Abstract
Net flux of cholesterol represents the difference between efflux and influx and can result in net cell-cholesterol accumulation, net cell-cholesterol depletion, or no change in cellular cholesterol content. We measured radiolabeled cell-cholesterol efflux and cell-cholesterol mass using cholesterol-normal and -enriched J774 and elicited mouse peritoneal macrophage cells. Net cell-cholesterol effluxes were observed when cholesterol-enriched J774 cells were incubated with 3.5% apolipoprotein (apo) B depleted human serum, HDL3, and apo A-I. Net cell-cholesterol influxes were observed when cholesterol-normal J774 cells were incubated with the same acceptors except apo A-I. When incubated with 2.5% individual sera, cholesterol mass efflux in free cholesterol (FC)-enriched J774 cells correlated with the HDL-cholesterol (HDL-C) concentrations (r2 = 0.4; P=0.003), whereas cholesterol mass influx in cholesterol-normal J774 cells correlated with the LDL cholesterol (LDL-C) concentrations (r2 = 0.6; P<0.0001) of the individual sera. A positive correlation was observed between measurements of [3H]cholesterol efflux and reductions in cholesterol mass (r2 = 0.4; P=0.001) in FC-enriched J774 cells. In conclusion, isotopic efflux measurements from cholesterol-normal or cholesterol-enriched cells provide an accurate measurement of relative ability of an acceptor to remove labeled cholesterol under a specific set of experimental conditions, i.e., efflux potential. Moreover, isotopic efflux measurements can reflect changes in cellular cholesterol mass if the donor cells are enriched with cholesterol.
Keywords: cholesterol-enriched, efflux, influx, reverse cholesterol transport
When cells in culture are incubated with serum, there is a flux of free cholesterol (FC) out of the cell (efflux) while simultaneously there is movement of lipoprotein-derived FC and esterified cholesterol (CE) into the cell (influx). The difference between influx and efflux is the net change in cell cholesterol content, or net flux. Net flux may result in net accumulation, net depletion, or no change in cell cholesterol content if influx and efflux are equal. The net flux of cholesterol plays a major role in maintaining cell cholesterol homeostasis, and this is particularly important for macrophages in the vessel wall where net influx results in the deposition of excess cholesterol and formation of macrophage-derived foam cells (1). Many investigations have quantitated cholesterol efflux using a variety of cell types and extracellular acceptors, which is relatively simple because only FC is released from cells. These studies have identified a number of pathways that mediate efflux (2) including 1) aqueous diffusion (3), 2) ATP binding cassette transporter A1 (ABCA1) (4), 3) ATP binding cassette transporter G1 (ABCG1) (5), and 4) scavenger receptor class B type I (SR-BI) (6). The influx of cholesterol from exogenous lipoproteins is much more difficult to quantitate because both FC and CE are incorporated by aqueous diffusion, SR-BI, classical LDL receptors, scavenger receptors, and pinocytosis (7).
Bidirectional cholesterol flux is influenced by the array of lipoproteins in serum, the combination of efflux pathways available to the cells, and the cholesterol status of the cells (8, 9). In the present study we have incubated cells with a number of different normolipemic human sera and quantitated the influence of each serum sample on fractional cholesterol efflux as well as on the net flux of cholesterol between cells and serum. Cholesterol mass and isotope flux studies were conducted on J774 (murine cell line) cells and mouse peritoneal macrophage (MPM) cells that contained both normal and elevated levels of cholesterol. The level of cell cholesterol influences flux in a number of ways, including modulating the expression level of cell proteins mediating efflux such as ABCA1, ABCG1, and SR-BI, and proteins, such as LDL and scavenger receptors, which participate in lipoprotein uptake (7, 10) or influx. The fractional efflux values were determined using cells prelabeled with [3H]cholesterol and net flux was measured directly by quantitating changes in cell cholesterol mass upon incubation with either human sera or apolipoprotein (apo)-B-depleted sera. Because a large body of data has been collected by following the movement of radiolabeled cholesterol, we compared values for isotopic efflux to changes in cell cholesterol mass so as to determine the reliability of isotopic measurements in predicting actual mass flux.
Materials and Methods
Materials
Tissue culture plastic wares were purchased from Corning (Corning, NY) and Falcon (Becton Dickinson Labware, Franklin Lakes, NJ). RPMI 1640, MEM, and PBS were purchased from Mediatech Cellgro (Manassas, VA). MEM buffered with 10 mM HEPES (pH 7.4) was prepared in the laboratory using HEPES purchased from Fisher Scientific (Newark, DE). FBS, gentamicin, trypsin EDTA, and the ACAT inhibitor (Sandoz 58-035) were all purchased from Sigma-Aldrich (St. Louis, MO). Organic solvents were purchased from Fisher Scientific (Newark, DE). BSA was purchased from Millipore (Kankakee, IL) and [1,2-3H]cholesterol was purchased from Perkin Elmer Analytical Sciences (Waltham, MA).
Preparation of lipoproteins and human sera
Human HDL3 and LDL were isolated by sequential ultracentrifugation from plasma obtained with approved consent from healthy, normolipemic individuals as previously described (HDL3, d = 1.12–1.21 g/mL and LDL, d = 1.006–1.063 g/mL) (11). The study protocol was reviewed and approved by the Institutional Review Board of The Children's Hospital of Philadelphia. To obtain acetylated low density lipoprotein (acLDL), LDL was modified using acetic anhydride as previously described (12). Apo A-I was purified from delipidated HDL using ethanol/diethyl ether followed by anion-exchange chromatography on a Q-Sepharose column. The apo A-I fractions were pooled, dialyzed against 5 mM NH4HCO3, lyophilized, and stored at −20°C. The apo A-I was resolubilized in 6 M guanidine hydrochloride and dialyzed extensively against saline (0.15 M NaCl, pH 7.4) prior to incubating with the cells. The human sera used in these studies were collected, with informed consent, from a group of 22 normolipemic individuals consisting of 13 females and 9 males ranging in age from 23 to 70 years. The sera had an average HDL cholesterol (HDL-C) level of 58 ± 15 mg/dl (31–100 mg/dl), LDL cholesterol (LDL-C) of 113 ± 22 mg/dl (62–160 mg/dl) and triglycerides of 120 ± 66 mg/dl (52–294 mg/dl). The 22 human serum samples were used individually or combined to obtain a pool of serum. To isolate the serum HDL fraction, the pool of serum or individual serum samples were depleted of apo-B-containing lipoproteins by precipitating them with polyethylene glycol (PEG) solution as described previously (13). Presence of PEG does not alter the efflux capacity of the extracellular acceptors. Because of the dilution of the sera by the precipitation reagents, 3.5% of the apo B depleted serum is equivalent to 2.5% whole serum.
Cell culture
J774 cells were plated in growth medium in 24-well plates at a density of 150,000 cells/well. All the samples were assayed in triplicate. Cholesterol-normal cells were labeled for 24 h with 2 μCi/ml [3H]cholesterol in medium supplemented with 1% FBS and 2 μg/ml ACAT inhibitor. Cholesterol-enriched cells were incubated with the same labeling medium plus 25 μg/ml acLDL. In some experiments, the ACAT inhibitor was added to ensure pools of radiolabeled CE would not be present because this could complicate the determination of the fractional release of labeled cholesterol from the cells. After labeling plus or minus cholesterol enrichment, the cells were washed twice with MEM HEPES and equilibrated for 18 h in RPMI medium containing 0.2% BSA. In the case of enriched J774 cells without ACAT inhibitor, the equilibration period was 2 h. Following these treatments the cells were incubated with indicated exogenous acceptors for 8 h to measure the labeled FC efflux and the cellular FC mass.
Elicited MPM cells were collected from C57Bl/6 mice and plated for 24 h to allow attachment of the MPM to the plastic. Nonadhering cells were removed and MPM cells were incubated with RPMI containing 2 μCi/ml [3H]cholesterol, 1% FBS, and 50 µg/ml acLDL for 24 h. This treatment enriched the cells with both FC and CE and produced a foam cell phenotype (CE ≥ FC). The washed monolayers were then equilibrated in RPMI medium containing 0.2% BSA for 18 h. Afterwards, the cells were incubated for 18 h with the indicated exogenous cholesterol acceptors.
The efflux of labeled cholesterol was quantified as the percentage of radiolabel in the media compared with that present in the cells prior to the incubation with cholesterol acceptors. The amount of radiolabel present in the cells was determined by extracting cell lipids with 2-propanol and measuring the [3H]cholesterol in the lipid extraction by liquid scintillation counting.
Protein and cholesterol mass determination
At the end of the experiment, the cell monolayers were washed with DPBS and cell lipid was extracted using 2-propanol containing 5 µg/ml of cholesteryl methyl ether (CME; Sigma, St. Louis, MO) as an internal standard for gas liquid chromatography (GLC) analysis. A fraction of the extracted lipid was used to measure total cholesterol radioactivity incorporated into cellular lipids using scintillation counting. The remaining lipid was prepared for GLC to measure cholesterol mass (FC and CE) as previously described (14). The extent of loss of cell FC and CE mass was calculated based on the cholesterol content of the cells at time zero, prior to the efflux phase. Cell protein was measured by the method described by Markwell et al. (15). Cell proteins were measured before the addition of acceptors (T0) and after the incubation period with serum or lipoproteins. There were no statistically significant changes in cell protein measurement between cells at T0 and cells at the end of efflux period.
Two-dimensional gel electrophoresis
Apo A-I containing subpopulations of HDL, particularly preβ HDL, were measured using immunoblotting and image analysis after separation of the particles using nondenaturing two-dimensional gel electrophoresis as previously described (13).
Statistical analysis
All statistical analyses were performed using GraphPad Prism (San Diego, CA) software. Data were presented as mean ± SD. Statistical significance was determined by unpaired t-tests. Pearson correlation (r2) was used to assess the correlation between the serum components and the % efflux of radiolabeled cholesterol or cholesterol mass. Significance was assessed at P ≤ 0.05.
Results
The flux of cell cholesterol is not only linked to the type and concentration of lipoproteins in serum but it is also a function of the array of transport proteins expressed by the cells. Although many manipulations can be done with cells in culture that will influence the expression level of transport proteins, we have compared cells prepared under two common growth conditions: cholesterol-normal (grown in the presence of FBS, which yields cells with normal levels of cholesterol) and cholesterol-enriched (grown in the presence of acLDL, which yields cells with excess cholesterol). For both conditions, we grew cells in the presence or absence of an ACAT inhibitor. Thus, in the absence of ACAT inhibitor, cholesterol accumulated as FC and CE, whereas in the presence of this inhibitor only the FC pool was expanded and there was no deposition of cellular CE. The expression level of efflux proteins is influenced by the cholesterol content of the cells. In cholesterol-normal J774 and MPM cells there are low levels of SR-BI, ABCA1, and ABCG1; however, enrichment of the cells with cholesterol produces an increase in the expression of both ABCA1 and ABCG1 together with a decrease in SR-BI (16). In addition to these pathways, our previous studies have demonstrated that the aqueous transfer pathway plays a large role in cholesterol efflux from cholesterol-normal cells (16).
Correlation between cholesterol flux and serum components
In our initial studies, we examined the correlation between fractional efflux and serum components (Table 1). The correlations were obtained using J774 cells enriched with either FC or both FC and CE. A comparison of the correlation between percent cholesterol flux and serum components determined for radiolabeled cholesterol efflux as well as cholesterol mass efflux are presented in Table 1. Although there is some similarity in correlations between the flux of either isotope or mass and serum components, the correlation patterns are not identical. It is probable that this is a reflection of the fact that mass changes reflect both the release of cell cholesterol and the uptake of lipoprotein cholesterol. At present, there is no information available on the efficiency of HDL subfractions in delivering cholesterol to cells. It will require the isolation and testing of individual subfractions to obtain such information. Even though the correlation coefficient of some HDL subfractions against percent efflux of radiolabeled cholesterol or cholesterol mass seems to be low, together, the HDL fraction contributes around 70–75% of the total labeled cholesterol efflux of whole serum in J774 cells.
TABLE 1.
Correlation between the HDL, apo A-I, and HDL subfractions vs. fractional efflux of cholesterol mass or label from J774 cells
| HDL Components/Subfractions | FC-Enriched |
FC- and CE-Enriched |
||
|---|---|---|---|---|
| % mass efflux | % label efflux | % mass efflux | % label efflux | |
| HDL | 0.2 | 0.4 | 0.3 | NS |
| Apo A-I | 0.3 | 0.3 | 0.4 | 0.2 |
| pre β−1 | NS | 0.3 | 0.4 | 0.2 |
| α-1 HDL | 0.2 | NS | 0.2 | NS |
| α-2 HDL | 0.2 | NS | 0.5 | NS |
| α-3 HDL | NS | 0.4 | NS | 0.4 |
| α-4 HDL | NS | 0.2 | NS | 0.2 |
| pre α−1 HDL | 0.2 | NS | NS | NS |
J774 cells were enriched with FC or FC and CE by exposure to acLDL with or without the presence of ACAT inhibitor respectively. The cell cholesterol mass at T0 (before the efflux phase) was 53.1 ± 1.9 µg total cholesterol/mg cell protein for FC-enriched cells, and 89.9 ± 4.2 µg total cholesterol/mg cell protein for FC- and CE-enriched cells. The experiment has been repeated twice in triplicate and the value used for correlation is the average of six values. Pearson correlations (r2) were assessed between the HDL, apo A-I, and HDL subfractions and the % radiolabeled cholesterol efflux or % cholesterol mass efflux. The r2 of the statistically significant correlations are presented in the table. The statistical significance of the correlations were assessed at P ≤ 0.05. NS = nonsignificant.
Net flux of cholesterol mass from J774 cells
The measurement of the efficiency of serum or isolated lipoproteins to mediate cell cholesterol efflux has been a valuable tool in elucidating the pathways and mechanisms involved in the removal of cell cholesterol. In addition, recent studies have demonstrated a relationship between efflux from macrophages and the deposition of lipids in vessels, as measured by intima media thickness (IMT) and angiography (17). Of prime importance with respect to understanding the process of reverse cholesterol transport (RCT) is net flux of cholesterol mass that occurs when cells are incubated with serum or isolated lipoproteins (18, 19). In the present study, we have quantitated net cholesterol mass flux by directly measuring the change in cell cholesterol mass upon incubation of both cholesterol-normal and cholesterol-enriched J774 macrophages with different acceptors. Table 2 demonstrates the changes in cell cholesterol mass when normal and enriched cells were exposed to a pool of 3.5% apo-B-depleted human serum (equivalent to 2.5% serum), isolated HDL3 (50 µg/ml), or apo A-I (25 µg/ml) for 8 h. As shown in Table 2, incubating cholesterol-enriched cells for 8 h resulted in a significant net reduction of cell cholesterol mass (net efflux). In contrast, if the starting cells contained the level of sterol normally observed when the cells were grown in FBS, exposure to the same acceptors resulted in a small net increase in cell cholesterol mass (net influx). As expected, there was no influx of cholesterol mass when lipid-free apo A-I was present as acceptor (Table 2).
TABLE 2.
Change in total cellular cholesterol mass in J774 cells
| Total Cholesterol Change(µg/mg cellular protein/8 h) |
|||
|---|---|---|---|
| Cholesterol-Enriched |
|||
| Cholesterol-Normal | FC-Enriched | FC- and CE-Enriched | |
| 3.5% Apo-B-depleted serum | +1.4 ± 0.3a | −16.7 ± 2.1a | −21.6 ± 3.2a |
| HDL3 (50 µg/ml) | +1.5 ± 0.6a | −12.7 ± 5.5a | −22.1 ± 4.3a |
| Apo A-I (25 µg/ml) | +0.6 ± 0.4 | −6.6 ± 2.3a | −19.4 ± 6.8a |
J774 cells were plated in growth medium for 24 h and labeled with medium containing 1% FBS and 2 µCi/ml ± 25 µg/ml acLDL for 24 h. The cells were equilibrated for 18 h in 0.2% BSA and the efflux was initiated by incubating the cells with 3.5% pooled apo-B-depleted human serum, HDL3 (50 µg/ml), and apo A-I (25 µg/ml). ACAT inhibitor was added in the medium for cholesterol-normal and FC-enriched cells. The cell cholesterol mass at T0 (before the efflux phase) was 6.4 ± 0.3 µg total cholesterol/mg cell protein for cholesterol-normal cells, 53.1 ± 1.9 µg total cholesterol/mg cell protein for FC-enriched cells, and 89.9 ± 4.2 µg total cholesterol/mg cell protein for FC- and CE-enriched cells. Values are presented as the difference in cholesterol mass between the treatment at the end of efflux period and their corresponding T0 values. Plus sign indicates increase in total cellular cholesterol content, whereas minus sign indicates decrease in total cellular cholesterol content. Each value represents the average of triplicate. The data are given as mean ± SD.
Statistically significant from their corresponding T0 cholesterol mass content.
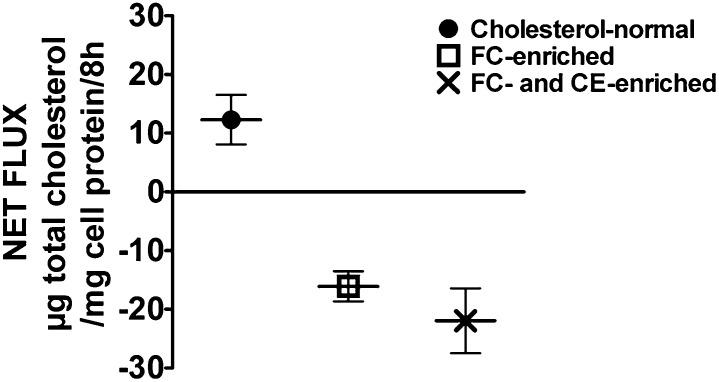
Because the net movement of cholesterol mass, either in (net influx) or out (net efflux) of cells, is a very relevant parameter resulting from exposure of cells to serum or lipoproteins, we determined the net cholesterol mass flux with both cholesterol-normal and cholesterol-enriched J774 cells upon exposure to a group of normal human sera. Net cholesterol mass flux values were determined directly by comparing the cell cholesterol content at the beginning of the incubation to that obtained following 8 h incubation with each serum sample. The results shown in Fig. 1 clearly illustrate that the flux of cell cholesterol mass is directly related to the starting cholesterol levels. With cells enriched with FC or both FC and CE there was a significant depletion of total cell cholesterol mass, whereas with cholesterol-normal cells there was a significant increase in total cell cholesterol mass upon exposure to 2.5% human serum.
Fig. 1.
Net flux of cholesterol mass in J774 cells when incubated with different individual sera. Net flux of cholesterol mass from J774 cells incubated with 22 individual serum samples presented in figure as the mean ± SD of 22 individual values (Each 22 value is an average of six determinations-triplicate from two experiments). There was a significant (P ≤ 0.5) depletion of total cell cholesterol mass in J774 cells enriched with cholesterol (both FC-enriched and FC- and CE-enriched cells), while there was a significant increase (P = 0.003) in total cellular cholesterol mass in cells that contain normal levels of cholesterol. The increase or decrease of cellular cholesterol mass were based on the initial cellular cholesterol content (T0) before the efflux phase.
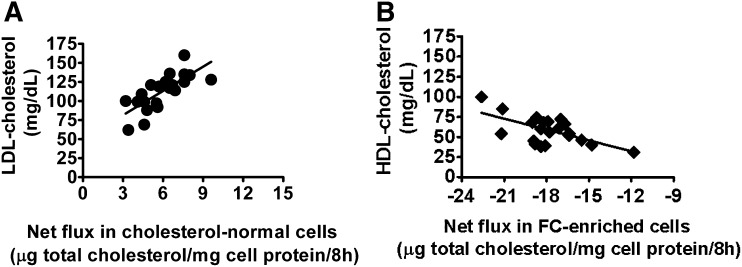
As illustrated in Fig. 2, when J774 cells were incubated with 22 different individual serum samples, the net increase in total cell cholesterol mass observed in cholesterol-normal cells correlates positively with the LDL-cholesterol concentrations of the individual sera (r2 = 0.6, P < 0.0001; Fig. 2, panel A). In contrast, the net loss of total cell cholesterol mass in FC-enriched J774 cells correlated negatively with the HDL-cholesterol concentrations of the sera (r2 = 0.4, P = 0.003; Fig. 2, panel B).
Fig. 2.
Correlation between net flux of cholesterol mass in cholesterol-normal/enriched- J774 cells and LDL-cholesterol (LDL-C) or HDL-cholesterol (HDL-C). Cholesterol-normal as well as free cholesterol-enriched J774 cells were incubated with 22 different individual serum samples and the correlations were assessed between the net flux of cholesterol mass in the cells and the serum lipid profiles of these 22 individuals. The net increase in total cell cholesterol mass in cholesterol-normal cells correlated positively (r2 = 0.6; P < 0.0001) with the LDL-C concentrations of the individual sera (A), while the net loss of total cellular cholesterol mass from FC-enriched J774 cells correlated negatively (r2 = 0.4; P = 0.003) with the HDL-C concentrations of the individual serum samples (B). Each 22 value is an average of six determinations-triplicate from two experiments.
Relationship between efflux of labeled cholesterol and reduction of cell cholesterol mass in J774 cells
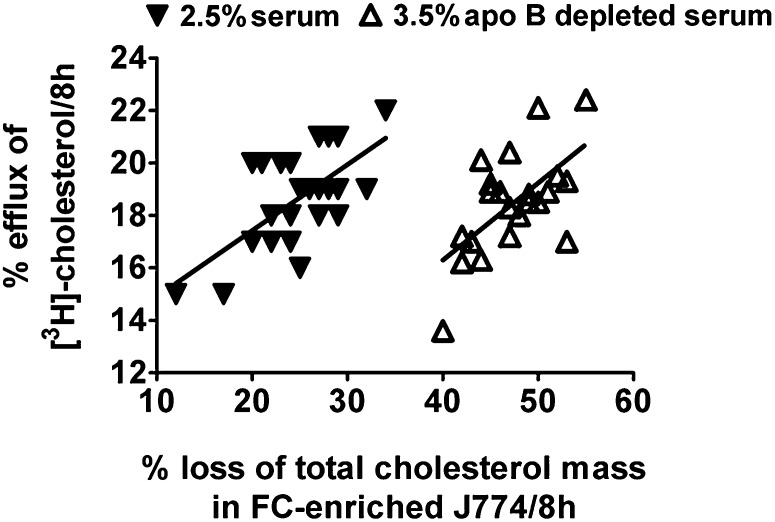
As discussed earlier, the great majority of studies on cell cholesterol flux have followed the release of radiolabeled cholesterol from the cells because the protocols for this type of experiment are straightforward and readily adapted to high-throughput assays (20). Using the data collected in the present study, we established the relationship between the fractional efflux of labeled cholesterol to the net reduction of cholesterol mass in J774 macrophages enriched with FC. As illustrated in Fig. 3, when the cells were enriched with FC and incubated with a group of 2.5% individual sera, there was a statistically significant positive correlation between the fraction of labeled cholesterol that was released and the percent reduction in cell cholesterol mass (r2 = 0.4, P = 0.001). There was also a good correlation between fractional efflux of labeled cholesterol and percent decrease in cell cholesterol mass (r2 = 0.4, P = 0.001) when 3.5% apo-B-depleted sera were used. However, the reductions in cell cholesterol mass upon exposure to the apo-B-depleted sera were greater than observed with whole sera, a result that can be attributed to the reduced influx of exogenous cholesterol upon removal of apo-B-containing lipoproteins.
Fig. 3.
Correlation between fractional efflux of labeled cholesterol and percent reduction of total cell cholesterol mass in free cholesterol-enriched J774 cells. J774 cells enriched with FC were incubated for 8 h with 2.5% human sera or 3.5% of the same sera following the removal of apo-B-containing lipoproteins. Significant correlation was seen between the fraction of labeled cholesterol that was released and the fractional reduction in total cell cholesterol mass when FC-enriched J774 cells were incubated with 2.5% serum (r2 = 0.4, P = 0.001) or apo-B-depleted serum (r2 = 0.4, P = 0.001) for 8 h. The fractional reduction in total cell cholesterol mass was greater upon exposure to apo-B-depleted serum compared with whole serum. Each 22 value is an average of six determinations-triplicate from two experiments.
Net flux of cholesterol mass from MPM cells
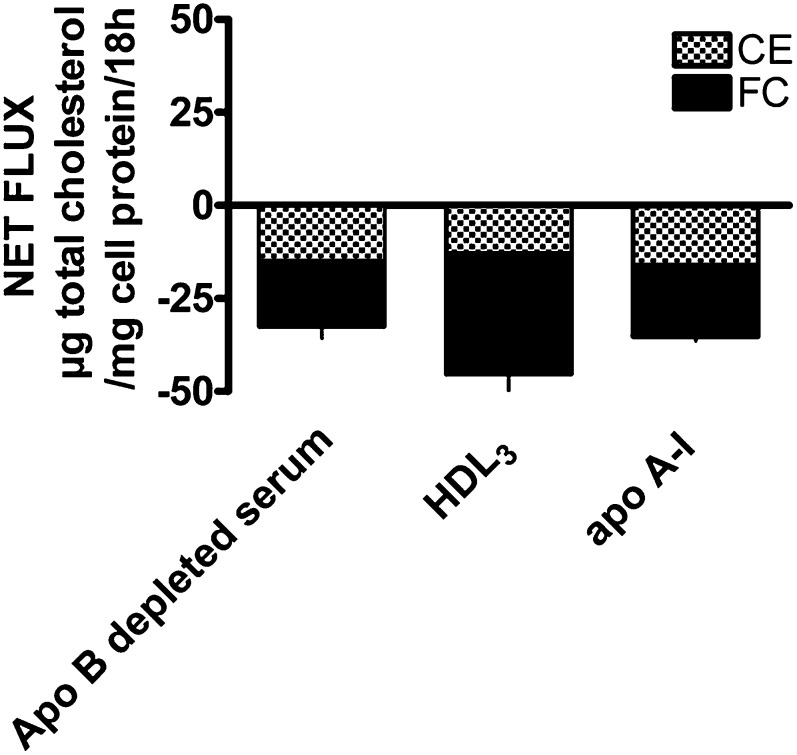
To demonstrate that the results obtained with J774 cells were not unique to this cell line, we measured net cholesterol mass and label flux using MPM grown in media containing 50 µg/ml of acLDL in the absence of an ACAT inhibitor (FC- and CE-enriched). Similar to cholesterol-enriched J774 cells, there was a significant reduction of total cell cholesterol mass (net efflux) when FC- and CE-enriched MPM cells (169.4 ± 19.4 µg total cellular cholesterol/mg cell protein, with 60% ± 1% CE) were incubated with 3.5% apo-B-depleted pooled human serum, 50 µg/ml of isolated HDL3, or 50 µg/ml of apo A-I for 18 h (Fig. 4). There was a significant correlation between the fractional loss of cholesterol mass and the change in radiolabeled cholesterol (r2 = 0.98, P < 0.001). The fractional loss was 23% when apo-B-depleted pooled serum was used and was similar for all of the cell cholesterol pools (TC, FC, and CE) because of their similar specific activity after 18 h of incubation. If an ACAT inhibitor was added together with the 3.5% apo-B-depleted pooled serum, the loss of total cell cholesterol mass was increased (data not shown).
Fig. 4.
Net flux of cholesterol mass in cholesterol-enriched MPM cells. The MPM cells enriched with FC and CE (169.4 ± 19.4 µg total cholesterol/mg cell protein with 60% ± 1% CE) were incubated with 3.5% apo-B-depleted pooled human serum, HDL3 (50 µg/ml), and apo A-I (50 µg/ml) for 18 h. There was a significant (P ≤ 0.5) net reduction of total cell cholesterol mass (net efflux) when incubated with these acceptors. Values are the average of six determinations- triplicate from two experiments.
Discussion
Various aspects of the movement of cholesterol between cells in culture and serum or isolated serum lipoproteins have been investigated. The majority of these investigations focused on the movement of FC (efflux) using radiolabeled cholesterol previously incorporated into the cells (20). The protocols for assaying the release of labeled cholesterol are relatively simple because only FC undergoes efflux and the assay can be easily adapted for high-throughput use. Data generated with this approach have been very valuable in identifying a number of pathways that participate in the release of cell cholesterol and the maintenance of cell cholesterol homeostasis. Thus, the measurement of the fractional release of labeled cell cholesterol can be used to assess the efficiency of serum or lipoproteins in stimulating efflux and to evaluate drugs or diets for their ability to modulate efflux, a process that is thought to be the first step in RCT. Indeed, recent investigations have provided evidence that the in vitro quantitation of cholesterol efflux from macrophages in culture to sera from human subjects can be associated with measurements of plaque size as determined by measures of IMT and angiography (17). However, because lipoproteins contain cholesterol, the incubation of cells with serum or isolated lipoproteins results in bidirectional flux between the cell and medium compartments. Thus, the measurement of labeled cholesterol release, or efflux, evaluates only one arm of this bidirectional process.
In the present study, we have measured the change in cell cholesterol mass when J774 macrophages or MPM were exposed to human serum or HDL obtained from normolipemic donors. We have used both J774 cells that were grown on FBS, and thus contain low levels of cholesterol, and J774 cells that have been exposed to acLDL to enrich the macrophages with both FC and CE, or only FC if an ACAT inhibitor was present. In additional experiments, we have used MPM enriched in both FC and CE.
Importance of cell cholesterol content on cholesterol mass flux
A number of both cellular and lipoprotein factors determine the net flux of cell cholesterol mass. Efflux is mediated by different pathways such as aqueous diffusion, SR-BI, ABCA1, and ABCG1 and the contribution of these pathways to efflux is influenced by cell type and cell cholesterol status. Even with a single cell type, the cholesterol status of the cells is a major determinant of the net flux of cholesterol mass. This is exemplified by Fig. 1, which illustrates that exposure of cholesterol-enriched J774 to 22 normolipemic human sera for 8 h results in the net depletion of cell cholesterol mass. In contrast, exposure of cholesterol-normal J774 to the same sera produced a net mass accumulation with all serum samples. The importance of cell cholesterol content on net mass flux is further exemplified by the data shown in Table 2, in which cholesterol-normal and -enriched J774 cells were exposed to a pool of 3.5% apo-B-depleted human serum, human HDL3 (50 µg/ml), and human apo A-I (25 µg/ml). The pool of 3.5% apo-B-depleted human serum produced a substantial reduction in cell cholesterol mass in cholesterol-enriched cells, whereas there was a small net increase in cell cholesterol mass with the cholesterol-normal cells. These data are consistent with the significant association between net efflux of cholesterol mass from FC-enriched cells and serum HDL-C levels (Fig. 2B) and the significant association of net influx of cholesterol mass to serum LDL-C levels seen with cholesterol-normal J774 cells (Fig. 2A). At the concentrations used in these experiments, both HDL3 and apo A-I produced net mass efflux from enriched cells (Table 2). However, apo A-I had no significant impact on the cell cholesterol content of cholesterol-normal cells.
If MPM containing elevated levels of both FC and CE are used for cholesterol mass studies (Fig. 4), the presence of the HDL fraction of serum during an 18 h incubation produces a net loss of cell cholesterol mass and the loss occurs equally from the FC and CE pools. If ACAT is blocked and an extracellular acceptor is present, the loss of total cholesterol is increased due to the inhibition of cholesterol reesterification and the accumulation and subsequent efflux of the newly generated FC (data not shown). If no acceptor is present, there is relatively little change in cell total cholesterol during an 18 h incubation; however, introduction of the ACAT inhibitor during the efflux phase shifts cholesterol mass from the CE pool into the FC pool without producing an appreciable change in the total cholesterol mass.
Relationship between percent labeled cholesterol efflux and cholesterol mass flux
The measurement of the release of radiolabeled cholesterol from cells to serum or lipoproteins has been extensively used to estimate the efflux efficiency of the acceptor particles. The extent to which the values obtained from isotopic efflux measurements reflect changes in the mass of cell cholesterol has not been well established. As shown in Fig. 3, there is a statistically significant association between fractional efflux of label and percent loss of cell cholesterol mass from enriched cells exposed to either 2.5% whole human sera or the apo-B-depleted sera. Although the apo B components of serum appear to be relatively inefficient in stimulating efflux, they do contribute significantly to the cellular uptake of both FC and CE, thus impacting on cell cholesterol mass. The agreement between measurement of flux based on labeled cholesterol versus cholesterol mass in J774 (Fig. 3) supports the conclusion that the use of cellular labeled cholesterol to assess the flux of cholesterol mass does provide reliable data on the flux of cell cholesterol mass if the cells are first cholesterol enriched.
Similar to the correlation between the fractional efflux of radiolabeled cholesterol and the percent loss of FC mass in J774 cells, there was a significant correlation between cholesterol mass and labeled cholesterol in the MPM exposed to apo-B-depleted pooled serum. In the case of MPM, the agreement between mass and isotope efflux was due to the fact that the specific activity of labeled FC and CE pools had come to an equilibrium under the conditions that we employed to enrich, label, and equilibrate the cells. If cholesterol-specific activity in FC and CE are not similar, isotopic release will not accurately predict mass changes. In addition, efflux of labeled cholesterol from cholesterol-normal cells will not predict mass flux because the difference between influx and efflux is generally not large and would not produce an appreciable change in the cholesterol mass in either the cell or media compartments. However, the measurement of release of labeled cholesterol does allow a comparison of the efficiency of various acceptors in mediating the efflux arm of the bidirectional flux.
Summary
There is recent evidence that the efflux efficiency of a given specimen of serum as measured in vitro using cholesterol-normal J774 macrophages is a reflection of its ability to reduce cell cholesterol from vessel wall cells as measured by IMT and angiography (17). The results presented here further suggest that in vitro assays of efflux efficiency may reflect the in vivo potential of a given serum to promote cholesterol homeostasis. If in vitro experiments are conducted exposing cholesterol-normal cells to HDL, cholesterol exchange will occur; however, if the cells contain excess cholesterol net movement of cholesterol mass will be achieved. In vivo this would result in the removal of cholesterol mass from foam cells, thus contributing to the regression of the atherosclerotic lesion. Enriching the macrophage cells with cholesterol allowed us to measure serum-induced cholesterol mass changes by 1) increasing the pool size of cellular cholesterol, and 2) altering the expression and array of cell cholesterol transporters. Thus, results from the present studies indicate that the fractional efflux of labeled cholesterol reflect the “efflux efficiency”, or as sometimes termed, “efflux capacity”, of a particular extracellular acceptor. Efflux efficiency/capacity can be defined as the relative ability of an acceptor to remove labeled cholesterol under a specific set of experimental conditions. In most cases, the efflux of labeled cholesterol does not represent the movement of cholesterol mass. The lack of an association between mass and isotopic values is a reflection of an exchange of cholesterol driven by the bidirectional flux process. The exception to this general phenomenon is if the acceptor is cholesterol-free where there is no bidirectional cholesterol flux. The present study expands our understanding of the relationship between radioisotopic flux and mass flux by showing that there is no association between changes in cell mass and isotope flux if the cells contain normal levels of cholesterol. However, we also demonstrate that there is an association between efflux of label and change in cell cholesterol mass if the cells are cholesterol enriched. This association is a reflection of greatly enhanced efflux from enriched cells while at the same time influx of exogenous cholesterol is relatively low. Thus, under most conditions, fractional release of label will provide an estimate of the “efflux efficiency” of an acceptor whereas actual mass movement has to be determined by measurement of changes in cell cholesterol mass in either the cells or incubation medium.
Acknowledgments
The authors acknowledge Sara Hayes of The Children's Hospital of Philadelphia for excellent technical assistance.
Footnotes
Abbreviations:
- ABCG1
- ATP binding cassette transporter G1
- AcLDL
- acetylated low density lipoprotein
- apo
- apolipoprotein
- CE
- cholesteryl ester
- FC
- free cholesterol
- GLC
- gas liquid chromatography
- HDL-C
- HDL-cholesterol
- IMT
- intima media thickness
- LDL-C
- LDL cholesterol
- MPM
- mouse peritoneal macrophage
- PEG
- polyethylene glycol
- RCT
- reverse cholesterol transport
- SR-BI
- scavenger receptor class B type I
- TC
- total cholesterol
This work was supported by National Institutes of Health grant, HL-22633 (G.H.R., M.L.L-M., G.L.W., S.S., D.D-S.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Part of the results in this manuscript were presented at a “meeting on high density lipoproteins (HDL)” held on June 19 and 20, 2009, in Newport, RI. The proceedings of this meeting have been published as a chapter in the book, “High Density Lipoproteins, Dyslipidemia, and Coronary Heart Disease” (Schaefer, E.J., editor; Springer Science+Business Media).
REFERENCES
- 1.Vainio S., Ikonen E. 2003. Macrophage cholesterol transport: a critical player in foam cell formation. Ann. Med. 35: 146–155. [DOI] [PubMed] [Google Scholar]
- 2.Jessup W., Kritharides L. 2008. Lipid metabolism: recent progress in defining the contributions of cholesterol transporters to cholesterol efflux in vitro and in vivo. Curr. Opin. Lipidol. 19: 212–214. [DOI] [PubMed] [Google Scholar]
- 3.Phillips M. C., Johnson W. J., Rothblat G. H. 1987. Mechanism and consequence of cellular cholesterol exchange and transfer. Biochim. Biophys. Acta. 906: 223–276. [DOI] [PubMed] [Google Scholar]
- 4.Tall A. R., Yvan-Charvet L., Terasaka N., Pagler T. A., Wang N. 2008. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 7: 365–375. [DOI] [PubMed] [Google Scholar]
- 5.Tarr P. T., Tarling E. J., Bojanic D. D., Edwards P. A., Baldan A. 2009. Emerging new paradigms for ABCG transporters. Biochim. Biophys. Acta. 1791: 584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji Y., Jian B., Wang N., Sun Y., de la Llera Moya M., Phillips M. C., Rothblat G. H., Swaney J. B., Tall A. R. 1997. Scavenger receptor B1 promotes high density lipoprotein-mediated cellular cholesterol efflux. J. Biol. Chem. 272: 20982–20985. [DOI] [PubMed] [Google Scholar]
- 7.Buono C., Anzinger J. J., Amar M., Kruth H. S. 2009. Fluorescent pegylated nanoparticles demonstrate fluid-phase pinocytosis by macrophages in mouse atheroslcerotic lesions. J. Clin. Invest. 119: 1373–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yancey P. G., Bortnick A. E., Kellner-Weibel G., de la Llera-Moya M., Phillips M. C., Rothblat G. H. 2003. Importance of different pathways of cellular cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 23: 712–719. [DOI] [PubMed] [Google Scholar]
- 9.Tall A. R., Coster P., Wang N. 2002. Regulation and mechanisms of macrophage cholesterol efflux. J. Clin. Invest. 110: 899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M. D., Kiss R. S., Franklin V., McBride H. M., Whitman S. C., Marcel Y. L. 2007. Different cellular traffic of LDL-cholesterol and acetylated LDL-cholesterol leads to distinct reverse cholesterol transport pathways. J. Lipid Res. 48: 633–645. [DOI] [PubMed] [Google Scholar]
- 11.Hatch F. T., Lees R. S. 1968. Practical methods for plasma lipoprotein analysis. Adv. Lipid Res. 6: 1–68. [PubMed] [Google Scholar]
- 12.McCloskey H. M., Rothblat G. H., Glick J. M. 1987. Incubation of acetylated low-density lipoprotein with cholesterol-rich dispersions enhances cholesterol uptake by macrophages. Biochim. Biophys. Acta. 921: 320–332. [DOI] [PubMed] [Google Scholar]
- 13.Asztalos B. F., de la Llera-Moya M., Dallal G. E., Horvath K. V., Schaefer E. J., Rothblat G. H. 2005. Differential effects of HDL subpopulations on cellular ABCA1- and SR-BI-mediated cholesterol efflux. J. Lipid Res. 46: 2246–2253. [DOI] [PubMed] [Google Scholar]
- 14.Klansek J. J., Yancey P. G., St.Clair R. W., Fischer R. T., Johnson W. J., Glick J. M. 1995. Cholesterol quantitation by GLC: artifactual formation of short-chain esters. J. Lipid Res. 36: 2261–2266. [PubMed] [Google Scholar]
- 15.Markwell M. A. K., Haas S. M., Bieber L. L., Tolbert N. E. 1978. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 87: 206–210. [DOI] [PubMed] [Google Scholar]
- 16.Adorni M. P., Zimetti F., Billheimer J. T., Wang N., Rader D. J., Phillips M. C., Rothblat G. H. 2007. The role of different pathways in the release of cholesterol from macrophages. J. Lipid Res. 48: 2453–2462. [DOI] [PubMed] [Google Scholar]
- 17.Khera A. V., Rodrigues A., de La Llera-Moya M., Rothblat G. H., Rader D. J. 2009. Serum cholesterol efflux capacity, a measure of HDL-C quality, varies according to coronary artery disease status independently of HDL-C quantity. Abstract, #1274. Circulation. 120: S469. [Google Scholar]
- 18.Zimetti F., Weibel G. K., Duong M. N., Rothblat G. H. 2006. Measurement of cholesterol bidirectional flux between cells and lipoproteins. J. Lipid Res. 47: 605–613. [DOI] [PubMed] [Google Scholar]
- 19.Yvan-Charvet L., Pagler T. A., Wang N., Senokuchi T., Brundert M., Li H., Rinninger F., Tall A. R. 2008. SR-BI inhibits ABCG1-stimulated net cholesterol efflux from cells to plasma HDL. J. Lipid Res. 49: 107–114. [DOI] [PubMed] [Google Scholar]
- 20.Rothblat G. H., de la Llera-Moya M., Favari E., Yancey P. G., Kellner-Weibel G. 2002. Cellular cholesterol flux studies: methodological considerations. Atherosclerosis. 163: 1–8. [DOI] [PubMed] [Google Scholar]