Abstract
GIP (glucose-dependent insulinotropic polypeptide) is a gastrointestinal hormone that regulates pancreatic islet function. Additionally, emerging evidence suggests an important physiological role for GIP in the regulation of adipocyte metabolism. In previous studies on the lipogenic effects of GIP, it was shown to increase adipocyte lipoprotein lipase (LPL) activity in both differentiated 3T3-L1 cells and human adipocytes through a pathway involving activation of protein kinase B (PKB)/Akt. In the current study, we examined the effects of GIP on LPL gene expression. GIP in the presence of insulin increased LPL gene expression in human adipocytes and LPL promoter activity in GIP receptor-expressing HEK-293 cells, and both effects were greatly reduced by the transcription inhibitor actinomycin D. Subsequent studies established that GIP increased phosphorylation of Serine 133 in cAMP-response element binding protein (CREB) and the nuclear localization of cAMP-responsive CREB coactivator 2 (TORC2) through a pathway involving phosphatidylinositol 3-kinase (PI3-K), PKB, and AMP-activated protein kinase (AMPK). However, in the presence of insulin, GIP failed to activate the cAMP/PKA pathway. Knockdown of CREB and TORC2 using RNA interference reduced LPL expression, supporting a functional regulatory role. GIP-induced phospho-CREB and TORC2 were shown to bind to a cAMP-response element (-II) site in the human LPL promoter and GIP increased protein-protein interactions of these two factors. The lipogenic effects of GIP in the presence of insulin are therefore at least partially mediated by upregulation of adipocyte LPL gene transcription through a pathway involving PI3-K/PKB/AMPK-dependent CREB/TORC2 activation.
Keywords: adenosine 3′,5′-cyclic monophosphate; glucose-dependent insulinotropic polypeptide; cAMP-response element binding protein; lipoprotein lipase; cAMP-responsive CREB coactivator 2
Glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) constitute the hormonal arm of the enteroinsular axis that conveys insulinotropic signals from the gut to pancreatic β-cells during a meal. It has been estimated that the combined effects of these incretin hormones contribute >60% of the total insulin secreted. Analogs of GIP (1, 2) and GLP-1 (3) have been shown to improve glucose homeostasis in animal models of diabetes and the long-acting incretin analogs (mimetics) exendin-4 (Exenatide) and liraglutide (1, 3–5), as well as highly selective inhibitors of the incretin-degrading enzyme dipeptidyl peptidase-IV (6–8), have been recently introduced as therapeutics. Because both GIP and GLP-1 also influence β-cell mass (2, 9–15), as well as various gastrointestinal and adipose tissue functions (2, 10), incretin-based drugs may demonstrate additional effects.
Although there has been great progress in understanding the mechanisms involved in regulating adipocyte metabolism (16–18), the roles of gastrointestinal hormones have not been extensively studied. GIP is released in response to triglyceride (TG) administration (19, 20) and there is strong evidence supporting a role for GIP in TG disposal in vivo. GIP promoted chylomicron-associated TG clearance in dogs (21) and lowered plasma TG responses to intraduodenal fat in rats (22), whereas immunoneutralization of endogenous GIP resulted in greater plasma TG levels following a fat load (22). In contrast, neither exogenous (23) nor endogenous (24) GIP influenced the removal of intravenously administered TGs alone, showing that chylomicron transport is necessary. Because GIP normally releases insulin during a meal and insulin is a major hormonal regulator of lipogenesis, part of the actions of GIP on fat metabolism in vivo is probably indirectly mediated via its β-cell action. However, a number of studies have shown that GIP exerts direct adipocyte actions (2, 25–28), including enhancement of lipoprotein lipase (LPL) activity (27–30). It was previously demonstrated that GIP in the presence of insulin increased the levels of LPL enzyme activity and TG accumulation in differentiated 3T3-L1 adipocytes and human subcutaneous adipocytes through a pathway involving increased phosphorylation of protein kinase B (PKB) and decreased LKB1 and AMP-activated protein kinase (AMPK) phosphorylation (27). However, it was unclear as to whether increases in LPL activity resulted from GIP-induced changes in transcription, translation, and/or posttranslational modification. In the current study, we show that GIP increases human adipocyte LPL gene transcription and that phosphorylation of cAMP-response element binding protein (CREB) (Ser133) and nuclear localization of TORC2 are involved in GIP-mediated trans-activation of the LPL gene.
EXPERIMENTAL PROCEDURES
Cell culture of human adipocytes
Subcutaneous human adipocytes from healthy nondiabetic women were obtained from Zen-Bio Inc. [Research Triangle Park, NC; n = 6; average body mass index, 27.9 kg/m2 (range: 25.7–29.9); average age, 40 years (range: 29–52)]. Institutional review board approval and informed consent for use of the adipose tissue were obtained from the patients by Zen-Bio Inc.
Lipoprotein lipase enzyme activity assays
A lipoprotein lipase activity assay kit (Roar Biomedical, Inc., NY) was used to measure enzyme activity according to the manufacturer's protocol. Enzyme activity is presented as relative activity normalized to protein concentration.
Quantitative RT-PCR
Total RNA was extracted from adipocytes and cDNA fragments were generated by reverse transcription. One hundred nanograms of cDNA were used in the real-time polymerase chain reaction (PCR) to measure LPL expression, whereas 10 ng cDNA were used in the 18S rRNA control PCR. The primer and probe sequences used for the amplification of LPL were as follows: forward primer, 5′- TAT GCA GAA GCC CCG AGT -3′; reverse primer, 5′- ATG AAG AGA TGA ATG GAG -3′; probe, 5′FAM- GAC ATT TAC CCG AAT GGA -3′ TAMRA. All reactions followed the typical sigmoidal reaction profile and cycle threshold was used as a measurement of amplicon abundance.
Preparation of nuclear extracts
Nuclear extracts were prepared from cells as described by Schreiber et al. (31). Briefly, cells were washed with PBS and scraped with 200 µl ice-cold buffer A (10 mM HEPES pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol, 0.1% Nonidet P40, and protease inhibitors). Following centrifugation, supernatants (cytoplasmic extracts) were collected and the resulting pellets were resuspended in 20 µl buffer B (20 mM HEPES, pH 7.9, 400 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 20% glycerol, and protease inhibitors) and incubated on ice for 10 min. After clarification of the mixture by centrifugation, the supernatants (nuclear extracts) were collected and subjected to Western blot analysis. Histone H3 and β-actin were used as nuclear and nonnuclear markers, respectively, to establish lack of cross-contamination between the fractions (data not shown).
Western blot analysis
Protein samples were separated on a 15% sodium dodecyl sulfate (SDS)/polyacrylamide gel and transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Mississauga, Ontario, Canada). Probing of the membranes was performed with phospho-PKB (Serine-473), phospho-PKB (Threonine-308), phospho-LKB1 (Serine-428), phospho-AMPK (Threonine-172), AMPK, phospho-CREB (Serine-133), CREB, TORC2, histone H3, β-actin, and LPL antibodies (Cell Signaling Technology, Beverly, MA; Santa Cruz Biotechnology, CA). Because appropriate phospho-TORC2 (Ser171) antibodies were not available, phospho-Serine/Threonine antibody immunoprecipitation and TORC2 antibody detection was applied (See Co-immunoprecipitation section below.). Immunoreactive bands were visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech) using horseradish peroxidase-conjugated IgG secondary antibodies.
Confocal microscopy
Immunocytochemical staining of treated adipocytes was performed using phospho-CREB (Serine-133) and TORC2 antibodies and visualized with Texas Red® dye conjugated anti-rabbit secondary antibody and Alexa fluor® 488 conjugated anti-mouse antibody. Cell nuclei were counterstained with DAPI (4′, 6-diamino-2-phenylindole) and imaged using a Zeiss laser scanning confocal microscope (Axioskop2). All imaging data were analyzed using the Northern Eclipse program (ver.6).
Transient transfection and luciferase assays
GIP receptor (GIPR) expressing HEK-293 cells (GIPR-HEK-293) (32) were cotransfected with 1 µg of human LPL promoter-luciferase construct (chr8+: 19840052 - 19841249; -992 to +206 of LPL gene, SwitchGear Genomics, Menlo Park, CA) and 1 µg of control vector, pCMV5 or the CREB expression plasmids, A-, M1- and Y/F- CREB. CREB constructs were kindly provided by Dr. C. Vinson (National Institutes of Health, MD) and Dr. M. Montminy (The Salk Institute, CA), respectively. Transfections were performed using LIPOFECTAMINE 2000TM reagent (Invitrogen) for 4 h according to the manufacturer's instructions. On the following day, cells were treated with GIP for the times indicated in the figures, washed with PBS, and lysed in reporter lysis buffer (Promega). Luciferase activities were measured using the Luciferase Assay System (Promega) and relative luciferase activity is expressed as the normalized luciferase activity per microgram of protein.
RNA interference knockdown of CREB and TORC2
To determine the effect of reducing levels of endogenous CREB and TORC2 on LPL expression, resuspended human adipocytes were transfected with a pool of 3 siRNAs each for CREB or TORC2 (Santa Cruz, sc-29281 and sc-45832) that resulted in ∼36 to ∼43% reduction of the respective mRNAs when applied as individual pools (data not shown). Following transfection, cells were replated and incubated for 72 h. The level of reduction in CREB and TORC2 protein expression was determined by Western blot hybridization using antibodies against phospho-CREB (Serine-133), CREB, TORC2, LPL, Histone H3, and β-actin.
cAMP measurements
Human adipocytes were incubated for 30 min with GIP or GLP-1 (100 nM) in the presence of insulin (1 nM) and IBMX (0.5 mM). cAMP concentrations in the cell extracts were determined using the Cyclic AMP EIA kit (Cayman Chemical Co., Ann Arbor, MI).
Co-immunoprecipitation
Following cell treatment, samples were immunoprecipitated with phospho-Serine/Threonine antibody, using protein A/G-agarose beads as indicated in figure legend. The precipitated products were then resolved by SDS-PAGE and probed with anti-TORC2 antibody.
Chromatin immunoprecipitation (ChIP)
Following treatment, cells were fixed to isolate intact chromatin. Phospho-CREB and TORC2 were respectively immunoprecipitated from intact chromatin using protein A/G-agarose beads and anti-phospho-CREB (Serine-133) or anti-TORC2 antibody. Bound DNA was eluted and amplified by PCR using oligonucleotides flanking the cAMP-response element (CRE)-I or CRE-II site of the human LPL promoter.
Statistical analysis
Data are expressed as mean ± SEM with the number of individual experiments presented in the figure legends. Significance was tested using ANOVA with Newman-Keul's post hoc test (P < 0.05) as indicated in the figure legends.
RESULTS
GIP but not GLP-1 increases LPL gene transcription in human adipocytes
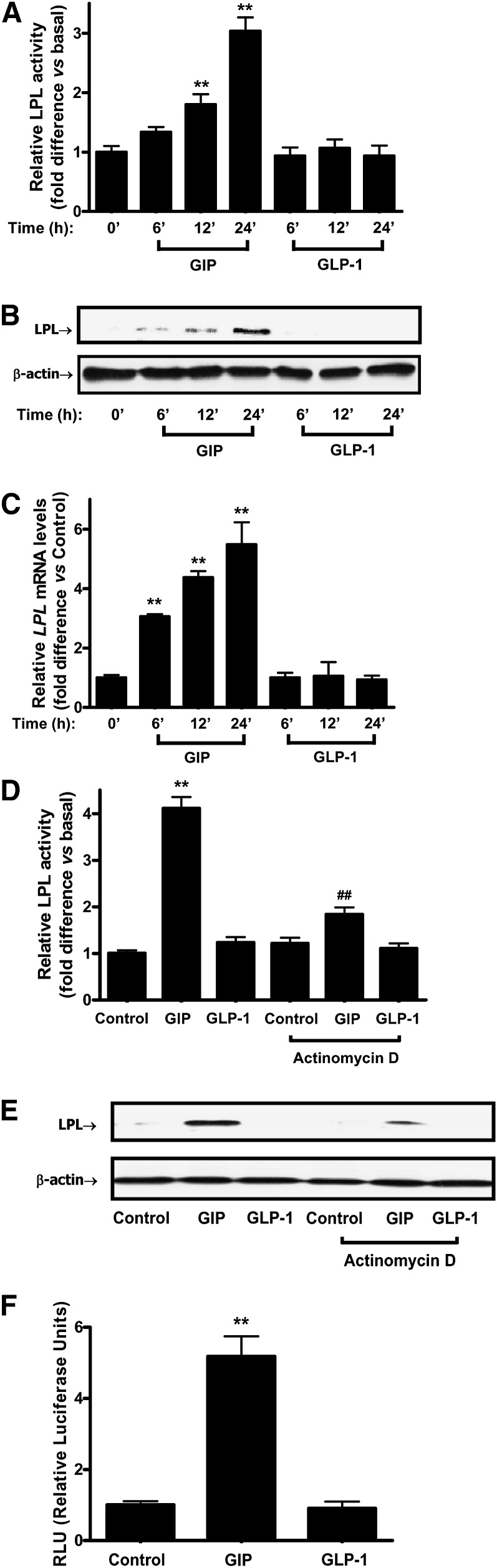
In agreement with our earlier study (27), treatment of human subcutaneous adipocytes with GIP (100 nM) in the presence of insulin (1 nM) resulted in ∼3.0-fold increases in LPL activity compared with basal, whereas there were no significant effects of GLP-1 (Fig. 1A).
Fig. 1.
GIP but not GLP-1 strongly increases LPL activity and expression in human adipocytes. Human adipocytes were serum starved in DMEM/Ham's F-12 medium (1:1, v/v) containing 0.1% BSA overnight and treated for the indicated periods of time with GIP or GLP-1 (100 nM) in the presence of insulin (1 nM). A: Effect of GIP or GLP-1 on LPL activity. LPL activity was determined as described in Materials and Methods. B: Effect of GIP or GLP-1 on LPL protein expression. Human adipocytes were treated as described above and Western blot analyses were performed using antibodies against LPL and β-actin. C: The effect of GIP and GLP-1 on LPL mRNA expression. Human adipocytes were treated as described above and real-time RT-PCR performed to quantify LPL mRNA levels; shown as the fold difference versus control normalized to 18S rRNA expression levels. D: Effects of transcription inhibition on GIP-mediated LPL activity. Human adipocytes were treated for 24 h with GIP or GLP-1 (100 nM) in the presence of transcription inhibitor, actinomycin D (5 μg/ml). LPL activity was determined as described in Materials and Methods. E: Effects of transcription inhibition on GIP-mediated LPL expression. Human adipocytes were treated as described above and Western blot analyses were performed using antibodies against LPL and β-actin. F: The effect of GIP and GLP-1 on LPL promoter activity. GIPR-HEK-293 cells were transfected with LPL promoter reporter construct (chr8+: 19840052 - 19841249; -992 to +206 of LPL gene, 1 µg) and then treated for 24 h with insulin (1 nM) plus GIP or GLP-1 (100 nM). The reporter activities are shown as the relative luciferase activity normalized to protein concentration. All data represent three independent experiments, each carried out in duplicate and Western blots are representative of n = 3. Significance was tested using ANOVA with Newman-Keul's post hoc test where ** represents P < 0.05 versus Control and ## represents P < 0.05 versus GIP.
We next investigated whether GIP-mediated LPL activation in human adipocytes was accompanied by corresponding changes in protein and mRNA levels. As shown in Fig. 1B and C, GIP in the presence of insulin (1 nM) but not GLP-1 increased LPL mRNA transcript levels as well as protein expression. These increases correlated well with LPL activity changes. Because GIP-mediated increases in LPL mRNA levels could potentially be due to changes in either gene transcription or mRNA stability, we next determined LPL activity in the presence or absence of the transcription inhibitor, actinomycin D. As shown in Fig. 1D and E, increases in both LPL activity and protein expression in response to GIP were greatly reduced by actinomycin D. To confirm that GIP was capable of directly stimulating LPL gene expression, GIPR-HEK-293 cells were transiently transfected with a human LPL promoter reporter construct and treated for 24 h with insulin (1 nM) plus GIP or GLP-1 (100 nM). Only GIP and not GLP-1 increased promoter activity (Fig. 1F). Taken together, these results strongly suggest that GIP-mediated increases in LPL activity were at least partially due to transcriptional activation of the LPL gene.
GIP but not GLP-1 modulates CREB phosphorylation and TORC2 nuclear localization in human adipocytes
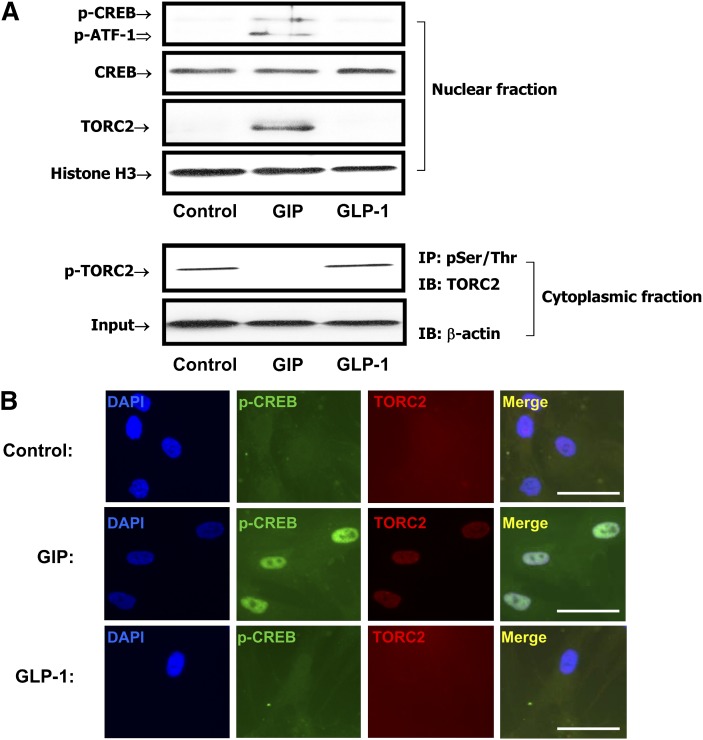
We next determined whether CREB played a role in GIP-mediated trans-activation of the LPL gene. This proposal was based on the fact that CREB activation had been implicated as an activator of LPL expression in other cell types (33, 34), although only a CRE-like sequence in reverse orientation had been identified in the proximal LPL promoter region (35). Additionally, we had previously shown that GIP-mediated reduction in phospho-Thr172 AMPK was linked to nuclear translocation of TORC2 and CREB activation in INS-1 (832/13) β-cells (14). Human adipocytes were therefore treated with GIP or GLP-1 (100 nM) and Western blot analyses were performed on nuclear extracts using antibodies against phospho-CREB Ser133 or TORC2. As shown in Fig. 2A, GIP but not GLP-1 increased the levels of phospho-Ser133 CREB and the nuclear localization of TORC2 and decreased the levels of cytoplasmic phospho-TORC2. Immunohistochemical staining and confocal imaging of adipocytes treated in an identical fashion confirmed that GIP increased both nuclear levels of phospho-CREB and TORC2 nuclear uptake (Fig. 2B).
Fig. 2.
GIP modulates CREB/TORC2 activity in human adipocytes. Human adipocytes were serum starved in DMEM/Ham's F-12 medium (1:1, v/v) containing 0.1% BSA overnight and treated for 24 h with GIP or GLP-1 (100 nM) in the presence of insulin (1 nM). A: Effects of GIP on phosphorylation of CREB and nuclear localization of TORC2. Nuclear extracts were isolated and Western blot analyses performed with antibodies against phosphorylated Ser133-CREB, CREB, TORC2, and Histone H3. Cytoplasmic extracts were immunoprecipitated (IP) with phospho-Serine/Threonine followed by immunoblotting (IB) for TORC2. Input represents one-tenth of total cytoplasmic extracts used in assay. B: Confocal microscopy. Human adipocytes were treated as described above. Immunocytochemical staining was performed using antibodies against phospho-CREB (Ser133, green) or TORC2 (red) and nuclei were stained with DAPI (blue). The scale bar indicates 20 μm and all imaging data were analyzed using the Northern Eclipse program (ver.6). Shown are representative of n = 3.
GIP-mediated trans-activation of LPL involves CREB phosphorylation
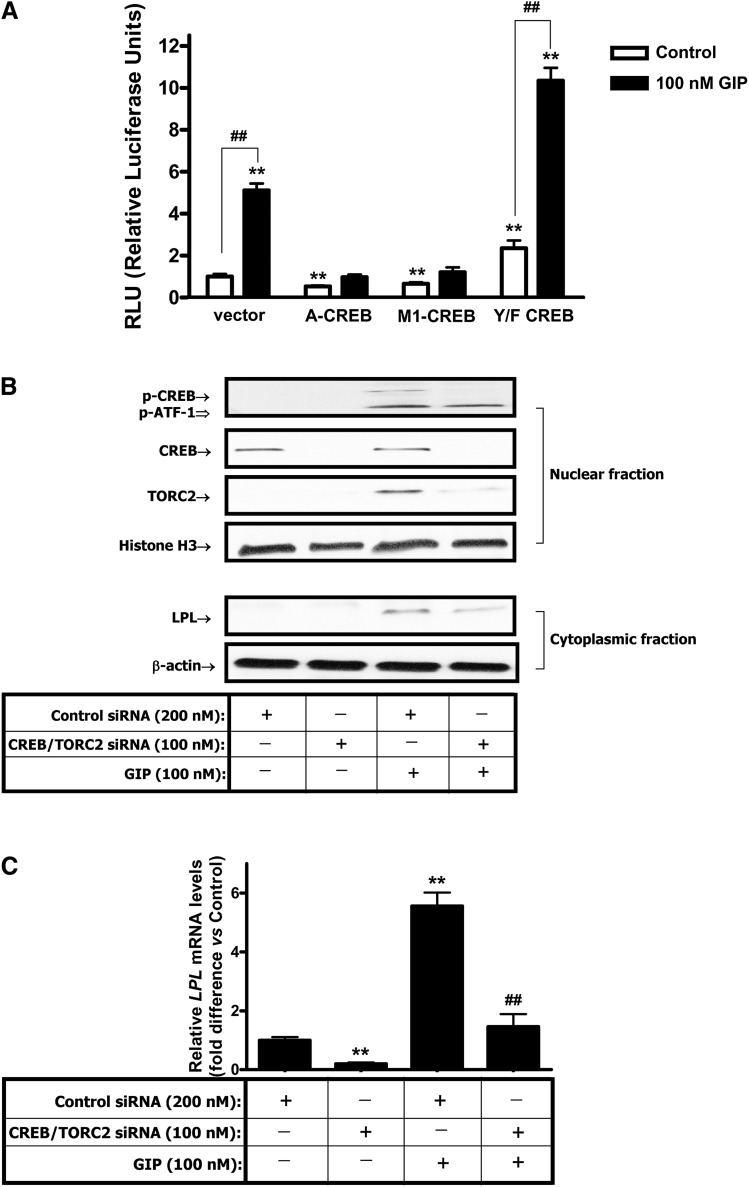
To establish that CREB phosphorylation plays a role in GIP-induced trans-activation of LPL, reporter analyses were performed using various CREB constructs cotransfected with the LPL promoter construct in GIPR-HEK-293 cells. A-CREB is an isoform of CREB that dimerizes with endogenous CREB, thus preventing its binding to the CRE (36). M1-CREB contains a S133A mutation, rendering CREB nonphosphorylatable on Ser 133; occupation of the CRE, thus blocking wild-type CREB binding (37). Y/F-CREB is a gain-of-function mutant containing a Tyr134Phe mutation, making CREB behave as a constitutive activator in vivo (38). Like wild-type-CREB, Y/F-CREB stimulates target gene expression via the Ser133-dependent recruitment of CBP and p300 (36). When A- or M1-CREB were cotransfected, basal levels of LPL promoter activity and GIP responsiveness of the LPL promoter was greatly decreased compared with naked vector-cotransfected control (P < 0.05). On the other hand, when Y/F-CREB was cotransfected and basal LPL promoter activity was increased and GIP-mediated activation of the LPL promoter was significantly potentiated compared with vector, cotransfected control was also significantly increased (Fig. 3A). These results strongly support a positive role for CREB in trans-activation of the LPL promoter. To establish functional involvement of CREB and TORC2 in the regulation of LPL expression in human adipocytes, RNA interference was employed. As shown in Fig. 3B and C, RNA interference treatment resulted in specific reductions in GIP-stimulated levels of phospho-CREB and TORC2 associated with reduced LPL protein expression. Combined CREB and TORC2 knockdown resulted in >70% reduction in GIP-stimulated LPL mRNA levels, demonstrating that they act as positive coordinate activators of LPL expression.
Fig. 3.
CREB/TORC2 are functionally involved in the regulation of LPL expression. A: Effects of different CREB constructs on GIP-responsiveness of the LPL promoter. GIPR-HEK-293 cells were cotransfected with an LPL promoter reporter construct (chr8+: 19840052 - 19841249; -992 to +206 of LPL gene, 2 µg), and various CREB constructs or control vector pCMV5 (1 µg). After transfection, cells were treated with insulin (1 nM) plus GIP (100 nM), and the reporter activities are shown as the relative luciferase activity normalized to protein concentration. All data represent three independent experiments, each carried out in triplicate. Significance was tested using ANOVA with Newman-Keuls post hoc test, where ** represents P < 0.05 versus untreated Vector Control, ## represents P < 0.05 versus respective untreated Control. B, C: RNAi-mediated suppression of CREB and TORC2 reduces GIP-stimulated LPL expression. B: Effects of CREB and TORC2 siRNAs on GIP-stimulated LPL protein expression. Human adipocytes were transfected with a pool of three siRNAs for CREB and TORC2 and incubated for 72 h. At 48 h after transfection, cells were treated with insulin (1 nM) plus GIP (100 nM) for 24 h and Western blot analyses were performed as described in Materials and Methods, using antibody against phospho-CREB (Serine-133), CREB, TORC2, LPL, Histone H3, and β-actin. C. Effects of CREB and TORC2 siRNAs on GIP-stimulated LPL mRNA expression. Human adipocytes were treated as described above and real-time RT-PCR performed to quantify LPL mRNA levels; shown as the fold difference versus control normalized to 18S rRNA expression levels. Significance was tested using ANOVA with Newman-Keul's post hoc test where ** represents P < 0.05 versus Control siRNA, ## represents P < 0.05 versus GIP.
PI3-K/PKB and AMPK are involved in GIP-mediated regulation of CREB/TORC2/LPL activation
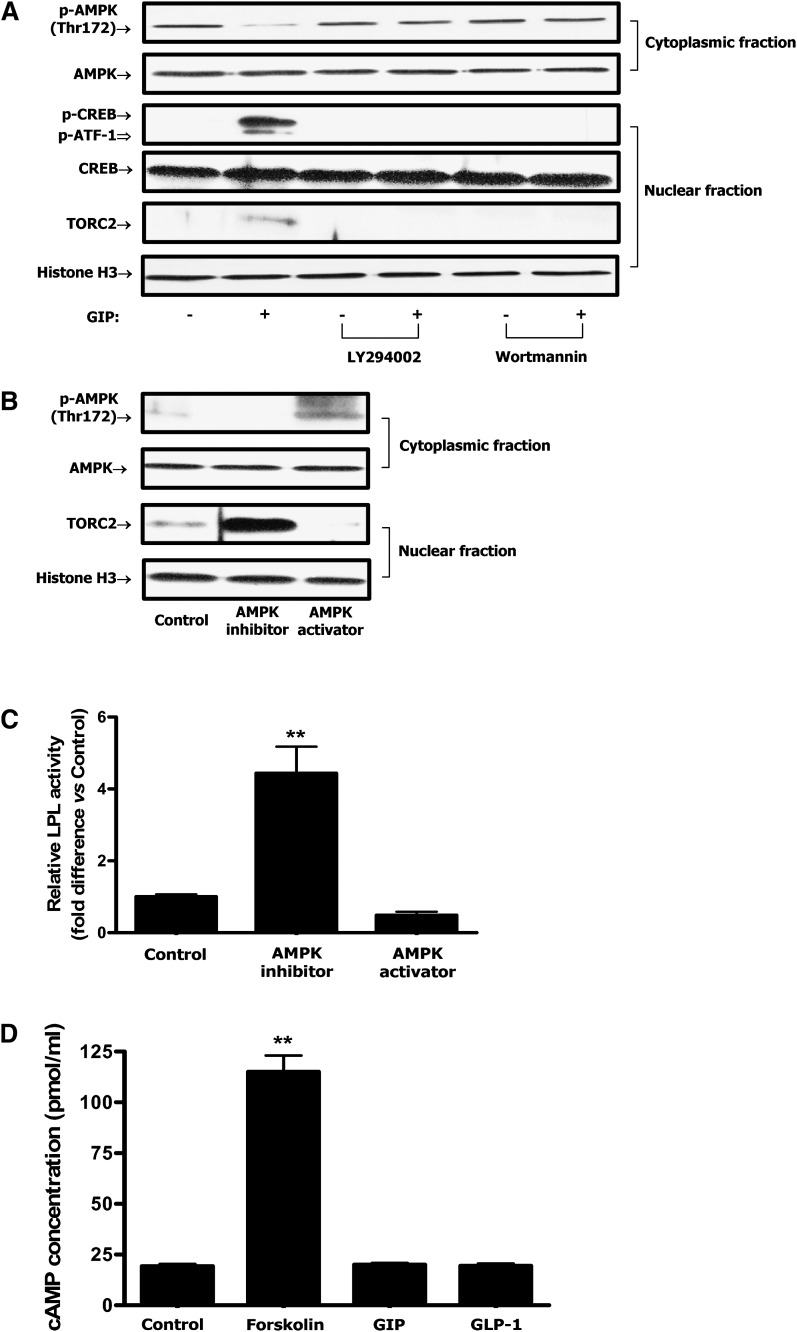
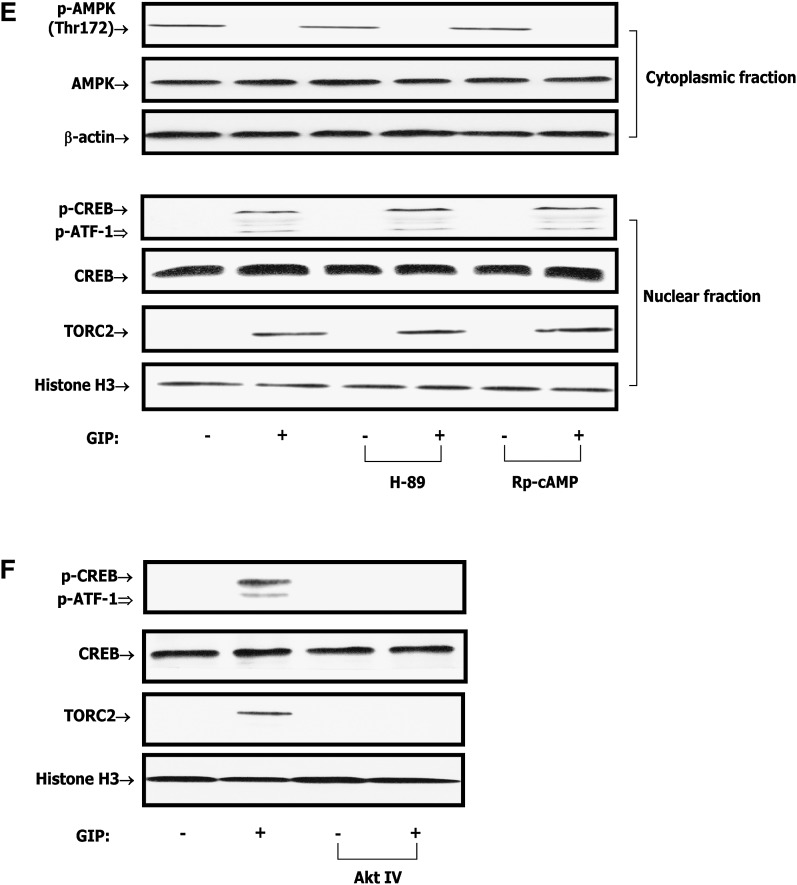
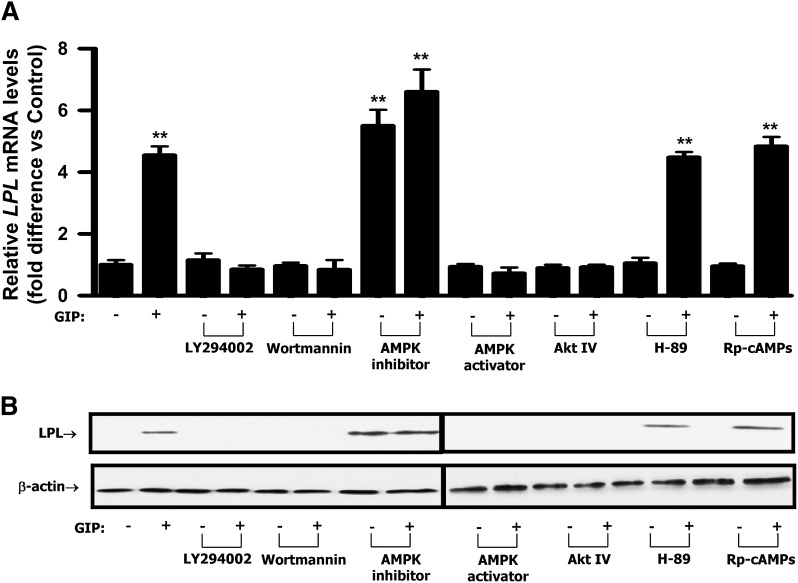
Because GIP-induced reductions in adipocyte phospho-AMPK Thr-172 were shown to be associated with increased phosphorylation of PKB Ser-473 (27), PI3-K was considered to be the likely upstream mediator. Inhibition of PI3-K with either of the two selective pharmacological inhibitors, LY294002 or wortmannin, abolished the effects of GIP on the phosphorylation of AMPK, CREB, and nuclear localization of TORC2 (Fig. 4A). In a subsequent study, treatment with the AMPK inhibitor, Compound C (6-[4-(2-Piperidin-1-yl-ethoxy)-phenyl)]3-pyridin-4-yl-pyrrazolo[1,5-a]pyrimidine), resulted in accumulation of TORC2 in the nucleus whereas treatment with the AMPK activator, (5-(3-(4-(2-(4-Fluorophenyl)ethoxy)phenyl)propyl)furan-2-carboxylic acid (FPPF) resulted in depletion of TORC2 from the nucleus (Fig. 4B). Additionally, AMPK inhibitor treatment was shown to increase LPL activity whereas the AMPK activator slightly decreased activity (Fig. 4C).
Fig. 4.
GIP regulates CREB/TORC2 through a pathway involving PI3-K/PKB/AMPK. Human adipocytes were serum starved in DMEM/Ham's F-12 medium (1:1, v/v) containing 0.1% BSA overnight and treated for 24 h with GIP (100 nM) in the presence of insulin (1 nM). A: Effect of PI3-K inhibition on GIP-mediated decreases in phospho-AMPK Thr172 and nuclear CREB/TORC2 activity. Human adipocytes were treated for 24 h with insulin (1 nM) plus GIP (100 nM) in the presence or absence of PI3-K inhibitors, LY 294002 (40 μM) or wortmannin (400 nM). B: Effect of AMPK modulation on CREB/TORC2 activity. Human adipocytes were treated for 24 h with insulin (1 nM) plus GIP (100 nM) in the presence or absence of AMPK inhibitor (Compound C, 6-[4-(2-Piperidin-1-yl-ethoxy)-phenyl)]3-pyridin-4-yl-pyrrazolo[1,5-a]pyrimidine, 40 μM) or AMPK activator (5-(3-(4-(2-(4-Fluorophenyl)ethoxy)phenyl)propyl)furan-2-carboxylic acid, FPPF, 50 μM). AMPK inhibitor or activator was added to cells during 1 h preincubation as well as GIP stimulation. Nuclear/cytoplasmic extracts were isolated from each sample and Western blot analyses were performed. TORC2 and histone H3 blots are from nuclear extracts and phospho-AMPK Thr172 and AMPK blots are from cytoplasmic extracts. C: Effect of AMPK modulation on LPL activity. Human adipocytes were treated as described above and LPL activity was determined as described in Materials and Methods. D: Effect of GIP or GLP-1 on cAMP accumulation. Human adipocytes were treated as described above and incubated with GIP or GLP-1 (100 nM) in the presence of insulin (1 nM) and IBMX (0.5 mM). cAMP concentrations determined in the cell extracts. E: Effect of PKA inhibition on GIP-mediated decreases in phospho-AMPK Thr172 and nuclear CREB/TORC2 activity. Human adipocytes were treated as described above and stimulated insulin (1 nM) plus GIP, GLP-1 (100 nM) in the presence or absence of H-89 or Rp-cAMPs. H-89 (10 μM) or Rp-cAMPs (200 μM) was added to cells during 1 h preincubation as well as during GIP stimulation. Nuclear/cytoplasmic extracts were isolated from each sample and Western blot analyses were performed with antibodies against phosphorylated Thr172-AMPK, AMPK, TORC2, and histone H3. F: Effect of PKB inhibition on GIP-mediated CREB/TORC2 activity. Human adipocytes were treated for 24 h with insulin (1 nM) plus GIP (100 nM) in the presence or absence of PKB inhibitor (Akt IV, 500 nM). Nuclear extracts were isolated from each sample and Western blot analyses were performed with antibodies against phosphorylated Ser133-CREB, CREB, and histone H3. Western blots are representative of n = 3. All data represent three independent experiments, each carried out in duplicate. Significance was tested using ANOVA with Newman-Keul's post hoc test where ** represents P < 0.05 versus Control.
Previously, we found that insulin suppresses the ability of GIP to activate PKA (25) and all experiments in the current study were performed in the presence of insulin. However, PKA/cAMP had been demonstrated to be involved in GIP-stimulated trafficking of TORC2 to the nucleus and phosphorylation of nuclear CREB in INS-1 β-cells (14). It was therefore considered important to reexamine the effect of GIP on the cAMP/PKA pathway in human adipocytes. Treatment of human adipocytes with forskolin (10 μM), a direct activator of adenylyl cyclase, resulted in ∼5.9-fold increase in cAMP concentration compared with control (Fig. 4D). By contrast, treatment with GIP or GLP-1 (100 nM) in the presence of insulin (1 nM) had no significant effects on cAMP levels (Fig. 4D). Additionally, the effects of GIP on AMPK and CREB/TORC2 were not ablated by the PKA inhibitors H-89 (10 μM) and Rp-cAMPs (200 μM), indicating that GIP in the presence of insulin did not modulate transcriptional activity of CREB/TORC2 via activation of the PKA/cAMP pathway (Fig. 4E). However, inhibition of PKB with the selective pharmacological inhibitor Akt IV abolished the effects of GIP on the phosphorylation of CREB and nuclear localization of TORC2 (Fig. 4F). In a subsequent study, PI3-K/PKB inhibitors and AMPK activator (FPPF) abolished the effects of GIP on LPL mRNA and protein expression. In contrast, an AMPK inhibitor (Compound C) was shown to mimic the effect of GIP on LPL expression. PKA inhibitors did not alter GIP-mediated effects (Fig. 5A, B). Together, these results strongly suggest that PI3-K/PKB and AMPK are upstream components of the GIP-mediated CREB/TORC2/LPL activation pathway in human adipocytes whereas cAMP/PKA does not play a role.
Fig. 5.
Effects of PI3-K, AMPK, PKA, or PKB inhibition on GIP-mediated LPL mRNA and protein expression. Human adipocytes were serum starved in DMEM/Ham's F-12 medium (1:1, v/v) containing 0.1% BSA overnight and treated for 24 h with GIP (100 nM) in the presence of insulin (1 nM). A: Effect of PI3-K, AMPK, PKA or PKB inhibition on GIP-mediated LPL transcription. Human adipocytes were treated for 24 h with insulin (1 nM) plus GIP (100 nM) in the presence or absence of PI3-K inhibitors (40 μM of LY 294002 or 400 nM of wortmannin), AMPK inhibitor (40 μM of Compound C) or AMPK activator (50 μM of FPPF), PKA inhibitors (10 μM of H-89 or 200 μM of Rp-cAMPs), or PKB inhibitor (500 nM of Akt IV). Real-time RT-PCR performed to quantify LPL mRNA levels; shown as the fold difference versus control normalized to 18S rRNA expression levels. B: Effect of PI3-K, AMPK, PKA, or PKB inhibition on GIP-mediated LPL expression. Human adipocytes were treated as described in A and Western blot analyses were performed using antibodies against LPL and β-actin. All data represent three independent experiments, each carried out in duplicate and Western blots are representative of n = 3. Significance was tested using ANOVA with Newman-Keul's post hoc test where ** represents P < 0.05 versus Control.
Direct interaction between phospho-CREB and TORC2 in the nucleus
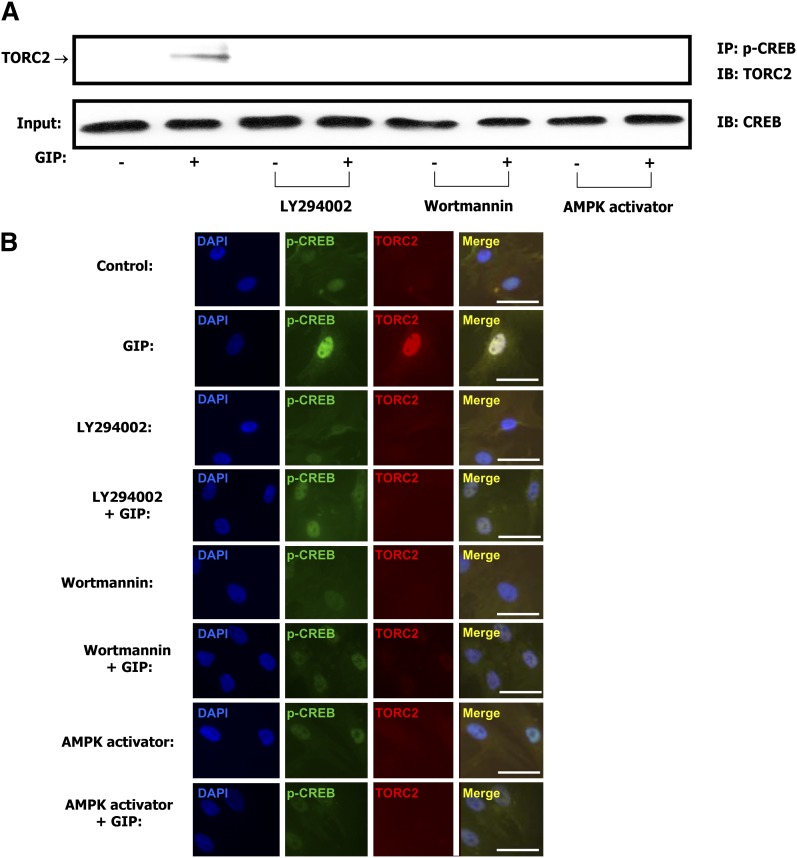
As shown in Fig. 6A, co-immunoprecipitation experiments demonstrated protein-protein interaction between phospho-CREB Ser133 and TORC2 in nuclear extracts from GIP-treated cells. Colocalization of phospho-CREB and TORC2 was also observed using confocal microscopy (Fig. 6B). Because the TORC N-terminal coiled-coil domain directly binds to the CREB basic-leucine zipper domain (39, 40) these results indicate increased interaction between the transcription factor and its coactivator in the nucleus. Both detectable protein-protein interaction and colocalization of phospho-CREB Ser133 and TORC2 were ablated by treatment with PI3K inhibitors or AMPK activation (Fig. 6A, B).
Fig. 6.
GIP increases protein-protein interaction between phospho-CREB and TORC2 in the nucleus. A: Coimmunoprecipitation. Human adipocytes were serum-starved in DMEM/Ham's F-12 medium (1:1, v/v) containing 0.1% BSA overnight and treated for 24 h with GIP (100 nM) in the presence of insulin (1 nM) and in the presence or absence of PI3-K inhibitors, LY 294002 (40 μM), wortmannin (400 nM) or AMPK activator (50 μM). Nuclear extracts were isolated from each sample and immunoprecipitated (IP) with phospho-CREB (Ser133) followed by immunoblotting (IB) for TORC2. Input represents one-tenth of total nuclear extract used in the coimmunoprecipitation assay. B: Confocal microscopy. Human adipocytes were treated as described above and fixed following stimulation with GIP (100 nM) in the presence or absence of PI3-K inhibitors or AMPK activator. Immunocytochemical staining was performed using antibodies against phospho-CREB (Ser133, green) or TORC2 (red) and nuclei were stained with DAPI (blue). The scale bar indicates 20 μm and all imaging data were analyzed using the Northern Eclipse program (ver.6). Shown are representative of n = 3.
Identification of a functional CRE in the human LPL promoter
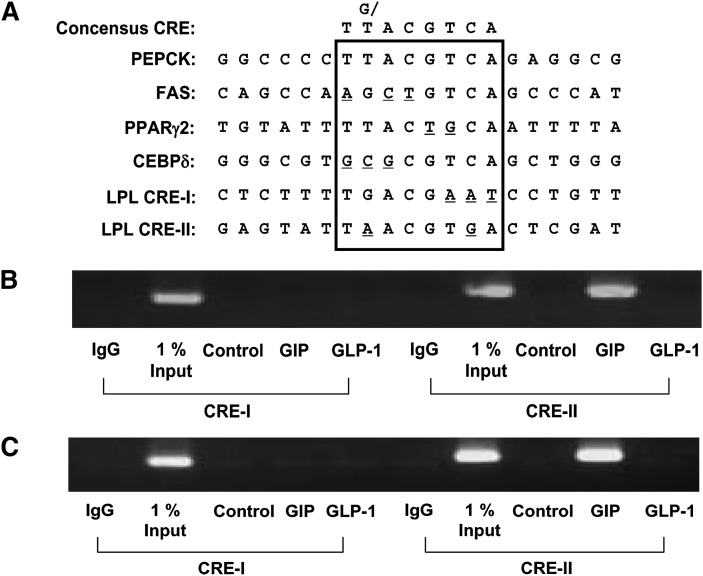
Before attempting to identify functional CREs in the LPL promoter, we evaluated the structures of known CREs in several adipocyte-expressing gene promoters including PEPCK, FAS, PPARγ2, and CEBPδ (Fig. 7A). These sites were selected based on their ability to mediate transcriptional regulation in response to cAMP mimetics, their participation in gene expression during adipogenesis, and their significant homology to the consensus CRE sequence. Using this information, analysis of the human LPL promoter sequence (chr8+: 19840052 - 19841249; -992 to +206 of LPL gene) using the MatInspector (Abteling Genetik, Braunschweig, Germany) program revealed the presence of two potential CREs that we have termed CRE-I and -II. Inverted CRE-I (TGACG AAT ) and CRE-II (T A ACGT G A) have 62.5% and 75% homology to the consensus CRE (TG/TACGTCA) (Fig. 7A). To identify the functional CREs responsible for GIP's action, direct phospho-CREB/TORC2 binding to the human LPL promoter was determined by measuring occupancy of CREs using ChIP assays. As shown in Fig. 7B and C, GIP but not GLP-1 increased both phospho-CREB and TORC2 binding to CRE-II of the LPL promoter, indicating that CRE-II is the functional cis-acting element of the human LPL promoter involved in responses to GIP.
Fig. 7.
Phospho-CREB/TORC2 binds CRE-II sequence of human LPL promoter. A: Identification of putative CRE sequences in the promoters of several adipocyte-specific genes. Potential CREs present in these promoters are indicated by the box-enclosed regions and consensus CRE sequences shown at the top of the figure. B, C: Representative gel of a ChIP assay for the binding of phospho-CREB (B) and TORC-2 (C) in the human LPL promoter. Human adipocytes were serum-starved in DMEM/Ham's F-12 medium (1:1, v/v) containing 0.1% BSA overnight and treated for 24 h with GIP (100 nM) in the presence of insulin (1 nM). Phospho-CREB and TORC2 were respectively immunoprecipitated from intact chromatin isolated from human adipocytes using anti-phospho-CREB (Ser 133) and anti-TORC2 antibody. Precipitated DNA fragments were analyzed by PCR using primers flanking the CRE-I and CRE-II sites in the LPL promoter. An isotype-matched IgG was used as negative control and 1% Input (PCR product of one-hundredth of the total isolated DNA used in the ChIP assay) as positive control, respectively. Shown are representative of n = 3.
DISCUSSION
The majority of studies on GIP have focused on its incretin action and, compared with its effects on the pancreatic islet, relatively little is known regarding the role of GIP in adipose tissue metabolism. GIP has been shown to stimulate FA synthesis from acetate in adipose tissue explants (41), as well as enhancing insulin-stimulated FA incorporation into adipose tissue (42). In previous studies, it was shown that GIP increased LPL activity and triglyceride levels in both 3T3-L1 cells and human adipocytes through a pathway involving increased phosphorylation of PKB and decreases in LKB1 and AMPK phosphorylation (27). The regulation of adipocyte LPL expression and action is complex, involving transcriptional, posttranscriptional, and translocation events (35, 43). The current study was therefore initiated in order to elucidate the mechanisms involved in GIP-mediated increases in LPL activity in human adipocytes. This is an important question because of the suggestion that GIPR-mediated effects in fat tissue are involved in the development of obesity associated with the consumption of energy-rich high-fat diets (2) and the potential for GIP and GIP analogs as therapeutics in Type 2 diabetes. GIP was found to increase LPL promoter activity (Fig. 1F) and in the presence of insulin both LPL mRNA and protein levels were increased in human adipocytes (Fig. 1B, C). Trans-activation of the LPL gene was shown to be a major contributor to GIP-mediated increases in LPL activity because responses were greatly reduced although not completely abolished by the transcription inhibitor actinomycin D (Fig. 1D). However, additional effects of GIP on posttranscriptional events including activation and translocation of preformed pools of enzyme may also contribute. In contrast, the second major incretin, GLP-1, exerted no effect on LPL gene expression. Evidence for the involvement of CREB in the regulation of LPL expression has been presented in studies on undifferentiated hepatoma cells (34) and the adrenal (35). Phosphorylation of Ser133 in CREB is important for the recruitment of CBP and p300 and association with TORC2 results in increased transcriptional activity. GIP treatment of human adipocytes was found to increase levels of phospho-Ser133 CREB and nuclear localization of TORC2 (Fig. 2A). A functional involvement of CREB and TORC2 in the regulation of LPL expression was demonstrated by RNA interference (Fig. 3B, C). Colocalization of phospho-CREB Ser133 and TORC2 was observed in immunocytochemical studies (Fig. 2B, 6B), and coimmunoprecipitation provided evidence for direct protein-protein interaction between CREB and TORC2 in nuclear extracts from GIP-treated cells (Fig. 6A). Database analysis of human LPL promoter sequences revealed the presence of two potential CREs here termed CRE-I and CRE-II. CRE-I (TGACGAAT) proved to have been earlier described as a ‘CRE-like sequence in reverse orientation’ in the proximal region of the human LPL promoter (35, 43, 44), whereas the CRE-II (TAACCGTGA) had not been previously identified. The human CRE-I and CRE-II sequences share 62.5% and 75% homology with the CRE consensus sequence. CRE-I was not found to be functional, but ChiP assays identified CRE-II as the functional CRE responsible for GIP action (Fig. 7). There is therefore strong evidence for a GIP-activated pathway in human adipocytes involving CREB-induced LPL gene expression. Interestingly, rat and mouse sequences situated at a similar site to the human CRE-I share only 37.5% homology with the consensus CRE whereas rat and mouse sequences corresponding to the human CRE-II share 62.5% and 50% homology, respectively (45). It is therefore likely that the mouse sequence is nonfunctional, as discussed further below.
The AMPK/TORC2/CREB pathway that mediated GIP action in the human adipocyte is similar to that shown to be involved in activation of Bcl2 gene expression in INS-1 (832/13) β-cells (14). However, whereas PKA was found to be a key upstream component of the activation pathway for TORC2 in β-cells (14), a PI3K/PKB-mediated pathway is a central player in the regulation of LPL gene expression in the human adipocyte (Fig. 8). PKB can also phosphorylate CREB (46, 47), and the selective PKB inhibitor Akt IV abolished the effects of GIP on LPL mRNA and protein expression as well as CREB phosphorylation (Figs. 4F, 5A, 5B). It is therefore likely that PKB plays a dual role, acting via TORC2 and direct phosphorylation of Ser133 CREB (Fig. 8). Activation of PKB has also been shown to be involved in GIP-induced enhancement of adipocyte glucose transport (48). However, activation of adenylyl cyclase is probably still important in GIP-mediated regulation of lipolysis in the absence of insulin because increased cAMP levels have been observed in isolated adipocytes, fat explants, and differentiated 3T3-L1 cells exposed to GIP (25, 49, 50). Although the significance of such a dual lipolytic/lipogenic action of GIP is currently unclear, as discussed elsewhere (2, 25), when insulin levels are low GIP may be important for priming β-cells for subsequent glucose stimulation by maintaining circulating FFAs at appropriate levels. Following nutrient ingestion, GIP stimulates insulin secretion, elevating insulin levels, resulting in inhibition of GIP's lipolytic action; the two hormones thus combining to stimulate lipogenesis.
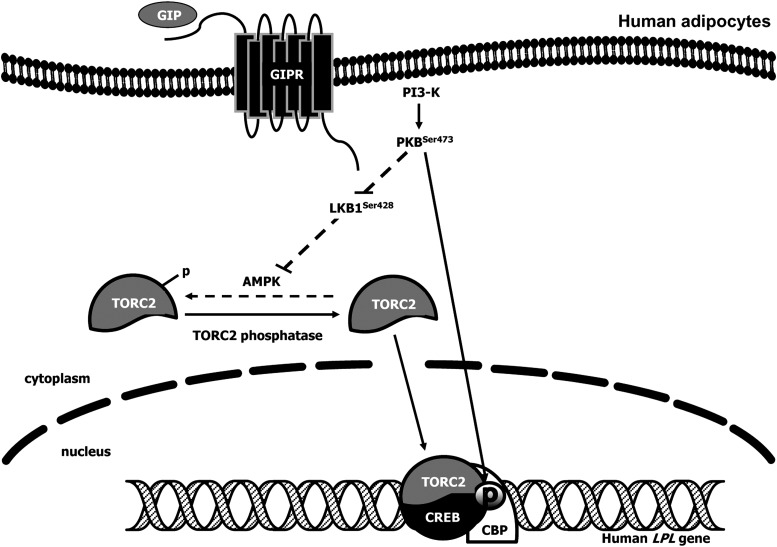
Fig. 8.
Proposed pathway by which GIP increases LPL activity in adipocytes. GIP receptor interaction results in PI3-K activation, increased phosphorylation of Ser473 and Thr308 in PKB, and reduced phosphorylation of Ser428 in LKB1 and Thr172 in AMPK. An associated decrease in AMPK phosphorylation leads to dephosphorylation of TORC2, thus allowing increased translocation from the cytoplasm and into the nucleus. PKB also increases phospho-CREB Ser133 levels in the nucleus. TORC2 complexes with phospho-CREB Ser133 in the nucleus and binds to CRE-II of the human LPL promoter, thus turning on the transcriptional machinery for up-regulation of LPL.
One question not addressed in the current study is the contribution of human resistin (FIZZ3) to GIP-induced responses. It was demonstrated earlier, that resistin mimicked the effects of GIP on the PKB/LKB1/AMPK/LPL pathway in 3T3-L1 adipocytes (28). Additionally RNAi-mediated knockdown of resistin resulted in attenuated effects of GIP on this pathway, suggesting that resistin is a key mediator of GIP-mediated LPL activation in the mouse (28). FIZZ3 is expressed in preadipocytes and adipocytes (51, 52), but only in low amounts, with monocytes/macrophages expressing the majority of FIZZ3 protein in adipose tissue (53). Because the mouse CRE-II in the LPL promoter shares only 50% homology with the consensus CRE, it is possible that it is nonfunctional, with the resistin-activated pathway responsible for regulating LPL gene expression in the mouse.
In summary, GIP in the presence of insulin increased LPL gene expression in human adipocytes. Using LPL promoter studies and ChIP assays it was established that GIP increased nuclear levels of TORC2 and its binding to phospho-CREB at a CRE-II site in the human LPL promoter. GIP-mediated effects involve a PI3-K/PKB/AMPK-dependent pathway. Although little is known regarding the in vivo actions of GIP on human adipose tissue metabolism, the current study provides evidence supporting a potentiating role in insulin-induced lipogenesis. Whether this physiological effect is exaggerated in conditions of excess nutrient intake and its potential contribution to the development of obesity is currently unclear.
Acknowledgments
The authors thank Dr. C. Vinson (National Institutes of Health, MD) and Dr. M. Montminy (The Salk Institute, CA) for M1-, A- and Y/F- CREB constructs.
Footnotes
Abbreviations:
- AMPK
- AMP-activated protein kinase
- CRE
- cAMP-response element
- CREB
- cAMP-response element binding protein
- GIP
- glucose-dependent insulinotropic polypeptide
- GIPR
- GIP receptor
- GLP-1
- glucagon-like peptide-1
- PI3-K
- phosphatidylinositol 3-kinase
- PKB
- protein kinase B
- TG
- triglyceride
- TORC2
- cAMP-responsive CREB coactivator 2
These studies were generously supported by funding to C.H.S.M. from the Canadian Diabetes Association.
References
- 1.Drucker D. J., Nauck M. A. 2006. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 368: 1696–1705. [DOI] [PubMed] [Google Scholar]
- 2.McIntosh C. H. S., Widenmaier S., Kim S. J. 2009. Glucose-dependent insulinotropic polypeptide (Gastric Inhibitory Polypeptide; GIP). Vitam. Horm. 80: 409–471. [DOI] [PubMed] [Google Scholar]
- 3.Davidson M. B., Bate G., Kirkpatrick P. 2005. Exenatide. Nat. Rev. Drug Discov. 4: 713–714. [DOI] [PubMed] [Google Scholar]
- 4.Harder H., Nielsen L., Tu D. T. T., Astrup A. 2004. The effect of liraglutide, a long-acting glucagon-like peptide 1 derivative, on glycemic control, body composition, and 24-h energy expenditure in patients with type 2 diabetes. Diabetes Care. 27: 1915–1921. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo R. A., Ratner R. E., Han J., Kim D. D., Fineman M. S., Baron A. D. 2005. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 28: 1092–1100. [DOI] [PubMed] [Google Scholar]
- 6.Deacon C. F. 2007. Dipeptidyl peptidase 4 inhibition with sitagliptin: a new therapy for type 2 diabetes. Expert Opin. Investig. Drugs. 16: 533–545. [DOI] [PubMed] [Google Scholar]
- 7.Rosenstock J., Baron M. A., Dejager S., Mills D., Schweizer A. 2007. Comparison of vildagliptin and rosiglitazone monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabetes Care. 30: 217–223. [DOI] [PubMed] [Google Scholar]
- 8.McIntosh C. H. S. 2008. Dipeptidyl peptidase IV inhibitors and diabetes therapy. Front. Biosci. 13: 1753–1773. [DOI] [PubMed] [Google Scholar]
- 9.Brubaker P. L., Drucker D. J. 2004. Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 145: 2653–2659. [DOI] [PubMed] [Google Scholar]
- 10.Drucker D. J. 2007. The role of gut hormones in glucose homeostasis. J. Clin. Invest. 117: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yusta B., Baggio L. L., Estall J. L., Koehler J. A., Holland D. P., Li H., Pipeleers D., Ling Z., Drucker D. J. 2006. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 4: 391–406. [DOI] [PubMed] [Google Scholar]
- 12.Ehses J. A., Casilla V. R., Doty T., Pospisilik J. A., Winter K. D., Demuth H. U., Pederson R. A., McIntosh C. H. S. 2003. Glucose-dependent insulinotropic polypeptide promotes beta-(INS-1) cell survival via cyclic adenosine monophosphate-mediated caspase-3 inhibition and regulation of p38 mitogen-activated protein kinase. Endocrinology. 144: 4433–4445. [DOI] [PubMed] [Google Scholar]
- 13.Kim S. J., Winter K., Nian C., Tsuneoka M., Koda Y., McIntosh C. H. S. 2005. Glucose-dependent insulinotropic polypeptide (GIP) stimulation of pancreatic beta-cell survival is dependent upon phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling, inactivation of the forkhead transcription factor Foxo1, and down-regulation of bax expression. J. Biol. Chem. 280: 22297–22307. [DOI] [PubMed] [Google Scholar]
- 14.Kim S. J., Nian C., Widenmaier S., McIntosh C. H. S. 2008. Glucose-dependent insulinotropic polypeptide mediated up-regulation of β-cell anti-apoptotic Bcl-2 gene expression is coordinated by cAMP-response element binding protein (CREB) and cAMP-responsive CREB coactivator 2. Mol. Cell. Biol. 28: 1644–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Widenmaier S. B., Ao Z., Kim S. J., Warnock G., McIntosh C. H. S. 2009. Suppression of p38 MAPK and JNK via Akt-mediated inhibition of apoptosis signal regulating kinase 1 constitutes a core component of the beta-cell pro-survival effects of glucose-dependent insulinotropic polypeptide. J. Biol. Chem. 284: 30372–30382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spiegelman B. M., Flier J. S. 2001. Obesity and the regulation of energy balance. Cell. 104: 531–543. [DOI] [PubMed] [Google Scholar]
- 17.MacDougald O. A., Mandrup S. 2002. Adipogenesis: forces that tip the scales. Trends Endocrinol. Metab. 13: 5–11. [DOI] [PubMed] [Google Scholar]
- 18.Attie A. D., Scherer P. E. 2009. Adipocyte metabolism and obesity. J. Lipid Res. 50: S395–S399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pederson R. A., Schubert H. E., Brown J. C. 1975. Gastric inhibitory polypeptide. Its physiologic release and insulinotropic action in the dog. Diabetes. 24: 1050–1056. [DOI] [PubMed] [Google Scholar]
- 20.Krarup T. 1988. Immunoreactive gastric inhibitory polypeptide. Endocr. Rev. 9: 122–134. [DOI] [PubMed] [Google Scholar]
- 21.Wasada T., McCorkle K., Harris V., Kawai K., Howard B., Unger R. H. 1981. Effect of gastric inhibitory polypeptide on plasma levels of chylomicron triglycerides in dogs. J. Clin. Invest. 68: 1106–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebert R., Nauck M., Creutzfeldt W. 1991. Effect of exogenous or endogenous gastric inhibitory polypeptide (GIP) on plasma triglyceride responses in rats. Horm. Metab. Res. 23: 517–521. [DOI] [PubMed] [Google Scholar]
- 23.Jorde R., Pettersen J. E., Burhol P. G. 1984. Lack of effect of exogenous or endogenous gastric inhibitory polypeptide on the elimination rate of Intralipid in man. Acta Med. Scand. 216: 19–23. [DOI] [PubMed] [Google Scholar]
- 24.Ohneda A., Kobayashi T., Nihei J. 1983. Effect of endogenous gastric inhibitory polypeptide (GIP) on the removal of triacylglycerol in dogs. Regul. Pept. 6: 25–32. [DOI] [PubMed] [Google Scholar]
- 25.McIntosh C. H. S., Bremsak I., Lynn F. C., Gill R., Hinke S. A., Gelling R., Nian C., McKnight G., Jaspers S., Pederson R. A. 1999. Glucose-dependent insulinotropic polypeptide stimulation of lipolysis in differentiated 3T3–L1 cells: wortmannin-sensitive inhibition by insulin. Endocrinology. 140: 398–404. [DOI] [PubMed] [Google Scholar]
- 26.Yip R. G., Wolfe M. M. 2000. GIP biology and fat metabolism. Life Sci. 66: 91–103. [DOI] [PubMed] [Google Scholar]
- 27.Kim S. J., Nian C., McIntosh C. H. S. 2007. Activation of lipoprotein lipase by glucose- dependent insulinotropic polypeptide in adipocytes. A role for a protein kinase B, LKB1, and AMP-activated protein kinase cascade. J. Biol. Chem. 282: 8557–8567. [DOI] [PubMed] [Google Scholar]
- 28.Kim S. J., Nian C., McIntosh C. H. S. 2007. Resistin is a key mediator of glucose-dependent insulinotropic polypeptide (GIP) stimulation of lipoprotein lipase (LPL) activity in adipocytes. J. Biol. Chem. 282: 34139–34147. [DOI] [PubMed] [Google Scholar]
- 29.Eckel R. H., Fujimoto W. Y., Brunzell J. D. 1979. Gastric inhibitory polypeptide enhanced lipoprotein lipase activity in cultured preadipocytes. Diabetes. 28: 1141–1142. [DOI] [PubMed] [Google Scholar]
- 30.Knapper J. M., Puddicombe S. M., Morgan L. M., Fletcher J. M. 1995. Investigations into the actions of glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1(7–36)amide on lipoprotein lipase activity in explants of rat adipose tissue. J. Nutr. 125: 183–188. [DOI] [PubMed] [Google Scholar]
- 31.Schreiber E., Matthias P., Muller M. M., Schaffner W. 1989. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 17: 6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim S. J., Choi W. S., Han J. S., Warnock G., Fedida D., McIntosh C. H. S. 2005. A novel mechanism for the suppression of a voltage-gated potassium channel by glucose-dependent insulinotropic polypeptide: protein kinase A-dependent endocytosis. J. Biol. Chem. 280: 28692–28700. [DOI] [PubMed] [Google Scholar]
- 33.Peinado-Onsurbe J., Staels B., Deeb S., Auwerx J. 1992. Lipoprotein lipase expression in undifferentiated hepatoma cells is regulated by progesterone and protein kinase A. Biochemistry. 31: 10121–10128. [DOI] [PubMed] [Google Scholar]
- 34.Staels B., Martin G., Martinez M., Albert C., Peinado-Onsurbe J., Saladin R., Hum D. W., Reina M., Vilaro S., Auwerx J. 1996. Expression and regulation of the lipoprotein lipase gene in human adrenal cortex. J. Biol. Chem. 271: 17425–17432. [DOI] [PubMed] [Google Scholar]
- 35.Merkel M., Eckel R. H., Goldberg R. H. 2002. Lipoprotein lipase: genetics, lipid uptake, and regulation. J. Lipid Res. 43: 1997–2006. [DOI] [PubMed] [Google Scholar]
- 36.Vinson C., Myakishev M., Acharya A., Mir A. A., Moll J. R., Bonovich M. 2002. Classification of human B-ZIP proteins based on dimerization properties. Mol. Cell. Biol. 22: 6321–6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez G. A., Montminy M. R. 1989. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 59: 675–680. [DOI] [PubMed] [Google Scholar]
- 38.Du K., Asahara H., Jhala U. S., Wagner B. L., Montminy M. 2000. Characterization of a CREB gain-of-function mutant with constitutive transcriptional activity in vivo. Mol. Cell. Biol. 20: 4320–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conkright M. D., Canettieri G., Screaton R., Guzman E., Miraglia L., Hogenesch J. B., Montminy M. 2003. TORCs: transducers of regulated CREB activity. Mol. Cell. 12: 413–423. [DOI] [PubMed] [Google Scholar]
- 40.Xu W., Kasper L. H., Lerach S., Jeevan T., Brindle P. K. 2007. Individual CREB-target genes dictate usage of distinct cAMP-responsive coactivation mechanisms. EMBO J. 26: 2890–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oben J., Morgan L., Fletcher J., Marks V. 1991. Effect of the entero-pancreatic hormones, gastric inhibitory polypeptide and glucagon-like polypeptide-1(7–36) amide, on fatty acid synthesis in explants of rat adipose tissue. J. Endocrinol. 130: 267–272. [DOI] [PubMed] [Google Scholar]
- 42.Beck B., Max J. P. 1986. Direct metabolic effects of gastric inhibitory polypeptide (GIP): dissociation at physiological levels of effects on insulin-stimulated fatty acid and glucose incorporation in rat adipose tissue. Diabetologia. 29: 68. [DOI] [PubMed] [Google Scholar]
- 43.Wang H., Eckel R. H. 2009. Lipoprotein lipase: from gene to obesity. Am. J. Physiol. Endocrinol. Metab. 297: E271–E288. [DOI] [PubMed] [Google Scholar]
- 44.Kirchgessner T. G., Chuat J. C., Heinzmann C., Etienne J., Guilhot S., Svenson K., Ameis D., Pilon C., D'Auriol L., Andalibi A., et al. 1989. Organization of the human lipoprotein lipase gene and evolution of the lipase gene family. Proc. Natl. Acad. Sci. USA 86: 9647–9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bey L., Etienne J., Tse C., Brault D., Noe L., Raisonnier A., Arnault F., Hamilton M. T., Galibert F. 1998. Cloning, sequencing and structural analysis of 976 base pairs of the promoter sequence for the rat lipoprotein lipase gene. Comparison with the mouse and human sequences. Gene. 209: 31–38. [DOI] [PubMed] [Google Scholar]
- 46.Du K., Montminy M. 1998. CREB is a regulatory target for the protein kinase Akt/PKB. J. Biol. Chem. 273: 32377–32379. [DOI] [PubMed] [Google Scholar]
- 47.Kato S., Ding J., Du K. 2007. Differential activation of CREB by Akt1 and Akt2. Biochem. Biophys. Res. Commun. 354: 1061–1066. [DOI] [PubMed] [Google Scholar]
- 48.Song D. H., Getty-Kaushik L., Tseng E., Simon J., Corkey B. E., Wolfe M. M. 2007. Glucose-dependent insulinotropic polypeptide enhances adipocyte development and glucose uptake in part through Akt activation. Gastroenterology. 133: 1796–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yip R. G., Boylan M. O., Kieffer T. J., Wolfe M. M. 1998. Functional GIP receptors are present on adipocytes. Endocrinology. 139: 4004–4007. [DOI] [PubMed] [Google Scholar]
- 50.Ebert R., Creutzfeldt W. 1987. Metabolic effects of Gastric Inhibitory Polypeptide. Front. Horm. Res. 16: 175–185. [Google Scholar]
- 51.McTernan P. G., Kusminski C. M., Kumar S. 2006. Resistin. Curr. Opin. Lipidol. 17: 170–175. [DOI] [PubMed] [Google Scholar]
- 52.Janke J., Engeli S., Gorzelniak K., Luft F. C., Sharma A. M. 2002. Resistin gene expression in human adipocytes is not related to insulin resistance. Obes. Res. 10: 1–5. [DOI] [PubMed] [Google Scholar]
- 53.Savage D. B., Sewter C. P., Klenk E. S., Segal D. G., Vidal-Puig A., Considine R. V., O'Rahilly S. 2001. Resistin/Fizz3 Expression in relation to obesity and peroxisome proliferator-activated receptor-γ action in humans. Diabetes. 50: 2199–2202. [DOI] [PubMed] [Google Scholar]