Abstract
The mechanisms involved in the development of alcoholic liver disease (ALD) are not well established. We investigated the involvement of acyl-CoA: diacylglycerol acyltransferase 2 (DGAT2) upregulation in mediating hepatic fat accumulation induced by chronic alcohol consumption. Chronic alcohol feeding caused fatty liver and increased hepatic DGAT2 gene and protein expression, concomitant with a significant suppression of hepatic MAPK/ERK kinase/extracellular regulated kinase 1/2 (MEK/ERK1/2) activation. In vitro studies demonstrated that specific inhibitors of the MEK/ERK1/2 pathway increased DGAT2 gene expression and triglyceride (TG) contents in HepG2 cells, whereas epidermal growth factor, a strong ERK1/2 activator, had the opposite effect. Moreover, chronic alcohol feeding decreased hepatic S-adenosylmethionine (SAM): S-adenosylhomocysteine (SAH) ratio, an indicator of disrupted transmethylation reactions. Mechanistic investigations revealed that N-acetyl-S-farnesyl-l-cysteine, a potent inhibitor of isoprenylcysteine carboxyl methyltransferase, suppressed ERK1/2 activation, followed by an enhanced DGAT2 expression and an elevated TG content in HepG2 cells. Lastly, we demonstrated that the beneficial effects of betaine supplementation in ALD were associated with improved SAM/SAH ratio, alleviated ERK1/2 inhibition, and attenuated DGAT2 upregulation. In conclusion, our data suggest that upregulation of DGAT2 plays an important role in the pathogenesis of ALD, and that abnormal methionine metabolism contributes, at least partially, to DGAT2 upregulation via suppression of MEK/ERK1/2 activation.
Keywords: alcohol, ERK1/2, DGAT2, betaine
Alcoholic liver disease (ALD) continues to be an important health problem in the United States. Although much progress has been made over the past two decades, the mechanisms involved in its initiation and progression remain to be fully understood. The disease is characterized by early steatosis, subsequent steatohepatitis (steatosis with inflammatory cell infiltration and necrosis), and, in some instances, progression to fibrosis and/or cirrhosis (1, 2). Excessive neutral fat accumulation in hepatocytes (steatosis) is the most common and earliest response of the liver to chronic alcohol consumption (3) and plays a critical role in disease progression. Hepatic steatosis results from an imbalance between intrahepatic triglyceride (TG) production and removal. Both uptake of free fatty acids (FAs) to the liver and de novo synthesis contribute to hepatic TG production, whereas FA β-oxidation and formation of very low density lipoprotein (VLDL) particles contribute to hepatic TG removal.
The final step and rate-limiting reaction in TG synthesis is catalyzed by acyl CoA:diacylglycerol acyltransferase (DGAT), which covalently joins a fatty acyl-CoA and a diacylglycerol (DG) molecule to form TG. In mammals, DGAT occurs in two isoforms, DGAT1 and DGAT2, from distinct gene families (4, 5). Although both isoforms are widely expressed and present at high levels in white adipose tissue, DGAT1 is most highly expressed in the small intestine, whereas DGAT2 is primarily expressed in the liver (4, 6). Evidence suggest that the two enzymes play different roles in TG metabolism, with DGAT2 participating in steatosis and DGAT1 in VLDL synthesis. Overexpression of liver-specific DGAT2 in mice results in hepatic steatosis (7). Conversely, inhibition of DGAT2 with antisense oligonucleotides reverses hepatic steatosis in ob/ob mice and in mice challenged with high-fat diet (8–10), suggesting that this enzyme plays a critical role in the development of fatty liver disease. Although strong evidence supports that fatty liver development induced by chronic alcohol consumption is secondary to increased de novo synthesis, the effect of alcohol exposure on DGAT regulation remains to be clarified.
Disrupted methionine metabolism plays a pathological role in ALD (11–15). S-adenosylmethionine (SAM) is the first product in the methionine metabolic pathway and a universal methyl donor. After donating its methyl group for transmethylation reaction, SAM is converted into S-adenosylhomocysteine (SAH), which is subsequently hydrolyzed to homocysteine and adenosine. In turn, SAH is a potent inhibitor of most known methyltransferases. Alcohol-induced abnormal methionine metabolism is characterized by disrupted transmethylation reactions due to decreased SAM and increased SAH levels and affects numerous signaling pathways involved in apoptosis, regeneration, and lipid metabolism. Among these is the Ras/ERK1/2 pathway. The extracellular signal-regulated kinases 1 and 2 (ERK1/2) are members of the mitogen-activated protein kinase (MAPK) family, whose activation results in cell growth, proliferation, survival, and inflammation (16). The activation of ERK1/2 is strongly dependent on the GTPase Ras, whose activity requires association with cellular membranes (17, 18). Ras plasma membrane association requires a series of posttranslational modifications of its carboxyl terminus, including farnesylation, proteolysis, and methylation of its CAAX sequence. The methylation reactions require SAM as a methyl group donor to produce methylated Ras. In this context, ERK1/2 activation is closely related to methionine metabolism.
We previously reported that ERK1/2 phosphorylation was reduced in the liver of alcohol-fed rats, and this is associated with hypercholesterolemia and reduced hepatic low density lipoprotein (LDL) receptor expression, suggesting that the ERK1/2 pathway participates in the regulation of hepatic lipid metabolism (19). In the present study, we conducted in vivo and in vitro experiments to investigate the role of DGAT2 upregulation in the development of hepatic steatosis in alcohol-fed mice and the potential mechanistic involvement of suppression of the MEK/ERK1/2 pathway in DGAT2 upregulation.
MATERIALS AND METHODS
Animals and treatments
Male C57BL/6 mice weighing 25 ± 0.5 g (means ± SD) were obtained from the Jackson Laboratory (Bar Harbor, ME). Studies were approved by the Institutional Animal Care and Use Committee, which is certified by the American Association of Accreditation of Laboratory Animal Care. Eight mice were randomly assigned to two groups and fed for 4 weeks with liquid diets containing (in percent of energy intake) 18% protein, 35% fat, 12% carbohydrate, and 35% either ethanol (alcohol-fed, AF) or an isocaloric maltose-dextrin mixture (pair-fed, PF), according to Lieber and De Carli (20). Food intake and body weight were recorded daily and weekly, respectively. For betaine supplementation, betaine (anhydrous; Sigma, St. Louis, MO) was supplemented in the alcohol-containing liquid diet (1%, w/v) and started simultaneously with AF diet. Mice were euthanized and plasma and liver tissue samples harvested at the end of the experiment.
Cells and culture conditions
HepG2 cells, a human hepatoma cell line, were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and cultured in DMEM containing 10% (v/v) fetal bovine serum, 2 mM glutamine, 5U/ml penicillin, and 50μg/ml streptomycin at 37°C in a humidified O2/CO2 (19:1) atmosphere.
Plasma biochemical assays
Plasma levels of alanine aminotransferases (ALT) and TG were assayed using commercially available kits (Infinity, Thermo Electron, Melbourne, Australia).
Liver TG measurements
For intrahepatic TG measurement, liver tissues (∼80 mg) were homogenized in 1.0ml NaCl (50 mM) solution and hepatic total lipids were extracted overnight in 10 ml heptane:isopropanol (3:2) mixture at 4°C. Hepatic TG content was determined by enzymatic colorimetric methods using a commercially available kit (Infinity, Thermo Electron, Melbourne, Australia).
Measurement of methionine metabolites
Liver tissue was homogenized and deproteinized in 4% metaphosphoric acid (1:8, m/v). The homogenates were centrifuged at 15,000 g for 10 min. SAM and SAH were determined via a high-performance liquid chromatography (HPLC) method using a 5-mm Hypersil C-18 column (250 × 4.6 mm). The mobile phase consisted of 40 mM ammonium phosphate, 8 mM heptane sulfonic acid [ion-pairing reagent (pH 5.0)], and 6% acetonitrile, and was delivered at a flow rate of 1.0ml/minute. SAM, SAH, betaine, and GSH were detected using a Waters 740 UV detector at 254nm. An internal standard, S-adenosylethionine, was added to all samples and standard solutions to a concentration of 100µM.
Measurement of intracellular TG content
To determine the intracellular TG content, HepG2 cells seeded in 24-well plates were washed twice with phosphate buffered saline (PBS) and cellular lipids were extracted by 1ml hexane:isopropanol (3:2) mixture. TG content was measured using a TG assay kit (Infinity, Thermo Electron, Melbourne, Australia). Cells undergoing the same treatment conditions were lysed in RIPA buffer for protein concentration determination and data normalization.
Suppression of DGAT2 expression by siRNA
RNA targeting the human DGAT2 gene and a control small interfering (si)RNA containing a scrambled sequence (Ambion, Austin, TX) were transfected by siPORT™ NeoFX™ Transfection Agent (Ambion, Austin, TX), according to the manufacturer's instructions.
Measurement of DGAT2 activity
The microsomal fraction from HepG2 cells was prepared as described previously (21). Because DGAT2 activity is inhibited at high concentrations of MgCl2 (100 mM), the DGAT assay was carried out in the presence of 8 mM and 100 mM MgCl2 and DGAT2 activity was calculated by subtracting the value obtained at 100 mM from the value obtained at 8 mM. Brief, 200µl of the reaction mixture containing 200µM sn-1,2-dioleoylglycerol (Sigma-Aldrich, Saint Louis, MO) in acetone, 30µM [14C]oleoyl-CoA (American Radiolabeled Chemicals, Saint Louis, MO), 8 mM MgCl2, 200µg BSA, and 10µg microsomal protein in 175 mM Tris-HCl buffer, pH 8.0 was incubated for 30 min at 25°C. After incubation, the reaction was stopped by adding 1.5ml stop solution [2-propanol-heptane-water (80:20:2, v/v)]. The top heptane phase was collected and washed with alkaline ethanol. An aliquot of the top heptane phase was used for radioactivity measurements using a Beckman LS 5801 counter.
Quantitative real-time RT-PCR
Total RNA from either frozen liver tissue or cultured cells was isolated with a phenol-chloroform extraction. For each sample, 1.0μg total RNA was reverse transcribed using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). The cDNA was amplified in MicroAmp Optical 96-well reaction plates with a SYBR Green PCR Master Mix (Applied Biosystems) on an Applied Biosystems Prism 7000 sequence detection system. Relative gene expression was calculated after nomalization by a house-keeping gene (mouse or human 18S rRNA).
Western blotting
Liver tissues were homogenized and HepG2 cells were lysed in RIPA buffer and the isolated proteins were separated by SDS polyacrylamide gel electrophoresis and transferred to 0.45 μm polyvinylidene difluoride membrane. After transfer, membranes were blocked in 5% BSA in PBS with 0.1% Tween 20 and probed with anti-DGAT2 (Novus Biologicals, Littleton, CO), anti-SREBP-1c (Santa Cruz Biotechnology, Santa Cruz, CA), anti-phospho-ERK1/2 or anti-ERK1/2 (Cell Signaling Technology, Danvers, MA) antibodies. Horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence substrate kit were used in detection of specific proteins.
Statistical analysis
All data are expressed as means ± SD. Statistical analysis was performed using a one-way ANOVA and further analyzed by Newman-Keuls test for statistical difference. Differences between treatments were considered to be statistically significant at P < 0.05.
RESULTS
Chronic alcohol exposure increased hepatic DGAT2 gene expression and protein production
Chronic alcohol consumption for 4 weeks caused fatty liver and liver injury as evidenced by significantly increased plasma ALT levels, increased liver weight versus body weight ratio, and elevated liver TG content in the alcohol-fed group (data not shown). Long-term AF increased DGAT2 gene (Fig. 1A) and protein expression (Fig. 1B, C) in the liver when compared with the PF group. We also examined the effect of AF on hepatic expression of sterol regulatory element binding protein-1c (SREBP-1c), the master regulator of de novo FA synthesis. In line with previous studies (22–24), AF significantly elevated SREBP-1c protein in the liver (Fig. 1D, E).
Fig. 1.
Chronic alcohol exposure increased hepatic DGAT2 gene expression and protein production. Male C57BL/6 mice were pair-fed liquid diets with or without ethanol for 4 weeks. Chronic alcohol exposure increased DGAT2 gene expression (A) and protein abundance (B) in the liver. C: Quantitative analysis of DGAT2 protein in the liver, normalized by corresponding GAPDH expression level. D: The protein abundance of SREBP-1c in the liver was augmented significantly in alcohol-fed mice; E: quantitative analysis of SREBP-1c protein expression in the liver, normalized by corresponding actin expression level. Data are means ± SD (n = 4). * P < 0.05. PF, pair-fed; AF, alcohol-fed.
Chronic alcohol exposure resulted in ERK1/2 suppression in the liver
To examine the effect of AF on MAPK activation in the liver, we conducted immunoblotting analysis using total liver tissue extracts from both PF and AF mice. As shown in Fig. 2, AF had no effect on c-Jun N-terminal kinases (JNK) activation (Fig. 2A), whereas the activation of p38 was minimally enhanced (Fig. 2B). However, AF resulted in a significant reduction in the phosphorylation of ERK1/2 (Fig. 2C, D), which was in line with our previous observation in rats (19). No changes in protein levels of the three members of the MAPK family were observed in the liver of AF animals when compared with PF controls.
Fig. 2.
Chronic alcohol exposure resulted in prominent ERK1/2 suppression in the liver. Male C57BL/6 mice were pair-fed liquid diets with or without ethanol for 4 weeks. Chronic alcohol exposure had no effect on JNK activation (A) and minimally enhanced p38 activation (B) in the liver. C: Chronic alcohol exposure decreased the phosphorylation levels of ERK1/2 in the liver; D: quantitative analysis of ERK1/2 phosphorylation in the liver, normalized by corresponding total ERK1/2 expression level. Data are means ± SD (n = 4). * P < 0.05. PF, pair-fed; AF, alcohol-fed.
Chronic alcohol exposure caused abnormal methionine metabolism in the liver
To determine the effects of AF on methionine metabolism in the liver, we measured SAM, SAH, betaine, and glutathione (GSH) concentrations in the liver via HPLC. In line with our previous reports, AF significantly decreased hepatic SAM levels, whereas hepatic SAH levels were significantly elevated, leading to a significantly decreased SAM/SAH ratio. Liver betaine levels decreased slightly in AF, whereas GSH levels in the liver significantly decreased (Table 1).
TABLE 1.
Changes in methionine metabolism in the liver
| PF | AF | AF+BT | |
|---|---|---|---|
| SAM (nmol/g liver) | 172.25 ± 15.95 | 115.93 ± 36.04a | 221.33 ± 36.69b |
| SAH (nmol/g liver) | 69.75 ± 8.62 | 219.33 ± 94.36a | 175.67 ± 51.40a |
| SAM/SAH | 2.48 ± 0.08 | 0.63 ± 0.35a | 1.30 ± 0.25ab |
| Betaine (mg/g liver) | 6.74 ± 1.11 | 4.89 ± 2.05 | 18.87 ± 8.76ab |
| GSH (mmol/g liver) | 4.66 ± 0.10 | 3.93 ± 0.22a | 4.49 ± 0.19b |
Data represent mean ± SD (N = 4). PF, pair-fed; AF, alcohol-fed; BT, betaine.
P < 0.05 compared with PF.
P < 0.05 compared with AF.
MEK/ERK1/2 inhibition leads to increased intracellular TG accumulation in hepatocytes
To determine if ERK1/2 inhibition was associated with increased TG accumulation in the liver, we treated HepG2 cells with two specific inhibitors of the MEK/ERK1/2 pathway, U0126 (10µM) and PD98059 (10µM), before challenging cells with oleic acid. Intracellular TG levels were assayed 20 h later. As shown in Fig. 3, both inhibitors significantly increased intracellular TG content (Fig. 3A). To examine whether TG accumulation could be prevented by enhancing ERK1/2 activation, we treated HepG2 cells with epidermal growth factor (EGF) (100ng/ml), an activator of ERK1/2 signaling, prior to oleic acid challenge. EGF treatment reduced intracellular TG content (Fig. 3B), which was associated with enhanced ERK1/2 activation (Fig. 3C).
Fig. 3.
MEK/ERK1/2 inhibition leads to increased intracellular TG accumulation in hepatocytes. HepG2 cells were pretreated with MEK/ERK1/2 inhibitors or activator for 2 h, and then challenged with 0.3 mM oleic acids for 20 h. A: MEK/ERK1/2 inhibitors, U0126 (10µM) or PD98059 (10µM), elevated intracellular TG content; whereas (B) EGF (100ng/ml), an activator of ERK1/2 signaling, reduced intracellular TG content in HepG2 cells. C: The time course for the effect of EGF on ERK1/2 activation. HepG2 cells were treated with EGF (100ng/ml) and cell lysates were collected at different time points. ERK1/2 activation was measured by detecting its phosphorylation. On the other hand, HepG2 cells were treated with U0126 (10µM) or EGF (100ng/ml) for 18 h, then the total RNA was isolated and DGAT2 gene expression was analyzed by real time RT-PCR. D: U0126 enhanced gene expression of DGAT2; conversely, (E) EGF inhibited the gene expression of DGAT2 in HepG2 cells. Further, the microsomes in HepG2 cells between untreated group and U0126-treated group were isolated for determining the DGAT2 activity. F: Application of U0126 to inhibit ERK1/2 activation elevated the DGAT2 activity significantly in HepG2 cells. All values are denoted as means ± SD from three or more independent batches of cells, *P < 0.05.
MEK/ERK1/2 suppression upregulated DGAT2 expression and activity in HepG2 cells
To test whether hepatic ERK1/2 inhibition affected DGAT2 expression, gene and protein expression and DGAT2 activity were measured. Exposure to the ERK1/2 inhibitor U0126 (10µM) resulted in an ∼5-fold increase in DGAT2 mRNA expression in HepG2 cells compared with the untreated group (Fig. 3D). In contrast, enhanced ERK1/2 activation by EGF (100ng/ml) decreased DGAT2 mRNA levels (Fig. 3E). Moreover, exposure of cells to U0126 to inhibit ERK1/2 activation significantly increased DGAT2 enzymatic activity (Fig. 3F). Furthermore, transfection of HepG2 cells with DGAT2 siRNA, which caused an 80% decrease in DGAT2 mRNA expression, significantly decreased intracellular TG levels (Fig. 4A, B). Accordingly, treatment with DGAT2 siRNA completely prevented U0126-induced intracellular TG increase in HepG2 cells (Fig. 4B), implying that upregulation of DGAT2 contributes to the increased hepatic TG content by ERK1/2 inhibition.
Fig. 4.
DGAT2 silencing prevented the elevation of TG content caused by MEK/ERK1/2 suppression in HepG2 cells. Transfection of siRNA for DGAT2 into HepG2 cells decreased DGAT2 mRNA levels (A), which prevented U0126 (10µM)-induced intracellular TG elevation (B). All values were denoted as means ± SD from three or more independent batches of cells, *P < 0.05.
Inhibition of methyltransferase suppressed MEK/ERK1/2 activation, enhanced DGAT2 expression, and elevated TG contents in HepG2 cells
In order to determine whether inhibition of transmethylation reactions was related with hepatic ERK1/2 inhibition and DGAT2 induction, we applied N-acetyl-S-farnesyl-l-cysteine (AFC), an inhibitor of SAM-dependent methyltransferases (specific for isoprenylcysteine carboxyl methyltransferase, ICMT), to HepG2 cells. Result showed that treatment with AFC (from 10µM to 40µM) for 24 h decreased ERK1/2 phosphorylation in a dose-dependent fashion (Fig. 5A). In addition, AFC significantly augmented intracellular TG levels (Fig. 5B) and DGAT2 gene expression (Fig. 5C) when compared with the untreated group.
Fig. 5.
Inhibition of methyltransferases suppressed MEK/ERK activation, enhanced DGAT2 expression, and elevated TG contents in HepG2 cells. A: AFC suppressed the phosphorylation level of ERK1/2. HepG2 cells were treated with methyltransferases inhibitor AFC (10µM, 20µM or 40µM) for 24 h and cell lysates were collected for detecting ERK1/2 phosphorylation. B: AFC increased the intracellular TG content. HepG2 cells were pretreated with AFC (40µM) for 2 h before challenged with 0.3 mM oleic acids. Intracellular TG contents were measured 20 h later. C: AFC decreased the gene expression of DGAT2 in HepG2 cells. HepG2 cells were treated with AFC (40µM) for 18 h and total RNA was isolate and DGAT2 gene expression was determined by real time RT-PCR. All values were denoted as means ± SD from three or more independent batches of cells, *P < 0.05.
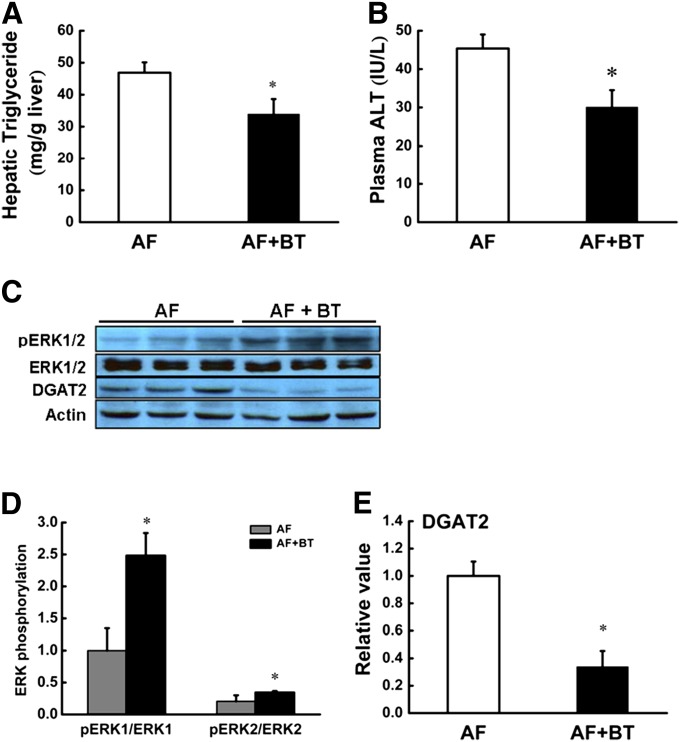
The beneficial effect of betaine in ALD was associated with improved methionine metabolism, alleviated ERK1/2 inhibition, and attenuated DGAT2 induction in the liver
Betaine exerts beneficial effects on fatty liver diseases by improving methionine metabolism. In an attempt to elucidate the mechanisms for the beneficial effect of betaine in ALD, betaine, at a dose of 1% (v/w), was supplemented in the alcohol-containing liquid diet. Four weeks later, we found that betaine supplementation prevented alcohol-induced fatty liver and liver injury, as evidenced by lowered TG content in the liver (Fig. 6A) and ALT levels in plasma (Fig. 6B), which were associated with significantly improved hepatic methionine metabolism (Table 1). Data showed that the beneficial effect of betaine supplementation was associated with alleviated ERK1/2 inhibition and attenuated DGAT2 induction (Fig. 6C–E) in the liver.
Fig. 6.
Betaine supplementation alleviated alcohol-induced ERK1/2 inhibition and DGAT2 induction in the liver. Male C57BL/6 mice were fed alcohol-containing liquid diets (AF) with or without betaine [1% (wt/vol)] supplementation for 4 weeks. A: Liver TG contents; B: Plasma ALT levels; C: Betaine supplementation attenuated alcohol-induced ERK1/2 inhibition and DGAT2 induction in the liver. Data are means ± SD (n = 4). D: Quantitative analysis of ERK1/2 phosphorylation in the liver, normalized by corresponding total ERK1/2 expression level. E: Quantitative analysis of DGAT2 expression in the liver, normalized by corresponding actin expression level. Data are means ± SD (n = 4). *P < 0.05. AF, alcohol-fed; BT, betaine.
DISCUSSION
Neutral fat (mainly TG) deposition is the initial stage of alcoholic fatty liver disease and plays a critical role in disease progression. Although the exact mechanisms remain elusive, it is generally accepted that increased hepatic de novo FA synthesis plays a critical role in the development of fatty liver. FA synthesis is centrally regulated by SREBP-1c, a nuclear transcription factor, via a subset of enzymes involved in de novo FA synthesis, including acetyl-coA carboxylase, fatty acid synthase, and stearoyl-CoA desaturase. Enhanced gene expression and transactivation of SREBP-1c in the liver and subsequently increased hepatic de novo FA synthesis by AF have been documented by several groups (22–24). However, the effects of alcohol consumption on the downstream steps in TG synthesis remain unclear. TG is synthesized from three FAs and glycerol through ester bonds. Among the complex metabolism network for TG synthesis in the liver, DGAT is a rate-limiting enzyme catalyzing the final step in TG synthesis by facilitating the linkage of sn-1,2-diacylglycerol with a long-chain fatty acyl-CoA. Overexpression of DGAT2 is associated with hepatic steatosis (7); suppression of DGAT2 with antisense oligonucleotides reverses diet-induced hepatic steatosis (8–10), suggesting that DGAT2 exerts an important role in the development of fatty liver diseases. The present study provides initial evidence showing that chronic alcohol consumption induces expression of DGAT2 at both the gene and protein level. Because FA for TG synthesis in the liver derives not only from endogenous de novo lipogenesis but also exogenous sources (e.g., dietary fat or white adipose tissue via lipolysis during fasting), it is conceivable that upregulation of DGAT2 by chronic alcohol consumption may play an important role in the development of alcohol-induced hepatic steatosis.
ERK1/2-regulated pathways play an important role in controlling lipid metabolism in the liver (25, 26). We and others previously reported that chronic alcohol exposure causes significant MEK/ERK1/2 suppression in the liver (19, 27, 28). In the present study, we demonstrate that alcohol-induced hepatic MEK/ERK1/2 suppression is associated with increased DGAT2 expression and protein production. In vitro studies reveal that specific inhibition of MEK/ERK1/2 activation increases gene expression and enzymatic activity of DGAT2 in HepG2 cells and causes intracellular TG accumulation. Conversely, enhanced ERK1/2 activation through EGF stimulation, slightly but significantly suppresses DGAT2 gene expression and prevents intracellular TG accumulation. Furthermore, our data show that elevation of intracellular TG content induced by MEK/ERK1/2 is inhibited by DGAT2 siRNA in HepG2 cells, indicating that DGAT2 upregulation contributes to increased TG synthesis by MEK/ERK1/2 inactivation. Because HepG2 cells have high N-ras activity, and thus high ERK1/2 activity (26, 29) compared with primary hepatocytes, it is conceivable that the decreased amplitude in DGAT2 gene expression by EGF in HepG2 cells was much less than the increased one triggered by ERK1/2 inhibitor U0126. Collectively, our data support the hypothesis that suppression of ERK1/2 activation by chronic alcohol exposure plays a critical role in upregulating DGAT2 in the liver. Our study suggests that the hepatic MEK/ERK1/2 pathway may be a therapeutic target for treatment of ALD. Further support for this hypothesis derived from a study showing that EGF, a strong ERK1/2 activator, confers beneficial effects on alcohol-induced fatty liver and liver injury (30).
ERK1/2 is a member of the MAPK family, whose activation results in cell growth, proliferation, survival, and inflammation. The results with regard to the effect of alcohol exposure on ERK1/2 activation are controversial, mainly due to the cell type usage and exposure duration. For instance, accumulating evidence indicates that chronic alcohol exposure activates the MEK/ERK1/2 pathway and potentiates endotoxin-stimulated ERK1/2 activation in Kupffer cells, resulting in increased synthesis of tumor necrosis factor and augmented inflammatory responses (31, 32). It has also been well documented that acute exposure to alcohol results in modest activation of ERK1/2 in hepatocytes (33), whereas chronic alcohol exposure suppresses both constitutive and growth factor-stimulated ERK1/2 activation in the liver (27, 28). The mechanisms involved in MEK/ERK1/2 inhibition by chronic alcohol exposure are not well understood and are probably multifactorial. The ethanol-induced lipid peroxidation product 4-hydroxy-2-nonenal has been reported to be an upstream modulator of ERK1/2 activation (27). Inhibition of the insulin receptor substrate 1 signaling pathway by chronic alcohol consumption may also contribute to suppressed ERK1/2 activation in the liver (28). The Ras/Raf/MEK/ERK1/2 pathway is the major signal transduction pathway for ERK1/2 activation, which is strongly dependent on the GTPase Ras, whose activity requires its association with cellular membranes (17, 18). Ras plasma membrane association requires a series of posttranslational modifications of its carboxyl terminus, including farnesylation, proteolysis, and methylation of its CAAX sequence. These modifications allow CAAX proteins to associate with cellular membranes and promote protein-protein interactions (34). The methylation reactions require SAM as a methyl group donor to produce methylated Ras, thus linking methionine metabolism to modulation of MEK/ERK1/2 activation. Indeed, intracellular SAH level is negatively related to ICMT activity (34). In addition, 3-Deazaadenosine, a potent inhibitor of S-adenosylhomocysteine hydrolase, whose inhibition induces intracellular SAH accumulation, prevents vascular smooth muscle cell proliferation and neointima formation by interfering with Ras methylation and thereby with mitogenic activation of ERK1/2 (36). Abnormalities in hepatic methionine metabolism are well documented in ALD. Decreased phosphatidylethanolamine methyltransferase activity due to disrupted transmethylation reactions and subsequently decreased phosphatidylcholine synthesis contributes to alcohol-induced fatty liver via suppressing VLDL formation and secretion. In agreement with this observation, improvement of methionine metabolism by betaine supplementation alleviates phosphatidylethanolamine methyltransferase suppression and attenuates alcoholic fatty liver (37). In line with previous studies, our results show that chronic alcohol exposure is associated with suppressed hepatic transmethylation reactions, evidenced by decreased SAM and increased SAH levels in the liver, leading to a significantly decreased SAM:SAH ratio. In the present study, we examined whether inhibition of methylation reactions could also modulate ERK1/2 activation in hepatocytes by treating HepG2 cells with AFC, a specific inhibitor of ICMT. Our results demonstrate that AFC exposure leads to ERK1/2 inhibition, which is associated with increased DGAT2 expression and elevated intracellular TG accumulation. Since ICMT is the methyltransferase specifically involved in posttranslational modification of Ras protein, these results suggest that ERK1/2 inhibition is a mechanistic link between abnormal methionine metabolism and DGAT2 upregulation in the liver. These observations also imply that the beneficial effects of betaine and folate supplementation in ALD may result from their ability to activate ERK1/2 and thereby attenuate DGAT2 upregulation by improving hepatic methionine metabolism. This is indeed validated by our study with betaine supplementation, in which we found that betaine supplementation improves liver injury and hepatic fat accumulation in AF mice, in parallel with improved methionine metabolism, alleviated hepatic ERK1/2 suppression, and attenuated hepatic DGAT2 overexpression.
Hepatic lipid metabolism is complex and dynamically regulated. Excessive TG accumulation in the liver originates from an imbalance between TG synthesis and removal via mitochondrial β-oxidation and VLDL secretion. In line with previous reports (22–24), in this study, we demonstrate increased SREBP-1c protein abundance in the liver of AF mice (Fig. 3D, E), an indication of enhanced de novo FA synthesis. Moreover, increased plasma FA levels suggest that FA from adipose tissue lipolysis contributes to the intrahepatic FA pool for TG synthesis. Although in the present study we did not directly measure the effect of chronic alcohol exposure on VLDL secretion and mitochondrial β-oxidation of FA, elevated plasma ketone bodies and fasting TG concentrations in AF mice provide indirect evidence of enhanced FA oxidation and VLDL secretion in the early stages of ALD. The elevated plasma TG content is in line with the observed hepatic DGAT1 expression (data not shown), which plays an important role in VLDL secretion (38). These data altogether suggest that both TG synthesis and disposal are enhanced in the early stage of alcoholic liver disease and that fatty liver develops when the capability of the liver to remove TG dose not compensate for the increased synthesis.
In conclusion, we demonstrate that upregulation of hepatic DGAT2 by chronic alcohol exposure contributes to the development of ALD. Abnormal hepatic methionine metabolism, specifically the suppression of transmethylation reactions, plays a mechanistic role in alcohol-induced DGAT2 upregulation by suppressing MEK/ERK1/2 activation. Therefore, inhibition of ERK1/2 activation represents a critical link between methionine metabolism and fatty liver development via upregulation of hepatic DGAT2 expression.
Acknowledgments
The authors thank Dr. Alan Diamond from the Department of Pathology, Dr. P. V. Subbaiah from the Department of Medicine, and Dr. Giamila Fantuzzi from the Department of Kinesiology and Nutrition, University of Illinois at Chicago, for their technical support, scientific advice, and generous editorial assistance.
Footnotes
Abbreviations:
- AF
- alcohol-fed
- AFC
- N-acetyl-S-farnesyl-L-cysteine
- ALD
- alcoholic liver disease
- ALT
- alanine aminotransferases
- DG
- diacylglycerol
- DGAT
- acyl-CoA:diacylglycerol acyltransferase
- EGF
- epidermal growth factor
- ERK1/2
- extracellular signal-regulated kinases 1 and 2
- GSH
- glutathione
- ICMT
- isoprenylcysteine carboxyl methyltransferase
- MAPK
- mitogen-activated protein kinase
- MEK
- MAPK/ERK kinase
- PF
- pair-fed
- SAH
- S-adenosylhomocysteine
- SAM
- S-adenosylmethionine
- SREBP-1c
- sterol regulatory element binding protein-1c
- TG
- triglyceride
This work was supported by the National Institutes of Health grants K01 AA015344 and R01 AA017442 (Z. S.), and the National Natural Science Foundation of China 81000168 and the China Postdoctoral Science Foundation 20100471022 (Z. W.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Lelbach W. K. 1966. Liver damage in chronic alcoholism. Results of a clinical, clinical-chemical and bioptic-histological study in 526 alcoholic patients during a low-calorie diet in an open drinking sanatorium. Acta Hepatosplenol. 13: 321–349. [PubMed] [Google Scholar]
- 2.Diehl A. M. 2002. Liver disease in alcohol abusers: clinical perspective. Alcohol. 27: 7–11. [DOI] [PubMed] [Google Scholar]
- 3.Purohit V., Russo D., Coates P. M. 2004. Role of fatty liver, dietary fatty acid supplements, and obesity in the progression of alcoholic liver disease: introduction and summary of the symposium. Alcohol. 34: 3–8. [DOI] [PubMed] [Google Scholar]
- 4.Cases S., Smith S. J., Zheng Y. W., Myers H. M., Lear S. R., Sande E., Novak S., Collins C., Welch C. B., Lusis A. J., et al. 1998. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA. 95: 13018–13023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cases S., Stone S. J., Zhou P., Yen E., Tow B., Lardizabal K. D., Voelker T., Farese R. V., Jr 2001. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J. Biol. Chem. 276: 38870–38876. [DOI] [PubMed] [Google Scholar]
- 6.Farese R. V., Jr, Cases S., Smith S. J. 2000. Triglyceride synthesis: insights from the cloning of diacylglycerol acyltransferase. Curr. Opin. Lipidol. 11: 229–234. [DOI] [PubMed] [Google Scholar]
- 7.Monetti M., Levin M. C., Watt M. J., Sajan M. P., Marmor S., Hubbard B. K., Stevens R. D., Bain J. R., Newgard C. B., Farese R. V., Sr, et al. 2007. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 6: 69–78. [DOI] [PubMed] [Google Scholar]
- 8.Choi C. S., Savage D. B., Kulkarni A., Yu X. X., Liu Z. X., Morino K., Kim S., Distefano A., Samuel V. T., Neschen S., et al. 2007. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J. Biol. Chem. 282: 22678–22688. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y., Millar J. S., Cromley D. A., Graham M., Crooke R., Billheimer J. T., Rader D. J. 2008. Knockdown of acyl-CoA:diacylglycerol acyltransferase 2 with antisense oligonucleotide reduces VLDL TG and ApoB secretion in mice. Biochim. Biophys. Acta. 1781: 97–104. [DOI] [PubMed] [Google Scholar]
- 10.Yu X. X., Murray S. F., Pandey S. K., Booten S. L., Bao D., Song X. Z., Kelly S., Chen S., McKay R., Monia B. P., et al. 2005. Antisense oligonucleotide reduction of DGAT2 expression improves hepatic steatosis and hyperlipidemia in obese mice. Hepatology. 42: 362–371. [DOI] [PubMed] [Google Scholar]
- 11.Halsted C. H., Villanueva J., Chandler C. J., Stabler S. P., Allen R. H., Muskhelishvili L., James S. J., Poirier L. 1996. Ethanol feeding of micropigs alters methionine metabolism and increases hepatocellular apoptosis and proliferation. Hepatology. 23: 497–505. [DOI] [PubMed] [Google Scholar]
- 12.Esfandiari F., You M., Villanueva J. A., Wong D. H., French S. W., Halsted C. H. 2007. S-adenosylmethionine attenuates hepatic lipid synthesis in micropigs fed ethanol with a folate-deficient diet. Alcohol. Clin. Exp. Res. 31: 1231–1239. [DOI] [PubMed] [Google Scholar]
- 13.Lee T. D., Sadda M. R., Mendler M. H., Bottiglieri T., Kanel G., Mato J. M., Lu S. C. 2004. Abnormal hepatic methionine and glutathione metabolism in patients with alcoholic hepatitis. Alcohol. Clin. Exp. Res. 28: 173–181. [DOI] [PubMed] [Google Scholar]
- 14.Song Z., Zhou Z., Uriarte S., Wang L., Kang Y. J., Chen T., Barve S., McClain C. J. 2004. S-adenosylhomocysteine sensitizes to TNF-alpha hepatotoxicity in mice and liver cells: a possible etiological factor in alcoholic liver disease. Hepatology. 40: 989–997. [DOI] [PubMed] [Google Scholar]
- 15.Song Z., Zhou Z., Deaciuc I., Chen T., McClain C. J. 2008. Inhibition of adiponectin production by homocysteine: a potential mechanism for alcoholic liver disease. Hepatology. 47: 867–879. [DOI] [PubMed] [Google Scholar]
- 16.Pearson G., Robinson F., Beers Gibson T., Xu B. E., Karandikar M., Berman K., Cobb M. H. 2001. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22: 153–183. [DOI] [PubMed] [Google Scholar]
- 17.Willumsen B. M., Christensen A., Hubbert N. L., Papageorge A. G., Lowy D. R. 1984. The p21 ras C-terminus is required for transformation and membrane association. Nature. 310: 583–586. [DOI] [PubMed] [Google Scholar]
- 18.Chiu V. K., Silletti J., Dinsell V., Wiener H., Loukeris K., Ou G., Philips M. R., Pillinger M. H. 2004. Carboxyl methylation of Ras regulates membrane targeting and effector engagement. J. Biol. Chem. 279: 7346–7352. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z., Yao T., Song Z. 2010. Chronic alcohol consumption disrupted cholesterol homeostasis in rats: down-regulation of low-density lipoprotein receptor and enhancement of cholesterol biosynthesis pathway in the liver. Alcohol. Clin. Exp. Res. 34: 471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lieber C. S., De Carli L. M. 1973. Ethanol dependence and tolerance: a nutritionally controlled experimental model in the rat. Res. Commun. Chem. Pathol. Pharmacol. 6: 983–991. [PubMed] [Google Scholar]
- 21.Ganji S. H., Tavintharan S., Zhu D., Xing Y., Kamanna V. S., Kashyap M. L. 2004. Niacin noncompetitively inhibits DGAT2 but not DGAT1 activity in HepG2 cells. J. Lipid Res. 45: 1835–1845. [DOI] [PubMed] [Google Scholar]
- 22.Shinohara M., Ji C., Kaplowitz N. 2010. Differences in betaine-homocysteine methyltransferase expression, endoplasmic reticulum stress response, and liver injury between alcohol-fed mice and rats. Hepatology. 51: 796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.You M., Liang X., Ajmo J. M., Ness G. C. 2008. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am. J. Physiol. Gastrointest. Liver Physiol. 294: G892–G898. [DOI] [PubMed] [Google Scholar]
- 24.Ji C., Chan C., Kaplowitz N. 2006. Predominant role of sterol response element binding proteins (SREBP) lipogenic pathways in hepatic steatosis in the murine intragastric ethanol feeding model. J. Hepatol. 45: 717–724. [DOI] [PubMed] [Google Scholar]
- 25.Kong W., Wei J., Abidi P., Lin M., Inaba S., Li C., Wang Y., Wang Z., Si S., Pan H., et al. 2004. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 10: 1344–1351. [DOI] [PubMed] [Google Scholar]
- 26.Tsai J., Qiu W., Kohen-Avramoglu R., Adeli K. 2007. MEK-ERK inhibition corrects the defect in VLDL assembly in HepG2 cells: potential role of ERK in VLDL-ApoB100 particle assembly. Arterioscler. Thromb. Vasc. Biol. 27: 211–218. [DOI] [PubMed] [Google Scholar]
- 27.Sampey B. P., Stewart B. J., Petersen D. R. 2007. Ethanol-induced modulation of hepatocellular extracellular signal-regulated kinase-1/2 activity via 4-hydroxynonenal. J. Biol. Chem. 282: 1925–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeon J. E., Califano S., Xu J., Wands J. R., De La Monte S. M. 2003. Potential role of PTEN phosphatase in ethanol-impaired survival signaling in the liver. Hepatology. 38: 703–714. [DOI] [PubMed] [Google Scholar]
- 29.Richards C. A., Short S. A., Thorgeirsson S. S., Huber B. E. 1990. Characterization of a transforming N-ras gene in the human hepatoma cell line HepG2: additional evidence for the importance of c-myc and ras cooperation in hepatocarcinogenesis. Cancer Res. 50: 1521–1527. [PubMed] [Google Scholar]
- 30.Deaciuc I. V., D'Souza N. B., Burikhanov R., Lee E. Y., Tarba C. N., McClain C. J., de Villiers W. J. 2002. Epidermal growth factor protects the liver against alcohol-induced injury and sensitization to bacterial lipopolysaccharide. Alcohol. Clin. Exp. Res. 26: 864–874. [PubMed] [Google Scholar]
- 31.Mandrekar P., Szabo G. 2009. Signalling pathways in alcohol-induced liver inflammation. J. Hepatol. 50: 1258–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thakur V., Pritchard M. T., McMullen M. R., Wang Q., Nagy L. E. 2006. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J. Leukoc. Biol. 79: 1348–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee Y. J., Aroor A. R., Shukla S. D. 2002. Temporal activation of p42/44 mitogen-activated protein kinase and c-Jun N-terminal kinase by acetaldehyde in rat hepatocytes and its loss after chronic ethanol exposure. J. Pharmacol. Exp. Ther. 301: 908–914. [DOI] [PubMed] [Google Scholar]
- 34.Kramer K., Harrington E. O., Lu Q., Bellas R., Newton J., Sheahan K. L., Rounds S. 2003. Isoprenylcysteine carboxyl methyltransferase activity modulates endothelial cell apoptosis. Mol. Biol. Cell. 14 : 848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winter-Vann A. M., Kamen B. A., Bergo M. O., Young S. G., Melnyk S., James S. J., Casey P. J. 2003. Targeting Ras signaling through inhibition of carboxyl methylation: an unexpected property of methotrexate. Proc. Natl. Acad. Sci. USA. 100: 6529–6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sedding D. G., Tröbs M., Reich F., Walker G., Fink L., Haberbosch W., Rau W., Tillmanns H., Preissner K. T., Bohle R. M., et al. 2009. 3-Deazaadenosine prevents smooth muscle cell proliferation and neointima formation by interfering with Ras signaling. Circ. Res. 104: 1192–1200. [DOI] [PubMed] [Google Scholar]
- 37.Kharbanda K. K., Mailliard M. E., Baldwin C. R., Beckenhauer H. C., Sorrell M. F., Tuma D. J. 2007. Betaine attenuates alcoholic steatosis by restoring phosphatidylcholine generation via the phosphatidylethanolamine methyltransferase pathway. J. Hepatol. 46: 314–321. [DOI] [PubMed] [Google Scholar]
- 38.Yamazaki T., Sasaki E., Kakinuma C., Yano T., Miura S., Ezaki O. 2005. Increased very low density lipoprotein secretion and gonadal fat mass in mice overexpressing liver DGAT1. J. Biol. Chem. 280: 21506–21514. [DOI] [PubMed] [Google Scholar]