubMed] [Google Scholar]
- 28.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome-c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. doi:10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, et al. Prevention of apoptosis by Bcl-2: release of cytochrome-c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. doi:10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 30.Chittenden T, Flemington C, Houghton AB, Ebb RG, Gallo GJ, Elangovan B, et al. A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. EMBO J. 1995;14:5589–5596. doi: 10.1002/j.1460-2075.1995.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelekar A, Thompson CB. Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol. 1998;8:324–330. doi: 10.1016/s0962-8924(98)01321-x. doi:10.1016/S0962-8924(98)01321-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.