1. Introduction
Down's syndrome (DS) is the most common aneuploidy diagnosed in liveborn babies (∼1/700 live births). While DS is typically associated with recognizable dysmorphic features, clinical anomalies exhibit variable expressivity. The DS phenotype includes many organ systems and affects all three embryonic germ layers. DS is the most frequent chromosomal cause of mental retardation, is a recognized genetic aetiology of Alzheimer disease, and is associated with congenital anomalies of the gastrointestinal and cardiac systems.1 It has been over 50 years since trisomy 21 was discovered as the genetic aetiology of DS.2 Despite progress in diagnosis and management of DS-related health problems, the definition of the pathogenetic mechanisms by which these gene dosage errors induce the DS phenotype and elucidation of DS genotype–phenotype correlations remains elusive. One of the barriers to progress in defining the underlying pathogenesis of this common genetic disorder has been the lack of a suitable animal model (reviewed in refs.3,4). This has been especially true for DS-associated cardiovascular malformations. Nearly half of the DS patients have a congenital heart malformation;5 the signature cardiac defect is atrioventricular septal defect (AVSD), also known as atrioventricular (AV) canal defect or endocardial cushion defect.6,7 The paper by Dunlevy et al.8 in the current issue addresses this problem.
2. What is an AVSD?
The AV junction is the area of the heart where atrial myocardium is inserted into the base of the ventricles. The arrangement of each atrium connected to its own ventricle via right and left AV junctions describes the normal heart. AVSD results from abnormal developmental processes which culminate in a common AV junction. AVSD is present in ∼45% of the children with DS.5 Variations in the arrangement of the leaflets of the common AV valve relative to each other, the septal structures, and the AV junction are frequent and in large measure explain the variation in AVSD anatomy.7
3. Developmental abnormalities result in AVSD
The primitive heart tube consists of an inner endocardial layer wrapped in an outer myocardial layer. During cardiac looping, endocardial cushions develop in both the AV canal and the outflow tract by extracellular matrix accumulation, known as cardiac jelly, and epithelial to mesenchymal transition. During this process, endothelial cells lining the endocardial cushions delaminate and move into the adjacent extracellular matrix-rich environment in order to populate the endocardial cushions with mesenchymal cells. Through processes of elongation and remodelling, cushions then develop into cardiac valves and contribute to septation of the outflow tract and the AV canal.9
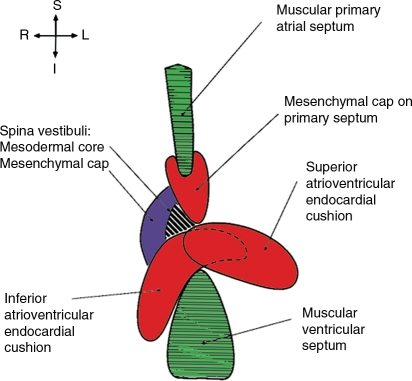
Previously, it was thought that AVSD, characterized by lack of normal AV septal structures, develop due to a problem with fusion of the AV endocardial cushions—hence the reference to AVSD as endocardial cushion defect. However, the formation of the AV septal area is complex, and several important structures contribute to its development (Figure 1). For example, AVSD may result from malalignment of atria over the ventricles due either to misalignment of the AV septal components or to looping defects in response to abnormal left–right patterning as seen in heterotaxy syndromes. AVSD may also result from abnormal development of the contributing myocardial structures. This is somewhat obvious, as the largest parts of the primary atrial septum and the ventricular septum are of myocardial origin, and myocardial signalling to the endocardial cushions is necessary for induction of epithelial to mesenchymal transition and for mesenchymal cell proliferation. Abnormal myocardial development may explain the aetiology of AVSD in Gata4 and Tbx5 mouse mutants.10 Finally, both endothelium-derived and non-endothelium-derived mesenchyme play a role in AV septal morphogenesis. Endothelium-derived mesenchyme is found at the tip of the atrial septum and in the developing AV cushions. Non-endothelium-derived mesenchyme in the dorsal mesenchymal protrusion, also known as spina vestibuli, also contributes to AV septation. Aberrant sonic hedgehog (Shh) signalling in the secondary heart field, including the dorsal mesenchymal protrusion, has been associated with murine AVSD.11 In addition, dysfunction of Ellis van Creveld syndrome gene (1) and Limbin or Ellis van Creveld syndrome 2 gene proteins in the dorsal mesenchymal protrusion is also a suggested aetiology for AVSD seen in Ellis van Creveld syndrome.12 Thus, it is time to move beyond the notion that the development in the AV canal reflects only appropriate fusion of the AV endocardial cushions.13
Figure 1.
Multiple tissues contribute to AV septation during development. In normal cardiac development, the AV septum is formed from myocardial septae (green), endothelially derived mesenchyme of the AV cushions and the cap of the primary atrial septum (red), and non-endothelially derived mesenchyme of the dorsal mesenchymal protrusion (aka spina vestibuli). The dorsal mesenchymal protrusion then differentiates into muscle at the AV junction (black and white stripes). Adapted from Webb et al.;13 used with permission.
4. The Tc1 mouse develops DS-like AVSD
Efforts to understand the developmental aetiology of the cardiac malformations of DS have been severely hampered by the absence of an accurate mouse model that exhibits the full range of DS phenotypes, in particular the profound disruptions resulting in AVSD. The paper by Dunlevy et al.8 presents the analysis of the cardiac malformations exhibited by embryos of the transchromosomic mouse line (Tc(Hsa21)1`TybEmcf (Tc1).14 A major strength of the study is the detailed cardiac phenotyping using high-resolution episcopic microscopy and 3D modelling. In this way, the investigators show that Tc1 embryos exhibit cardiac defects similar to those seen in DS, particularly an AVSD with a common AV canal balanced between the left and right ventricles. This is a unique accomplishment in a mouse model of aneuploidy. In contrast, no comparable cardiac defects were detected in embryos of the more limited mouse trisomy model Ts1Rhr, indicating that trisomy of the region syntenic to the DS Critical Region is insufficient to yield DS-like cardiac abnormalities. Further, while previous studies found AVSD in the Ts16 mouse,15 the common AV canal was not balanced between the two ventricles in the Ts16 model. The authors conclude that the Tc1 mouse line provides a suitable model for studying the underlying genetic causes of the DS AVSD cardiac phenotype; they speculate that an abnormality in the dorsal mesenchymal protrusion underlies the AVSD seen in DS. Interestingly, frequencies of cardiac malformations, ranging from 38 to 55% in the Tc1 mouse, were dependent on strain background.
5. Significance
Despite amazing advances in the diagnosis and treatment of congenital cardiac malformations, mortality and morbidity remain significant concerns. One of the major barriers to progress in the diagnosis and novel therapeutic strategies has been the lack of animal models of viable congenital heart malformations. Despite the mosaic and variable distribution of cells containing the Hsa21 transchromosome seen in the Tc1 line, the model is a step in the right direction and provides a new way to ‘look Down the AV canal'.
Conflict of interest: none declared.
Funding
This work was supported by the National Institutes of Health (HL69712 to D.W.B.).
References
- 1.Torfs CP, Christianson RE. Anomalies in Down syndrome individuals in a large population-based registry. Am J Med Genet. 1998;77:431–438. [PubMed] [Google Scholar]
- 2.Lejeune J, Gautier M, Turpin R. Study of somatic chromosomes from 9 mongoloid children. C R Hebd Seances Acad Sci. 1959;248:1721–1722. [PubMed] [Google Scholar]
- 3.Delabar JM, Aflalo-Rattenbac R, Creau N. Developmental defects in trisomy 21 and mouse models. Sci World J. 2006;6:1945–1964. doi: 10.1100/tsw.2006.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Megarbane A, Ravel A, Mircher C, Sturtz F, Grattau Y, Rethore MO, et al. The 50th anniversary of the discovery of trisomy 21: the past, present, and future of research and treatment of Down syndrome. Genet Med. 2009;11:611–616. doi: 10.1097/GIM.0b013e3181b2e34c. [DOI] [PubMed] [Google Scholar]
- 5.Freeman SB, Taft LF, Dooley KJ, Allran K, Sherman SL, Hassold TJ, et al. Population-based study of congenital heart defects in Down syndrome. Am J Med Genet. 1998;80:213–217. [PubMed] [Google Scholar]
- 6.Anderson RH, Ho SY, Falcao S, Daliento L, Rigby ML. The diagnostic features of atrioventricular septal defect with common atrioventricular junction. Cardiol Young. 1998;8:33–49. doi: 10.1017/s1047951100004613. [DOI] [PubMed] [Google Scholar]
- 7.Mahle WT, Shirali GS, Anderson RH. Echo-morphological correlates in patients with atrioventricular septal defect and common atrioventricular junction. Cardiol Young. 2006;16(Suppl. 3):43–51. doi: 10.1017/s1047951106000758. [DOI] [PubMed] [Google Scholar]
- 8.Dunlevy L, Bennett M, Slender A, Lana-Elola E, Tybulewicz VL, Fisher EMC, et al. Down's syndrome-like cardiac developmental defects in embryos of the transchromosomic Tc1 mouse. Cardiovasc Res. 2010;88:287–295. doi: 10.1093/cvr/cvq193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinton RB, Jr, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, et al. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98:1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- 10.Clark KL, Yutzey KE, Benson DW. Transcription factors and congenital heart defects. Annu Rev Physiol. 2006;68:97–121. doi: 10.1146/annurev.physiol.68.040104.113828. [DOI] [PubMed] [Google Scholar]
- 11.Goddeeris MM, Rho S, Petiet A, Davenport CL, Johnson GA, Meyers EN, et al. Intracardiac septation requires hedgehog-dependent cellular contributions from outside the heart. Development. 2008;135:1887–1895. doi: 10.1242/dev.016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sund KL, Roelker S, Ramachandran V, Durbin L, Benson DW. Analysis of Ellis van Creveld syndrome gene products: implications for cardiovascular development and disease. Hum Mol Genet. 2009;18:1813–1824. doi: 10.1093/hmg/ddp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webb S, Brown NA, Anderson RH. Formation of the atrioventricular septal structures in the normal mouse. Circ Res. 1998;82:645–656. doi: 10.1161/01.res.82.6.645. [DOI] [PubMed] [Google Scholar]
- 14.O'Doherty A, Ruf S, Mulligan C, Hildreth V, Errington ML, Cooke S, et al. An aneuploid mouse strain carrying human chromosome 21 with Down syndrome phenotypes. Science. 2005;309:2033–2037. doi: 10.1126/science.1114535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson RH, Webb S, Brown NA. The mouse with trisomy 16 as a model of human hearts with common atrioventricular junction. Cardiovasc Res. 1998;39:155–164. doi: 10.1016/s0008-6363(98)00037-6. [DOI] [PubMed] [Google Scholar]