Abstract
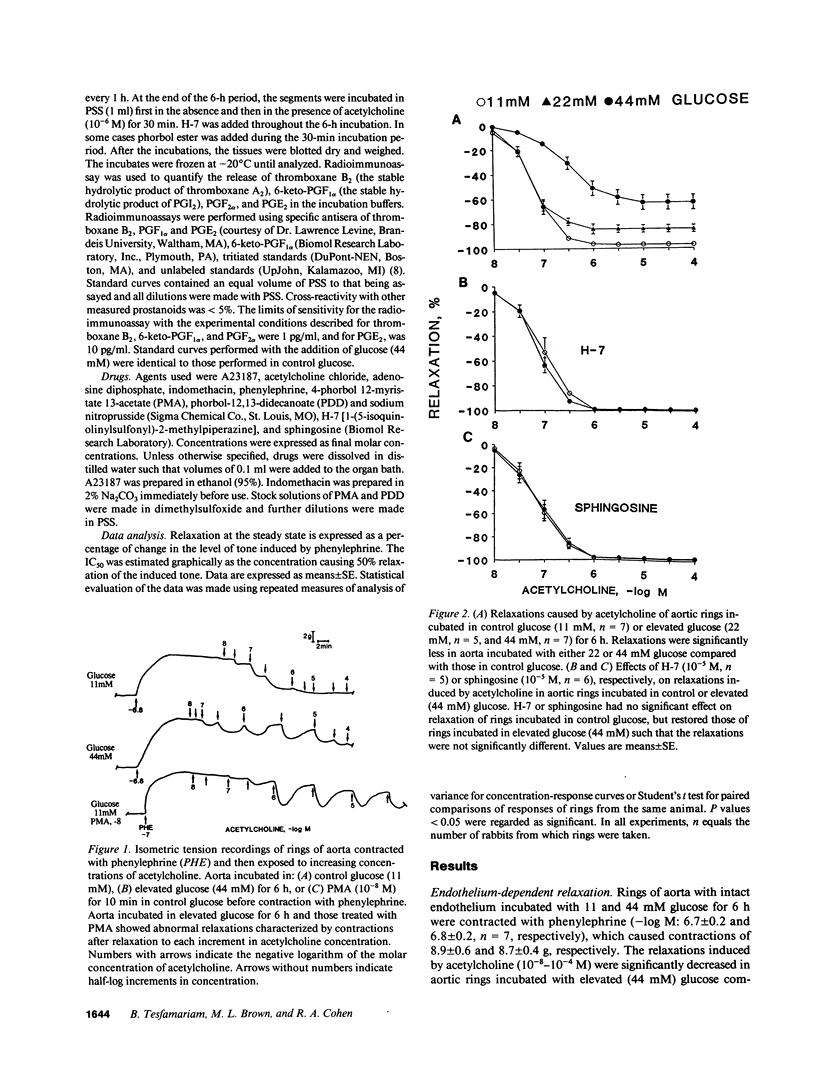
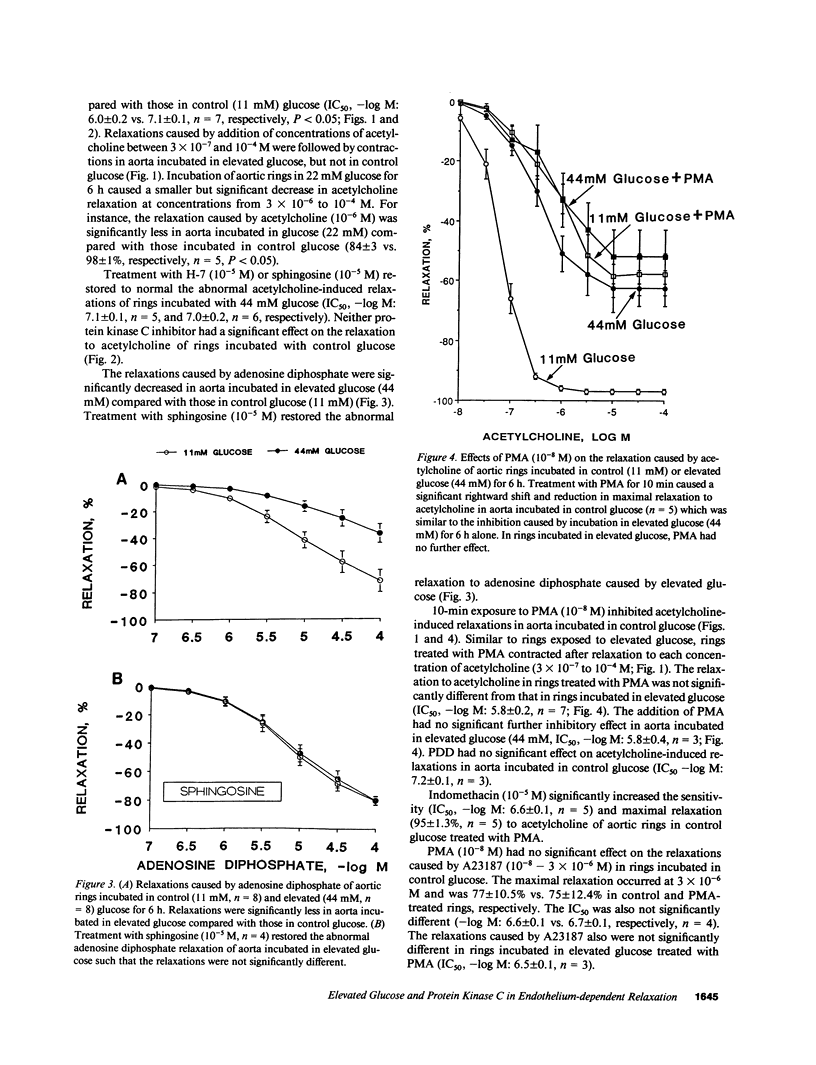
A possible relationship between protein kinase C activation and impaired receptor-mediated endothelium-dependent relaxation in diabetes mellitus was examined in isolated aorta from normal rabbit exposed to elevated glucose. Aorta treated for 10 min with 4-phorbol 12-myristate 13-acetate (PMA), a protein kinase C activator, showed decreased relaxations to the endothelium-dependent vasodilator, acetylcholine, similar to normal aorta exposed to elevated glucose (22 and 44 mM) for 6 h. Relaxations to the receptor-independent endothelium-dependent vasodilator, A23187, and those caused by the direct smooth muscle vasodilator, sodium nitroprusside, were unaffected by treatment with PMA or exposure to elevated glucose. Indomethacin increased relaxations to acetylcholine of aorta treated with PMA indicating a role for vasoconstrictor prostanoids. PMA caused a significant increase in basal and acetylcholine-stimulated release of vasoconstrictor prostanoids including thromboxane A2 from aortic segments with, but not without endothelium. Protein kinase C inhibitors, H-7 or sphingosine, restored the abnormal acetylcholine-induced relaxations as well as suppressed the abnormal release of prostanoids in aorta exposed to elevated glucose. These findings suggest that the dysfunction of receptor-mediated endothelium-dependent relaxation associated with exposure to elevated glucose is due to increased production of vasoconstrictor prostanoids by the endothelium as a consequence of protein kinase C activation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron C. B., Cunningham M., Strauss J. F., 3rd, Coburn R. F. Pharmacomechanical coupling in smooth muscle may involve phosphatidylinositol metabolism. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6899–6903. doi: 10.1073/pnas.81.21.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cherry P. D., Gillis C. N. Antagonism of acetylcholine-mediated relaxation of rabbit pulmonary arteries by phorbol myristate acetate. J Pharmacol Exp Ther. 1988 Nov;247(2):542–546. [PubMed] [Google Scholar]
- Craven P. A., Patterson M. C., DeRubertis F. R. Role for protein kinase C in the modulation of glomerular PGE2 production by angiotensin II. Biochem Biophys Res Commun. 1988 May 16;152(3):1481–1489. doi: 10.1016/s0006-291x(88)80453-4. [DOI] [PubMed] [Google Scholar]
- Demolle D., Boeynaems J. M. Role of protein kinase C in the control of vascular prostacyclin: study of phorbol esters effect in bovine aortic endothelium and smooth muscle. Prostaglandins. 1988 Feb;35(2):243–257. doi: 10.1016/0090-6980(88)90091-3. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Ishizuka T., Hoffman J., Cooper D. R., Watson J. E., Pushkin D. B., Farese R. V. Glucose-induced synthesis of diacylglycerol de novo is associated with translocation (activation) of protein kinase C in rat adipocytes. FEBS Lett. 1989 Jun 5;249(2):234–238. doi: 10.1016/0014-5793(89)80630-1. [DOI] [PubMed] [Google Scholar]
- Jakubowski J. A., Stampfer M. J., Vaillancourt R., Deykin D. Cumulative antiplatelet effect of low-dose enteric coated aspirin. Br J Haematol. 1985 Aug;60(4):635–642. doi: 10.1111/j.1365-2141.1985.tb07467.x. [DOI] [PubMed] [Google Scholar]
- Lee T. S., MacGregor L. C., Fluharty S. J., King G. L. Differential regulation of protein kinase C and (Na,K)-adenosine triphosphatase activities by elevated glucose levels in retinal capillary endothelial cells. J Clin Invest. 1989 Jan;83(1):90–94. doi: 10.1172/JCI113889. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lewis M. J., Henderson A. H. A phorbol ester inhibits the release of endothelium-derived relaxing factor. Eur J Pharmacol. 1987 Jun 4;137(2-3):167–171. doi: 10.1016/0014-2999(87)90218-4. [DOI] [PubMed] [Google Scholar]
- Meraji S., Jayakody L., Senaratne M. P., Thomson A. B., Kappagoda T. Endothelium-dependent relaxation in aorta of BB rat. Diabetes. 1987 Aug;36(8):978–981. doi: 10.2337/diab.36.8.978. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Oyama Y., Kawasaki H., Hattori Y., Kanno M. Attenuation of endothelium-dependent relaxation in aorta from diabetic rats. Eur J Pharmacol. 1986 Dec 2;132(1):75–78. doi: 10.1016/0014-2999(86)90013-0. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Kurtz A., Bauer C. Role of phospholipase C and protein kinase C in vasoconstrictor-induced prostaglandin synthesis in cultured rat renal mesangial cells. Biochem J. 1986 Feb 15;234(1):125–130. doi: 10.1042/bj2340125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Desiderio D., Luisi A., Johns A., Sybertz E. J. Phorbol dibutyrate inhibits release and action of endothelium-derived relaxing factor(s) in canine blood vessels. J Pharmacol Exp Ther. 1989 Jun;249(3):858–863. [PubMed] [Google Scholar]
- Saenz de Tejada I., Goldstein I., Azadzoi K., Krane R. J., Cohen R. A. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989 Apr 20;320(16):1025–1030. doi: 10.1056/NEJM198904203201601. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B., Brown M. L., Deykin D., Cohen R. A. Elevated glucose promotes generation of endothelium-derived vasoconstrictor prostanoids in rabbit aorta. J Clin Invest. 1990 Mar;85(3):929–932. doi: 10.1172/JCI114521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinheimer G., Wagner B., Osswald H. Interference of phorbolesters with endothelium-dependent vascular smooth muscle relaxation. Eur J Pharmacol. 1986 Nov 4;130(3):319–322. doi: 10.1016/0014-2999(86)90285-2. [DOI] [PubMed] [Google Scholar]
- Wu K. K., Hatzakis H., Lo S. S., Seong D. C., Sanduja S. K., Tai H. H. Stimulation of de novo synthesis of prostaglandin G/H synthase in human endothelial cells by phorbol ester. J Biol Chem. 1988 Dec 15;263(35):19043–19047. [PubMed] [Google Scholar]
- de Nucci G., Gryglewski R. J., Warner T. D., Vane J. R. Receptor-mediated release of endothelium-derived relaxing factor and prostacyclin from bovine aortic endothelial cells is coupled. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2334–2338. doi: 10.1073/pnas.85.7.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]



