Abstract
Low-fat diets have been shown to increase plasma concentrations of lipoprotein(a) [Lp(a)], a preferential lipoprotein carrier of oxidized phospholipids (OxPLs) in plasma, as well as small dense LDL particles. We sought to determine whether increases in plasma Lp(a) induced by a low-fat high-carbohydrate (LFHC) diet are related to changes in OxPL and LDL subclasses. We studied 63 healthy subjects after 4 weeks of consuming, in random order, a high-fat low-carbohydrate (HFLC) diet and a LFHC diet. Plasma concentrations of Lp(a) (P < 0.01), OxPL/apolipoprotein (apo)B (P < 0.005), and OxPL-apo(a) (P < 0.05) were significantly higher on the LFHC diet compared with the HFLC diet whereas LDL peak particle size was significantly smaller (P < 0.0001). Diet-induced changes in Lp(a) were strongly correlated with changes in OxPL/apoB (P < 0.0001). The increases in plasma Lp(a) levels after the LFHC diet were also correlated with decreases in medium LDL particles (P < 0.01) and increases in very small LDL particles (P < 0.05). These results demonstrate that induction of increased levels of Lp(a) by an LFHC diet is associated with increases in OxPLs and with changes in LDL subclass distribution that may reflect altered metabolism of Lp(a) particles.
Keywords: lipid, atherosclerosis, cardiovascular risk, low density lipoprotein, diet, oxidized lipids
Oxidized phospholipids (OxPLs) play an important role in inflammation and in progression of atherosclerosis (1, 2). Levels of OxPL on apolipoprotein (apo)B-100 (OxPL/apoB) lipoproteins can be measured with the monoclonal antibody E06, which binds OxPL but not native phospholipids (3). OxPL/apoB levels are elevated in patients with acute coronary syndromes (4, 5), and in coronary (6), carotid, and peripheral arterial disease (7) and predict death and myocardial infarction in unselected populations (8). Recently, Bergmark et al. (9) showed that lipoprotein(a) [Lp(a)] is the preferential lipoprotein carrier of OxPL in human plasma, and other studies (4, 5, 10–12) have shown strong associations between OxPL/apoB and Lp(a).
Elevated plasma Lp(a) concentrations are associated with increased risk of cardiovascular disease (CVD) (13, 14), and there is increasingly strong data that they may be causal in the etiology of myocardial infarction (15, 16). Lp(a) consists of an LDL-like particle covalently attached to apo(a) by a single disulfide bond (17). Plasma Lp(a) levels vary in the population from nearly undetectable to >100 mg/dl. This heterogeneity is mainly determined by variation at the apo(a) gene locus (18) and is inversely related to the number of kringle IV repeats (19). Additional single nucleotide polymorphisms (SNPs) such as the recently described LPA I4399M and rs10455872 SNPs, which are associated with marked elevation of Lp(a) levels, coronary artery disease, and myocardial infarction (20–24), are associated with even further heterogeneity in Lp(a) levels.
Although the variability in Lp(a) concentration is largely genetically determined, environmental influences and pharmacological therapies may also affect Lp(a) levels. In particular, low-fat diets have been shown to result in small but significant increases in Lp(a) (25, 26). Furthermore, such diets can induce inverse changes in levels of large versus small LDL as well as in levels of medium versus very small LDL particles (27). In the present study, we tested the impact of a combined low-fat high-carbohydrate (LFHC) diet on plasma Lp(a) concentrations and whether any changes are related to changes in both OxPL and LDL subclasses.
METHODS
Study design and experimental diets
All subjects underwent a diet intervention (randomized crossover design) involving 4 weeks of a high-fat low-carbohydrate (HFLC) diet and 4 weeks of an LFHC diet. The HFLC diet was designed to provide 40% of energy from fat (13.0% saturated, 11.0% monounsaturated, 13.8% polyunsaturated, and 3.4% trans), 45% carbohydrate, and 15% protein. The LFHC diet was designed to provide 20% of energy from fat (4.9% saturated, 9.9% monounsaturated, 5.1% polyunsaturated, and 2.4% trans), 65% carbohydrate, and 15% protein. There were no differences in dietary cholesterol and the ratio of simple:complex carbohydrate was ∼50:50 between the diets (Table 1). Nutrient composition was calculated using the Minnesota Nutrition Data System software, version 2.1, developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN.
TABLE 1.
Macronutrient composition of the diets
| HFLC | LFHC | |
|---|---|---|
| % of energy | ||
| Protein | 15 | 15 |
| Carbohydrate | 45 | 65 |
| Fat | 40 | 20 |
| Saturated | 13 | 5 |
| Monounsaturated | 11 | 10 |
| Polyunsaturated | 14 | 5 |
| Trans | 3 | 2 |
Each diet was normalized to cholesterol content and ratio of simple versus complex carbohydrate (50/50).
Study participants
Subjects were recruited from an extensive database and the community at large through telephone and written materials. The study participants were healthy non-smoking men and women at least 20 years old who had been free of chronic disease in the last 5 years, were not taking medication known to affect lipid metabolism, and had a resting blood pressure <160/105 mmHg. Other eligibility criteria included a body weight <130% of ideal, total cholesterol and LDL cholesterol less than the 95th percentile for age and sex, triglyceride <500 mg/dl, blood glucose <126 mg/dl, and plasma Lp(a) levels >3 mg/dl. Men (n = 61) and women (n = 2) were combined for analysis because the results were not different after exclusion of the two women participants. We previously reported lipoprotein fractionation data in a subset (n = 33) of the individuals (28).
Registered dietitians instructed the participants on the experimental diets at a group orientation class and contacted the subjects weekly to maintain diet adherence and weight maintenance. Four-day food records (Thursday through Sunday) were used to assess compliance with the experimental diets. All subjects were compliant, with estimated daily deviations averaging < 5% of total calories. Nutrient analysis of the 4-day food record was performed by Nutrition Data System software. Subjects were asked to refrain from alcohol and maintain their regular level of physical activity throughout the study. All subjects gave informed consent under a protocol approved by the Institutional Review Boards at Children's Hospital Oakland, CA and EO Lawrence Berkeley National Laboratory, University of California, Berkeley, CA.
Laboratory measurements
Blood samples were obtained after an overnight fast after the experimental diets (week 4). Plasma samples were prepared within 2 h of collection from venous blood collected in tubes containing Na2EDTA (1.4 g/L) and a preservative cocktail of protease and bacterial inhibitors. Plasma was kept at 4°C throughout processing. Plasma total cholesterol and triglyceride concentrations were determined by enzymatic procedures on an Express 550 Plus analyzer (Ciba Corning, Oberlin, OH). These measurements were consistently in control as monitored by the standardization program of the Centers for Disease Control-National Heart, Lung and Blood Institute. HDL cholesterol was measured after dextran sulfate precipitation of plasma (29), and LDL cholesterol was calculated from the formula of Friedewald et al. (30). Apo A-I and apoB were measured by immunoturbidometric assay (Bacton Assay Systems, San Marcos, CA, and Express 550 Plus analyzer). Lp(a) plasma concentrations were measured in triplicate by a sandwich assay with polyclonal antibodies (International Immunology Corp.).
Chemiluminescent ELISA was used to measure the content of OxPL per apoB-100 lipoprotein using the murine monoclonal antibody E06, which binds to the phosphocholine headgroup of oxidized but not native phospholipids (4). A 1:50 dilution of plasma in phosphate-buffered saline was added to microtiter wells coated with murine monoclonal antibody MB47, which specifically equally binds all apoB-100 [LDL, Lp(a), intermediate density lipoprotein (IDL), VLDL] particles (31, 32). Under these conditions, a saturating amount of apoB-100 was added to each well and consequently an equal number of apoB particles are captured in each well for all assays. The content of OxPL per apoB was then determined with biotinylated E06 as previously described (4, 5, 10, 33). Thus, by design, the OxPL/apoB measurement is independent of apoB or LDL cholesterol levels. The apoB in the denominator of the OxPL/apoB does not represent the plasma apoB-100 level but the amount of apoB-100 captured on each microtiter well plate. Because most of the OxPL are present on Lp(a) in this assay format, as previously shown (9), the OxPL/apoB measure primarily detects OxPL on Lp(a).
We also measured OxPL directly on Lp(a) [OxPL-apo(a)] particles using a variation of the chemiluminescent ELISA technique for OxPL/apoB. Unlike apoB levels, which differ by ∼3-5-fold among subjects, Lp(a) plasma levels can range ∼1000-fold among subjects, and therefore it is not possible to saturate the microtiter well plates with Lp(a) in subjects with low Lp(a) levels (∼20 mg/dl), which represents the majority of patients. Therefore, it is impossible to develop an OxPL/apo(a) assay in such patients that reflects the OxPL content per apo(a) particle like the OxPL/apoB assay. Instead, the assay was performed at a nonsaturating dilution of plasma (1:2500), which is in the linear range of Lp(a) levels in this assay format. Murine monoclonal antibody LPA4, which binds apo(a) (10), was plated on microtiter well plates (5 µg/ml) and used to capture Lp(a) from plasma. OxPL was then detected with E06 as described above. We report this assay as OxPL-apo(a) as relative light units (RLUs) of E06 immunoreactivity, which closely reflects the total OxPL content on Lp(a). Unlike the OxPL/apoB assay, this assay does not detect the amount of OxPL per apo(a) particle or the small amount of OxPL that may be present on non-Lp(a) apoB particles; therefore, the data are complementary (9).
Mass concentrations of plasma lipoprotein subclasses were measured by analytic ultracentrifugation and estimated as a function of Svedberg flotation rate (Sf): large VLDL (VLDL I: Sf 60–400), small VLDL (VLDL II: Sf 20–60), IDL (Sf 12–20), and LDL subclasses, ranging from largest and most buoyant to smallest and most dense, large (LDL I: Sf 7–12), medium (LDL II: Sf 5–7), small (LDL III: Sf 3–5), and very small (LDL IV: Sf 0–3) LDL (34). Nondenaturing polyacrylamide gradient gel electrophoresis with lipid staining of plasma was performed as described previously for determination of peak LDL and Lp(a) particle diameters (34–36).
Statistical analysis
JMP statistical software (version 7.0; SAS Institute, Inc.) was used for statistical analysis. Data are presented as means ± SD. Shapiro-Wilk test was used to test the skewness of the distribution. Non-normally distributed variables (triglyceride, apoA-I, apoB, Lp(a), OxPL/apoB, LDL size, and lipoprotein subclass mass concentrations) were log-transformed before analysis. Paired t-test was used to evaluate differences in lipids, lipoproteins, and apolipoproteins between the HFLC diet and the LFHC diet. Wilcoxon signed rank test was used to evaluate differences in OxPL on Lp(a) between the HFLC and the LFHC diet. Spearman's correlation coefficients were used to evaluate the relationship between diet-induced changes in lipids, lipoproteins, apolipoproteins, Lp(a), and OxPL/apoB. Multivariate model analysis was used to determine factors contributing to the diet-induced change in Lp(a). The independent variables were changes in OxPL/apoB, triglyceride, LDL cholesterol, HDL cholesterol, apoB, and apoA-I. P-values < 0.05 were considered statistically significant.
RESULTS
The baseline characteristics of the participants are presented in Table 2. As shown in Table 3, plasma concentrations of triglycerides (P < 0.0001), apoB (P < 0.05), Lp(a) (P < 0.01), OxPL/apoB (P < 0.005), and OxPL-apo(a) (P < 0.05) were significantly higher with the LFHC diet than with the HFLC diet. In contrast, total cholesterol (P = 0.06), LDL cholesterol (P < 0.05), HDL cholesterol (P < 0.0001), and apoA-1 (P < 0.0001) were lower on the LFHC diet.
TABLE 2.
Subject characteristics
| Male/Female | 61/2 |
| Age (yr) | 47.9 ± 11.2 |
| BMI (kg/m ) | 26.7 ± 2.7 |
| Waist (cm) | 94.4 ± 2.7 |
| Waist:Hip ratio | 0.91 ± 0.05 |
| Systolic BP (mmHg) | 121.1 ± 13.6 |
| Diastolic BP (mmHg) | 75.6 ± 8.7 |
| Total cholesterol (mg/dl) | 199.7 ± 30.6 |
| LDL cholesterol (mg/dl) | 128.1 ± 29.5 |
| HDL cholesterol (mg/dl) | 41.4 ± 7.5 |
| Triglyceride (mg/dl) | 152.4 ± 82.8 |
| ApoA-I (mg/dl) | 111.1 ± 12.9 |
| ApoB (mg/dl) | 99.0 ± 18.8 |
| Lipoprotein(a) (mg/dl) | 17.2 ± 11.0; 13.6 (8.2, 24.5)a |
Values are means ± SD. BMI, body mass index; BP, blood pressure; LDL, low density lipoprotein; HDL, high density lipoprotein; Apo, apolipoprotein.
Median (interquartile range).
TABLE 3.
Plasma measurements during high-fat low-carbohydrate (HFLC) and low-fat high-carbohydrate (LFHC) diets
| HFLC Diet(n = 63) | LFHC Diet(n = 63) | Δ Diet | P | |
|---|---|---|---|---|
| OxPL/apoB (RLUs) | 4259.6 ± 3351.3 | 4853.9 ± 3591.6 | 450.8 ± 1801.3 | <0.005 |
| OxPL-apo(a) (RLUs)a | 1091.4 ± 532.2 | 1282.1 ± 526.7 | 178.2 ± 656.4 | <0.05 |
| Lp(a) (mg/dl) | 17.8 ± 12.8 | 19.9 ± 13.7 | 2.17 ± 4.5 | <0.01 |
| Triglyceride (mg/dl) | 134.0 ± 100.8 | 165.4 ± 96.2 | 31.4 ± 84.9 | <0.0001 |
| Total cholesterol (mg/dl) | 192.0 ± 34.5 | 187.0 ± 31.7 | −5.0 ± 21.3 | 0.06 |
| LDL cholesterol (mg/dl) | 124.0 ± 31.3 | 117.3 ± 30.7 | −6.6 ± 22.0 | < 0.05 |
| HDL cholesterol (mg/dl) | 41.1 ± 7.7 | 37.0 ± 7.4 | −4.1 ± 3.8 | <0.0001 |
| ApoA-I (mg/dl) | 105.6 ± 12.2 | 100.5 ± 13.5 | −5.0 ± 8.3 | <0.0001 |
| ApoB (mg/dl) | 94.4 ± 22.0 | 99.7 ± 20.5 | 5.2 ± 17.4 | <0.05 |
Values are means ± SD. Non-normally distributed variables were log transformed before analysis.Apo, apolipoprotein; Lp(a), lipoprotein(a); OxPL/apoB, oxidized phospholipids per apolipoprotein B-100; OxPL-apo(a), oxidized phospholipids on Lp(a); RLU, relative light unit. (Δ; 20% low-fat diet minus 40% high-fat diet).
n = 57.
Plasma lipoprotein mass concentrations (mg/dl) and LDL and Lp(a) peak particle diameters (Å) are shown in Table 4. Compared with the HFLC diet, large and small VLDL significantly increased on the LFHC diet. Large and medium LDL decreased significantly, whereas small and very small LDL increased on the LFHC diet compared with the HFLC diet. As expected, the LFHC diet significantly decreased mean LDL peak particle diameter from 262 to 257 Å. However, mean Lp(a) peak particle diameter did not differ between the diets.
Table 4.
Plasma lipoprotein mass concentrations (mg/dl) and size (Å) during high-fat low-carbohydrate (HFLC) and low-fat high-carbohydrate (LFHC) diets
| HFLC Diet(n = 63) | LFHC Diet(n = 63) | P | |
|---|---|---|---|
| VLDL | |||
| VLDL I (Sf 60-400) | 29.2 ± 33.6 | 39.6 ± 35.7 | < 0.005 |
| VLDL II (Sf 20-60) | 52.6 ± 25.3 | 62.0 ± 27.0 | < 0.001 |
| IDL (Sf 12-20) | 36.1 ± 16.4 | 35.8 ± 15.7 | NS |
| LDL | |||
| LDL I (Sf 7-12) | 102.9 ± 37.3 | 82.6 ± 35.1 | < 0.0001 |
| LDL II (Sf 5-7) | 102.2 ± 31.1 | 88.4 ± 35.2 | < 0.001 |
| LDL III (Sf 3-5) | 60.6 ± 33.9 | 73.6 ± 34.8 | < 0.0001 |
| LDL IV (Sf 0-3) | 12.8 ± 10.3 | 18.0 ± 14.3 | < 0.005 |
| LDL peak particle diameter (Å)a | 261.6 ± 9.5 | 256.5 ± 8.3 | < 0.0001 |
| Lp(a) peak particle diameter (Å)a | 306.3 ± 10.3 | 307.7 ± 9.0 | NS |
Values are means ± SD. Non-normally distributed variables were log transformed before analysis.
n = 42.
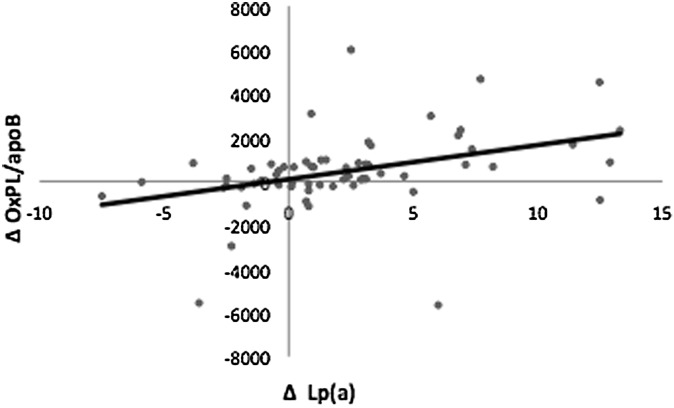
Table 5 shows that diet-induced changes in Lp(a) were significantly correlated with changes in OxPL/apoB (r = 0.49, P < 0.0001) and LDL cholesterol (r = 0.40, P < 0.005). In contrast, there were no significant associations between diet-induced changes in Lp(a) and plasma triglyceride, total cholesterol (P = 0.08), HDL cholesterol, apoB (P = 0.07), and apoA-I. Increases in Lp(a) with the LFHC diet were strongly related to increases in OxPL/apoB (P < 0.0001) (Fig. 1). Multivariate model analysis showed the associations between diet-induced changes in Lp(a) and changes in OxPL/apoB were independent of changes in plasma triglyceride, total cholesterol, LDL cholesterol, HDL cholesterol, apoA-I, and apoB (data not shown). Furthermore, increases in OxPL/apoB with the LFHC diet were positively associated with LDL cholesterol (P < 0.01), apoB (P < 0.05), and total cholesterol (P = 0.06) (data not shown).
TABLE 5.
Spearman's correlations between diet-induced changes in Lp(a) and lipids, lipoproteins, and apolipoproteins
| Δ Lp(a) | |
|---|---|
| Δ OxPL/apoB | 0.492b |
| Δ Triglyceride | −0.207 |
| Δ Total cholesterol | 0.219 |
| Δ LDL cholesterol | 0.396a |
| Δ LDL I | 0.129 |
| Δ LDL II | 0.336a |
| Δ LDL III | 0.179 |
| Δ LDL IV | −0.300a |
| Δ HDL cholesterol | −0.086 |
| Δ ApoA-I | −0.013 |
| Δ ApoB | 0.234 |
Apo, apolipoprotein; Lp(a), lipoprotein(a); OxPL/apoB, oxidized phospholipids per apolipoprotein B-100. (Δ; 20% low-fat diet minus 40% high-fat diet).
P < 0.05.
P < 0.0001.
Fig. 1.
Spearman's correlation between diet-induced changes in lipoprotein(a) [Lp(a)] (mg/dl) and oxidized phospholipids per apolipoprotein B-100 (OxPL/apoB) n = 62, r = 0.49, P < 0.0001. RLU, relative light unit.
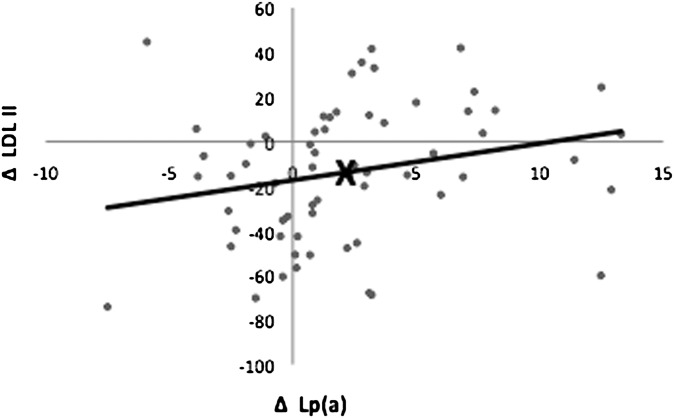
We found a reciprocal relationship between the diet-induced changes in medium and very small LDL (r = −0.46, P < 0.0005) and large and small LDL (r = −0.31, P < 0.05) (data not shown). The diet-induced changes in Lp(a) were positively correlated to changes with medium LDL (P < 0.01) (Table 5) (Fig. 2) and negatively with changes in very small LDL (P < 0.05) (Table 5). Note that as shown in Table 4, despite the positive correlation between Lp(a) and medium LDL and the increase in Lp(a) with LFHC, there was a mean reduction in medium LDL from 102.2 to 88.4 mg/dl with this diet. Furthermore, 15.6% of the variance of the diet-induced changes in Lp(a) was explained by changes in medium and very small LDL. Adjustment for changes in levels of medium and very small LDL resulted in an increase of the mean change in Lp(a) from 2.17 mg/dl to 2.85 mg/dl.
Fig. 2.
Spearman's correlation between diet-induced changes in lipoprotein(a) [Lp(a)] (mg/dl) and medium-size LDL II particles (mg/dl). n = 62, r = 0.34, P < 0.01. The value corresponding to mean changes in Lp(a) and LDL II is shown by ×.
Lp(a) peak particle diameter was positively correlated with LDL peak particle diameter during the LFHC diet (P < 0.01) but not on the HFLC diet (P = 0.12) (data not shown) and diet-induced changes in peak particle diameter of LDL and Lp(a) were not correlated (P = 0.97) (Table 4).
DISCUSSION
The results of this dietary intervention study utilizing a randomized crossover design demonstrate that an LFHC diet increases levels of both Lp(a) and OxPL/apoB, and that this response involves a diet-induced increase in OxPL on Lp(a) particles. Furthermore, the correlated changes in Lp(a) and OxPL/apoB were independent of changes in plasma triglycerides, total cholesterol, LDL cholesterol, HDL cholesterol, apoA-1, and apoB.
The measurement of OxPL on apoB-100 particles in the circulation has generated novel insights into the atherogenicity of Lp(a) (33). Initially, the OxPL/apoB assay using murine monoclonal antibody E06 was designed to measure “minimally modified LDL”. However, it was subsequently learned from multiple clinical and experimental studies that most of the E06-detectable OxPLs are actually present on Lp(a) particles rather than LDL (33). Biochemical and immunological studies on purified Lp(a) have suggested that ∼90% of OxPL associated with apoB lipoproteins are actually present on Lp(a), both on the apo(a) component and in the lipid phase (9, 11, 37). In fact, OxPL from copper oxidized LDL may be transferred to Lp(a) in a time- and temperature-dependent manner (9). Currently, the source of OxPL that ultimately is present on Lp(a), the sites of binding, and the relative content of OxPL on various Lp(a) particles is not yet fully determined and awaits further mechanistic studies.
It is clearly demonstrated that OxPL/apoB levels, which reflect primarily OxPL on Lp(a), are elevated in coronary (6), carotid, and femoral atherosclerosis (7) and enhance the predictive value of the Framingham Risk Score in predicting future death, myocardial infarction, and stroke (8). Thus, they may serve as a useful biomarker for identifying subjects at high cardiovascular risk. However, changes in the OxPL/apoB measurements in response to therapeutic interventions are less well established. Interestingly and seemingly paradoxically, we have noted an increase in OxPL/apoB levels as well as Lp(a) in several studies with different statins. For example, we have noted significant increases of ∼10–50% in OxPL/apoB and Lp(a) with various doses of pravastatin and atorvastatin in the MIRACL (4) and REVERSAL (38) trials, in children with familial hypercholesterolemia (39), and in subjects with hypercholesterolemia (40).
Diets low in total and saturated fat have been shown to increase plasma Lp(a) levels (25, 26, 41) and there is evidence that this effect may be specific for saturated fat (41). Increases in both Lp(a) and OxPL/apoB with no significant changes in LDL cholesterol or apoB were previously reported in a study of 37 women who consumed diets with reduced fat and high or low vegetable content. However, the baseline comparison diet in this study was not controlled and its composition estimated from 4-day food records indicated multiple differences in average macronutrient composition compared with the experimental diets and a range of fat intakes (36 ± 6%) that overlapped those of the experimental diets (31%), making it difficult to assess specific dietary influences on Lp(a) and OxPL/apoB response (26).
Notably, we have demonstrated that increases in OxPL/apoB occur in response to low-fat low-cholesterol diets in rabbits, which do not have Lp(a) and where apoB rather than Lp(a) is the OxPL carrier. We have made similar observations in cynomolgous monkeys, which have circulating Lp(a) but whose Lp(a) does not bind OxPL (42). In both the rabbit and primate studies, the increase in OxPL/apoB in plasma was associated with reduced content of OxPL in the vessel wall along with concomitant evidence of reduced oxidative stress (43). Although the mechanistic underpinnings of these changes cannot be determined from these studies, in composite they suggest that increases in plasma OxPL/apoB in response to therapeutic measures, perhaps including diet, may reflect a beneficial response within the artery wall leading to reduced OxPL content. In support of improved vascular health, a recent analysis from the MIRACL trial showed that individuals with the largest increases in OxPL/apoB were those who were felt to benefit the most from statins, such as younger patients with fewest risk factors (44). Clinical studies are underway to evaluate whether increases in OxPL/apoB in response to statins predict improved clinical outcomes.
Plasma levels of Lp(a) are thought to be determined primarily by the rate of hepatic synthesis and secretion of apo(a), which then binds to newly secreted apoB-containing lipoproteins, generating Lp(a) (45). Clearance of Lp(a), on the other hand, is not as well understood but studies suggest that the LDL receptor is not involved. Therefore, increased Lp(a) in response to diet is likely to result from altered hepatocyte handling of apo(a) and apoB synthesis. Low-fat diets are recommended to reduce risk of coronary heart disease, but LFHC diets have been shown to induce a more atherogenic lipoprotein profile in healthy individuals by increasing small dense LDL particles (Table 3), reducing HDL cholesterol, and increasing plasma triglyceride levels (Table 4) (27). LFHC diets result in increased concentrations of triglyceride-rich lipoproteins (TRLs) (46) and a reciprocal shift from medium-size LDL particles to very small LDL (27) (Table 4). The increase in plasma Lp(a) levels with an LFHC diet may be due to changes in TRL metabolism because apo(a) has been associated with TRLs and this TRL-apo(a) association has been suggested to be atherogenic (28, 47–49). Furthermore, the increase in plasma Lp(a) on an LFHC is associated with increased plasma apoC-III bound to apoB-containing lipoproteins and with the enrichment of TRL-apo(a) with apoC-III (28).
Increases in Lp(a) after the LFHC diet were positively correlated with changes in medium LDL particles, suggesting that the metabolism of Lp(a) and medium LDL may be coordinately regulated. Thus, the decrease in medium LDL and the increase in Lp(a) are proportionally less after the LFHC diet. Furthermore, we have shown an inverse correlation between diet-induced changes of Lp(a) and very small LDL such that the larger increase in very small LDL with an LFHC diet is associated with a smaller increase in Lp(a) (P < 0.05). Sixteen percent of the variance in diet-induced changes in Lp(a) was explained by the reciprocal changes in medium and very small LDL particles. Moreover, the strength of this relationship may have been underestimated due to the fact that the Sf 0–3 fraction including very small LDL also includes a portion of plasma Lp(a) particles (50). After adjusting for changes in medium and very small LDL, the diet-induced increase in Lp(a) was substantially greater (mean 2.85 mg/dl vs. 2.17 mg/dl), suggesting the shift in metabolism of these two LDL subclasses may be diverting the formation or increasing the catabolism or clearance of Lp(a), hence limiting the diet-induced increase in Lp(a)-associated OxPL.
One limitation is that the study population was primarily made up of men. Another concern is that there was no washout period between the two diets; however, we (51) have previously shown a dietary intervention of 4 weeks is sufficient to induce stabilization of plasma lipids and lipoproteins.
In conclusion, the results demonstrate that an LFHC diet results in increased plasma Lp(a) and its associated oxidized phospholipids and suggest that these increases may be modified by changes in LDL metabolism leading to altered LDL subclass distribution. Additional studies are needed to understand the physiological and clinical impact of such changes.
Acknowledgments
The authors thank the study participants and Robin S. Rawlings for subject recruitment and clinical assistance, Joe Orr and Bahareh Sahami for laboratory support, and Harriett S. Fernstrom for assistance in dietary intervention.
Footnotes
Abbreviations:
- apo
- apoliporotein
- CVD
- cardiovascular disease
- HFLC
- high-fat low-carbohydrate
- LFHC
- low-fat high-carbohydrate
- Lp(a)
- lipoprotein(a)
- OxPL
- oxidized phospholipid
- RLU
- relative light unit
- SNP
- single nucleotide polymorphism
- TRL
- triglyceride-rich lipoprotein
REFERENCES
- 1.Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. 1989. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 320: 915–924. [DOI] [PubMed] [Google Scholar]
- 2.Navab M., Berliner J. A., Watson A. D., Hama S. Y., Territo M. C., Lusis A. J., Shih D. M., Van Lenten B. J., Frank J. S., Demer L. L., et al. 1996. The Yin and Yang of oxidation in the development of the fatty streak. A review based on the 1994 George Lyman Duff Memorial Lecture. Arterioscler. Thromb. Vasc. Biol. 16: 831–842. [DOI] [PubMed] [Google Scholar]
- 3.Friedman P., Horkko S., Steinberg D., Witztum J. L., Dennis E. A. 2002. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids. Importance of Schiff base formation and aldol condensation. J. Biol. Chem. 277: 7010–7020. [DOI] [PubMed] [Google Scholar]
- 4.Tsimikas S., Witztum J. L., Miller E. R., Sasiela W. J., Szarek M., Olsson A. G., Schwartz G. G. 2004. High-dose atorvastatin reduces total plasma levels of oxidized phospholipids and immune complexes present on apolipoprotein B-100 in patients with acute coronary syndromes in the MIRACL trial. Circulation. 110: 1406–1412. [DOI] [PubMed] [Google Scholar]
- 5.Tsimikas S., Bergmark C., Beyer R. W., Patel R., Pattison J., Miller E., Juliano J., Witztum J. L. 2003. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J. Am. Coll. Cardiol. 41: 360–370. [DOI] [PubMed] [Google Scholar]
- 6.Tsimikas S., Brilakis E. S., Miller E. R., McConnell J. P., Lennon R. J., Kornman K. S., Witztum J. L., Berger P. B. 2005. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N. Engl. J. Med. 353: 46–57. [DOI] [PubMed] [Google Scholar]
- 7.Tsimikas S., Kiechl S., Willeit J., Mayr M., Miller E. R., Kronenberg F., Xu Q., Bergmark C., Weger S., Oberhollenzer F., et al. 2006. Oxidized phospholipids predict the presence and progression of carotid and femoral atherosclerosis and symptomatic cardiovascular disease: five-year prospective results from the Bruneck study. J. Am. Coll. Cardiol. 47: 2219–2228. [DOI] [PubMed] [Google Scholar]
- 8.Kiechl S., Willeit J., Mayr M., Viehweider B., Oberhollenzer M., Kronenberg F., Wiedermann C. J., Oberthaler S., Xu Q., Witztum J. L., et al. 2007. Oxidized phospholipids, lipoprotein(a), lipoprotein-associated phospholipase A2 activity, and 10-year cardiovascular outcomes: prospective results from the Bruneck study. Arterioscler. Thromb. Vasc. Biol. 27: 1788–1795. [DOI] [PubMed] [Google Scholar]
- 9.Bergmark C., Dewan A., Orsoni A., Merki E., Miller E. R., Shin M. J., Binder C. J., Horkko S., Krauss R. M., Chapman M. J., et al. 2008. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J. Lipid Res. 49: 2230–2239. [DOI] [PubMed] [Google Scholar]
- 10.Tsimikas S., Lau H. K., Han K. R., Shortal B., Miller E. R., Segev A., Curtiss L. K., Witztum J. L., Strauss B. H. 2004. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation. 109: 3164–3170. [DOI] [PubMed] [Google Scholar]
- 11.Edelstein C., Pfaffinger D., Hinman J., Miller E., Lipkind G., Tsimikas S., Bergmark C., Getz G. S., Witztum J. L., Scanu A. M. 2003. Lysine-phosphatidylcholine adducts in kringle V impart unique immunological and potential pro-inflammatory properties to human apolipoprotein(a). J. Biol. Chem. 278: 52841–52847. [DOI] [PubMed] [Google Scholar]
- 12.Tsimikas S., Clopton P., Brilakis E. S., Marcovina S. M., Khera A., Miller E. R., de Lemos J. A., Witztum J. L. 2009. Relationship of oxidized phospholipids on apolipoprotein B-100 particles to race/ethnicity, apolipoprotein(a) isoform size, and cardiovascular risk factors: results from the Dallas Heart Study. Circulation. 119: 1711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong V. W., Cremer P., Eberle E., Manke A., Schulze F., Wieland H., Kreuzer H., Seidel D. 1986. The association between serum Lp(a) concentrations and angiographically assessed coronary atherosclerosis. Dependence on serum LDL levels. Atherosclerosis. 62: 249–257. [DOI] [PubMed] [Google Scholar]
- 14.Danesh J., Collins R., Peto R. 2000. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation. 102: 1082–1085. [DOI] [PubMed] [Google Scholar]
- 15.Kamstrup P. R., Tybjaerg-Hansen A., Steffensen R., Nordestgaard B. G. 2009. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 301: 2331–2339. [DOI] [PubMed] [Google Scholar]
- 16.Erqou S., Kaptoge S., Perry P. L., Di Angelantonio E., Thompson A., White I. R., Marcovina S. M., Collins R., Thompson S. G., Danesh J. 2009. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 302: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scanu A. M., Fless G. M. 1990. Lipoprotein (a). Heterogeneity and biological relevance. J. Clin. Invest. 85: 1709–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boerwinkle E., Leffert C. C., Lin J., Lackner C., Chiesa G., Hobbs H. H. 1992. Apolipoprotein(a) gene accounts for greater than 90% of the variation in plasma lipoprotein(a) concentrations. J. Clin. Invest. 90: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lackner C., Cohen J. C., Hobbs H. H. 1993. Molecular definition of the extreme size polymorphism in apolipoprotein(a). Hum. Mol. Genet. 2: 933–940. [DOI] [PubMed] [Google Scholar]
- 20.Chasman D. I., Shiffman D., Zee R. Y., Louie J. Z., Luke M. M., Rowland C. M., Catanese J. J., Buring J. E., Devlin J. J., Ridker P. M. 2009. Polymorphism in the apolipoprotein(a) gene, plasma lipoprotein(a), cardiovascular disease, and low-dose aspirin therapy. Atherosclerosis. 203: 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luke M. M., Kane J. P., Liu D. M., Rowland C. M., Shiffman D., Cassano J., Catanese J. J., Pullinger C. R., Leong D. U., Arellano A. R., et al. 2007. A polymorphism in the protease-like domain of apolipoprotein(a) is associated with severe coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 27: 2030–2036. [DOI] [PubMed] [Google Scholar]
- 22.Shiffman D., O'Meara E. S., Bare L. A., Rowland C. M., Louie J. Z., Arellano A. R., Lumley T., Rice K., Iakoubova O., Luke M. M., et al. 2008. Association of gene variants with incident myocardial infarction in the Cardiovascular Health Study. Arterioscler. Thromb. Vasc. Biol. 28: 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tregouet D. A., Konig I. R., Erdmann J., Munteanu A., Braund P. S., Hall A. S., Grosshennig A., Linsel-Nitschke P., Perret C., DeSuremain M., et al. 2009. Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nat. Genet. 41: 283–285. [DOI] [PubMed] [Google Scholar]
- 24.Clarke R., Peden J. F., Hopewell J. C., Kyriakou T., Goel A., Heath S. C., Parish S., Barlera S., Franzosi M. G., Rust S., et al. 2009. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N. Engl. J. Med. 361: 2518–2528. [DOI] [PubMed] [Google Scholar]
- 25.Ginsberg H. N., Kris-Etherton P., Dennis B., Elmer P. J., Ershow A., Lefevre M., Pearson T., Roheim P., Ramakrishnan R., Reed R., et al. 1998. Effects of reducing dietary saturated fatty acids on plasma lipids and lipoproteins in healthy subjects: the DELTA Study, protocol 1. Arterioscler. Thromb. Vasc. Biol. 18: 441–449. [DOI] [PubMed] [Google Scholar]
- 26.Silaste M. L., Rantala M., Alfthan G., Aro A., Witztum J. L., Kesaniemi Y. A., Horkko S. 2004. Changes in dietary fat intake alter plasma levels of oxidized low-density lipoprotein and lipoprotein(a). Arterioscler. Thromb. Vasc. Biol. 24: 498–503. [DOI] [PubMed] [Google Scholar]
- 27.Berneis K. K., Krauss R. M. 2002. Metabolic origins and clinical significance of LDL heterogeneity. J. Lipid Res. 43: 1363–1379. [DOI] [PubMed] [Google Scholar]
- 28.Shin M. J., Blanche P. J., Rawlings R. S., Fernstrom H. S., Krauss R. M. 2007. Increased plasma concentrations of lipoprotein(a) during a low-fat, high-carbohydrate diet are associated with increased plasma concentrations of apolipoprotein C–III bound to apolipoprotein B-containing lipoproteins. Am. J. Clin. Nutr. 85: 1527–1532. [DOI] [PubMed] [Google Scholar]
- 29.Warnick G. R., Nguyen T., Albers A. A. 1985. Comparison of improved precipitation methods for quantification of high-density lipoprotein cholesterol. Clin. Chem. 31: 217–222. [PubMed] [Google Scholar]
- 30.Friedewald W. T., Levy R. I., Fredrickson D. S. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18: 499–502. [PubMed] [Google Scholar]
- 31.Young S. G., Smith R. S., Hogle D. M., Curtiss L. K., Witztum J. L. 1986. Two new monoclonal antibody-based enzyme-linked assays of apolipoprotein B. Clin. Chem. 32: 1484–1490. [PubMed] [Google Scholar]
- 32.Young S. G., Witztum J. L., Casal D. C., Curtiss L. K., Bernstein S. 1986. Conservation of the low density lipoprotein receptor-binding domain of apoprotein B. Demonstration by a new monoclonal antibody, MB47. Arteriosclerosis. 6: 178–188. [DOI] [PubMed] [Google Scholar]
- 33.Tsimikas S., Witztum J. L. 2008. The role of oxidized phospholipids in mediating lipoprotein(a) atherogenicity. Curr. Opin. Lipidol. 19: 369–377. [DOI] [PubMed] [Google Scholar]
- 34.Krauss R. M., Burke D. J. 1982. Identification of multiple subclasses of plasma low density lipoproteins in normal humans. J. Lipid Res. 23: 97–104. [PubMed] [Google Scholar]
- 35.Dreon D. M., Fernstrom H. A., Williams P. T., Krauss R. M. 2000. Reduced LDL particle size in children consuming a very-low-fat diet is related to parental LDL-subclass patterns. Am. J. Clin. Nutr. 71: 1611–1616. [DOI] [PubMed] [Google Scholar]
- 36.McNamara J. R., Campos H., Adolphson J. L., Ordovas J. M., Wilson P. W., Albers J. J., Usher D. C., Schaefer E. J. 1989. Screening for lipoprotein[a] elevations in plasma and assessment of size heterogeneity using gradient gel electrophoresis. J. Lipid Res. 30: 747–755. [PubMed] [Google Scholar]
- 37.Edelstein C., Philips B., Pfaffinger D., Scanu A. M. 2009. The oxidized phospholipids linked to human apolipoprotein(a) do not derive from circulating low-density lipoproteins and are probably of cellular origin. FASEB J. 23: 950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi S. H., Chae A., Miller E., Messig M., Ntanios F., DeMaria A. N., Nissen S. E., Witztum J. L., Tsimikas S. 2008. Relationship between biomarkers of oxidized low-density lipoprotein, statin therapy, quantitative coronary angiography, and atheroma: volume observations from the REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering) study. J. Am. Coll. Cardiol. 52: 24–32. [DOI] [PubMed] [Google Scholar]
- 39.Rodenburg J., Vissers M. N., Wiegman A., Miller E. R., Ridker P. M., Witztum J. L., Kastelein J. J., Tsimikas S. 2006. Oxidized low-density lipoprotein in children with familial hypercholesterolemia and unaffected siblings: effect of pravastatin. J. Am. Coll. Cardiol. 47: 1803–1810. [DOI] [PubMed] [Google Scholar]
- 40.Ky B., Burke A., Tsimikas S., Wolfe M. L., Tadesse M. G., Szapary P. O., Witztum J. L., FitzGerald G. A., Rader D. J. 2008. The influence of pravastatin and atorvastatin on markers of oxidative stress in hypercholesterolemic humans. J. Am. Coll. Cardiol. 51: 1653–1662. [DOI] [PubMed] [Google Scholar]
- 41.Berglund L., Lefevre M., Ginsberg H. N., Kris-Etherton P. M., Elmer P. J., Stewart P. W., Ershow A., Pearson T. A., Dennis B. H., Roheim P. S., et al. 2007. Comparison of monounsaturated fat with carbohydrates as a replacement for saturated fat in subjects with a high metabolic risk profile: studies in the fasting and postprandial states. Am. J. Clin. Nutr. 86: 1611–1620. [DOI] [PubMed] [Google Scholar]
- 42.Tsimikas S., Aikawa M., Miller F. J., Jr, Miller E. R., Torzewski M., Lentz S. R., Bergmark C., Heistad D. D., Libby P., Witztum J. L. 2007. Increased plasma oxidized phospholipid:apolipoprotein B-100 ratio with concomitant depletion of oxidized phospholipids from atherosclerotic lesions after dietary lipid-lowering: a potential biomarker of early atherosclerosis regression. Arterioscler. Thromb. Vasc. Biol. 27: 175–181. [DOI] [PubMed] [Google Scholar]
- 43.Aikawa M., Sugiyama S., Hill C. C., Voglic S. J., Rabkin E., Fukumoto Y., Schoen F. J., Witztum J. L., Libby P. 2002. Lipid lowering reduces oxidative stress and endothelial cell activation in rabbit atheroma. Circulation. 106: 1390–1396. [DOI] [PubMed] [Google Scholar]
- 44.Fraley A. E., Schwartz G. G., Olsson A. G., Kinlay S., Szarek M., Rifai N., Libby P., Ganz P., Witztum J. L., Tsimikas S. 2009. Relationship of oxidized phospholipids and biomarkers of oxidized low-density lipoprotein with cardiovascular risk factors, inflammatory biomarkers, and effect of statin therapy in patients with acute coronary syndromes: results from the MIRACL (Myocardial Ischemia Reduction With Aggressive Cholesterol Lowering) trial. J. Am. Coll. Cardiol. 53: 2186–2196. [DOI] [PubMed] [Google Scholar]
- 45.Anuurad E., Boffa M. B., Koschinsky M. L., Berglund L. 2006. Lipoprotein(a): a unique risk factor for cardiovascular disease. Clin. Lab. Med. 26: 751–772. [DOI] [PubMed] [Google Scholar]
- 46.Parks E. J., Hellerstein M. K. 2000. Carbohydrate-induced hypertriacylglycerolemia: historical perspective and review of biological mechanisms. Am. J. Clin. Nutr. 71: 412–433. [DOI] [PubMed] [Google Scholar]
- 47.Hoppichler F., Kraft H. G., Sandholzer C., Lechleitner M., Patsch J. R., Utermann G. 1996. Lipoprotein(a) is increased in triglyceride-rich lipoproteins in men with coronary heart disease, but does not change acutely following oral fat ingestion. Atherosclerosis. 122: 127–134. [DOI] [PubMed] [Google Scholar]
- 48.Bersot T. P., Innerarity T. L., Pitas R. E., Rall S. C., Jr, Weisgraber K. H., Mahley R. W. 1986. Fat feeding in humans induces lipoproteins of density less than 1.006 that are enriched in apolipoprotein [a] and that cause lipid accumulation in macrophages. J. Clin. Invest. 77: 622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaubatz J. W., Hoogeveen R. C., Hoffman A. S., Ghazzaly K. G., Pownall H. J., Guevara J., Jr, Koschinsky M. L., Morrisett J. D. 2001. Isolation, quantitation, and characterization of a stable complex formed by Lp[a] binding to triglyceride-rich lipoproteins. J. Lipid Res. 42: 2058–2068. [PubMed] [Google Scholar]
- 50.Albers J. J., Chen C. H., Aladjem F. 1972. Human serum lipoproteins. Evidence for three classes of lipoproteins in S fO-2. Biochemistry. 11: 57–63. [DOI] [PubMed] [Google Scholar]
- 51.Krauss R. M., Blanche P. J., Rawlings R. S., Fernstrom H. S., Williams P. T. 2006. Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia. Am. J. Clin. Nutr. 83: 1025–1031. [DOI] [PubMed] [Google Scholar]