Abstract
We determined the contribution of the combination of FEN1 10154G>T with the most significant association in the analysis of plasma arachidonic acid (AA, 20:4ω6) and the APOA5-1131T>C on phospholipid ω6PUFA and coronary artery disease (CAD). Patients with CAD (n = 807, 27–81 years of age) and healthy controls (n = 1123) were genotyped for FEN1 10154G>T and APOA5-1131T>C. We found a significant interaction between these two genes for CAD risk (P = 0.007) adjusted for confounding factors. APOA5-1131C allele carriers had a higher CAD risk [odds ratio (OR):1.484, 95% confidence interval (CI):1.31–1.96; P = 0.005] compared with APOA5-1131TT individuals in the FEN1 10154GG genotype group but not in the FEN1 10154T allele group (OR:1.096, 95%CI:0.84–1.43; P = 0.504). Significant interactions between these two genes were also observed for the AA proportion (P = 0.04) and the ratio of AA/linoleic acid (LA, 18:2ω6) (P = 0.004) in serum phospholipids of controls. The APOA5-1131C allele was associated with lower AA (P = 0.027) and AA/LA (P = 0.014) only in controls carrying the FEN1 10154T allele. In conclusion, the interaction between these genes suggests that the FEN1 10154T variant allele decreases AA and AA/LA in the serum phospholipids of carriers of the APOA5-1131C allele, but contributes no significant increase in CAD risk for this population subset despite their increased triglylcerides and decreased apoA5.
Keywords: arachidonic acid, linoleic acid, CAD risk, apolipoprotein A5
The apolipoprotein A5 gene (APOA5) is predominantly expressed in the liver and secreted into the blood where it resides on HDL and VLDL particles (1). Despite its very low levels in plasma, apoA5 appears to facilitate the interaction between circulating lipoprotein particles and proteoglycans on the vascular wall with the subsequent activation of proteoglycan-bound lipoprotein lipase (2–4). The APOA5 -1131T>C single nucleotide polymorphism (SNP) has been reported to be functional and is associated with reduced apoA5 concentration or activity (5). In particular, the APOA5 -1131C variant allele modulates the effects of dietary PUFA intake on fasting triglyceride levels, lipoprotein particle size (6), and obesity (7) and PUFA-APOA5 interactions are specific for dietary ω6 PUFAs. The fatty acid composition of serum phospholipids mirrors dietary fatty acid intake over previous weeks and also reflects endogenous fatty acid metabolism (8–11). However, an interaction of the APOA5 -1131T>C SNP with the gene associated with intrinsic phospholipid PUFA metabolism has not been studied.
The key enzymes in PUFA metabolism are delta 5 desaturase (D5D) and delta 6 desaturase, which are encoded by the fatty acid desaturase 1 and 2 (FADS1 and FADS2) genes, respectively (12–14). These two genes are located in the desaturase gene cluster on chromosome 11 (11q12-13.1). A recent genome-wide association study for plasma PUFAs showed strong evidence for association with this region of chromosome 11 (15). The most significant association was between the SNP rs174537 (flap endonuclease 1, FEN1 10154G>T) near FADS1 and the analysis of arachidonic acid (AA, 20:4ω6). AA is synthesized primarily in the liver and then mobilized to inflammatory cells via blood lipoproteins (16). AA is the precursor of prostaglandins, leukotrienes, and related compounds, all of which have important roles in inflammation (17–19). Thus, the FEN1 10154G>T SNP may represent a candidate gene of importance in coronary artery disease (CAD). Therefore, we hypothesized that genetic variation at the APOA5 -1131 locus has an interactive effect with genetic variation at the FEN1 10154 locus on serum phospholipid ω6 PUFA metabolism and CAD. Our aim was to determine the contribution of the combination of the FEN1 10154G>T and APOA5 -1131T>C SNPs on serum phospholipid ω6 PUFA metabolism and CAD.
SUBJECTS AND METHODS
Study participants
Study participants were enrolled in a clinical study conducted by the Laboratory of Clinical Nutrigenetics/Nutrigenomics (project#: 2010-0015017 and M10642120002-06N4212-00210) at Yonsei University. Study participants ranged in age from 27 to 81 years old. Participants were recruited from the Cardiovascular Genome Center, Yonsei University Severance Hospital, Seoul, Korea. Control participant exclusion criteria were any diagnosis of vascular disease, diabetes mellitus (DM), cancer (clinically or by anamnesis), renal disease, liver disease, thyroid disease, and acute or chronic inflammatory disease. No participant was taking any drugs or supplements. Inclusion criteria of patients with CAD were: a) angiographically confirmed CAD with ≥50% occlusion of one or more major coronary arteries, b) myocardial infarction confirmed according to the World Health Organization criteria for symptoms, enzyme elevation, or electrocardiographic changes, c) absence of nonatherogenic occlusion such as osteal stenosis and spasm, d) no orthopedic limitations or any diagnosis of DM, liver disease, renal disease, thyroid, or pituitary disease, and e) no acute or chronic inflammatory disease. In total, 1,930 genetically unrelated Koreans were included; 807 CAD patients and 1,123 control participants. Written informed consent was obtained from all participants, and the protocol was approved by the Institutional Review Board of Yonsei University.
Anthropometric parameters, BP, and blood collection
Body weight and height were measured unclothed and without shoes in the morning for the calculation of body mass index (BMI) (kg/m2). Blood pressure (BP) was measured in the left arm of seated patients with an automatic blood pressure monitor (TM-2654, A and D, Tokyo, Japan) after a 20 min rest. After a 12 h fasting period, venous blood specimens were collected in EDTA-treated and plain tubes, centrifuged to produce plasma or serum, and stored at −70°C until analysis.
Genotyping of FEN1 10154G>T and APOA5 -1131T>C
Genomic DNA was extracted from 5 ml whole blood with a commercially available DNA isolation kit (WIZARDR Genomic DNA purification kit, Promega, Madison, WI). Genotyping of FEN1 10154G>T was performed with the Taqman assay (Applied Biosystems, Foster City, CA). Genotyping of APOA5 -1131T>C was performed with SNP-ITTM assays and single primer extension technology (SNPstream 25KTM System, Orchid BioSciences, NJ). Colorimetric reactions were detected with an enzyme-linked immunosorbent assay (ELISA) reader, and the genotype was determined with QCReviewTM software.
Serum lipid profile and fasting glucose
Fasting total cholesterol and triglycerides were measured using commercially available kits on a Hitachi 7150 Autoanalyzer (Hitachi Ltd., Tokyo, Japan). After precipitation of serum chylomicrons with dextran sulfate magnesium, the concentrations of LDL- and HDL-cholesterol in the supernatants were measured enzymatically. LDL cholesterol was indirectly estimated in participants with serum triglyceride concentrations less than 400 mg/ml with the Friedewald formula. Fasting glucose levels were measured with a glucose oxidase method and a Beckman Glucose Analyzer (Beckman Instruments, Irvine, CA).
Plasma apoA5 concentration
Plasma concentrations of apoA5 were measured with an enzyme immunoassay (Human Apolipoprotein A ELISA kit, Millipore, MO). The resulting color reaction was read at 450 nm on a Victor2 plate reader (Perkin Elmer Life Sciences, Turka, Finland).
Fatty acid composition in serum phospholipids
The fatty acid composition in serum phospholipids was analyzed by gas chromatography (HP 7890A, Hewlett-Packard) with the modified method of Folch et al. (20) and Lepage and Roy (21). Individual fatty acids were identified by comparing their retention times with those of standard fatty acid methyl esters and quantitated according to the peak areas relative to the total area (total fatty acid area was set at 100%).
Plasma ox-LDL and LDL particle size
Plasma oxidized LDL (ox-LDL) was measured using an enzyme immunoassay (Mercodia, Uppsala, Sweden). Particle size distribution of LDL (d1.019–1.063 g/ml) isolated by sequential flotation ultracentrifugation was examined with a pore-gradient lipoprotein system (CBS Scientific, CA) with commercially available nondenaturing polyacrylamide slab gels containing a linear gradient of 2–16% acrylamide (Alamo Gels Inc., San Antonio, TX). Standards of latex beads (34 nm), thyroglobulin (17 nm), apoferritin (12.2 nm), and catalase (10.4 nm) were used to estimate the relative migration rates of each band. Gels were scanned with a GS-800 Calibrated Imaging Densitometer (Bio-Rad, Graz, Austria). LDL particle size was calculated with reference to the relative migration value of the standards.
Urinary 8-epi-PGF2α and plasma MDA
Urine was collected in polyethylene bottles containing 1% butylated hydroxytoluene after 12 h of fasting. The bottles were immediately covered with aluminum foil and stored at –70°C until analysis. The compound 8-epi-prostaglandin F2α (8-epi-PGF2α) was measured with an enzyme immunoassay (BIOXYTECH urinary 8-epi-PGF2αTM Assay kit, OXIS International Inc., OR). Urinary creatinine was determined with the alkaline picrated (Jeffe) reaction. Urinary 8-epi-PGF2α concentrations were expressed as pmol/mmol creatinine. Plasma malondialdehyde (MDA) was measured from thiobarbituric acid-reactive substances (TBARS Assay Kit, Zepto Metric Inc.).
Serum hs-CRP and TNF-α concentration
Serum hs-CRP (C-reactive protein) concentrations were measured with an Express+ autoanalyzer (Chiron Diagnostics Co., Walpole, MA) with a commercially available, high-sensitivity CRP-Latex (II) X2 kit (Seiken Laboratories Ltd., Tokyo, Japan). Serum tumor necrosis factor-α (TNF-α) concentrations were measured with a Bio-plex human cytokine panel (Bio-Rad, CA) using a Quantikine ELISA kit (Human TNF-α, R and D Systems, Minneapolis, MN).
Assessment of dietary intake
Dietary intake was assessed with a 24 h recall method and semi-quantitative food frequency questionnaire. Dietary energy values and nutrient content were calculated using the Computer Aided Nutritional analysis program (CAN-pro 2.0. Korean Nutrition Society, Seoul, Korea). Total energy expenditure was calculated from activity patterns including basal metabolic rate, physical activity over a 24-h period, and specific dynamic action of food. The base metabolic rate of each participant was calculated with the Harris-Benedict equation.
Statistical analysis
Statistical analyses were performed with SPSS version 12.0 for Windows (Statistical Package for the Social Sciences, SPSS Inc., Chicago, IL). Hardy-Weinberg Equilibrium and linkage disequilibrium tests were examined using the Haploviewver 4.1 (Broad Inst., MA). The association of CAD with genotype was calculated using the odds ratio (OR) [95% Confidence intervals (CIs)] for a chi-square test or logistic regression model after adjusting for confounding factors. The Student's t-test was used to compare parameters between the two groups. General linear model analysis was also used to compare groups after adjusting for confounding factors. We determined whether each variable was normally distributed before statistical testing and then logarithmic transformation was performed on skewed variables. Frequency was tested with the chi-square test. Pearson correlation coefficients were used to examine relationships between variables. For descriptive purposes, mean values are presented using untransformed and unadjusted values. Results are the means ± SE or percentage. A two-tailed value of P < 0.05 was considered statistically significant.
RESULTS
Characteristics of controls and patients with CAD
General characteristics of controls and patients with CAD are shown in Table 1. Patients with CAD had a lower prevalence of current smoking (20.1% vs. 24.4%; P = 0.025) and alcohol drinking (54.8% vs. 64.1%; P < 0.001) than controls. Lipid-lowering drugs (LLD), antihypertensive drugs, and antiplatelet drugs were used more frequently in patients than in controls (64.4% vs. 0%, P < 0.001; 88.7% vs. 0%; P < 0.001; 91.8% vs. 0%; P < 0.001, respectively). After adjusting for sex, age, BMI, cigarette smoking, alcohol consumption, and systolic and diastolic blood pressure, CAD patients had lower concentrations of apoA5, total cholesterol, LDL cholesterol, HDL cholesterol, and ox-LDL; higher concentrations of glucose, triglyceride, CRP, MDA, and 8-epi-PGF2α; and smaller LDL particle size than controls. CAD patients had a lower proportion of linoleic acid (LA, 18:2ω6), γ-linolenic acid (20:3ω6), and eicosadienoic acid (20:2ω6), a higher proportion of dihomo-γ-linolenic acid (DGLA, 20:3ω6) and AA (20:4ω6), and a higher ratio of AA/LA in serum phospholipids than controls after adjusting for confounding factors (Table 1).
TABLE 1.
Anthropometric and biochemical parameters of controls and patients with CAD
| Control (n = 1123) | CAD (n = 807) | |
|---|---|---|
| Male/Female, (%) | 78.4/21.6 | 81.5/18.5 |
| Age (yr) | 56.1 ± 0.23 | 56.9 ± 0.34 |
| BMI (kg/m2) | 24.1 ± 0.07 | 25.1 ± 0.10 |
| Blood pressure (mm Hg) | ||
| Systolic | 124.1 ± 0.46 | 127.2 ± 0.60 |
| Diastolic | 78.4 ± 0.32 | 77.3 ± 0.36 |
| Fasting glucose (mg/dl)a | 86.6 ± 0.24 | 88.8 ± 0.44* |
| Triglycerides (mg/dl)a | 128.9 ± 2.22 | 147.3 ± 2.80*** |
| ApoA5 (ng/ml)a | 225.1 ± 3.55 | 197.4 ± 5.02*** |
| Total-cholesterol (mg/dl) | 195.5 ± 0.99 | 166.2 ± 1.38*** |
| LDL-cholesterol (mg/dl) | 117.9 ± 0.91 | 90.7 ± 1.25*** |
| HDL-cholesterol (mg/dl)a | 51.7 ± 0.42 | 45.5 ± 0.39*** |
| LDL particle size (nm) | 23.76 ± 0.02 | 23.47 ± 0.03*** |
| TNF-α (pg/ml)a | 5.38 ± 0.10 | 5.65 ± 0.43 |
| hs-CRP (mg/dl)a | 1.14 ± 0.06 | 2.08 ± 0.15*** |
| Malondialdehyde (nmol/ml)a | 9.18 ± 0.09 | 10.5 ± 0.17*** |
| 8-epi-PGF2α (pg/mg creatinine)a | 1296.4 ± 18.2 | 1417.5 ± 27.0*** |
| Ox-LDL (μ/l)a | 63.5 ± 0.75 | 59.5 ± 0.89*** |
| FA composition (%) in serum PL | ||
| Total polyunsaturated ω-6 FA | 20.6 ± 0.19 | 20.6 ± 0.20 |
| 18:2(ω-6) | 12.9 ± 0.13 | 12.1 ± 0.13*** |
| 18:3(ω-6)a | 0.27 ± 0.01 | 0.24 ± 0.01 |
| 20:2(ω-6)a | 0.63 ± 0.05 | 0.36 ± 0.01*** |
| 20:3(ω-6) | 1.61 ± 0.03 | 1.86 ± 0.03*** |
| 20:4(ω-6) | 4.84 ± 0.07 | 5.72 ± 0.10*** |
| Total polyunsaturated ω-3 FA | 5.39 ± 0.09 | 5.51 ± 0.12 |
| 18:3(ω-3)a | 0.16 ± 0.00 | 0.17 ± 0.01 |
| 20:3(ω-3)a | 0.09 ± 0.01 | 0.08 ± 0.00* |
| 20:5(ω-3)a | 1.28 ± 0.03 | 1.44 ± 0.05 |
| 22:5(ω-3)a | 0.60 ± 0.01 | 0.60 ± 0.01 |
| 22:6(ω-3)a | 3.25 ± 0.06 | 3.21 ± 0.07 |
| 20:4(ω-6)/20:3(ω-6) | 3.17 ± 0.04 | 3.21 ± 0.06* |
| AA/LA | 0.38 ± 0.01 | 0.48 ± 0.01 |
| APOA5 -1131T>C, n(%) | ||
| TT | 566 (50.4) | 363 (45.0) |
| TC | 455 (40.5) | 367 (45.5) |
| CC | 102 (9.1) | 77 (9.5) |
| FEN1 10154 G>T, n(%) | ||
| GG | 516 (45.9) | 399 (49.4) |
| GT | 494 (44.0) | 330 (40.9) |
| TT | 113 (10.1) | 78 (9.7) |
Mean ± SE. P-values were tested with a paired t-test. *P < 0.05, **P < 0.01, ***P < 0.001; adjusted for sex, age, BMI, cigarette smoking, alcohol consumption, and systolic and diastolic BP.
Logarithmic transformation.
When we subdivided patients with CAD into two groups, those not treated with LLD (n = 286) and those treated with LLD (n = 521), those not treated with LLD showed higher concentrations of total cholesterol (183.2 ± 2 vs. 156.9 ± 2 mg/dl; P < 0.001) and LDL cholesterol (106.8 ± 2 vs. 82.0 ± 1 mg/dl; P < 0.001) than patients treated with LLD.
Distribution of FEN1 10154G>T and APOA5 -1131T>C polymorphisms
Genotype frequencies of FEN1 10154G>T and APOA5 -1131T>C SNPs did not deviate from Hardy-Weinberg Equalibrium expectation. The genotype distribution and allele frequency of the FEN1 10154G>T SNP between controls and patients were comparable. As shown in Table 1, the genotype distribution of APOA5 -1131T>C in controls and patients differed (P = 0.058), and the -1131C allele was more frequent among CAD patients (55%) than controls (49.6%; P = 0.019). Therefore, the presence of the APOA5 -1131C allele increased the OR of CAD risk after adjustment for confounders (OR = 1.274; CI = 1.052-1.544, P = 0.013).
Genotype combinations for the two SNPs were constructed to evaluate interaction between the genes and CAD risk. Table 2 shows OR estimations of CAD risk for the APOA5 -1131T>C polymorphism in stratified analyses by FEN1 10154G>T genotype. We found a statistically significant interaction between APOA5 -1131T>C and FEN1 10154G>T on CAD risk (P = 0.007) after adjusting for confounders. The APOA5 -1131C minor allele was associated with a 48.4% increase in CAD risk (P = 0.005) in participants carrying the FEN1 10154GG genotype relative to homozygous carriers of the wild-type alleles for each SNP (FEN1 10154GG+APOA5 -1131TT). In contrast, the APOA5 -1131C minor allele was not associated with CAD risk (P = 0.524) in participants carrying the FEN1 10154T allele when compared with the concomitant carrier status for the FEN1 10154T allele and the APOA5 -1131TT genotype (FEN1 10154T allele+APOA5 -1131TT) (Table 2).
TABLE 2.
Risk of CAD depending on the APOA5 –1131 T>C and FEN1 10154 G>T polymorphisms
| FEN1 10154 G>T | |||||
|---|---|---|---|---|---|
| GG |
GT+TT |
Pb for interaction between APOA5 and FEN1 | |||
| APOA5−1131 T>C | Adjusted OR (95% CI) | Pa | Adjusted OR (95% CI) | Pa | |
| TT | 1 | 0.005 | 1 | 0.504 | 0.007 |
| TC+CC | 1.484 (1.13–1.96) | 1.096 (0.84–1.43) | |||
CI, Confidence interval, OR, odds ratio.
P-value for the genotype obtained from the corresponding logistic regression model after adjusting for age, BMI, sex, cigarette smoking, alcohol consumption, and systolic and diastolic BP.
P for the interaction from the corresponding logistic regression model after adjusting for age, BMI, sex, cigarette smoking, alcohol consumption, and systolic and diastolic BP.
ApoA5, lipid profiles, LDL particle size, lipid peroxides, and ω6 PUFA in serum phospholipids according to APOA5 -1131T>C and FEN1 10154G>T polymorphisms
After adjusting for confounders, C allele carriers of APOA5 -1131T>C showed lower apoA5, higher triglyceride, lower HDL cholesterol, and smaller LDL particle size than TT participants in both the control and CAD group (Table 3). Controls with the APOA5 -1131C allele showed lower DGLA (20:3ω6) in serum phospholipids. No genotype differences in levels of LDL, total cholesterol, lipid peroxides, and ω3 fatty acids in serum phospholipids were observed for APOA5 -1131T>C (data not shown). Controls with the FEN1 10154T allele showed lower total cholesterol, MDA, ox-LDL, and DGLA than those with GG (Table 4). CAD patients carrying the FEN1 10154T allele showed larger LDL particle size and a trend toward a decrease in 8-epi-PGF2α. Minor T allele carriers of FEN1 10154G>T showed higher LA (18:2ω6), lower AA (20:4ω6), and lower ratio of AA/DGLA and AA/LA than GG participants in both the control and CAD groups. No genotype differences in apoA5, triglycerides, or ω3 fatty acids in serum phospholipids were observed for FEN1 10154G>T (data not shown).
TABLE 3.
Associations of the APOA5 -1131T>C genotypes with plasma apoA5, lipid profiles, and ω6 PUFA in serum phospholipids
| Control (n = 1123) |
CAD (n = 807) |
|||
|---|---|---|---|---|
| APOA5 TT(n = 566) | APOA5 C allele (n = 557) | APOA5 TT(n = 363) | APOA5 C allele (n = 444) | |
| ApoA5 (ng/ml)a | 238.2 ± 5.21 | 213.7 ± 4.78*** | 213.1 ± 7.70 | 185.0 ± 6.50*** |
| Triglyceride (mg/dl)a | 118.3 ± 2.56 | 139.6 ± 3.58*** | 136.8 ± 3.51 | 156.0 ± 4.17*** |
| HDL-cholesterol (mg/dl)a | 52.8 ± 0.59 | 50.7 ± 0.59** | 46.2 ± 0.58 | 44.9 ± 0.52* |
| LDL particle size (nm) | 23.8 ± 0.03 | 23.7 ± 0.03*** | 23.6 ± 0.04 | 23.4 ± 0.04*** |
| 18:2(ω6) | 12.9 ± 0.17 | 12.8 ± 0.18 | 12.0 ± 0.18 | 12.3 ± 0.19 |
| 20:3(ω6) | 1.69 ± 0.04 | 1.53 ± 0.03** | 1.89 ± 0.05 | 1.84 ± 0.04 |
| 20:4(ω6) | 4.95 ± 0.10 | 4.74 ± 0.10 | 5.65 ± 0.15 | 5.77 ± 0.13 |
| 20:4(ω6)/20:3(ω6) | 3.11 ± 0.06 | 3.23 ± 0.06 | 3.11 ± 0.08 | 3.29 ± 0.08 |
| AA/LA | 0.39 ± 0.01 | 0.37 ± 0.01 | 0.48 ± 0.01 | 0.49 ± 0.01 |
Mean ± SE. P-values were tested with a paired t-test, *P < 0.05, **P < 0.01, ***P < 0.001; adjusted for sex, age, BMI, cigarette smoking, alcohol consumption, and systolic and diastolic BP.
Logarithmic transformation.
TABLE 4.
Associations of the FEN1 10154 G>T genotypes with serum lipids, lipid peroxides, and ω6 PUFA in serum phospholipids
| Control (n = 1123) |
CAD (n = 807) |
|||
|---|---|---|---|---|
| FEN1 10154GG (n = 516) | FEN1 10154T allele (n = 607) | FEN1 10154 GG n = 399) | FEN1 10154T allele (n = 408) | |
| Total-cholesterol (mg/dl) | 197.6 ± 1.48 | 193.6 ± 1.34* | 164.4 ± 1.91 | 168.0 ± 1.99 |
| LDL particle size (nm) | 23.7 ± 0.03 | 23.8 ± 0.03 | 23.4 ± 0.03 | 23.5 ± 0.04** |
| Malondialdehyde (nmol/ml)a | 9.41 ± 0.15 | 8.98 ± 0.11* | 10.6 ± 0.27 | 10.4 ± 0.21 |
| 8-epi-PGF2α (pg/mg creatinine)a | 1316.4 ± 28.3 | 1279.2 ± 23.6 | 1458.7 ± 41.2 | 1377.9 ± 35.0 |
| Ox-LDL (μ/L)a | 65.0 ± 1.13 | 62.2 ± 1.01* | 58.2 ± 1.17 | 60.7 ± 1.33 |
| 18:2(ω6) | 12.4 ± 0.18 | 13.2 ± 0.17** | 11.6 ± 0.18 | 12.7 ± 0.19*** |
| 20:3(ω6) | 1.67 ± 0.04 | 1.56 ± 0.03* | 1.82 ± 0.04 | 1.91 ± 0.05 |
| 20:4(ω6) | 5.36 ± 0.11 | 4.46 ± 0.09*** | 6.17 ± 0.15 | 5.22 ± 0.12*** |
| 20:4(ω6)/20:3(ω6) | 3.33 ± 0.06 | 3.05 ± 0.06*** | 3.52 ± 0.08 | 2.87 ± 0.07*** |
| AA/LA | 0.43 ± 0.01 | 0.34 ± 0.01*** | 0.54 ± 0.01 | 0.42 ± 0.01*** |
Mean ± SE. P-values were tested with a paired t-test, *P < 0.05, **P < 0.01, ***P < 0.001 adjusted for sex, age, BMI, cigarette smoking, alcohol consumption, and systolic and diastolic BP.
Logarithmic transformation.
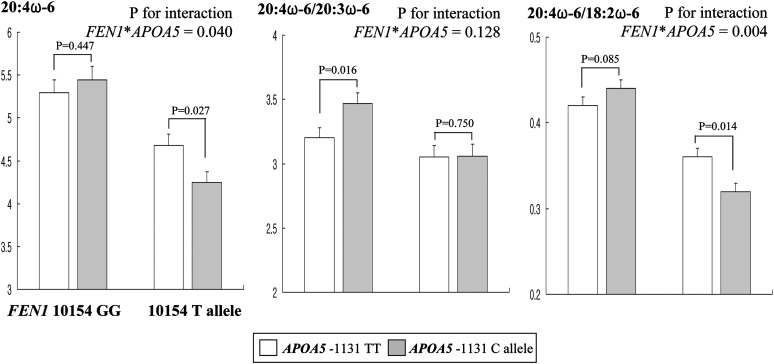
Genotype combinations for the two SNPs were constructed to evaluate interactions between the genes for serum lipid profiles, LDL particle size, lipid peroxides, and ω6 PUFA in serum phospholipids. Except for serum phospholipid AA, no significant interactions were observed between APOA5 -1131T>C and FEN1 10154G>T for these variables (data not shown). A significant interaction between APOA5 -1131T>C and FEN1 10154G>T was observed for AA in serum phospholipids (P = 0.04) and the AA/LA ratio (P = 0.004) in healthy controls (Fig. 1). Significantly lower AA (P = 0.029) was observed in controls with the minor C allele for APOA5 -1131T>C who also carried the FEN1 10154 minor T allele. Significantly lower AA/LA ratios (P = 0.014) were observed in controls with the APOA5 -1131C minor allele who also carried the FEN1 10154 minor T allele. In contrast, a trend toward an increase in the AA/LA ratio (P = 0.085) was observed in controls with the APOA5 -1131C minor allele who also carried the FEN1 10154 GG genotype. Although an interaction between the two genes for the 20:4(ω6)/20:3(ω6) ratio failed to reach statistical significance (P = 0.128), the APOA5 -1131C allele was associated with a higher 20:4(ω6)/20:3(ω6) ratio (P = 0.016) only in individuals carrying the FEN1 10154T allele. The FEN1 10154T allele was associated with lower 20:3(ω6) (P = 0.005) only in individuals carrying the APOA5 -1131C allele (Fig. 1).
Fig. 1.
Serum phospholipid arachidonic acid (AA, 20:4ω6) and the ratio of 20:4ω6/20:3ω6 and 20:4ω6/18:2ω6 by APOA5 -1131T>C and FEN1 10154G>T genotype combination in healthy controls. Mean ± SE. P for interaction was tested by multiple logistic regression analysis after adjusting for sex, age, BMI, cigarette smoking, alcohol consumption, and systolic and diastolic BP.
Relation of serum phospholipid AA with apoA5, lipid peroxides, and TNF-α
Pearson correlation analysis showed that the AA proportion in serum phospholipids was positively correlated with ox-LDL (r = 0.177, P < 0.001), MDA (r = 0.153, P = 0.002), 8-epi-PGF2α (r = 0.122, P = 0.007), and TNF-α (r = 0.187, P < 0.001) in healthy controls. In addition, 8-epi-PGF2α was positively correlated with TNF-α (r = 0.094, P = 0.037) and CRP (r = 0.175, P < 0.001) in healthy controls. In CAD patients, AA was negatively correlated with apoA5 (r = −0.146, P = 0.030) and positively correlated with 8-epi-PGF2α (r = 0.126, P = 0.018).
DISCUSSION
We found an interaction between APOA5 -1131T>C and FEN1 10154G>T for CAD risk. The interaction effect between these two genes revealed that carriers of the APOA5 -1131C allele, despite their increased triglycerides and decreased apoA5, were not associated with CAD risk when carrying the FEN1 10154T variant allele as compared with participants homozygous for the APOA5 -1131T allele. This circumstance may mask or reduce the risk estimation of CAD if the interaction between APOA5 -1131T>C and FEN1 10154G>T is not considered. This finding partly explains the highly controversial results regarding the association of the APOA5 -1131C variant allele with CAD risk despite the general consistency regarding its relationship with higher triglyceride and lower apoA5 concentrations.
ApoA5 is an important regulator of triglyceride-rich lipoprotein metabolism (22). In controls with the APOA5 -1131C allele, higher triglycerides, smaller LDL particle size, and lower levels of apoA5 and serum phospholipid DGLA (20:3ω6) are consistent with the suggestion of a role for apoA5 in VLDL assembly (3, 23) or triglyceride hydrolysis (24). Previous studies have shown interaction effects between dietary ω6 PUFA and APOA5 -1131T>C when determining fasting triglyceride, lipoprotein particle size (6), postprandial triglyceride (2, 25, 26), BMI, and obesity risk (7). Although PUFA levels in phospholipids are known to be determined by a combination of dietary intake and metabolic efficiency (15, 27–29), an interaction between APOA5 -1131T>C and FEN1 10154G>T polymorphisms on ω6 PUFA metabolism and CAD has not been previously reported. FEN1 10154G>T near FADS1 was found to be the SNP with the most significant association in the analysis of AA (20:4ω6), the long-chain ω6 derivative of LA (18:2ω6), in a recent genome-wide association study of plasma PUFAs (15).
Martinelli et al. (30) showed that a higher ratio of AA/LA is an independent risk factor for CAD, and a graded increase in CAD risk is related to the carrier status of FADS haplotypes associated with a higher desaturase activity. Conversely, a possible causality link between vascular disease and lower desaturase activity also has been suggested (31). However, exaggerated desaturation and elongation generally have been reported to characterize CAD patients and individuals with major risk factors for CAD (30, 32–35). In addition, AA in adipose tissue was found to be associated with acute myocardial infarction (36, 37), and this association was not related to dietary intake of ω6 PUFA including LA (37). In this study, CAD patients also showed higher AA and AA/LA in serum phospholipids, but there was no significant difference in the proportion of energy intake derived from fat and PUFA intake between control and CAD groups. Furthermore, fat intake did not differ according to genotype combination in either controls or CAD patients. Therefore, the difference in ω6 PUFAs in serum phospholipids between controls and CAD patients was shown not to be derived from dietary fat intake but may be due to intrinsic phospholipid metabolism. In part, not only AA, but also its precursors including LA and DGLA in phospholipids, are known to be genetically determined (15, 29).
An interaction effect between APOA5 -1131T>C and FEN1 10154G>T was also found in serum phospholipid AA and AA/LA in controls. In the FEN1 10154GG genotype group, APOA5 -1131C allele carriers showed higher activity of D5D (AA/DGLA) and a trend toward an increase in AA/LA. In contrast, in the FEN1 10154T minor allele group, the APOA5 -1131C allele was associated with a lower AA/LA ratio. The mechanism of this interaction cannot be determined from our experimental approach. However, the FEN1 10154T allele is associated with higher LA and lower AA in plasma PUFA (15), suggesting that this variant allele may reduce D5D expression and the elongation-desaturation process of converting LA to AA. Therefore, the interaction effect between the two genes may be partly due to lower activity of D5D in controls with the FEN1 10154T variant allele who also carry the APOA5 -1131C allele, and the result may be lower DGLA. This may also partly explain our current observation that the lowest AA and AA/LA in serum phospholipids was found in controls carrying minor alleles at both genes, despite their increased serum triglyceride levels.
AA, a precursor of eicosanoids including prostaglandins and leukotrienes, is liberated from the hydrolysis of the sn-2 position of glycerophospholipids (38). Radical peroxidation of AA produces a family of prostaglandin F2-isomers called F2-isoprostanes (39). One such F2-isoprostane is 8-epi-PGF2α, a sensitive and independent risk marker for CAD (40–42) that is probably released into biological fluids through a phospholipase-mediated pathway and consequently excreted in urine. Here, we found a positive correlation between serum phospholipid AA and urinary excretion of 8-epi-PGF2α in both controls and CAD patients. In addition, serum phospholipid AA was positively correlated with ox-LDL, a strong predictor for CAD (43), as well as MDA and TNF-α in controls. Interestingly, we also found a positive relationship between 8-epi-PGF2α, TNF-α, and CRP. These results support the previous suggestion that decreased synthesis and thus, availability of AA, mitigates the inflammatory response by altering AA metabolism, for example, by decreasing eicosanoid levels (11).
Several points should be considered when interpreting our findings. First, our study measured D5D activity rather than concentration. PUFA levels were also expressed as a percent of total FAs in serum phospholipids rather than an absolute concentration. Therefore, we could detect relative differences in PUFA levels and D5D activity but were unable to decipher the mechanisms, which depend on the absolute values. Second, our results share the general limitations of other cross-sectional observational studies in that we evaluated association, not prospective prediction. Finally, we specifically focused on a representative group of Korean adults aged 27–81 years. This selection was based on the knowledge that CAD is greatly influenced by genetic factors at younger ages. Our controls had normal fasting glucose levels (<100 mg/dl) and were not taking any medications or functional foods. Therefore, our data cannot be generalized to other ethnic groups or other populations. Despite these limitations, we found an interaction between APOA5 -1131T>C and FEN1 10154G>T polymorphisms on AA and AA/LA in serum phospholipids as well as on CAD risk. The observed interaction between these genes suggests that the FEN1 10154T variant allele decreases AA and AA/LA in serum phospholipids in carriers of the APOA5 -1131C allele, contributing to an insignificant increase in CAD risk for this subset of the population, despite their increased triglyceride and decreased apoA5 levels.
Acknowledgments
The authors thank the research volunteers who participated in the studies described in this report. We also thank the researchers at DNA Link Ltd. for their technical help in DNA extraction and genotyping.
Footnotes
- 8-epi-PGF2α
- 8-epi-prostaglandin F2α
- AA
- arachidonic acid
- apo
- apolipoprotein
- BMI
- body mass index
- BP
- blood pressure
- CAD
- coronary artery idsease
- CI
- confidence interval
- CRP
- C-reactive protein
- D5D
- delta 5 desaturase
- DGLA
- dihomo-γ-linolenic acid
- DM
- diabetes mellitus
- LA
- linoleic acid
- LLD
- lipid-lowering drug
- MDA
- malondialdehyde
- OR
- odds ratio
- ox-LDL
- oxidized LDL
- SNP
- single nucleotide polymorphism
- TNF
- tumor necrosis factor
This work was supported by the National Research Foundation, Ministry of Education, Science and Technology (Mid-career Researcher Program: 2010-0015017, M10642120002-06N4212-00210 and C00048), Seoul, Korea, and Korea Health 21 R&D Projects, Ministry of Health & Welfare (A000385), Seoul, Korea. None of the authors have any conflicts of interest in relation to the materials presented in this paper.
REFERENCES
- 1.van der Vliet H. N., Sammels M. G., Leegwater A. C., Levels J. H., Reitsma P. H., Boers W., Chamuleau R. A. 2001. Apolipoprotein A-V: a novel apolipoprotein associated with an early phase of liver regeneration. J. Biol. Chem. 276: 44512–44520. [DOI] [PubMed] [Google Scholar]
- 2.Merkel M., Loeffler B., Kluger M., Fabig N., Geppert G., Pennacchio L. A., Laatsch A., Heeren J. 2005. Apolipoprotein AV accelerates plasma hydrolysis of triglyceride-rich lipoproteins by interaction with proteoglycan-bound lipoprotein lipase. J. Biol. Chem. 280: 21553–21560. [DOI] [PubMed] [Google Scholar]
- 3.Schaap F. G., Rensen P. C., Voshol P. J., Vrins C., van der Vliet H. N., Chamuleau R. A., Havekes L. M., Groen A. K., van Dijk K. W. 2004. ApoAV reduces plasma triglycerides by inhibiting very low density lipoprotein-triglyceride (VLDL-TG) production and stimulating lipoprotein lipase-mediated VLDL-TG hydrolysis. J. Biol. Chem. 279: 27941–27947. [DOI] [PubMed] [Google Scholar]
- 4.Pennacchio L. A., Rubin E. M. 2003. Apolipoprotein A5, a newly identified gene that affects plasma triglyceride levels in humans and mice. Arterioscler. Thromb. Vasc. 23: 529–534. [DOI] [PubMed] [Google Scholar]
- 5.Jang Y., Paik J. K., Hyun Y. J., Chae J. S., Kim J. Y., Choi J. R., Lee S. H., Shin D. J., Ordovas J. M., Lee J. H. 2009. The apolipoprotein A5–1131T>C promoter polymorphism in Koreans: association with plasma APOA5 and serum triglyceride concentrations, LDL particle size and coronary artery disease. Clin. Chim. Acta. 402: 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai C. Q., Corella D., Demissie S., Cupples L. A., Adiconis X., Zhu Y., Parnell L. D., Tucker K. L., Ordovas J. M. 2006. Dietary intake of n-6 fatty acids modulates effect of apolipoprotein A5 gene on plasma fasting triglycerides, remnant lipoprotein concentrations, and lipoprotein particle size: the Framingham Heart Study. Circulation. 113: 2062–2070. [DOI] [PubMed] [Google Scholar]
- 7.Corella D., Lai C. Q., Demissie S., Cupples L. A., Manning A. K., Tucker K. L., Ordovas J. M. 2007. APOA5 gene variation modulates the effects of dietary fat intake on body mass index and obesity risk in the Framingham Heart Study. J. Mol. Med. 85: 119–128. [DOI] [PubMed] [Google Scholar]
- 8.Plakké T., Berkel J., Beynen A. C., Hermus R. J., Katan M. B. 1983. Relationship between the fatty acid composition of the diet and that of the subcutaneous adipose tissue in individual human subjects. Hum. Nutr. Appl. Nutr. 37: 365–372. [PubMed] [Google Scholar]
- 9.Dougherty R. M., Galli C., Ferro-Luzzi A., Iacono J. M. 1987. Lipid and phospholipid fatty acid composition of plasma, red blood cells, and platelets and how they are affected by dietary lipids: a study of normal subjects from Italy, Finland, and the USA. Am. J. Clin. Nutr. 45: 443–455. [DOI] [PubMed] [Google Scholar]
- 10.Vessby B. 2003. Dietary fat, fatty acid composition in plasma and the metabolic syndrome. Curr. Opin. Lipidol. 14: 15–19. [DOI] [PubMed] [Google Scholar]
- 11.Vessby B., Uusitupa M., Hermansen K., Riccardi G., Rivellese A. A., Tapsell L. C., Nälsén C., Berglund L., Louheranta A., Rasmussen B. M., et al. 2001. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: the KANWU Study. Diabetologia. 44: 312–319. [DOI] [PubMed] [Google Scholar]
- 12.Marquardt A., Sto¨hr H., White K., Weber B. H. 2000. cDNA cloning, genomic structure, and chromosomal localization of three members of the human fatty acid desaturase family. Genomics. 66: 175–183. [DOI] [PubMed] [Google Scholar]
- 13.Cho H. P., Nakamura M. T., Clarke S. D. 1999. Cloning, expression, and nutritional regulation of the mammalian delta-6 desaturase. J. Biol. Chem. 274: 471–477. [DOI] [PubMed] [Google Scholar]
- 14.Cho H. P., Nakamura M., Clarke S. D. 1999. Cloning, expression, and fatty acid regulation of the human delta-5 desaturase. J. Biol. Chem. 274: 37335–37339. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T., Shen J., Abecasis G. R., Kisialiou A., Ordovas J. M., Guralnik J. M., Singleton A., Bandinelli S., Cherubini A., Arnett D., et al. 2009. Genome-wide association study of plasma polyunsaturated fatty acids in the InCHIANTI Study. PLoS Genet. 5: e1000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obukowicz M. G., Raz A., Pyla P. D., Rico J. G., Wendling J. M., Needleman P. 1998. Identification and characterization of a novel delta6/delta5 fatty acid desaturase inhibitor as a potential anti-inflammatory agent. Biochem. Pharmacol. 55: 1045–1058. [DOI] [PubMed] [Google Scholar]
- 17.Malerba G., Schaeffer L., Xumerle L., Klopp N., Trabetti E., Biscuola M., Cavallari U., Galavotti R., Martinelli N., Guarini P., et al. 2008. SNPs of the FADS gene cluster are associated with polyunsaturated fatty acids in cohort of patients with cardiovascular disease. Lipids. 43: 289–299. [DOI] [PubMed] [Google Scholar]
- 18.Funk C. D. 2001. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 294: 1871–1875. [DOI] [PubMed] [Google Scholar]
- 19.Brash A. R. 2001. Arachidonic acid as a bioactive molecule. J. Clin. Invest. 107: 1339–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 21.Lepage G., Roy C. C. 1986. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 27: 114–120. [PubMed] [Google Scholar]
- 22.Rensen P. C., van Dijk K. W., Havekes L. M. 2005. Apolipoprotein AV: low concentration, high impact. Arterioscler. Thromb. Vasc. 25: 2445–2447. [DOI] [PubMed] [Google Scholar]
- 23.Weinberg R. B., Cook V. R., Beckstead J. A., Martin D. D., Gallagher J. W., Shelness G. S., Ryan R. O. 2003. Structure and interfacial properties of human apolipoprotein A-V. J. Biol. Chem. 278: 34438–34444. [DOI] [PubMed] [Google Scholar]
- 24.Grosskopf I., Baroukh N., Lee S. J., Kamari Y., Harats D., Rubin E. M., Pennacchio L. A., Cooper A. D. 2005. APOA5 deficiency results in marked hypertriglyceridemia attributable to decreased lipolysis of triglyceride-rich lipoproteins and removal of their remnants. Arterioscler. Thromb. Vasc. 25: 2573–2579. [DOI] [PubMed] [Google Scholar]
- 25.Marcais C., Verges B., Charriere S., Pruneta V., Merlin M., Billon S., Perrot L., Drai J., Sassolas A., Pennacchio L. A., et al. 2005. Apoa5 Q139X truncation predisposes to late-onset hyperchylomicronemia due to lipoprotein lipase impairment. J. Clin. Invest. 115: 2862–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang Y., Kim J. Y., Kim O. Y., Lee J. E., Cho H., Ordovas J. M., Lee J. H. 2004. The -1131T->C polymorphism in the apolipoprotein A5 gene is associated with postprandial hypertriacylglycerolemia; elevated small, dense LDL concentrations; and oxidative stress in nonobese Korean men. Am. J. Clin. Nutr. 80: 832–840. [DOI] [PubMed] [Google Scholar]
- 27.Emken E. A., Adlof R. O., Gulley R. M. 1994. Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochim. Biophys. Acta. 1213: 277–288. [DOI] [PubMed] [Google Scholar]
- 28.Di Stasi D., Bernasconi R., Marchiali R., Marfisi R. M., Rossi G., Rognoni G., Sacconi M. T. 2004. Early modifications of fatty acid composition in plasma phospholipids, platelets and mononucleates of healthy volunteers after low doses of n-3 polyunsaturated fatty acids. Eur. J. Clin. Pharmacol. 60: 183–190. [DOI] [PubMed] [Google Scholar]
- 29.Schaeffer L., Gohlke H., Müller M., Heid I. M., Palmer L. J., Kompauer I., Demmelmair H., Illig T., Koletzko B., Heinrich J. 2006. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum. Mol. Genet. 15: 1745–1756. [DOI] [PubMed] [Google Scholar]
- 30.Martinelli N., Girelli D., Malerba G., Guarini P., Illig T., Trabetti E., Sandri M., Friso S., Pizzolo F., Schaeffer L., et al. 2008. FADS genotypes and desaturase activity estimated by the ratio of arachidonic acid to linoleic acid are associated with inflammation and coronary artery disease. Am. J. Clin. Nutr. 88: 941–949. [DOI] [PubMed] [Google Scholar]
- 31.Das U. N. 2007. A defect in the activity of Delta6 and Delta5 desaturases may be a factor in the initiation and progression of atherosclerosis. Prostaglandins Leukot. Essent. Fatty Acids. 76: 251–268. [DOI] [PubMed] [Google Scholar]
- 32.Glew R. H., Okolie H., Huang Y. S., Chuang L. T., Suberu O., Crossey M., VanderJagt D. J. 2004. Abnormalities in the fatty-acid composition of the serum phospholipids of stroke patients. J. Natl. Med. Assoc. 96: 826–832. [PMC free article] [PubMed] [Google Scholar]
- 33.Warensjo E., Riserus U., Vessby B. 2005. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia. 48: 1999–2005. [DOI] [PubMed] [Google Scholar]
- 34.Warensjo E., Ohrvall M., Vessby B. 2006. Fatty acid composition and estimated desaturase activities are associated with obesity and lifestyle variables in men and women. Nutr. Metab. Cardiovasc. Dis. 16: 128–136. [DOI] [PubMed] [Google Scholar]
- 35.Warensjo E., Sundstrom J., Lind L., Vessby B. 2006. Factor analysis of fatty acids in serum lipids as a measure of dietary fat quality in relation to the metabolic syndrome in men. Am. J. Clin. Nutr. 84: 442–448. [DOI] [PubMed] [Google Scholar]
- 36.Kark J. D., Kaufmann N. A., Binka F., Goldberger N., Berry E. M. 2003. Adipose tissue n-6 fatty acids and acute myocardial infarction in a population consuming a diet high in polyunsaturated fatty acids. Am. J. Clin. Nutr. 77: 796–802. [DOI] [PubMed] [Google Scholar]
- 37.Baylin A., Campos H. 2004. Arachidonic acid in adipose tissue is associated with nonfatal acute myocardial infarction in the central valley of Costa Rica. J. Nutr. 134: 3095–3099. [DOI] [PubMed] [Google Scholar]
- 38.Stafforini D. M., Sheller J. R., Blackwell T. S., Sapirstein A., Yull F. E., McIntyre T. M., Bonventre J. V., Prescott S. M., Roberts L. J. 2006. Release of free F2-isoprostanes from esterified phospholipids is catalyzed by intracellular and plasma platelet-activating factor acetylhydrolases. J. Biol. Chem. 281: 4616–4623. [DOI] [PubMed] [Google Scholar]
- 39.Voss P., Siems W. 2006. Clinical oxidation parameters of aging. Free Radic. Res. 40: 1339–1349. [DOI] [PubMed] [Google Scholar]
- 40.Schwedhelm E., Bartling A., Lenzen H., Tsikas D., Maas R., Brümmer J., Gutzki F. M., Berger J., Frölich J. C., Böger R. H. 2004. Urinary 8-iso-prostaglandin F2alpha as a risk marker in patients with coronary heart disease: a matched case-control study. Circulation. 109: 843–848. [DOI] [PubMed] [Google Scholar]
- 41.Wolfram R., Oguogho A., Palumbo B., Sinzinger H. 2005. Enhanced oxidative stress in coronary heart disease and chronic heart failure as indicated by an increased 8-epi-PGF(2alpha). Eur. J. Heart Fail. 7: 167–172. [DOI] [PubMed] [Google Scholar]
- 42.Vassalle C., Petrozzi L., Botto N., Andreassi M. G., Zucchelli G. C. 2004. Oxidative stress and its association with coronary artery disease and different atherogenic risk factors. J. Intern. Med. 256: 308–315. [DOI] [PubMed] [Google Scholar]
- 43.Huang H., Mai W., Liu D., Hao Y., Tao J., Dong Y. 2008. The oxidation ratio of LDL: a predictor for coronary artery disease. Dis. Markers. 24: 341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]