Abstract
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is an independent cardiovascular risk factor. We investigated the plasma levels of Lp-PLA2 activity and mass as a function of plasma lipid levels, LDL subclass profile, and oxidative stress in patients with β-thalassemia. Thirty-five patients with β-thalassemia major (β-TM) and 25 patients with β-thalassemia intermedia (β-TI) participated in the study. Lp-PLA2 activity and mass were measured in total plasma, in apolipoprotein (apo)B-depleted plasma (HDL-Lp-PLA2), and in LDL subclasses. Lp-PLA2 activity produced and secreted from peripheral blood monocytes in culture was also determined. Patients with β-thalassemia are characterized by a predominance of small-dense LDL particles, increased oxidative stress, and very high plasma levels of Lp-PLA2 mass and activity, despite low LDL-cholesterol levels. A significant positive correlation between plasma Lp-PLA2 activity or mass and 8-isoprostane (8-epiPGF2a) and ferritin levels as well as intima-media thickness (IMT) values was observed. An increase in secreted and cell-associated Lp-PLA2 activity from monocytes in culture was observed in both patient groups. The HDL-Lp-PLA2 activity and mass as well as the ratio of HDL-Lp-PLA2/plasma Lp-PLA2 were significantly higher in both patient groups compared with the control group. In conclusion, patients with β-thalassemia exhibit high plasma Lp-PLA2 levels, attributed to increased enzyme secretion from monocytes/macrophages and to the predominance of sdLDL particles in plasma. Plasma Lp-PLA2 is correlated with carotid IMT, suggesting that this enzyme may be implicated in premature carotid atherosclerosis observed in β-thalassemia.
Keywords: low density lipoprotein, high density lipoprotein, oxidative stress, atherosclerosis
Platelet-activating factor (PAF) is a pro-inflammatory phospholipid that may be implicated in atherogenesis. PAF is degraded by PAF-acetylhydrolase, an enzyme that exhibits a Ca2+-independent phospholipase A2 activity (1). PAF-acetylhydrolase also degrades oxidized phospholipids, which are formed during oxidation of low-density lipoprotein (LDL) and may play key roles in vascular inflammation and atherosclerosis (2). Four intracellular (Ia, Ib, II, and erythrocyte forms) and one secreted (plasma form) isoenzymes have been described. Among the intracellular forms, the erythrocyte PAF-acetylhydrolase is a distinct 25 kDa protein that is found primarily in the cytosol (3). The plasma form of PAF-acetylhydrolase is complexed to lipoproteins (1); thus, it is also referred to as lipoprotein-associated phospholipase A2 (Lp-PLA2) (4). Lp-PLA2 is primarily associated with LDL; however, a small proportion of the circulating enzyme is also associated with high-density lipoprotein (HDL) (1). Lp-PLA2 is produced by hematopoietic cells, primarily from monocytes/macrophages (5, 6), and it is located with and highly expressed by macrophages within the necrotic core and the fibrotic cap of advanced rupture-prone plaques (7, 8). The Lp-PLA2 associated with LDL is the major determinant of plasma enzyme levels, and most studies over past years suggest that this enzyme plays a pro-inflammatory role in the artery wall thus promoting vascular inflammation and atherosclerosis (9, 10). The pro-inflammatory and pro-atherogenic role of plasma Lp-PLA2 is primarily attributed to the fact that during the hydrolysis of oxidized phospholipids, this enzyme generates lysophosphatidylcholine and oxidized free fatty acids, both of which exhibit pro-atherogenic activities (4). In this regard, a substantial body of peer-reviewed studies in Caucasian populations has supported Lp-PLA2 as a new, independent cardiovascular risk factor (11).
In contrast to the Lp-PLA2 associated with LDL, several lines of evidence suggest that HDL-associated Lp-PLA2 (HDL-Lp-PLA2), although present at low levels, may contribute to the anti-atherogenic effects of this lipoprotein. However, the clinical value of HDL-Lp-PLA2 as an inhibitor of the atherosclerotic process needs further investigation (1).
Beta-thalassemias result from either reduced synthesis (i.e., thalassemia intermedia, [β-TI]) or complete absence (i.e., thalassemia major, [β-TM]) of structurally normal β-globin subunits of hemoglobin. Patients with β-TI require sporadic, if any, blood transfusions during life, whereas patients with the major phenotype are on a regular transfusion program from their first months after birth (12). Thalassemic patients are at risk for iron overload due to increased gastrointestinal iron absorption (in β-TI) and frequent blood transfusions (in β-TM). Furthermore, a high incidence of strokes and thromboembolic episodes, despite rarity of coronary artery disease, has been found and accounted for by interplay of profound endothelial activation, immunomodulation, and a characteristic, rather anti-atherogenic, plasma lipid profile (13–15). In accordance with the observations above, we previously demonstrated a premature carotid artery disease as well as a globally disturbed vasorelaxation in β-thalassemic patients (16).
According to previously published results, the plasma Lp-PLA2 activity in β-thalassemia/hemoglobin E patients is significantly increased, a phenomenon that could be attributed to the increased oxidative stress observed in these patients (17). Therefore, the aim of the present study was to investigate the plasma levels of Lp-PLA2 activity and mass as a function of plasma lipid levels, LDL subclass profile, and oxidative stress and to further elucidate the mechanisms that may underline the alterations of the enzyme observed in patients with β-thalassemia.
MATERIALS AND METHODS
Study design
Thirty-five patients with β-TM, who were on a regular blood transfusion regimen since their first years of life, participated in the study. All patients were receiving desferrioxamine (approximately 40–50 mg/kg) subcutaneously overnight and ascorbic acid orally. Compliance with chelation therapy was considered optimal if patients were >90% adherent to the instructions given by the hematologists. Twenty-five patients with β–TI, who were either not transfused or who received only sporadic blood transfusions (i.e., less than four times each year), were also included in the study. All β-TI patients were instructed to receive chelation therapy with desferrioxamine if serum ferritin levels exceeded 2000 ng/ml. Serum ferritin levels in both patient groups were determined three to five times each year. All patients who participated in the study were free from diabetes mellitus and cardiac disease and demonstrated an echocardiographically normal bi-ventricular systolic function. For comparison, 30 healthy normolipidemic volunteers, who were matched with the patient group for age, sex, body surface area, and smoking habit, also participated in the study (control group). Post hoc carotid artery ultrasonography was obtained in all patients with β-TM; however, this was not possible for the rest of the participating study subjects due to logistic constraints. As we previously described (16), the left and right common carotid arteries were examined in multiple directions. We used the beginning of the dilatation of the carotid bulb as a reference point for measurement of the intima-media thickness (IMT). IMT was defined as the distance between the boundaries of lumen-intima and media-adventitia interfaces at the far common carotid artery wall. The average IMT of the six measurements in each patient was obtained from the frozen magnified images of the screen display. Plaque was defined as a focal structure encroaching into the arterial lumen at least 0.5 mm or 50% of the surrounding IMT. The ethics committee of the University Hospital of Patras approved the research protocol, and written informed consent was obtained from all subjects.
Biochemical measurements
Antecubital venous blood samples were obtained after 12 h of fasting. Serum total cholesterol, HDL-cholesterol, triglyceride glucose, and ferritin levels as well serum aminotransferase levels, were determined on the Olympus AU560 Clinical Chemistry analyzer (Hamburg, Germany) as previously described Serum LDL-cholesterol levels were calculated using the Friedewald formula (16, 18). Serum lipoprotein [a] (Lp[a]) levels were measured by an enzyme immunoassay method (Macra Lp[a], Terumo Medical Corporation Diagnostic Division, Elkton, MD) (18). The levels of high-sensitivity tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were measured in duplicate by the quantitative sandwich enzyme-linked immunoassay technique (ELISA, R and D Systems, Minneapolis, MN) (16). Creatinine levels in urine were measured by the Jaffe' method (19). Hematologic parameters were also determined in all subjects participating in the study (16). Products of lipid peroxidation in lipoprotein subclasses were detected through the measurement of thiobarbituric acid-reactive substances (TBARS) by using a spectrophotometric assay, essentially as previously described (20).
LDL subclass analysis
LDL subclass analysis was performed electrophoretically by use of high-resolution 3% polyacrylamide gel tubes and the Lipoprint LDL System (Quantimetrix, Redondo Beach, CA) as we previously described (21). After electrophoresis, very low-density lipoprotein (VLDL) remained in the origin [retention factor (Rf) = 0.0], whereas HDL migrated to the front (Rf = 1.0). In between, several bands can be detected: MID bands C, B, and A, which correspond mainly to intermediate-density lipoprotein (IDL), as well as up to seven LDL bands. The LDL-1 and LDL-2 bands correspond to large, buoyant LDL particles, whereas bands LDL-3 to LDL-7 correspond to small dense LDL (sdLDL) particles. We determined the cholesterol mass of each apolipoprotein (apo)B-lipoprotein subfraction, the mean LDL particle size (in Å), and the proportion (%) of the cholesterol mass of sdLDL particles over the total LDL-cholesterol mass (21).
Subfractionation of plasma apoB-containing lipoproteins
ApoB-containing lipoproteins were fractionated by isopycnic density gradient ultracentrifugation as previously described (22). Total plasma was subjected to ultracentrifugation, and 30 fractions of 0.4 ml each were collected and analyzed for their protein content. Equal volumes of gradient fractions 1–12, corresponding to apoB-containing lipoproteins, were pooled to form the following subfractions: VLDL-IDL (d = 1.019 g/ml), LDL-1 (d = 1.019–1.023 g/ml), LDL-2 (d = 1.023–1.029 g/ml), LDL-3 (d = 1.029 –1.039 g/ml), LDL-4 (d = 1.039–1.050 g/ml), and LDL-5 (d = 1.050–1.063 g/ml) (22). All subfractions were dialyzed extensively at 4°C in 10 mmol/l phosphate-buffered saline, pH 7.4 (PBS) containing 2 mmol/l EDTA, filter-sterilized, and maintained at 4°C under nitrogen until analysis. Under these storage conditions, no oxidation was observed in any LDL subfraction, as revealed by agarose gel electrophoresis and the determination of the TBARS values (0.9 ± 0.4 malondialdehyde equivalents per mg of protein for all lipoprotein preparations).
Preparation of erythrocyte lysates
Erythrocyte lysates were prepared as previously described (19). In brief, venous blood samples were collected using acid citrate as anticoagulant and centrifuged at 1500 g for 20 min. The supernatant plasma along with the buffy coat was removed by aspiration. The remaining erythrocyte pellet was washed three times with a 4.2 mM HEPES buffer solution pH 7.4. A small portion of this suspension was used for the erythrocyte count. The remaining suspension was centrifuged as above, and the sedimented erythrocytes were lysed by mixing with 5 vol of a 7 mM sodium phoshate buffer solution (pH 7.4) for 20 min at −4°C (19). The erythrocyte lysate was stored at −80°C until analysis.
Measurement of Lp-PLA2 activity and mass
Lp-PLA2 activity in total plasma, in apoB-depleted plasma (after the sedimentation of all apoB-containing lipoproteins with dextran sulfate-magnesium chloride [HDL-Lp-PLA2 activity]), and in LDL subclasses was determined by the trichloroacetic acid precipitation procedure using [3H]PAF (100 μM final concentration) as a substrate (18, 22). Fifty microliters of total plasma (diluted 1:50, v/v with HEPES buffer [pH 7.4]), or the apoB-depleted plasma (diluted 1:3, v/v with HEPES), or 5 µg protein of each LDL subclass were used as a source of the enzyme. The reaction was performed for 10 min at 37°C, and Lp-PLA2 activity was expressed as nmol PAF degraded per min per ml of plasma or mg of total protein. Lp-PLA2 activity was also determined in the supernatants and cell lysates of monocytes in culture as previously described (23). In some experiments, Lp-PLA2 activity was determined in 90 microliters of erythrocyte lysates. The reaction was performed for 10 min at 37°C, and enzyme activity was expressed as nmol PAF degraded per min per 109 cells (19).
Lp-PLA2 mass was determined by a dual monoclonal antibody immunoassay standardized to recombinant Lp-PLA2 (PLAC test, diaDexus), following the manufacturer instructions (24). Lp-PLA2 mass was measured in plasma and in apoB-depleted plasma prepared as above (HDL-Lp-PLA2 mass), as well as in LDL subclasses as previously described (21). We used 10 µl of undiluted plasma or apoB-depleted plasma or 10–20 µg of total protein from each LDL subfraction as the source of the enzyme. Lp-PLA2-specific activity was expressed as a ratio of the enzyme activity to the enzyme mass (nmol/ng/min) (21). Finally, we determined the Lp-PLA2 mass in 10–50 microliters of erythrocytes, monocyte lysates, or monocyte supernatants.
Determination of Lp-PLA2 activity associated with platelet microparticles in plasma
Plasma-containing microparticles were prepared as previously described (25). Blood samples from patient groups and the control group were drawn into tubes containing acid-citrate dextrose as an anticoagulant (Vacutainer, Becton Dickinson) and centrifuged at 150 g for 10 min to obtain platelet-rich plasma. Platelet-rich plasma was then mixed with 0.1% EDTA/saline, at a ratio of 3:2 (v/v) and then centrifuged at 1,500 g for 20 min to obtain the plasma-containing microparticles. To verify the presence of platelet microparticles, flow cytometry was performed using annexin-V-PE, which recognizes the population of all microparticles that express anionic phospholipids, and anti-CD41a-FITC, which is specific for platelet microparticles (PMP) (25). The Lp-PLA2 activity associated with PMPs in plasma was then measured by a captured ELISA method as previously described using ELISA plates coated with either the monoclonal antibody anti-CD61, which specifically recognizes the β3 integrin of the platelet integrin αbβ3 located on PMPs, or the isotype-matched mouse monoclonal IgG (25).
Isolation and culture of peripheral blood monocytes
Peripheral blood from patients with β-thalassemia as well as from the control group was drawn into EDTA-containing tubes. Blood was centrifuged, and monocytes were isolated from the buffy coats as previously described (23). Cells from each donor were cultured and grown in duplicate in 24-well plastic tissue culture dishes (1 × 106 cells/well) with RPMI medium [L-glutamine, 40 µg/ml gentamycin, and 1% Nutridoma (Boehringer Mannheim, Germany)] (23). After 48 h of culture, the cell supernatants were recovered. The cell layers were washed twice with PBS, and then detached and lysed by the addition of 0.2 ml of a lysis solution (1% EDTA and 0.1% Triton X-100). Supernatants and cell lysates were centrifuged (500 g for 10 min at 4°C), stored at 4°C, and then analyzed for Lp-PLA2 activity and lactate dehydrogenase activity (Boehringer Mannheim) within 24 h of collection (23, 26). Cell lysates were further analyzed for their protein content, which was determined with the Lowry method (26). Viability under all culture conditions was determined by trypan blue dye exclusion and the absence of lactate dehydrogenase release; viability was >95%.
Measurement of PON1 activities
Paraoxonase 1 (PON1) activities in serum were measured using paraoxon as a substrate (for paraoxonase activity) and phenyl acetate as a substrate (for arylesterase activity). Both PON1 activities were determined in the presence of 2 mM Ca2+ in 100 mM Tris-HCl buffer (pH 8.0) for paraoxon and in 20 mM Tris-HCl buffer (pH 8.0) for phenyl acetate (26).
Determination of 8-isoprostanes
To estimate the oxidative stress in all study subjects, plasma and urine levels of 8-isoprostane (8-epiPGF2a) were determined. Measurement of 8-epiPGF2a levels in plasma samples (expressed as pg/ml) was carried out by means of a competitive enzyme immunoassay (commercial 8-isoprostane EIA kit, Cayman Chemicals, Ann Arbor, MI), following manufacturer instructions as previously described (27). The 8-epiPGF2a levels in urine were determined by the same technique and were expressed as ng/mg creatinine.
Statistical analysis
Data are presented as the mean (SD), except for nongaussian-distributed variables, which are presented as the median (range). Statistical analyses were performed using ANOVA, followed by least significant difference (LSD) test for comparisons between individual groups. The nonparametric Mann-Whitney U test and the Kruskal-Wallis test were applied to discriminate differences in nongaussian-distributed variables. Correlations between Lp-PLA2 and other parameters determined in the study were estimated using linear regression analysis and Spearman's rank correlation coefficients (for nongaussian-distributed variables), while Yates's corrected chi-square test was used for differences in proportions. P < 0.05 was considered significant.
RESULTS
Characteristics of the study population
The clinical and biochemical characteristics of the study population are shown in Table 1. Both thalassemic patients groups exhibited elevated serum ferritin levels compared with the control group. Liver biopsy was performed in 12 patients. Histologically, hepatitis and cirrhosis were found in 4 patients and 1 patient, respectively. Moreover, severe hepatic siderosis could be shown in 2 patients out of 12 patients. Nineteen (55%) patients were on optimal desferrioxamine therapy, whereas history of splenectomy, endocrinopathy, and active or healed hepatitis was evident in a significant minority of them. Both patient groups demonstrated significantly lower serum total-, LDL-, and HDL-cholesterol levels and increased concentrations of both serum triglycerides and liver transaminases compared with the control group, whereas no difference in these parameters were observed between β-TI and β-TM patients. No differences in serum Lp(a) levels or glucose levels were observed among the groups. The atherogenic ratio, LDL-cholesterol to HDL-cholesterol, was significantly lower in both patient groups compared with the control group (Table 1). The pro-inflammatory cytokines IL-6 and TNF-α were significantly higher in both patient groups compared with the control group, the β-TM patients exhibiting higher levels of both compared with the β-TI patients (Table 1). Finally on ultrasonography, the mean IMT values in β-TM patients were 0.50 ± 0.14 mm, in accordance with our recently reported values on these patients (16).
TABLE 1.
Clinical and biochemical parameters of the study groups
| Control (n = 30) | β-TI (n = 25) | β-TM (n = 35) | |
|---|---|---|---|
| Age (yrs) | 33 ± 9 | 36 ± 12 | 27 ± 7 |
| Male sex, n (%) | 16 (53) | 16 (64) | 17 (49) |
| Body surface area (m ) | 1.71 ± 0.19 | 1.67 ± 0.16 | 1.66 ± 0.18 |
| Systolic blood pressure (mm Hg) | 111 ± 12 | 111 ± 20 | 106 ± 13 |
| Diastolic blood pressure (mm Hg) | 67 ± 8 | 67 ± 12 | 68 ± 7 |
| Heart rate (beats/minute) | 74 ± 10 | 80 ± 8 | 74 ± 10 |
| Smoking, n (%) | 14 (40) | 13 (52) | 14 (40) |
| Hb, pretransfusional (mg/dl) | 13.6 ± 1.4 | 9.3 ± 1.0a | 10.3 ± 0.9a |
| Serum ferritin over the last year (ng/ml) | 122 ± 65d | 1568 ± 1343a | 1975 ± 1635a |
| Splenectomy, n (%) | 0 | 10 (40) | 6 (17) |
| Hepatitis C, active or healed, n (%) | 0 | 4 (16) | 12 (34) |
| History of hypothyroidism, n (%) | 0 | 12 (48) | 15 (42) |
| History of hypoparathyroidism, n (%) | 0 | 8 (32) | 6 (17) |
| History of hypogonadism, n (%) | 0 | 9 (36) | 14 (40) |
| Glucose (mg/dl) | 94 ± 14 | 98 ± 12 | 99 ± 13 |
| Aspartate aminotransferase (U/l) | 19 ± 6 | 31 ± 11b | 41 ± 22bc |
| Alanine aminotransferase (U/l) | 21 ± 11 | 44 ± 21b | 54 ± 39bc |
| Total cholesterol (mg/dl) | 196 ± 41 | 144 ± 49a | 120 ± 34a |
| LDL-cholesterol (mg/dl) | 130 ± 40 | 70 ± 36a | 61 ± 27a |
| HDL-cholesterol (mg/dl) | 51 ± 14 | 31 ± 11a | 32 ± 10a |
| LDL-cholesterol / HDL-cholesterol | 2.5 ± 0.4 | 2.2 ± 0.3a | 1.9 ± 0.2a |
| Triglycerides (mg/dl) | 82 ± 39 | 204 ± 80a | 155 ± 61a |
| Lp(a) (mg/dl) | 8 (1-24) | 10 (4-28) | 6 (1-26) |
| IL-6 (pg/ml) | 1.3 ± 0.9 | 2.4 ± 0.8b | 3.3 ± 1.5bc |
| TNF-α (pg/ml) | 1.8 ± 0.7 | 2.6 ± 0.9b | 3.5 ± 1.5bc |
| Total plasma Lp-PLA2 activity (nmol/ml/min) | 43 ± 5 | 88 ± 17a | 113 ± 20ac |
| Total plasma Lp-PLA2 mass (ng/ml) | 250 ± 53 | 665 ± 99a | 869 ± 96ac |
| HDL-Lp-PLA2 activity (nmol/ml/min) | 3.8 ± 0.8 | 12.9 ± 4.9a | 18.1 ± 4.8ac |
| HDL-Lp-PLA2 mass (ng/ml) | 84 ± 21 | 331 ± 36a | 476 ± 45ac |
Values are means ± SD, except for Lp(a), where the value represents the median (range). β-TI, β-thalassemia intermedia; β-TM, β-thalassemia major; HDL-Lp-PLA2, HDL-associated Lp-PLA2; IL-6; interleukin-6; Lp-PLA2; lipoprotein-associated phospholipase A2; TNF-α, tumor necrosis factor-α.
P < 0.001 compared with control values.
P < 0.01 compared with control values.
P < 0.01 compared with β-TI patients.
Single measurement for each control subject.
Apolipoprotein B-containing lipoprotein subclasses
Both β-TI and β-TM patient groups had higher plasma levels of VLDL-cholesterol and lower levels of IDL-cholesterol and buoyant LDL-cholesterol compared with the control group (Table 2). Furthermore, β-TI and β-TM patients had significantly higher levels of sdLDL-cholesterol and a higher proportion of sdLDL, whereas the mean LDL size was lower compared with the control group. No differences were observed between the β-TI and β-TM patient groups regarding these parameters (Table 2).
TABLE 2.
ApoB-containing lipoprotein subclasses in patients with β-thalassemia and the control group
| Control | β-TI | β-TM | |
|---|---|---|---|
| Parameter | |||
| VLDL-cholesterol, mmol/l | 1.2 ± 0.2 | 1.6 ± 0.3a | 1.7 ± 0.3a |
| IDL-cholesterol, mmol/l | 1.7 ± 0.3 | 1.3 ± 0.2a | 1.2 ± 0.3a |
| Buoyant LDL-cholesterol, mmol/l | 3.6 ± 0.7 | 2.8 ± 0.8a | 2.6 ± 0.9a |
| sdLDL-cholesterol, mmol/l | 0.20 ± 0.08 | 0.43 ± 0.10b | 0.52 ± 0.30b |
| sdLDL proportion, % | 3.6 ± 0.9 | 9.5 ± 2.1b | 12.0 ± 4.1b |
| Mean LDL size, Å | 269 ± 4 | 259 ± 3b | 258 ± 4b |
Data represent the mean ± SD values. IDL, intermediate density lipoprotein; LDL, low density lipoprotein, sdLDL, small dense LDL; VLDL, very low density lipoprotein.
P < 0.01 compared with control values.
P < 0.001 compared with control values.
Plasma Lp-PLA2 activity and mass
Total plasma Lp-PLA2 activity and the enzyme mass were significantly higher in both patient groups compared with the control group, the β-TM patients having significantly higher enzyme activity and mass compared with β-TI patients (Table 1). Lp-PLA2 activity and mass in thalassemic patients were positively correlated with 8-epiPGF2a levels in serum (r = 0.245, P < 0.03 and r = 0.288, P < 0.03, respectively) and in urine (r = 0.310, P < 0.02 and r = 0.328, P < 0.02, respectively). A positive correlation was also observed between Lp-PLA2 activity and mass with serum ferritin levels (Fig. 1A, B), whereas no correlation was found between Lp-PLA2 activity or mass and LDL-cholesterol (Fig. 1C, D), total cholesterol, or Lp(a) levels in either patient group. A significant correlation between plasma Lp-PLA2 activity and mass with IMT values in β-TM patients was observed (r = 0.440, P < 0.01 and r = 0.463, P < 0.01, respectively). Importantly, the enzyme-specific activity in total plasma (calculated as the activity-to-mass ratio) in both patient groups was significantly lower (P < 0.05) compared with the control group (in nmol/ng/min, 0.17 ± 0.03 for the control group versus 0.13 ± 0.03 for β-TI and 0.13 ± 0.02 for β-TM patients). Similarly, the HDL-Lp-PLA2 activity and mass were significantly higher compared with the control group, the β-TM patients having significantly higher enzyme activity and mass compared with β-TI patients (Table 1), whereas in both patient groups, the enzyme-specific activity was significantly lower (P < 0.05) compared with the control group (0.045 ± 0.01 for the control group versus 0.039 ± 0.02 for β-TI and 0.038 ± 0.01 for β-TM patients). Finally, the ratio of HDL-Lp-PLA2 mass to total plasma Lp-PLA2 mass was significantly higher (P < 0.001) in both patient groups compared with the control group (0.11 ± 0.01 for the control group versus 0.14 ± 0.02 for β-TI and 0.15 ± 0.02 for β-TM patients). Similarly, the ratio of HDL-Lp-PLA2 activity to total plasma Lp-PLA2 activity was significantly higher (P < 0.001) in both patient groups compared with the control group (0.26 ± 0.03 for the control group versus 0.52 ± 0.04 for β-TI and 0.65 ± 0.04 for β-TM patients).
Fig. 1.
Correlations between serum ferritin levels and Lp-PLA2 activity (A) or mass (B) as well as between serum LDL-cholesterol levels and Lp-PLA2 activity (C) or mass (D) in patients with β-thalassemia. Lp-PLA2, lipoprotein-associated phospholipase A2.
Lp-PLA2 activity and mass in apolipoprotein B-containing lipoprotein subfractions
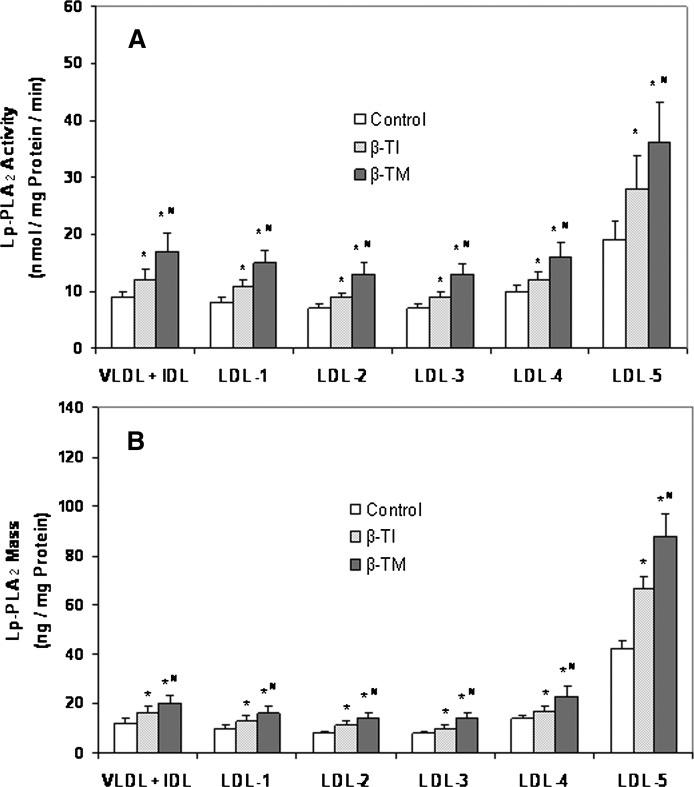
The higher Lp-PLA2 activity and mass found in plasma of β-TM and β-TI patients, despite their lower plasma levels of LDL-cholesterol, led us to determine the enzyme parameters described above in the apolipoprotein B (apoB)-containing lipoprotein subfractions prepared by an isopycnic density gradient ultracentrifugation method. The Lp-PLA2 activity and mass were preferentially associated with the small dense LDL-5 subfraction in all groups (Fig. 2). Importantly, β-TM and β-TI patients exhibited higher enzyme activity and mass in all lipoprotein subfractions compared with the control group, the β-TM patients having significantly higher enzyme activity and mass compared with β-TI patients (Fig. 2).
Fig. 2.
Bar graphs illustrating the Lp-PLA2 activity (A) and mass (B) associated with apoB-containing lipoprotein subspecies in patients with β-thalassemia. Lipoprotein subspecies were fractionated by isopycnic density gradient ultracentrifugation. Enzymatic activity was determined by the trichloroacetic acid precipitation procedure and enzyme mass by use of a dual monoclonal antibody immunoassay standardized to recombinant Lp-PLA2. Values represent the mean ± SD. *P < 0.005 compared with the control group and #P < 0.02 compared with β-TI patients. apoB, apolipoprotein B; β-TI, β-thalassemia intermedia; β-TM, β-thalassemia major; Lp-PLA2, lipoprotein-associated phospholipase A2.
The PAF-acetylhydrolase of erythrocytes does not contribute to the pool of plasma Lp-PLA2 of patients with β-thalassemia
The elevated Lp-PLA2 activity and mass in plasma of patients with β-thalassemia prompted us to study the source(s) from which this high amount of enzyme derives. We initially searched whether hemolysis, a phenomenon that occurs in these patients, could contribute to the increased Lp-PLA2 in plasma. Indeed, erythrocytes contain a PAF-acetylhydrolase type that exhibits similar substrate specificity, but it is structurally different to that of Lp-PLA2 (3). The enzyme activity determined in erythrocyte lysates prepared from blood of five subjects from each group was similar among the study groups (in nmol/109 cells/min, 73 ± 8 for the control group, 70 ± 10 for β-TI patients, and 75 ± 11 for β-TM patients). As expected, the enzyme activity in erythrocyte lysates was sensitive to sulfhydryl reagents (28), as it was significantly reduced by 75 ± 5% in all groups after treatment with 1 mM 5,5′-dithiobis (2-nitrobenzoic acid, DTNB) for 30 min at 37°C. Furthermore, as previously reported (28), the enzyme activity in erythrocyte lysates was susceptible to proteolysis, as it was significantly reduced by 90 ± 10% in all groups after treatment with 0.25 mg/ml Trypsin for 1 h at 37°C. By contrast, the plasma Lp-PLA2 activity in all studied groups was resistant to these treatments. Additionally, we were not able to detect Lp-PLA2 mass in erythrocyte lysates, indicating that the PLAC test does not detect the PAF-acetylhydrolase type that exists in erythrocytes. Overall, these results suggest that the increase in Lp-PLA2 mass and activity in plasma of β-TM and β-TI patients is not due to the existence in plasma of the enzyme type derived from erythrocytes.
Lp-PLA2 activity associated with platelet microparticles in plasma of patients with β-thalassemia
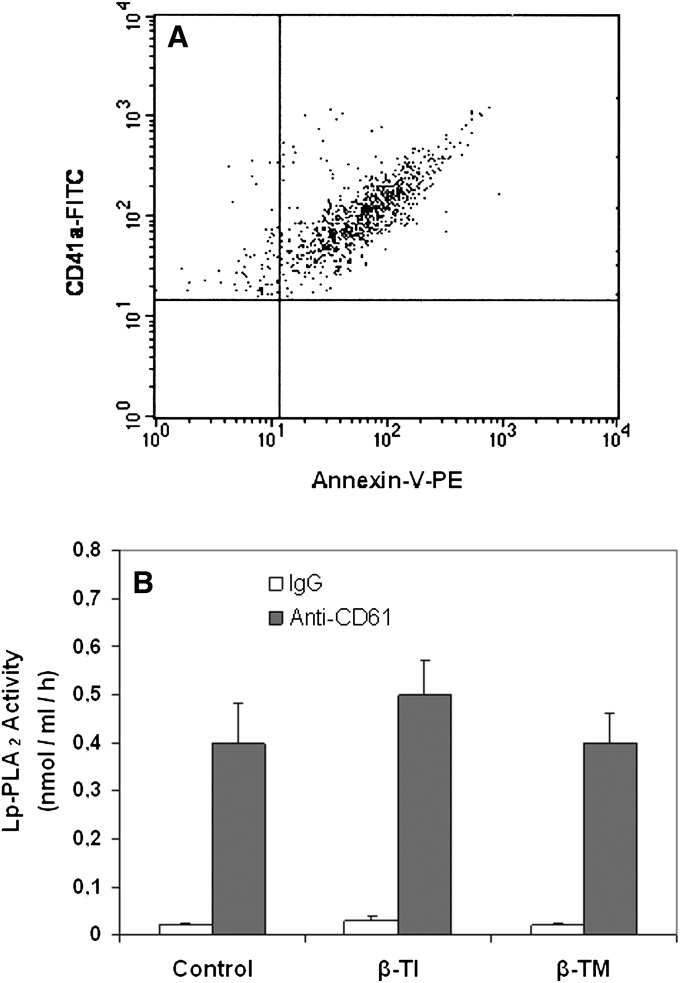
As previously shown, another source of the Lp-PLA2 in plasma could be platelets that secrete this enzyme associated with platelet microplates (PMP) (25). By using a previously described ELISA method, we determined the PMP-associated enzyme in the plasma of β-TM and β-TI patients and the control group. Representative flow cytometric profile of PMPs in plasma of β-TM patients is illustrated in Fig. 3A. Similar profiles were obtained in plasma of β-TI patients and the control group. As shown in Fig. 3B, The PMP-associated enzyme activity was similar among all groups, suggesting that the increase in plasma Lp-PLA2 observed in β-TM and β-TI patients is not due to an increase in enzyme secretion from the platelets of these patients.
Fig. 3.
A: Representative flow cytometric dot plot illustrating the PMPs in plasma of β-TM patients. PMPs were labeled with annexin-V-PE and anti-CD41a-FITC. B: Lp-PLA2 activity associated with PMPs in plasma of patients with β-thalassemia. ELISA plates were coated with the monoclonal antibody anti-CD61 or the isotype-matched mouse monoclonal IgG. Coating was performed overnight at 4°C. The amount of 100 μl of the plasma-containing microparticles were placed into the wells and incubated for 2 h at 37°C. The plate was washed three times with a HEPES buffer supplemented with 0.01% EDTA (Lp-PLA2 assay buffer), and then the Lp-PLA2 assay was performed in each well using the trichloroacetic acid precipitation procedure. Data represent the mean ± SD. apoB, apolipoprotein B; β-TI, β-thalassemia intermedia; β-TM, β-thalassemia major; Lp-PLA2, lipoprotein-associated phospholipase A2; PMP, platelet microparticle.
Lp-PLA2 secretion from adherent monocytes in culture
It has been shown that adherent monocytes produce and secrete Lp-PLA2 during differentiation into macrophages, and these cells represent the major source of the plasma Lp-PLA2 activity (5, 6). To investigate whether the increase in plasma- and HDL-Lp-PLA2 activity of patients with β-thalassemia was due to an increase in enzyme secretion from monocytes in culture, we studied the spontaneous enzyme secretion from monocytes of all groups. As shown in Fig. 4A, there was an increase in total (secreted plus cell-associated) Lp-PLA2 activity in both patient groups compared with the control group at 48 h of culture (P < 0.01), and this increase mainly reflected secreted enzyme activity (Fig. 4B). Importantly the total and secreted enzyme activity in β- patients was significantly higher compared with that observed in β- I patients (P < 0.03) (Fig. 4A, B). In preliminary experiments, we attempted to determine the enzyme mass in the supernatants and cell lysates using the PLAC test. However, we were not able to detect any mass in our samples, possibly due to the fact that the Lp-PLA2 mass was lower than the 1.2 ng/ml detection limit of the kit (24).
Fig. 4.
Increased spontaneous production and secretion of Lp-PLA2 activity from peripheral blood monocytes in culture in patients with β-thalassemia. Peripheral blood monocytes from each participant in the study were cultured and grown as described in “Materials and Methods.” Cultures were performed in duplicate for each donor. Total (A) and secreted (B) Lp-PLA2 activity was determined at 48 h of culture by the trichloroacetic acid precipitation procedure. Lines represent the mean from all donors of each studied group. Lp-PLA2, lipoprotein-associated phospholipase A2; β-TI, β-thalassemia intermedia; β-TM, β-thalassemia major.
Paraoxonase-1 activities and 8-epiPGF2a levels
The serum paraoxonase-1 (PON1) activities toward paraoxon and phenylacetate were significantly lower in both patient groups compared with the control group, whereas no difference was observed between patient groups (Table 3). Furthermore, both patient groups had significantly higher levels of serum and urine 8-isoprostane (8-epiPGF2a), an established marker of lipid peroxidation, compared with the control group. The β-TM patients had significantly higher levels of 8-epiPGF2a compared with β-TI patients (Table 3).
TABLE 3.
PON1 activities and 8-epiPGF2a levels in the study population
| Control | β-TI | β-TM | |
|---|---|---|---|
| PON1 activity toward paraoxon (U/l) | 77.4 ± 35.1 | 37.8 ± 29.1a | 51.7 ± 28.7a |
| PON1 activity toward phenylacetate (U/ml) | 66.6 ± 7.3 | 39.4 ± 3.6a | 54.1 ± 8.7a |
| Serum 8-epiPGF2a (pg/ml) | 75.2 ± 27.3 | 98.1 ± 30.2a | 125.0 ± 35.8bc |
| Urine 8-epiPGF2a (ng/mg creatinine) | 0.48 ± 0.16 | 0.65 ± 0.22a | 0.80 ± 0.29bc |
Data represent the mean ± SD values. 8-epiPGF2a, 8-isoprostanes; β-TI, β-thalassemia intermedia; β-TM, β-thalassemia major PON1, paraoxonase 1.
P < 0.01 compared with control values.
P < 0.001 compared with control values.
P < 0.001 compared with β-TI values.
DISCUSSION
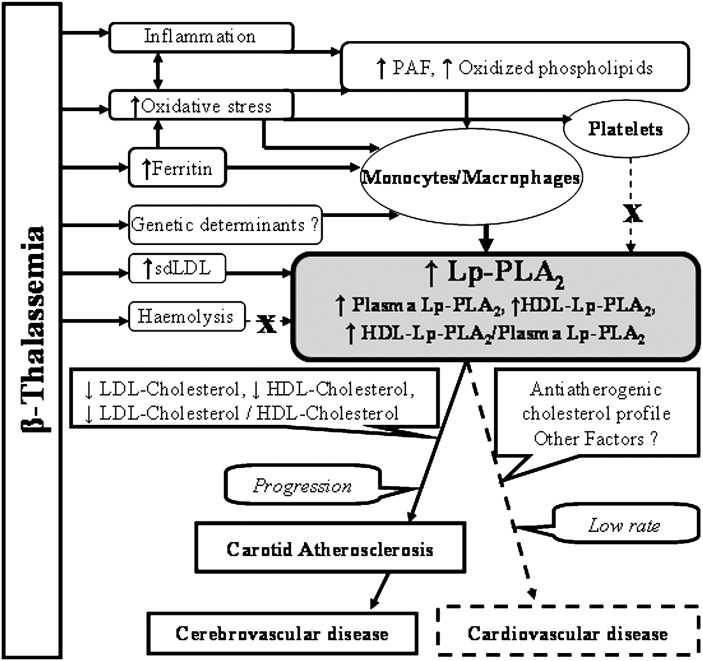
The present study shows for the first time that patients with β-thalassemia exhibit very high plasma levels of Lp-PLA2 mass and activity, these parameters being significantly higher in β-TM compared with β-TI patients. Major determinants of Lp-PLA2 levels in plasma are LDL levels and the rate of LDL clearance from the circulation (18, 26). In the present study, both patient groups exhibited low LDL-cholesterol levels; this is the first report of a population with low LDL-cholesterol levels exhibiting such high plasma levels of Lp-PLA2. Notably, the LDL-cholesterol or the cholesterol levels in thalassemic patients are not correlated with enzyme mass or activity, in contrast to expectations based on the results from most studies including those from our group, published to date (18, 26). As previously shown, the majority of the LDL-associated Lp-PLA2 is bound to atherogenic sdLDL particles in both normolipidemic and dyslipidemic populations (18, 22, 26, 29). Our results revealed that both patient groups are characterized by a predominance of sdLDL particles, a finding that is consistent with their elevated fasting triglyceride levels (30). Furthermore, sdLDL particles (LDL-5) carry the majority of LDL-associated Lp-PLA2. Thus the predominance of sdLDL particles enriched in Lp-PLA2 in plasma of β-thalassemic patients could contribute to the high enzyme mass and activity observed in these patients, despite their low LDL-cholesterol levels (Fig. 5).
Fig. 5.
Possible mechanisms underlining the increase of plasma Lp-PLA2 in patients with β-thalassemia and relationship to atherosclerotic diseases. β-thalassemia is characterized by increased inflammation and oxidative stress, increased plasma levels of ferritin and sdLDL, and increased hemolysis. The increased oxidative stress induces (possibly through the generation of PAF and oxidized phospholipids) platelet and monocyte/macrophage activation. The latter may also be attributed to the increased ferritin levels and possibly to unknown genetic factors. The activated monocyte/macrophages as well the increased levels of sdLDL (but not the oxidative stress) induces platelet activation, or the increased hemolysis may account for the increased plasma levels of Lp-PLA2 observed in β-thalassemia despite the relatively anti-atherogenic cholesterol profile (low LDL-cholesterol levels and low ratio of LDL-cholesterol/HDL-cholesterol). The high plasma Lp-PLA2 levels in the presence of a relatively anti-atherogenic cholesterol profile in patients with β-thalassemia may contribute to premature carotid atherosclerosis and cerebrovascular disease but not to coronary artery disease. Lp-PLA2, lipoprotein-associated phospholipase A2; PAF, platelet-activating factor; sdLDL, small dense LDL.
The increased hemolysis occurring in β-thalassemia could also contribute to the elevated plasma enzyme observed in these patients. However, this hypothesis is unlikely because the method used to determine enzyme mass in plasma did not detect erythrocyte PAF-acetylhydrolase in the lysates of these cells, despite the relatively high enzyme activity, and the plasma enzyme in our patients did not exhibit any sensitivity to treatments with sulfhydryl reagents or proteases, in contrast to the erythrocyte enzyme. A source of Lp-PLA2 in plasma is platelets that secrete this enzyme associated with PMPs (25). Platelets in β-thalassemia undergo a state of oxidative stress, leading to their activation (31). Thus platelets could contribute to the elevation in plasma Lp-PLA2 of our patients because they exhibited increased oxidative stress, indicated by the elevation of serum and urine 8-epiPGF2a, an established marker of lipid peroxidation correlated with Lp-PLA2 activity and mass. However this hypothesis is also unlikely because the Lp-PLA2 activity associated with PMP in the plasma of our patients was not different compared with the control group.
In contrast to platelets, monocytes/macrophages, the major cellular source of plasma Lp-PLA2, may significantly contribute to the elevation in plasma enzyme observed in β-thalassemia because increased Lp-PLA2 production and secretion from monocytes in culture was observed in both patient groups (Fig. 5). This phenomenon could be at least partially due to the increased oxidative stress attributed to the high plasma iron turnover observed in patients with β-thalassemia (32, 33). Indeed, the plasma ferritin levels, a measure of body and macrophage iron content and a potent pro-oxidant, are a potential modulator of Lp-PLA2 activity in plasma (34). Consistent with the above suggestion, the results of the present study show that increased plasma ferritin levels in β-thalassemic patients are strongly correlated with Lp-PLA2 activity and mass.
Patients with β-thalassemia exhibit increased inflammation (32) that may also contribute to the elevation in plasma Lp-PLA2. In this regard, it has been reported that the only pro-inflammatory mediator that stimulates the expression and secretion of Lp-PLA2 by monocyte/macrophages is PAF (35). As we (36) and others (37) have shown, PAF and structurally related oxidized phospholipids are formed during LDL oxidation; these molecules being elevated in patients with increased oxidative stress (38). Increased plasma levels of various lipid peroxidation products and marked LDL oxidative modification have been reported in thalassemic patients (33). Thus a contributory role in the enhanced secretion of Lp-PLA2 from monocytes/macrophages observed in our patients may be played by pro-inflammatory phospholipids, such as PAF and structurally related oxidized phospholipids formed under conditions of increased oxidative stress, a hypothesis that needs further investigation (Fig. 5). Some of the β-thalassemic patients exhibit liver disease or hypothyroidism that might have influenced the plasma Lp-PLA2 levels. However when we excluded these patients from the statistical analysis, the differences in Lp-PLA2 levels among studied groups were not altered (data not shown). Finally, differences in genetic expression of Lp-PLA2 between patients with β-thalassemia and the control group that may lead to increased Lp-PLA2 expression and secretion from patient monocytes may not be excluded (Fig. 5).
An important observation of our study is that the specific activity of Lp-PLA2 (i.e., the activity expressed per enzyme mass) is lower in our patients compared with the control group. As the enzyme is susceptible to oxidative inactivation (reviewed in Ref. 1), the decreased Lp-PLA2-specific activity could be attributed to the increased oxidative stress observed in these patients. Hence, we may suggest that the enhanced production and secretion of new active enzyme from monocytes/macrophages could counteract the oxidative stress-induced partial enzyme inhibition, thereby resulting in an overall increase in plasma Lp-PLA2 activity.
Similar to the total plasma Lp-PLA2, the HDL-Lp-PLA2 also is elevated in both patient groups, despite the low HDL-cholesterol levels, and it also could be primarily attributed to the enhanced enzyme secretion from monocytes/macrophages. Furthermore, the specific HDL-Lp-PLA2 activity is decreased, possibly due to its partial inactivation from the increased oxidative stress observed in these patients. The increased oxidative stress could also be responsible for the reduced PON1 activities observed in our patients. Indeed, like Lp-PLA2, PON1 is also susceptible to oxidative inactivation (39).
The findings of the present study may be clinically important because patients with β-thalassemia exhibit endothelial dysfunction, premature carotid atherosclerosis (16), and high incidence of stroke despite their very uncommon rates of coronary artery disease (13, 16). Lp-PLA2 is independently associated with endothelial dysfunction (40) and is highly expressed in carotid artery plaques (41). The significant correlation of plasma Lp-PLA2 concentrations with carotid artery IMT values found in our β-TM patients implies that this enzyme may be implicated in premature carotid atherosclerosis observed in β-thalassemia. Clinical studies have demonstrated that increased plasma Lp-PLA2 levels are associated with high incidence of stroke (42). Our β-thalassemic patients exhibit high plasma Lp-PLA2 levels but low LDL-cholesterol levels and low atherogenic LDL-cholesterol/HDL-cholesterol ratio. They also present with high levels of anti-atherogenic HDL-Lp-PLA2 and ratio of HDL-Lp-PLA2 to total plasma enzyme, which could be a potential anti-atherogenic marker in patients with dyslipidemia (18). Considering that dyslipidemias have long been associated with coronary artery disease (but not with cerebrovascular disease (43)) and that carotid artery disease could be a possible cause of stroke in cardiac disease-free patients with β-thalassemia (44), we suggest that the high plasma Lp-PLA2 levels in the presence of a relatively anti-atherogenic cholesterol profile may contribute to the premature carotid atherosclerosis and high incidence of stroke (but not coronary artery disease) observed in patients with β-thalassemia (13, 16) (Fig. 5).
In conclusion, patients with β-thalassemia exhibit high plasma Lp-PLA2 levels, which could be primarily attributed to increased enzyme production and secretion from monocytes/macrophages as well as to the predominance of sdLDL particles in plasma. This phenomenon may be a feature characteristic of β-thalassemia because patients with other types of anemia, such as sickle cell anemia, have similarities to the Lp-PLA2 activity of the control group (44). The high plasma Lp-PLA2 levels in the presence of a relatively anti-atherogenic cholesterol profile may contribute to premature carotid atherosclerosis but not to coronary artery disease in patients with β-thalassemia, a hypothesis that needs further investigation.
Footnotes
Abbreviations:
- 8-epiPGF2a
- 8-isoprostane
- apoB
- apolipoprotein B
- β-TI
- β-thalassemia intermedia
- β-TM
- β-thalassemia major
- IDL
- intermediate-density lipoprotein
- IL-6
- interleukin-6
- IMT
- intima-media thickness
- Lp-PLA2
- lipoprotein-associated phospholipase A2
- Lp(a)
- lipoprotein(a)
- PAF
- platelet-activating factor
- PMP
- platelet microparticle
- PON1
- paraoxonase 1
- sdLDL
- small dense LDL
- TBARS
- thiobarbituric acid-reactive substances
- TNF-α
- tumor necrosis factor-α
REFERENCES
- 1.Tellis C. C., Tselepis A. D. 2009. The role of lipoprotein-associated phospholipase A2 in atherosclerosis may depend on its lipoprotein carrier in plasma. Biochim. Biophys. Acta. 1791: 327–338. [DOI] [PubMed] [Google Scholar]
- 2.Davis B., Koster G., Douet L. J., Scigelova M., Woffendin G., Ward J. M., Smith A., Humphries J., Burnand K. G., Macphee C. H., et al. 2008. Electrospray ionization mass spectrometry identifies substrates and products of lipoprotein-associated phospholipase A2 in oxidized human low density lipoprotein. J. Biol. Chem. 283: 6428–6437. [DOI] [PubMed] [Google Scholar]
- 3.Stafforini D. M., Rollins E. N., Prescott S. M., McIntyre T. M. 1993. The platelet-activating factor acetylhydrolase from human erythrocytes. Purification and properties. J. Biol. Chem. 268: 3857–3865. [PubMed] [Google Scholar]
- 4.MacPhee C. H., Moores K. E., Boyd H. F., Dhanak D., Ife R. J., Leach C. A., Leake D. S., Milliner K. J., Patterson R. A., Suckling K. E., et al. 1999. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase, generates two bioactive products during the oxidation of low-density lipoprotein: use of a novel inhibitor. Biochem. J. 338: 479–487. [PMC free article] [PubMed] [Google Scholar]
- 5.Asano K., Okamoto S., Fukunaga K., Shiomi T., Mori T., Iwata M., Ikeda Y., Yamaguchi K. 1999. Cellular source(s) of platelet-activating-factor acetylhydrolase activity in plasma. Biochem. Biophys. Res. Commun. 261: 511–514. [DOI] [PubMed] [Google Scholar]
- 6.Stafforini D. M., Elstad M. R., McIntyre T. M., Zimmerman G. A., Prescott S. M. 1990. Human macrophages secret platelet-activating factor acetylhydrolase. J. Biol. Chem. 265: 9682–9687. [PubMed] [Google Scholar]
- 7.Hakkinen T., Luoma J. S., Hiltunen M. O., Macphee C. H., Milliner K. J., Patel L., Rice S. Q., Tew D. G., Karkola K., Yla-Herttuala S. 1999. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase, is expressed by macrophages in human and rabbit atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 19: 2909–2917. [DOI] [PubMed] [Google Scholar]
- 8.Kolodgie F. D., Burke A. P., Skorija K. S., Ladich E., Kutys R., Makuria A. T., Virmani R. 2006. Lipoprotein-associated phospholipase A2 protein expression in the natural progression of human coronary atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 26: 2523–2529. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y., Zhang P., Zhang L., Osman H., Mohler E. R., 3rd, Macphee C., Zalewski A., Postle A., Wilensky R. L. 2007. Role of lipoprotein-associated phospholipase A2 in leukocyte activation and inflammatory responses. Atherosclerosis. 191: 54–62. [DOI] [PubMed] [Google Scholar]
- 10.Lavi S., McConnell J. P., Rihal C. S., Prasad A., Mathew V., Lerman L. O., Lerman A. 2007. Local production of lipoprotein-associated phospholipase A2 and lysophosphatidylcholine in the coronary circulation: association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation. 115: 2715–2721. [DOI] [PubMed] [Google Scholar]
- 11.Garza C. A., Montori V. M., McConnell J. P., Somers V. K., Kullo I. J., Lopez-Jimenez F. 2007. Association between lipoprotein-associated phospholipase A2 and cardiovascular disease: a systematic review. Mayo Clin. Proc. 82: 159–165. [DOI] [PubMed] [Google Scholar]
- 12.Hahalis G., Alexopoulos D., Kremastinos D. T., Zoumbos N. C. 2005. Heart failure in beta-thalassemia syndromes: a decade of progress. Am. J. Med. 118: 957–967. [DOI] [PubMed] [Google Scholar]
- 13.Ladis V., Chouliaras G., Berdousi H., Kanavakis E., Kattamis C. 2005. Longitudinal study of survival and causes of death in patients with thalassemia major in Greece. Ann. N. Y. Acad. Sci. 1054: 445–450. [DOI] [PubMed] [Google Scholar]
- 14.Taher A., Isma'eel H., Cappellini M. D. 2006. Thalassemia intermedia: revisited. Blood Cells Mol. Dis. 37: 12–20. [DOI] [PubMed] [Google Scholar]
- 15.Taher A., Isma'eel H., Mehio G., Bignamini D., Kattamis A., Rachmilewitz E. A., Cappellini M. D. 2006. Prevalence of thromboembolic events among 8,860 patients with thalassaemia major and intermedia in the Mediterranean area and Iran. Thromb. Haemost. 96: 488–491. [PubMed] [Google Scholar]
- 16.Hahalis G., Kremastinos D. T., Terzis G., Kalogeropoulos A. P., Chrysanthopoulou A., Karakantza M., Kourakli A., Adamopoulos S., Tselepis A. D., Grapsas N., et al. 2008. Global vasomotor dysfunction and accelerated vascular aging in beta-thalassemia major. Atherosclerosis. 198: 448–457. [DOI] [PubMed] [Google Scholar]
- 17.Phumala Morales N., Cherlermchoung C., Fucharoen S., Chantharaksri U. 2007. Paraoxonase and platelet-activating factor acetylhydrolase activities in lipoproteins of beta-thalassemia/hemoglobin E patients. Clin. Chem. Lab. Med. 45: 884–889. [DOI] [PubMed] [Google Scholar]
- 18.Tsimihodimos V., Karabina S. A., Tambaki A. P., Bairaktari E., Miltiadous G., Goudevenos J. A., Cariolou M. A., Chapman M. J., Tselepis A. D., Elisaf M. 2002. Altered distribution of platelet-activating factor-acetylhydrolase activity between LDL and HDL as a function of the severity of hypercholesterolemia. J. Lipid Res. 43: 256–263. [PubMed] [Google Scholar]
- 19.Papavasiliou E. C., Gouva C., Siamopoulos K. C., Tselepis A. D. 2005. Erythrocyte PAF-acetylhydrolase activity in various stages of chronic kidney disease: effect of long-term therapy with erythropoietin. Kidney Int. 68: 246–255. [DOI] [PubMed] [Google Scholar]
- 20.Glantzounis G. K., Tselepis A. D., Tambaki A. P., Trikalinos T. A., Manataki A. D., Galaris D. A., Tsimoyiannis E. C., Kappas A. M. 2001. Laparoscopic surgery-induced changes in oxidative stress markers in human plasma. Surg. Endosc. 15: 1315–1319. [DOI] [PubMed] [Google Scholar]
- 21.Gazi I., Lourida E. S., Filippatos T., Tsimihodimos V., Elisaf M., Tselepis A. D. 2005. Lipoprotein-associated phospholipase A2 activity is a marker of small, dense LDL particles in human plasma. Clin. Chem. 51: 2264–2273. [DOI] [PubMed] [Google Scholar]
- 22.Tselepis A. D., Dentan C., Karabina S. A., Chapman M. J., Ninio E. 1995. PAF-degrading acetylhydrolase is preferentially associated with dense LDL and VHDL-1 in human plasma. Catalytic characteristics and relation to the monocyte-derived enzyme. Arterioscler. Thromb. Vasc. Biol. 15: 1764–1773. [DOI] [PubMed] [Google Scholar]
- 23.Tselepis A. D., Karabina S. A., Stengel D., Piedagnel R., Chapman M. J., Ninio E. 2001. N-linked glycosylation of macrophage-derived PAF-AH is a major determinant of enzyme association with plasma HDL. J. Lipid Res. 42: 1645–1654. [PubMed] [Google Scholar]
- 24.Hoogeveen R. C., Ballantyne C. M. 2005. PLAC test for identification of individuals at increased risk for coronary heart disease. Expert Rev. Mol. Diagn. 5: 9–14. [DOI] [PubMed] [Google Scholar]
- 25.Mitsios J. V., Vini M. P., Stengel D., Ninio E., Tselepis A. D. 2006. Human platelets secrete the plasma type of platelet-activating factor acetylhydrolase primarily associated with microparticles. Arterioscler. Thromb. Vasc. Biol. 26: 1907–1913. [DOI] [PubMed] [Google Scholar]
- 26.Tsimihodimos V., Karabina S. A., Tambaki A. P., Bairaktari E., Goudevenos J. A., Chapman M. J., Elisaf M., Tselepis A. D. 2002. Atorvastatin preferentially reduces LDL-associated platelet-activating factor acetylhydrolase activity in dyslipidemias of type IIA and type IIB. Arterioscler. Thromb. Vasc. Biol. 22: 306–311. [DOI] [PubMed] [Google Scholar]
- 27.Dounousi E., Papavasiliou E., Makedou A., Ioannou K., Katopodis K. P., Tselepis A., Siamopoulos K. C., Tsakiris D. 2006. Oxidative stress is progressively enhanced with advancing stages of CKD. Am. J. Kidney Dis. 48: 752–760. [DOI] [PubMed] [Google Scholar]
- 28.Stafforini D. M., Prescott S. M., McIntyre T. M. 1991. Platelet-activating factor acetylhydrolase in human erythrocytes. Methods Enzymol. 197: 411–425. [DOI] [PubMed] [Google Scholar]
- 29.Karabina S. A., Liapikos T. A., Grekas G., Goudevenos J., Tselepis A. D. 1994. Distribution of PAF-acetylhydrolase activity in human plasma low-density lipoprotein subfractions. Biochim. Biophys. Acta. 1213: 34–38. [DOI] [PubMed] [Google Scholar]
- 30.Chapman M. J. 2007. Metabolic syndrome and type 2 diabetes: lipid and physiological consequences. Diab. Vasc. Dis. Res. 4(Suppl 3): S5–S8. [DOI] [PubMed] [Google Scholar]
- 31.Amer J., Fibach E. 2004. Oxidative status of platelets in normal and thalassemic blood. Thromb. Haemost. 92: 1052–1059. [DOI] [PubMed] [Google Scholar]
- 32.Walter P. B., Fung E. B., Killilea D. W., Jiang Q., Hudes M., Madden J., Porter J., Evans P., Vichinsky E., Harmatz P. 2006. Oxidative stress and inflammation in iron-overloaded patients with beta-thalassaemia or sickle cell disease. Br. J. Haematol. 135: 254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Domenica Cappellini M., Tavazzi D., Duca L., Marelli S., Fiorelli G. 2000. Non-transferrin-bound iron, iron-related oxidative stress and lipid peroxidation in beta-thalassemia intermedia. Transfus. Sci. 23: 245–246. [DOI] [PubMed] [Google Scholar]
- 34.Tsimikas S., Willeit J., Knoflach M., Mayr M., Egger G., Notdurfter M., Witztum J. L., Wiedermann C. J., Xu Q., Kiechl S. 2009. Lipoprotein-associated phospholipase A2 activity, ferritin levels, metabolic syndrome, and 10-year cardiovascular and non-cardiovascular mortality: results from the Bruneck study. Eur. Heart J. 30: 107–115. [DOI] [PubMed] [Google Scholar]
- 35.Cao Y., Stafforini D. M., Zimmerman G. A., McIntyre T. M., Prescott S. M. 1998. Expression of plasma platelet-activating factor acetylhydrolase is transcriptionally regulated by mediators of inflammation. J. Biol. Chem. 273: 4012–4020. [DOI] [PubMed] [Google Scholar]
- 36.Liapikos T. A., Antonopoulou S., Karabina S. P., Tsoukatos D. C., Demopoulos C. A., Tselepis A. D. 1994. Platelet-activating factor formation during oxidative modification of low-density lipoprotein when PAF-acetylhydrolase has been inactivated. Biochim. Biophys. Acta. 1212: 353–360. [DOI] [PubMed] [Google Scholar]
- 37.Heery J. M., Kozak M., Stafforini D. M., Jones D. A., Zimmerman G. A., McIntyre T. M., Prescott S. M. 1995. Oxidatively modified LDL contains phospholipids with platelet-activating factor-like activity and stimulates the growth of smooth muscle cells. J. Clin. Invest. 96: 2322–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsimikas S., Brilakis E. S., Miller E. R., McConnell J. P., Lennon R. J., Kornman K. S., Witztum J. L., Berger P. B. 2005. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N. Engl. J. Med. 353: 46–57. [DOI] [PubMed] [Google Scholar]
- 39.Aviram M., Rosenblat M., Bisgaier C. L., Newton R. S., Primo-Parmo S. L., La Du B. N. 1998. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J. Clin. Invest. 101: 1581–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang E. H., McConnell J. P., Lennon R. J., Barsness G. W., Pumper G., Hartman S. J., Rihal C. S., Lerman L. O., Lerman A. 2006. Lipoprotein-associated phospholipase A2 is an independent marker for coronary endothelial dysfunction in humans. Arterioscler. Thromb. Vasc. Biol. 26: 106–111. [DOI] [PubMed] [Google Scholar]
- 41.Herrmann J., Mannheim D., Wohlert C., Versari D., Meyer F. B., McConnell J. P., Gossl M., Lerman L. O., Lerman A. 2009. Expression of lipoprotein-associated phospholipase A(2) in carotid artery plaques predicts long-term cardiac outcome. Eur. Heart J. 30: 2930–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorelick P. B. 2008. Lipoprotein-associated phospholipase A2 and risk of stroke. Am. J. Cardiol. 101: 34F–40F. [DOI] [PubMed] [Google Scholar]
- 43.Shahar E., Chambless L. E., Rosamond W. D., Boland L. L., Ballantyne C. M., McGovern P. G., Sharrett A. R. 2003. Plasma lipid profile and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 34: 623–631. [DOI] [PubMed] [Google Scholar]
- 44.Oh S. O., Ibe B. O., Johnson C., Kurantsin-Mills J., Raj J. U. 1997. Platelet-activating factor in plasma of patients with sickle cell disease in steady state. J. Lab. Clin. Med. 130: 191–196. [DOI] [PubMed] [Google Scholar]