Abstract
Serum amyloid A (SAA) is an acute-phase protein mainly associated with HDL. To study the role of SAA in mediating changes in HDL composition and metabolism during inflammation, we generated mice in which the two major acute-phase SAA isoforms, SAA1.1 and SAA2.1, were deleted [SAA knockout (SAAKO) mice], and induced an acute phase to compare lipid and apolipoprotein parameters between wild-type (WT) and SAAKO mice. Our data indicate that SAA does not affect apolipoprotein A-I (apoA-I) levels or clearance under steady-state conditions. HDL and plasma triglyceride levels following lipopolysaccharide administration, as well as the decline in liver expression of apoA-I and apoA-II, did not differ between both groups of mice. The expected size increase of WT acute-phase HDL was surprisingly also seen in SAAKO acute-phase HDL despite the absence of SAA. HDLs from both mice showed increased phospholipid and unesterified cholesterol content during the acute phase. We therefore conclude that in the mouse, SAA does not impact HDL levels, apoA-I clearance, or HDL size during the acute phase and that the increased size of acute-phase HDL in mice is associated with an increased content of surface lipids, particularly phospholipids, and not surface proteins. These data need to be transferred to humans with caution due to differences in apoA-I structure and remodeling functions.
Keywords: inflammation, acute phase, apolipoprotein A-I, serum amyloid A-deficient mice, high-density lipoprotein characterization
The study of inflammation and atherosclerosis is relevant given that the atherosclerotic process itself constitutes a chronic inflammation. Furthermore, inflammatory diseases such as rheumatoid arthritis are associated with remarkable acceleration of atherosclerosis (1). A plethora of metabolic changes affect lipids and lipoproteins during inflammation. Typically, VLDL and serum triglycerides (TGs) tend to be increased (2). Most notable are the structural and metabolic alterations of HDL (3). In practically all species, there is a significant decrease in HDL cholesterol and apolipoprotein A-1 (apoA-I) levels (2, 3). The mouse is somewhat resistant to these changes. The degree to which differences in apoA-I structure or the absence of remodeling functions such as cholesteryl ester transfer protein (CETP) is involved needs to be considered. The hallmark of acute-phase HDL (AP HDL) is its high content of serum amyloid A (SAA) protein, which, during the acute phase, can become the major apolipoprotein of HDL (3). In the mouse, SAA exists as two major acute-phase isoforms, namely, SAA1.1 and SAA2.1 (4, 5). These are hepatically produced in response to cytokine stimulation (5). Two other members of the SAA family exist, with SAA4 being a minor constitutive apolipoprotein of HDL (6) and SAA3 mostly induced in extra-hepatic tissues, but not significantly present on AP HDL (7).
The role of SAA in altered lipoprotein and particularly HDL metabolism during inflammation has been a focus for some time (8). Studies have been confounded by the fact that induction of SAA by inflammatory mediators also induces the totality of the acute-phase response, thus making it difficult to dissect out the specific role of SAA. We have generated mice in which the two major acute-phase SAA isoforms, SAA1.1 and SAA2.1, were deleted [SAA knockout (SAAKO) mice]. An acute phase was induced with lipopolysaccharide (LPS) in wild-type (WT) and SAAKO mice, and lipid and apolipoprotein parameters were compared. Data indicated that the absence of SAA does not influence plasma lipid or HDL levels during inflammation or the clearance of apoA-I under steady-state conditions. AP HDL from WT and SAAKO mice exhibited a similar increase in size, notwithstanding the absence of SAA on the latter. Data will be presented that this is probably due to the enhanced phospholipid content of the SAAKO AP HDL.
MATERIALS AND METHODS
Generation of SAAKO mice
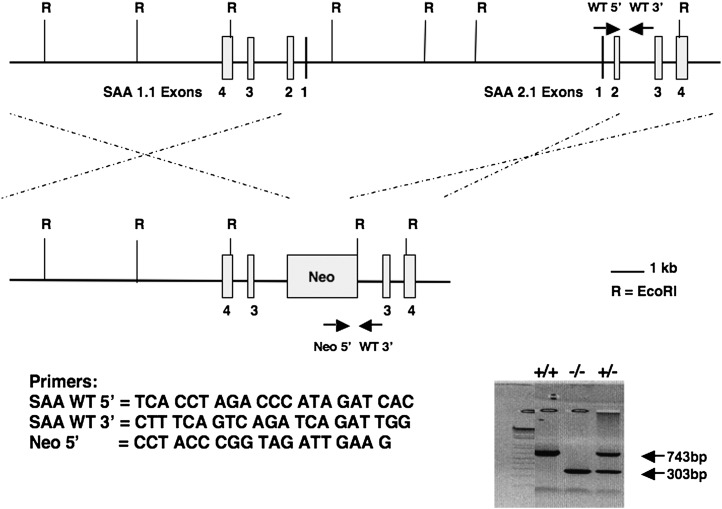
Targeted deletion of the SAA1.1 and SAA2.1 genes was performed by InGenious Targeting Laboratory, Inc. (Stony Brook, NY) using embryonic stem cells derived from C57BL/6N and 129SVEV mice. SAA1.1 and SAA2.1 are located approximately 9 kb apart in opposite orientation on chromosome 7. An approximately 21.3 kb region was used to construct the targeting vector, which contains a short homology arm extending ∼2.8 kb and a long homology arm extending ∼8.4 kb on the 5′ and 3′ sides, respectively, of a Lox/FRT-flanked neocassette. The neocassette replaced ∼10.1 kb of the SAA1.1 and SAA2.1 genes, including exons 1 and 2 of each gene. Heterozygote animals were received and bred to generate the SAA1.1/SAA2.1+/+ (WT) and SAA1.1/SAA2.1−/− (SAAKO) mice used for these studies. Chimeric mice used for experiments were 8–12 weeks of age and maintained in a pathogen-free facility under equal light-dark cycles with free access to water and food.
An acute-phase response was elicited by intraperitoneal injection of 1–6 µg LPS (Escherichia coli 0111:B4, Sigma Chemical Co.) per g body weight, as per legends. After 24 h, the mice were humanely euthanized, and livers were collected, as well as plasma for lipid analyses and preparation of HDL. Plasma lipids were measured using enzymatic kits (Wako Chemicals). All procedures were approved by the Veterans Affairs Medical Center Institutional Animal Care and Use Committee (Assurance number A3506-01).
HDL isolation, characterization, and radiolabeling
HDL (d = 1.063–1.21 g/ml) was isolated from mouse plasma by sequential density gradient ultracentrifugation (3). For a typical HDL preparation, plasma from 15–25 mice (equal numbers of male and female mice) was pooled. HDL was dialyzed against 150 mM NaCl and 0.01% EDTA, sterile filtered, and stored under argon gas at 4°C. Protein concentrations were determined by the method of Lowry et al. (9). Characterization of HDL was by SDS-PAGE (4–20% polyacrylamide SDS gels), gradient gel electrophoresis (4–20% nondenaturing polyacrylamide gels on which HDL was electrophoresed for 3.5 h at 200 V, 4°C), and agarose gel electrophoresis (10). HDL apolipoproteins were iodinated according to the method of Bilheimer, Eisenberg, and Levy (11).
The apolipoprotein composition of HDL was determined by densitometric scanning of Coomassie-stained SDS gels (4–20% polyacrylamide gradient) on which 5 µg aliquots of control or AP HDL from WT or SAAKO mice were analyzed. The combined values of all the apolipoproteins in a lane were assigned an arbitrary value of 100, and the individual apolipoproteins were expressed as a percentage of the total value.
Agarose gel electrophoresis
The electrophoretic mobility of the HDL was determined as described for LDL (10). Agarose gels (1%) were cast in 60 mM barbital buffer (pH 8.6). Sample loading was 5 µg HDL per lane, and electrophoresis was carried out for 90 min at 100 V in barbital buffer. (agarose, Biorad 162-0125; barbital buffer, Sigma B5934). Visualization was by staining with Sudan Black B.
Electrofocusing
Aliquots of 7 µl mouse plasma were subjected to electrofocusing as previously described (12), using an ampholine gradient consisting of 20% (v/v) ampholines pH 3–10, 40% (v/v) ampholines pH 4–6.5, and 40% (v/v) ampholines pH 7–9 (Pharmacia LKB Biotechnology, Inc). Electrofocused samples were subjected to immunochemical analysis as described (12).
Real-time PCR
Total RNA was isolated from mouse liver using TRIzol Reagent (Invitrogen) according to the manufacturer's protocol. RNA samples were treated with DNase I (Roche) for 30 min at 37°C and then purified with the RNeasy Mini Kit (QIAGEN). RNA (2 µg) was reverse transcribed into cDNA using a reverse transcription system (Promega). After 4-fold dilution, 5 µl was used as a template for quantitive real-time (RT)-PCR. Amplification was done for 40 cycles using Power SYBR Green PCR master Mix Kit (Applied Biosystems). Quantification was performed in duplicate using the standard curve method and normalizing to GAPDH. The primers used for various genes are as follows: mSAA, NM_009117: forward 5′-agacaaatacttccatgctcgg-3′, reverse 5′-catcactgattttctcagcagc-3′, 86 bp (specific for mouse SAA1.1 and mouse SAA2.1); mapoAI, NM_009692: forward 5′-aacagctgaacctgaatctcctgg-3′, reverse 5′-ctgcaccttctgtttcacttcctc-3′, 176 bp; mapoAII, NM_013474: forward 5′-tgtagcctggaaggagctttggtt-3′, reverse 5′-tcagctgctcgtgtgtcttctcaa-3′, 178 bp.
ApoA-I quantitation
Murine apoA-I was purified by gel filtration from delipidated mouse HDL as described (13) and used as a standard for quantitative immunoblotting (14). Briefly, aliquots of mouse plasma (3 µl of 1:10 dilution) were subjected to SDS-PAGE using a 4–20% acrylamide gel. A standard curve was obtained from the same gel using four different amounts of purified mouse apoA-I. Plasma samples and purified mouse apoA-I were transferred to a polyvinylidene fluoride membrane and immunoblotted using rabbit anti-mouse apoA-I (Biodesign International; Saco, ME). Quantitation was by densitometric scanning of Western blots (Kodak 1D; New Haven, CT).
ApoA-I turnover
ApoA-I turnover studies were performed essentially as described (15). Briefly, AP HDL was isolated from WT and SAAKO mice 24 h after injection of LPS (3 µg/g), and the apolipoproteins were radiolabeled by iodination (11). Radiolabeled AP HDL (30 µg HDL diluted in 100 µl saline; ∼3.5 × 106 cpm) was injected via the tail vein into autologous mice 24 h after injection of LPS (3 µg/g). This dose was employed to minimize animal stress during the extended experiment. Recipient mice had free access to food and water throughout the study. Blood samples (∼50 µl) were collected at the indicated time points from the retro-orbital sinus. Plasma samples, now containing radiolabeled HDL, were counted in a γ counter (Packard Cobra II auto γ), and aliquots of approximately equivalent cpm were subjected to electrophoresis in a 5–20% acrylamide SDS gel. The stained and dried gels were subjected to autoradiography to identify the radiolabeled apolipoprotein bands. ApoA-I bands were excised and counted in a γ counter. ApoA-I decay curves were generated by correcting the apoA-I-associated radioactivity for volume of plasma loaded on the gel and expressing the radioactivity at each time point as a percentage of the radioactivity determined 3 min after tracer injection.
Statistical analyses
Data presented are the mean ± SD. Statistical analyses to compare the differences between groups were carried out using one-way ANOVA with the Holm-Sidak multiple comparison test (SigmaStat 3.5, GmbH; Germany). Significance was set at *P < 0.05, **P < 0.01, and ***P < 0.001.
RESULTS
Generation of SAAKO mice
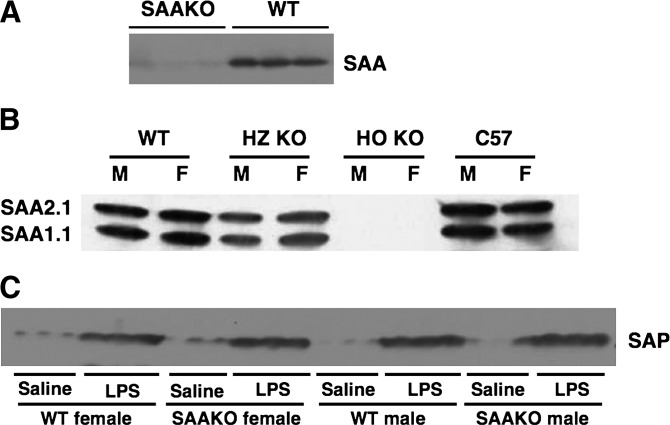
The genes encoding the murine SAA family are located on a 45 kb region of chromosome 7 (16). The two major mouse acute-phase SAAs, SAA1.1 and SAA2.1, are located in opposite orientation approximately 9.0 kb apart. The proximity of the genes was a contraindication for attempting to generate a double-knockout mouse through homologous crossover by breeding mice lacking only SAA1.1 with mice lacking only SAA2.1. A strategy was pursued to construct a single conventional targeting vector in which the neocassette replaced ∼21.3 kb of the SAA1.1 and SAA2.1 genes including exons 1 and 2 of both oppositely oriented genes (Fig. 1). This strategy proved to be successful, and mice in which the two major acute-phase SAAs, SAA1.1 and SAA2.1, were deleted (SAAKO mice) showed the expected lack of SAA in plasma after the administration of LPS (Fig. 2A). The absence of both SAA isoforms was confirmed by isoelectric focusing (Fig. 2B). Because plasma SAAs tend to plateau with high doses of LPS, a dose of 1 µg/g/body weight was employed to discriminate between heterozygous knockout mice and WT mice. The former had less of each of the two major SAA isoforms compared with the WT mice, whereas there was no significant difference in plasma SAA between WT male and female mice (Fig. 2B).
Fig. 1.
Targeting construct for generation of serum amyloid A (SAA) knockout (SAAKO) mice. The genomic organization of the SAA1.1 and SAA2.1 genes is depicted. Exons 1 and 2 of both genes were replaced with a neomycin resistance gene cassette to produce the double-deficient construct. EcoRI restriction sites are shown (R), as well as the location and direction of the PCR primers used for screening (arrows). Primer sequences and PCR banding patterns are presented (gel inset). WT, wild type.
Fig. 2.
Plasma from SAAKO mice lacks SAA. Plasma was collected from mice lacking both the SAA1.1 and the SAA2.1 genes (SAAKO) as well as from wild-type littermates (WT) 24 h after administration of lipopolysaccharide (LPS) (1 µg/g). A: Aliquots (5 µl) from three mice from each group were subjected to SDS-PAGE, followed by blotting for SAA using rabbit anti-mouse SAA antisera (De Beer laboratory). B: Aliquots (7 µl) collected from male (M) and female (F) homozygous SAA knockout mice (HO KO), heterozygous SAA knockout mice (HZ KO), and wild-type (WT) littermates were subjected to IEF, as set out in Materials and Methods, followed by blotting for SAA (rabbit anti-mouse SAA antisera; De Beer laboratory). Each lane represents plasma from a single mouse. C: Plasma was collected from WT and SAAKO mice 24 h after administration of LPS (6 µg/g), and aliquots (0.5 µl) from three mice from each group were subjected to SDS-PAGE, followed by blotting for serum amyloid P (SAP) using rabbit anti-mouse SAP antibody (gift of Dr. Mark Pepys). Each lane represents plasma from a single mouse.
The serum amyloid P (SAP) component is the classical cytokine-induced acute-phase member of the pentraxin family in the mouse, whereas C-reactive protein is the human homolog. These two molecules share ∼70% amino acid identity. As an independent measure of the induction of the acute-phase response in WT and SAAKO mice, we compared SAP in the plasma of control WT and SAAKO mice as well as inflamed mice (Fig. 2C), indicating that LPS-injected SAAKO mice express levels of SAP similar to those of inflamed WT mice.
Impact of SAA on plasma lipid levels
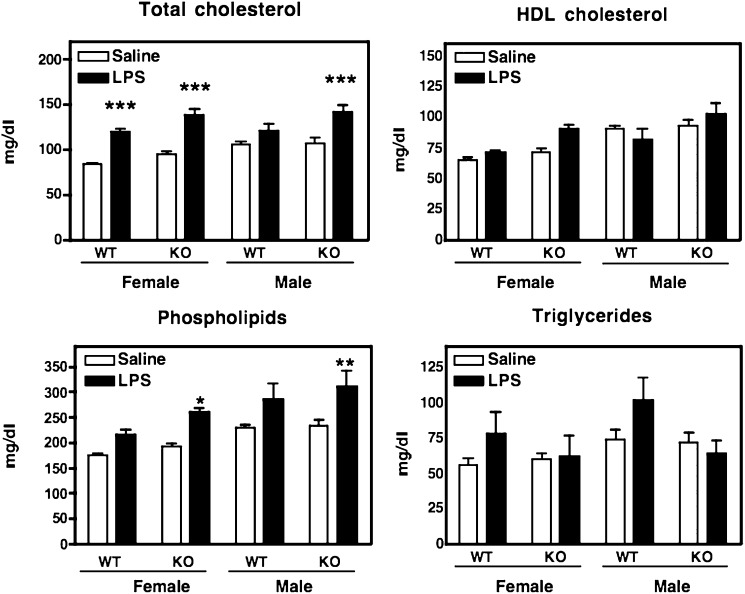
The changes in plasma lipids in WT and SAAKO mice during an acute-phase response were assessed (Fig. 3). The data were compiled from two separate experiments in which mice were injected with saline or LPS (6 µg/g). In female WT mice, LPS elicited a significant increase in plasma cholesterol consistent with previously published mouse studies (2, 17). This increase was, however, not observed in male WT mice. Interestingly, the ability of LPS to increase plasma cholesterol was not dependent on SAA, inasmuch as both male and female SAAKO mice exhibited a significant increase in plasma cholesterol levels. A tendency for HDL cholesterol levels to increase in mice injected with LPS has been reported (2, 17), and we found a similar though not significant tendency after LPS administration in all groups except WT male mice. In accordance with previous reports (2, 17), plasma TG levels tended to increase in all groups except male SAAKO mice during the acute phase, but significance was not attained. Plasma phospholipids also increased during the acute phase, reaching significance only in SAAKO mice (females P < 0.05 and males P < 0.01 compared with 0 h), but not in WT mice. In summary, LPS elicited only modest effects on plasma lipids in WT mice. The absence of SAA did not influence these lipid parameters in a meaningful way except for phospholipids, which were significantly increased in male and female SAAKO mice injected with LPS compared with LPS-treated WT mice.
Fig. 3.
Plasma lipids of SAAKO and WT mice after intraperitoneal injection of LPS. Plasma was collected from male (M) and female (F) WT and SAAKO mice 24 h after intraperitoneal administration of saline or 6 µg/g LPS for the determination of plasma lipid concentrations. Values represent the mean ± SEM. * P < 0.05; ** P < 0.01; *** P < 0.001, saline versus LPS for each group (male WT and SAAKO, female WT and SAAKO); n = 10–21.
Impact of SAA on hepatic apoA-I expression and apoA-I plasma levels
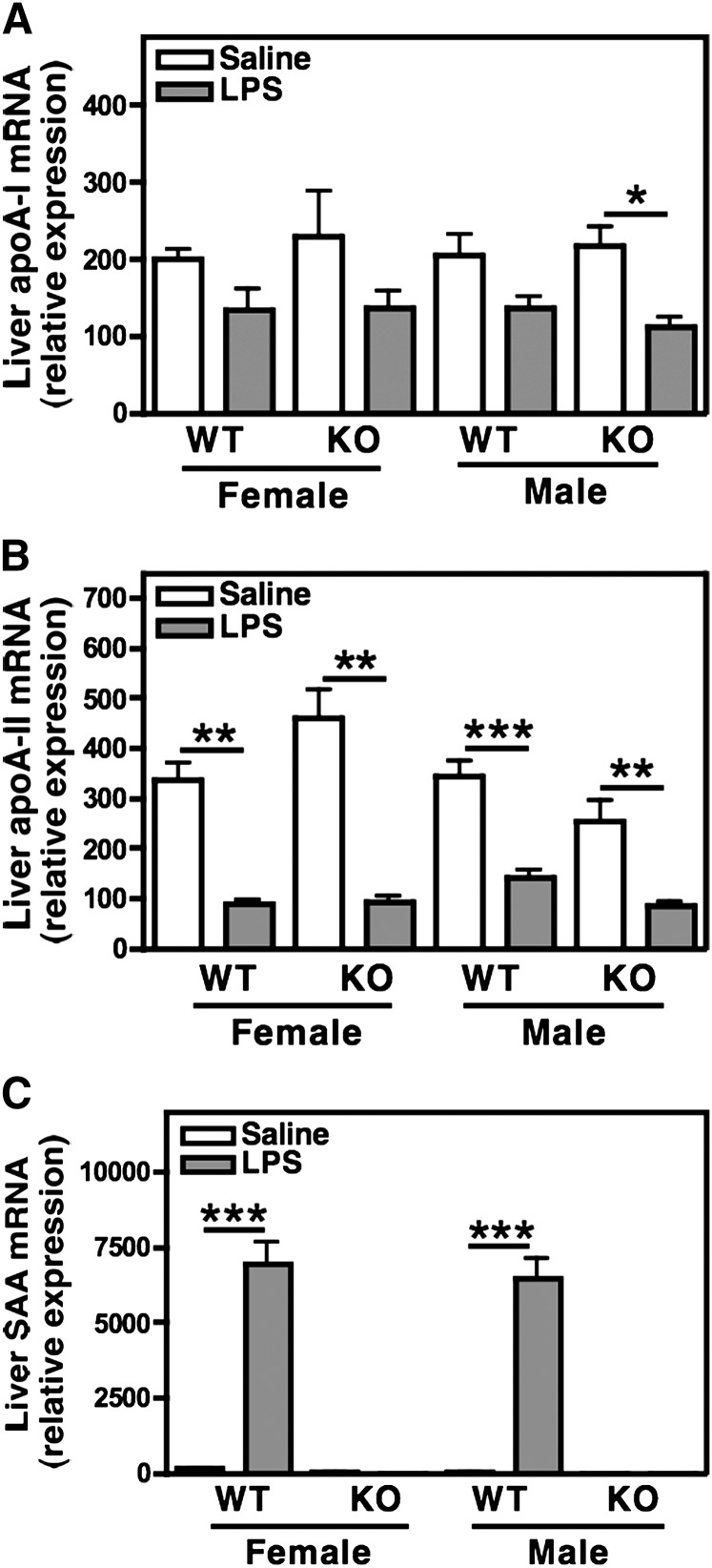
Because the liver is the main site of apolipoprotein synthesis, we examined the hepatic expression of the major HDL apolipoproteins, apoA-I, apoA-II, and SAA by quantitative RT-PCR. Hepatic apoA-II expression declined significantly during the acute phase (2). ApoA-I expression declined and just reached significance in SAAKO males (P < 0.05). There was no difference in the expression of these apolipoproteins between WT and SAAKO mice (Fig. 4A, B). Hepatic SAA expression was also investigated by quantitative RT-PCR using primers that recognized both murine SAA1.1 and SAA2.1. SAA mRNA was virtually undetectable in the livers of control WT mice but increased dramatically following LPS administration, in keeping with SAA being a major acute-phase reactant. As expected, SAA mRNA could not be detected in SAAKO mice (Fig. 4C).
Fig. 4.
Hepatic expression of apolipoprotein A-I (apoA-I), apoA-II, and SAA during the acute phase. Hepatic RNA was isolated from male and female SAAKO and WT mice 24 h after induction of an acute-phase response by LPS injection (6 µg/g). ApoA-I (A), apoA-II (B), and SAA (C) mRNA. Quantitive real-time PCR was performed using gene-specific primers as set out in Materials and Methods, and data were normalized to GAPDH. Values represent the mean ± SEM; n = 5. * P < 0.05; ** P < 0.01; *** P < 0.001, saline versus LPS.
Plasma apoA-I concentrations are presented in Table 1. Saline-injected female mice tended to have lower apoA-I levels compared with male mice. ApoA-I levels were not significantly altered by LPS treatment in female mice whether WT or SAAKO. In contrast, LPS treatment significantly lowered apoA-I levels in male mice, but this effect was not dependent on SAA.
TABLE 1.
Plasma apoA-1 concentrations
| ApoA-I mg/ml | ||
|---|---|---|
| Saline | LPS | |
| WT M | 1.61 ± 0.26 (n = 11) | 1.38 ± 0.23* (n = 11) |
| WT F | 1.49 ± 0.35 (n = 10) | 1.35 ± 0.30 (n = 10) |
| KO M | 1.74 ± 0.32 (n = 9) | 1.46 ± 0.29* (n = 9) |
| KO F | 1.39 ± 0.52 (n = 9) | 1.38 ± 0.47 (n = 9) |
apoA-I, apolipoprotein A-I; LPS, lipopolysaccharide; M, male; F, female; WT, wild type; KO, knockout. * P < 0.05.
Characterization of WT and SAAKO HDL
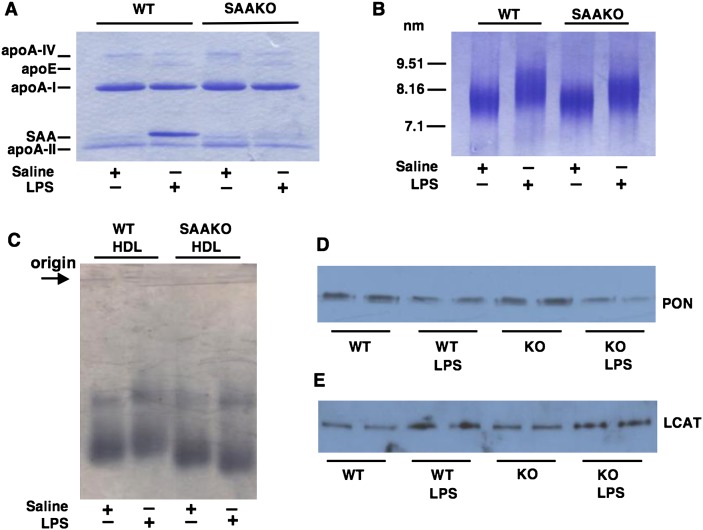
To investigate HDL apolipoprotein composition, HDL (d 1.063–1.21 g/ml) was isolated by sequential ultracentrifugation from the plasma of SAAKO and WT mice 24 h after the injection of saline (normal HDL) or 6 µg/g LPS (AP HDL) (Fig. 5A). HDL apolipoprotein composition was assessed by densitometric scanning of Coomassie-stained gels as set out in MATERIALS AND METHODS. Whereas SAA constituted approximately 37% of protein in WT AP HDL, the AP HDL from SAAKO mice had, as expected, no SAA. The increased SAA content of AP HDL from WT mice was offset by a decrease in apoA-I content (69% vs. 45%; N HDL vs. AP HDL) and apoA-II content (21% vs. 13%; N HDL vs. AP HDL). No significant differences were observed in the apoA-I content of N HDL and AP HDL of SAAKO mice (66% vs. 68%, respectively), or in the apoA-II content (21% vs. 19%, respectively). AP HDL from both WT and SAAKO mice contained more apoE and less apoA-IV than N HDL (Fig. 5A).
Fig. 5.
Characterization of HDL. HDL (d 1.063–1.21 g/ml) was isolated by sequential ultracentrifugation from the plasma of 15–20 SAAKO or WT mice 24 h after the intraperitoneal injection of saline or LPS (6 µg/g). A: HDL apolipoproteins were separated by SDS-PAGE on a 4–20% acrylamide gel. B: HDL size was compared by nondenaturing gel electrophoresis using a 4–20% acrylamide gel. Visualization for both gels was by Coomassie staining, and loading was 5 µg HDL/lane. C: The relative electrophoretic mobility of HDL was determined by agarose gel electrophoresis as described in Materials and Methods. D: The paraoxonase (PON) content of HDL was determined by Western blot analysis of equal quantities of HDL protein using a rabbit anti-rat PON antibody (abcam ab40775). E: The LCAT content of HDL was determined by Western blot analysis of equal quantities of HDL protein using a rabbit anti-human LCAT antibody (abcam ab786). Loading was 10 µg HDL/lane.
HDLs isolated by ultracentrifugation from WT and SAAKO mice were also analyzed by nondenaturing gradient gel electrophoresis (Fig. 5B). No apparent difference in the size of HDLs from untreated WT and SAAKO mice was observed. However, as shown previously for SAA-containing human HDL (3), AP HDL from WT mice migrated as larger-sized particles compared with N HDL from WT mice. Surprisingly, AP HDL from SAAKO mice also appeared larger, despite the lack of SAA. The slower migration of AP HDL from SAAKO mice was not due to charge, inasmuch as this HDL migrated somewhat faster than N HDL from SAAKO mice on agarose gels (Fig. 5C).
We additionally investigated the impact of LPS on minor HDL apolipoproteins in WT and SAAKO mice. Western blot analysis confirmed the known acute-phase decline in paraoxonase (PON) (Fig. 5D). LCAT was enriched in AP HDL (Fig. 5E). However, no significant differences with respect to these minor HDL apolipoproteins were noted between WT and SAAKO HDLs.
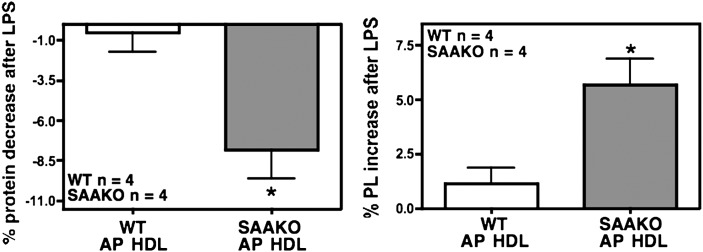
We next compared the protein and lipid content of N HDL and AP HDL from WT as well as SAAKO mice isolated from mice 24 h after injection of saline or LPS (6 µg/g) (Table 2 and Fig. 5A). In SAAKO mice, there was a significant decrease in the protein content of HDL during the acute phase. WT and SAAKO HDL exhibited changes in core lipid during inflammation. TG increased, which was significant for SAAKO HDL. Both HDLs showed similar reductions in core cholesteryl ester (CE) that reached significance in WT HDL. The major lipid components of the HDL surface, phospholipid and free cholesterol, increased in response to LPS treatment in both WT and SAAKO mice. FC increase was significant in both types of HDL, but PL increase was significant only in SAAKO HDL. In summary, the acute-phase response brings about alterations in HDL composition (decreased protein and CE content; increased FC, TG, and PL content) in both WT and SAAKO mice. The analyses of multiple HDL preparations indicate the effect of LPS to be a decrease in protein and an increase in PL content of HDL that is significantly more pronounced in SAAKO mice compared with WT mice (Fig. 6).
TABLE 2.
Composition of N and AP HDL from WT and SAAKO mice
| WT | WT + LPS | KO | KO + LPS | |
|---|---|---|---|---|
| % | % | % | % | |
| Prot | 46.5 ± 0.81 | 46.0 ± 0.44 | 46.87 ± 1.60 | 39.9 ± 0.54** |
| FC | 3.5 ± 0.17 | 5.6 ± 0.28*** | 3.2 ± 0.11 | 6.8 ± 0.21*** |
| CE | 16.0 ± 1.04 | 12.9 ± 0.60* | 16.9 ± 0.28 | 13.5 ± 1.41 |
| TG | 1.2 ± 0.31 | 1.5 ± 0.11 | 1.0 ± 0.15 | 1.9 ± 0.19* |
| PL | 32.9 ± 0.71 | 34.0 ± 0.73 | 32.3 ± 1.64 | 38.0 ± 1.24* |
HDL was isolated from plasma obtained 24 h after injection of WT and serum amyloid A knockout (SAAKO) mice with LPS (6 μg/g). Control mice received no LPS. Protein (Prot) concentration was determined by the method of Lowry. Total cholesterol (TC), free cholesterol (FC), phospholipids (PL), and triglycerides (TG) were determined enzymatically using commercial kits (WAKO Chemicals, Richmond,VA). Cholesteryl ester (CE) was calculated as the difference between TC and FC. Values represent the mean ± SEM of four HDL preparations for each permutation. AP HDL, acute-phase HDL. * P < 0.05; ** P < 0.01; *** P < 0.001, control versus LPS.
Fig. 6.
The absence of SAA significantly accentuates the HDL protein decrease and HDL phospholipid increase during the acute phase. Various batches of HDL (d 1.063–1.21 g/ml) were isolated by sequential ultracentrifugation from SAAKO or WT mice 24 h after the intraperitoneal injection of saline or LPS (6 µg/g body weight) for compositional analyses of N and acute-phase HDL (AP HDL). HDL protein was measured by the method of Lowry et al. (9), and HDL phospholipids were enzymatically determined with a commercial kit. WT HDL, n = 4; SAAKO HDL, n = 4; * P < 0.05. Values represent mean ± SEM.
Impact of SAA on apoA-I clearance
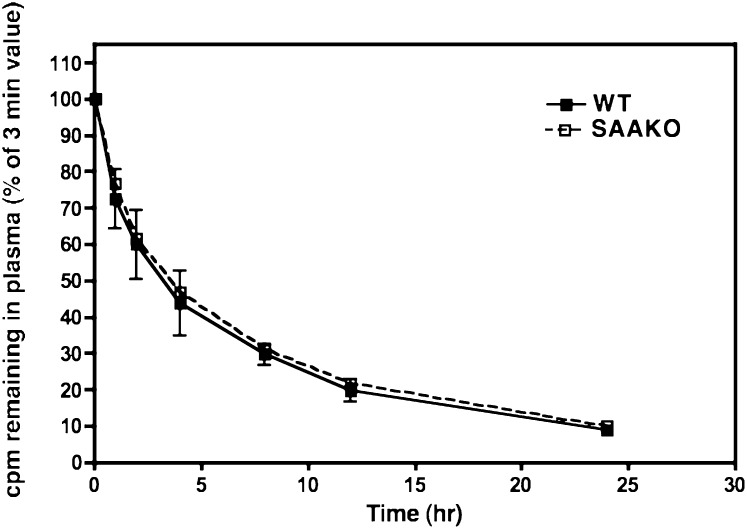
To determine whether the presence of SAA influences the clearance of apoA-I, apoA-I clearance experiments were performed essentially as described (15). In these experiments, radiolabeled autologous AP HDLs were injected into WT and SAAKO mice 24 h after the LPS induction of an acute-phase response. The period from 24 h to 48 h after LPS administration provides a reasonable steady-state condition in which SAA concentration is relatively stable. Die-away curves of total plasma radioactivity were similar for WT and SAAKO mice (data not shown). Plasma decay curves of apoA-I (Fig. 7) indicated no difference in the disappearance of apoA-I from AP HDL, irrespective of the presence of SAA. We thus conclude that SAA does not affect the clearance of apoA-I under steady-state inflammatory conditions.
Fig. 7.
SAA does not affect the plasma clearance of apoA-I under steady-state inflammatory conditions. 125I-labeled autologous AP HDL (30 µg) was administered intravenously to WT and SAAKO mice 24 h after injection of LPS (3 µg/g). Blood samples were collected at selected times up to 24 h. Plasma samples were processed as set out in Materials and Methods, and the data were plotted with GraphPad Prism 4. The y axis represents apoA-I radioactivity isolated by gel electrophoresis. Values (mean and SD) are expressed as a percentage of the first time point collected at 3 min. SAAKO, n = 5; WT, n = 5.
DISCUSSION
We explored the role that acute-phase SAA could play in the altered lipoprotein profiles characteristic of inflammation (2, 3, 17). Studies in the past were difficult to interpret, because inflammatory mediators impact lipoprotein metabolism in a number of ways distinct from SAA induction. We therefore developed an SAAKO mouse in which the two major acute-phase isoforms (SAA1.1 and SAA2.1) were deleted. Data presented here using SAAKO mice show that the absence of SAA during the acute phase does not impact HDL size, HDL levels, hepatic apoA-I expression, plasma apoA-I levels, or the clearance rate of apoA-I. Changes during the acute phase are probably due to cytokines affecting synthesis and enzymes/receptors interacting with HDL, rather than SAA per se. However, SAAKO HDLs exhibit a significantly increased PL and decreased protein content. The increased surface lipid components, particularly PL, may compensate for the decreased protein content found in SAAKO AP HDL.
The SAA proteins are a polymorphic family of apolipoproteins associated with HDL (4). Three subtypes can be identified. We have deleted the two cytokine-induced major acute-phase isoforms that are hepatically produced and can increase 1,000-fold to become the major apolipoprotein on HDL (3, 12). These two isotypes differ in only 9 of 103 amino acid residues. SAA1.1 seems to be metabolically the most dynamic, inasmuch as it is more rapidly cleared than SAA2.1. SAA3 is produced by extrahepatic cells, mostly macrophages and adipocytes, and is present in the plasma at very low concentrations unlikely to impact HDL remodeling (7). SAA4 is a minor constitutive HDL apolipoprotein not induced by cytokines and is probably not relevant in the inflammatory state (6).
The total absence of both major acute-phase SAA isoforms in the plasma was confirmed by immunoblotting and isoelectric focusing (Fig. 2A, B). Analysis of AP HDL isolated from SAAKO and WT mice showed the expected changes in apolipoprotein content of the HDLs. AP HDL from SAAKO mice lacked SAA, and the SAA present on AP HDL from WT mice was offset by a reduction in apoA-I content, whereas the apoA-II content did not vary significantly between the genotypes (Fig. 5A). It is notable that hepatic apoA-II mRNA is significantly reduced by inflammation with this gene seemingly more responsive to cytokines than is the gene encoding apoA-I. The apoA-I mRNA decline in WT and SAAKO males and females is similar. However, the decline in apoA-I protein is more noted in males than in females. This could be due to gender differences in HDL catabolism. In both WT and SAAKO HDL, similar decreases in apoA-IV and increases in apoE content were evident. The increase in apoE is intriguing in that it was reported that even though mRNA for this apolipoprotein is significantly decreased by LPS, more protein is present in the plasma (18). It is clear that this increase is not impacted by the absence of SAA. LPS treatment resulted in similar increases in total plasma cholesterol levels in WT and SAAKO mice. An increase in plasma cholesterol during the acute phase has been reported by others and is probably based on the fact that LPS stimulates hepatic cholesterol synthesis with modest increases in LDL and VLDL (19). Analyses of the composition of the HDL, shown in Table 2, and of the plasma lipids, provided in Fig. 3, indicated that with LPS, non-HDL phospholipids increased similarly in WT and SAAKO mice. This is partially due to a modest but similar increase in LDL induced by LPS, as others have shown (20). In addition, we have investigated the protein:PL ratio in LDL and VLDL from WT and SAAKO mice from a number of ultracentrifugal preparations. A consistent decline in this ratio in both LDL and VLDL, similar in WT and SAAKO mice, was noted. The increase in PL content of lipoproteins during the acute phase therefore applies to a broader spectrum of particles than just HDL. TG levels in WT mice showed a tendency to increase with LPS without reaching significance. SAAKO mice seemed less responsive than WT mice. Measurement of plasma TG in individual mice prior to and after LPS (6 µg/g) administration again indicated an overall tendency to increase during the acute phase, seemingly more pronounced in WT than in SAAKO mice, without reaching significance in any group. This phenomenon merits further study with larger numbers of mice. It is notable that the most profound decreases in HDL cholesterol and apoA-I occur in species that also display remarkable increases in TG levels during inflammation (17). The C57BL/6 mouse is relatively resistant to these changes (17). Plasma apoA-I levels declined significantly in male mice after LPS administration but not in females (Table 1). HDL cholesterol levels were similar in WT and SAAKO mice and did not decline with LPS administration, in keeping with similar findings in mice by others (17), and is in distinct contrast to the profound declines in apoA-I and HDL cholesterol associated with inflammation that occur in species such as rabbits and humans (3, 17). It is important to consider the extent to which mouse and human HDL differ to explain the relative resistance to changes in HDL and TG levels in mice. It is reasonable to presume that during inflammation, HDL remodeling is altered by a number of factors in addition to SAA that can impact both the core and the surface of the particle (21). Such factors include secretory phospholipase A2-IIA (22) or CETP (23), both absent in the mouse. They have been shown to have the capacity to remodel the particle so that apoA-I moves to a lipid-poor form (14). Further, the structure and conformation of mouse and human apoA-I differ. Whereas our data suggest that mouse SAA does not readily impact mouse apoA-1, the situation in humans could be different. SAA also does not seem to impact the presence of either PON or LCAT on HDL. It is notable that whereas LCAT declines commensurately with HDL levels in species responsive to LPS, in the mouse, where HDL levels are maintained, LCAT is even slightly increased on HDL.
Some substrains of C57BL/6 mice like C57BL/10ScCr and C57BL/6JKB2-mnd (24) have mutations in TLR4, which results in blunted responses to LPS. The mice in our experiments are C57BL/6N and 129SVEV chimeras that did not harbor such mutations.
The SAAKO mouse allowed for studies to evaluate the impact of SAA on apoA-I clearance during inflammation under steady-state conditions. Initial in vitro data suggested that the more-lipophilic SAA could readily displace apoA-I from HDL and was proposed as a mechanism that reduced apoA-I levels during inflammation (3). Subsequent studies utilizing adenoviral vector overexpression of SAA in the absence of an acute-phase response indicated that apoA-I levels were not influenced by SAA (25). It was, however, possible that liberated apoA-I could rapidly reaccumulate cholesterol and maintain HDL levels. Our data definitely show that, at least in the mouse, the presence of SAA does not impact apoA-I clearance. In human or other species that have CETP and sPLA2-IIA, the situation could be different, inasmuch as they could destabilize HDL with subsequent displacement of apoA-I by SAAs.
Previous studies showed that SAA-bearing acute-phase human HDL is larger than normal HDL. The presence of SAA on HDL may increase the width of the HDL shell (3). This increase in size was thought to be due to the hydrophilic C-terminal region of SAA extending from the HDL surface (26). We were surprised to note that the SAAKO AP HDL, despite the lack of SAA, migrated as larger particles on nondenaturing gradient gels run to equilibrium, similar in size to AP HDL from WT mice (Fig. 5B). HDL was enriched in surface components (FC and PL) during the acute phase, which was more pronounced for SAAKO HDL (Table 2). On the other hand, the core CE decreased during the acute phase and was not compensated for by the small TG increase. We therefore speculate that such changes in lipid composition during the acute phase could render oval-shaped particles that would migrate with a larger Stokes radius in a nondenaturing gel. Alternatively, the proteins could be rearranged on the surface of the HDL so as to migrate as an apparently larger particle. It is also feasible that conformational changes in the shape of the HDL surface, e.g., bulging or blebbing, could accommodate an increase in surface FC and PL without a significant increase in core content. The marginal increase in phospholipid content of AP HDL in WT mice was more pronounced in the SAAKO mice. Likewise, following LPS administration, the protein content of AP HDL declined to a greater extent in SAAKO mice than in WT mice. The decrease in protein content of AP HDL appeared to be matched by a commensurate increase in AP HDL phospholipids. These changes in HDL composition differ from those in human acute-phase HDL, which is both larger and more dense than normal HDL (3). The density was thought to result from increased SAA content of particles, with SAA increasing the Stokes radius of the particle to increase the apparent size.
In the human context, the influence of sPLA2-IIA, absent in certain mouse strains, also needs to be considered. This major acute-phase enzyme acts preferentially on HDL, resulting in more dense, relatively phospholipid-depleted particles (21). We suggest that any increase in phospholipid content of AP HDL in humans, putatively mediated by LPS, may be obscured by sPLA2-IIA actions. The presence of SAA on HDL may also limit the extent to which phospholipids can accumulate in the HDL shell. Thus, in the absence of SAA, the shell may be altered, in that the phospholipid content is increased.
The literature has suggested that SAA might directly impact lipid and apolipoprotein metabolism during inflammation. The centrality of HDL in lipid metabolism and the drastic remodeling of this particle during inflammation give credence to this. Data presented here using the SAAKO mouse provide little evidence for a direct effect of SAA on HDL levels or HDL catabolism following LPS administration, although SAAKO and WT AP HDL showed significant compositional differences, particularly with respect to PL and protein content.
Footnotes
Abbreviations:
- AP HDL
- acute-phase HDL
- apoA-I
- apolipoprotein A-I
- CETP
- cholesteryl ester transfer protein
- LPS
- lipopolysaccharide
- PON
- paraoxonase
- SAA
- serum amyloid A
- SAP
- serum amyloid P
- WT
- wild type
This work was supported by National Institutes of Health Grants PO1HL-086670 and P20 RR-021954 and VA Merit Review (FCdB). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Chung C. P., Oeser A., Raggi P., Gebretsadik T., Shintani A. K., Sokka T., Pincus T., Avalos I., Stein C. M. 2005. Increased coronary-artery atherosclerosis in rheumatoid arthritis: relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 52: 3045–3053. [DOI] [PubMed] [Google Scholar]
- 2.Khovidhunkit W., Kim M. S., Memon R. A., Shigenaga J. K., Moser A. H., Feingold K. R., Grunfeld C. 2004. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J. Lipid Res. 45: 1169–1196. [DOI] [PubMed] [Google Scholar]
- 3.Coetzee G. A., Strachan A. F., van der Westhuyzen D. R., Hoppe H. C., Jeenah M. S., de Beer F. C. 1986. Serum amyloid A-containing human high density lipoprotein 3. Density, size, and apolipoprotein composition. J. Biol. Chem. 261: 9644–9651. [PubMed] [Google Scholar]
- 4.de Beer M. C., de Beer F. C., Gerardot C. J., Cecil D. R., Webb N. R., Goodson M. L., Kindy M. S. 1996. Structure of the mouse Saa4 gene and its linkage to the serum amyloid A gene family. Genomics. 34: 139–142. [DOI] [PubMed] [Google Scholar]
- 5.Sipe J. D. 2000. Serum amyloid A: from fibril to function. Current status. Amyloid. 7: 10–12. [DOI] [PubMed] [Google Scholar]
- 6.Whitehead A. S., de Beer M. C., Steel D. M., Rits M., Lelias J. M., Lane W. S., de Beer F. C. 1992. Identification of novel members of the serum amyloid A protein superfamily as constitutive apolipoproteins of high density lipoprotein. J. Biol. Chem. 267: 3862–3867. [PubMed] [Google Scholar]
- 7.Chiba T., Han C. Y., Vaisar T., Shimokado K., Kargi A., Chen M. H., Wang S., McDonald T. O., O'Brien K. D., Heinecke J. W., et al. 2009. Serum amyloid A3 does not contribute to circulating SAA levels. J. Lipid Res. 50: 1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Westhuyzen D. R., de Beer F. C., Webb N. R. 2007. HDL cholesterol transport during inflammation. Curr. Opin. Lipidol. 18: 147–151. [DOI] [PubMed] [Google Scholar]
- 9.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275. [PubMed] [Google Scholar]
- 10.Gowri M. S., Van der Westhuyzen D. R., Bridges S. R., Anderson J. W. 1999. Decreased protection by HDL from poorly controlled type 2 diabetic subjects against LDL oxidation may be due to the abnormal composition of HDL. Arterioscler. Thromb. Vasc. Biol. 19: 2226–2233. [DOI] [PubMed] [Google Scholar]
- 11.Bilheimer D. W., Eisenberg S., Levy R. I. 1972. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim. Biophys. Acta. 260: 212–221. [DOI] [PubMed] [Google Scholar]
- 12.de Beer M. C., de Beer F. C., McCubbin W. D., Kay C. M., Kindy M. S. 1993. Structural prerequisites for serum amyloid A fibril formation. J. Biol. Chem. 268: 20606–20612. [PubMed] [Google Scholar]
- 13.Strachan A. F., Shephard E. G., Bellstedt D. U., Coetzee G. A., van der Westhuyzen D. R., de Beer F. C. 1989. Human serum amyloid A protein. Behaviour in aqueous and urea-containing solutions and antibody production. Biochem. J. 263: 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahangiri A., de Beer M. C., Noffsinger V., Tannock L. R., Ramaiah C., Webb N. R., van der Westhuyzen D. R., de Beer F. C. 2009. HDL remodeling during the acute phase response. Arterioscler. Thromb. Vasc. Biol. 29: 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rashid S., Trinh D. K., Uffelman K. D., Cohn J. S., Rader D. J., Lewis G. F. 2003. Expression of human hepatic lipase in the rabbit model preferentially enhances the clearance of triglyceride-enriched versus native high-density lipoprotein apolipoprotein A-I. Circulation. 107: 3066–3072. [DOI] [PubMed] [Google Scholar]
- 16.Butler A., Whitehead A. S. 1996. Mapping of the mouse serum amyloid A gene cluster by long-range polymerase chain reaction. Immunogenetics. 44: 468–474. [DOI] [PubMed] [Google Scholar]
- 17.Cabana V. G., Lukens J. R., Rice K. S., Hawkins T. J., Getz G. S. 1996. HDL content and composition in acute phase response in three species: triglyceride enrichment of HDL a factor in its decrease. J. Lipid Res. 37: 2662–2674. [PubMed] [Google Scholar]
- 18.Li L., Thompson P. A., Kitchens R. L. 2008. Infection induces a positive acute phase apolipoprotein E response from a negative acute phase gene: role of hepatic LDL receptors. J. Lipid Res. 49: 1782–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feingold K. R., Hardardottir I., Memon R., Krul E. J., Moser A. H., Taylor J. M., Grunfeld C. 1993. Effect of endotoxin on cholesterol biosynthesis and distribution in serum lipoproteins in Syrian hamsters. J. Lipid Res. 34: 2147–2158. [PubMed] [Google Scholar]
- 20.McGillicuddy F. C., de la Llera Moya M., Hinkle C. C., Joshi M. R., Chiquoine E. H., Billheimer J. T., Rothblat G. H., Reilly M. P. 2009. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 119: 1135–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Beer F. C., de Beer M. C., van der Westhuyzen D. R., Castellani L. W., Lusis A. J., Swanson M. E., Grass D. S. 1997. Secretory non-pancreatic phospholipase A2: influence on lipoprotein metabolism. J. Lipid Res. 38: 2232–2239. [PubMed] [Google Scholar]
- 22.Kennedy B. P., Payette P., Mudgett J., Vadas P., Pruzanski W., Kwan M., Tang C., Rancourt D. E., Cromlish W. A. 1995. A natural disruption of the secretory group II phospholipase A2 gene in inbred mouse strains. J. Biol. Chem. 270: 22378–22385. [DOI] [PubMed] [Google Scholar]
- 23.Hayek T., Chajek-Shaul T., Walsh A., Agellon L. B., Moulin P., Tall A. R., Breslow J. L. 1992. An interaction between the human cholesteryl ester transfer protein (CETP) and apolipoprotein A-I genes in transgenic mice results in a profound CETP-mediated depression of high density lipoprotein cholesterol levels. J. Clin. Invest. 90: 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bihl F., Lariviere L., Qureshi S. T., Flaherty L., Malo D. 2001. LPS-hyporesponsiveness of mnd mice is associated with a mutation in Toll-like receptor 4. Genes Immun. 2: 56–59. [DOI] [PubMed] [Google Scholar]
- 25.Hosoai H., Webb N. R., Glick J. M., Tietge U. J., Purdom M. S., de Beer F. C., Rader D. J. 1999. Expression of serum amyloid A protein in the absence of the acute phase response does not reduce HDL cholesterol or apoA-I levels in human apoA-I transgenic mice. J. Lipid Res. 40: 648–653. [PubMed] [Google Scholar]
- 26.Turnell W., Sarra R., Glover I. D., Baum J. O., Caspi D., Baltz M. L., Pepys M. B. 1986. Secondary structure prediction of human SAA1. Presumptive identification of calcium and lipid binding sites. Mol. Biol. Med. 3: 387–407. [PubMed] [Google Scholar]