Abstract
Proprotein convertase, subtilisin/kexin type 9 (PCSK9), a key regulator of plasma LDL-cholesterol (LDL-c) and cardiovascular risk, is produced in liver and secreted into plasma where it binds hepatic LDL receptors (LDLR), leading to their degradation. PCSK9 is transcriptionally activated by sterol response element-binding protein (SREBP)-2, a transcription factor that also activates all genes for cholesterol synthesis as well as the LDLR. Here we investigated the relationship between plasma PCSK9 levels and the lathosterol-to-cholesterol ratio, a marker of cholesterol biosynthesis, in 18 healthy subjects during a 48 h fast. In all individuals, plasma PCSK9 levels declined steadily during the fasting period, reaching a nadir at 36 h that was ∼58% lower than levels measured in the fed state (P < 0.001). Similarly, the lathosterol-to-cholesterol ratio declined in parallel with plasma PCSK9 concentrations during the fast, reaching a nadir at 36 h that was ∼28% lower than that measured in the fed state (P = 0.024). In summary, fasting has a marked effect on plasma PCSK9 concentrations, which is mirrored by measures of cholesterol synthesis in humans. Inasmuch as cholesterol synthesis and PCSK9 are both regulated by SREBP-2, these results suggest that plasma PCSK9 levels may serve as a surrogate marker of hepatic SREBP-2 activity in humans.
Keywords: PCSK9, cholesterol synthesis, LDL-cholesterol
Proprotein convertase, subtilisin/kexin type 9 (PCSK9) is a key regulator of plasma LDL-cholesterol (LDL-c) levels and cardiovascular risk (1). PCSK9 is a serine protease that is primarily made in liver and secreted into plasma where it binds hepatic LDL receptors (LDLR), leading to their degradation (1). Inasmuch as PCSK9 regulates the level of liver LDLRs and thereby plasma LDL-cholesterol (LDL-c) levels, individuals with gain-of-function missense mutations in PCSK9 have plasma LDL-c concentrations similar to those with the more common form of familial hypercholesterolemia that results from mutations in the LDLR (2). Such individuals develop early atherosclerosis and cardiovascular events (2). Conversely, individuals who are heterozygous for loss-of-function mutations in PCSK9 have on average 28% lower plasma LDL-c levels and enjoy an 88% reduction in cardiovascular events (3).
The LDLR and PCSK9 are transcriptionally regulated by sterol response element-binding protein (SREBP)-2, a transcription factor that also activates all genes required for cholesterol biosynthesis (4–6). The regulation of SREBP-2 and PCSK9 levels has been studied primarily using hepatocyte culture systems and rodent models; however, there is a paucity of physiologic data in humans. To date, studies of the in vivo regulation of plasma PCSK9 have focused on intervention investigations using lipid-lowering agents. Several groups have demonstrated that hydroxymethylgutaryl (HMG)-CoA reductase inhibitors increase levels of circulating PCSK9 in humans, an intervention known to enhance SREBP-2 levels in livers of rodents (7–13). Fenofibrate also increased PCSK9 concentrations in humans, although the mechanism is less well-defined (13, 14). Data on humans measuring the effects of fasting on circulating PCSK9 levels is not currently available. In light of the potential of PCSK9 as a therapeutic target for the treatment of hypercholesterolemia, we investigated the effect of fasting on circulating levels of plasma PCSK9 and its relationship to cholesterol synthesis in healthy human volunteers.
RESEARCH DESIGN AND METHODS
Protocol
Participants.
Recruitment for this study occurred at University of Texas Southwestern Medical Center (UTSW) via campus posters and word-of-mouth. Healthy individuals aged 18–65 were allowed to participate in the study. Subjects who had comorbid conditions, who were pregnant or breastfeeding, or who weighed less than 110 lbs (50 kg) were excluded. After a screening evaluation to determine fitness for enrollment, subjects were admitted to the Clinical and Translational Research Center (CTRC) at UTSW for two days.
The protocol and consent forms were approved by the UTSW Institutional Review Board, and all participants provided written informed consent prior to enrollment.
Design.
The study consisted of a two-day inpatient admission to the CTRC during which participants were fasted for 48 h. On protocol day 0, subjects ate a self-prepared lunch at 12:00 and then reported to the CTRC at 16:00 for admission. Subjects remained fasted from the time of admission on protocol day 0 until 12:00 on protocol day 2. During the fast, the individuals had free access only to water. At 4 h intervals during this protocol, 3 ml of blood was drawn from each subject's intravenous catheter for analysis.
Analysis of blood samples
Blood was collected in nonheparinized, EDTA-containing tubes and immediately centrifuged to isolate plasma. The isolated plasma was divided into three equal aliquots and pipetted into micofuge tubes for storage. Samples were then immediately frozen and maintained at −80°C, after which they were thawed once and analyzed.
Plasma concentrations of PCSK9 were determined as previously described (8). Lathosterol levels were measured by gas-liquid chromatography as described (15). Plasma glucose, cholesterol, triglycerides (TG), and high density lipoprotein cholesterol (HDL-c) concentrations were determined using a Vitros 250 spectrophotmetric analyzer (Ortho-Clinical Diagnostics, Rochester, NY). A commercially available enzyme-linked immunosorbent assay kit was used to measure plasma insulin concentrations (Millipore, Billerica, MA). Total plasma ketone concentrations were determined using a commercial kit (Wako Chemicals, Richmond, VA).
Statistical analysis
Statistical analyses were performed using SigmaStat 3.0 (SPSS, Inc., Chicago, IL). The significance of variable trends over the period of observation was determined using repeated measures ANOVA. The correlations of variables between subjects were calculated using the mean value for repeated observations over the fasting period (16). The correlations of variables within subjects were calculated using ANCOVA (ANCOVA) (17). Unless otherwise indicated, values are presented as mean and standard deviation. Statistical significance was taken at P < 0.05.
RESULTS
Subjects
The study population was composed of 18 individuals whose characteristics are presented in Table 1. The population was evenly divided between men and women. Subjects tended to be young, Caucasian, and lean. No subjects were underweight, five subjects were overweight, and three subjects were obese. Fasting glucose and glycosylated hemoglobin values were normal in all subjects. Among the subjects studied, fasting lipid profiles were generally normal, although three subjects had cholesterol levels > 200 mg/dl, and two subjects had triglycerides > 150 mg/dl. No subjects were taking any medication, including diuretics, β blockers, statins, fibrates, fish oil, or hormone supplements, that affected lipid metabolism.
TABLE 1.
Characteristics of study participants
| Characteristic | Value |
|---|---|
| Number | 18 |
| Age (years) | 29 ± 13 |
| Gender ratio (male:female) | 9:9 |
| Ethnicity/race (n) | |
| Non-Hispanic White | 13 |
| Non-Hispanic Black | 3 |
| Hispanic | 1 |
| Asian | 1 |
| Height (cm) | 173 ± 8 |
| Weight (kg) | 78 ± 16 |
| Body mass index (kg/m ) | 26 ± 5 |
| Glucose (mg/dl) | 88 ± 6 |
| Hemoglobin A1c (%) | 5.1 ± 0.3 |
| Total cholesterol (mg/dl) | 173 ± 31 |
| HDL-c (mg/dl) | 52 ± 18 |
| LDL-c (mg/dl) | 104 ± 25 |
| Triglycerides (mg/dl) | 84 ± 59 |
Values are mean ± SD. Conversions: triglycerides, HDL-c, and LDL-c (mg/dl) × 0.02586 = mmol/l; glucose (mg/dl) × 0.05551 = mmol/l. HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol.
Intervention
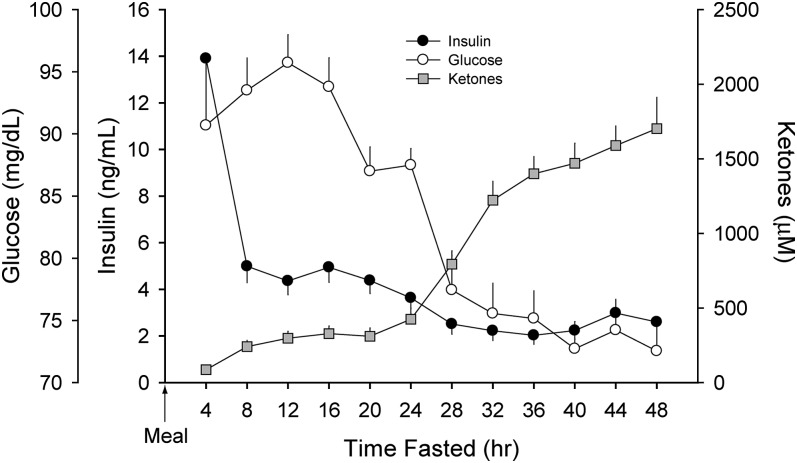
To verify that subjects were fasting, data for plasma glucose, insulin, and total ketones were measured (Fig. 1). Glucose levels peaked at 96 ± 10 mg/dl at 12 h postprandial and then declined rapidly, reaching a nadir at 40 h (73 ± 9 mg/dl; P = 0.015). Plasma insulin followed a similar pattern, although the peak value occurred at 0–4 h fasted followed by a rapid decline to a nadir at 28–32 h. As expected, plasma ketones increased modestly after 8 h of fasting and then rapidly increased after 24 h.
Fig. 1.
Plasma glucose, insulin, and total ketone concentrations during a 48 h fast. Data (mean ± SEM) were derived from 18 healthy subjects (9 men and 9 women described in Table 1) who underwent an observed 48 h fast. Glucose levels peaked 12 h postprandial and then declined rapidly, reaching a nadir at 40 h fasted (P = 0.015). Plasma insulin levels peaked during the initial 4 h of fasting followed by a rapid decline to a nadir by 28–32 h (P < 0.001). Plasma ketones increased modestly after 8 h of fasting and then rapidly increased after 24 h (P < 0.001).
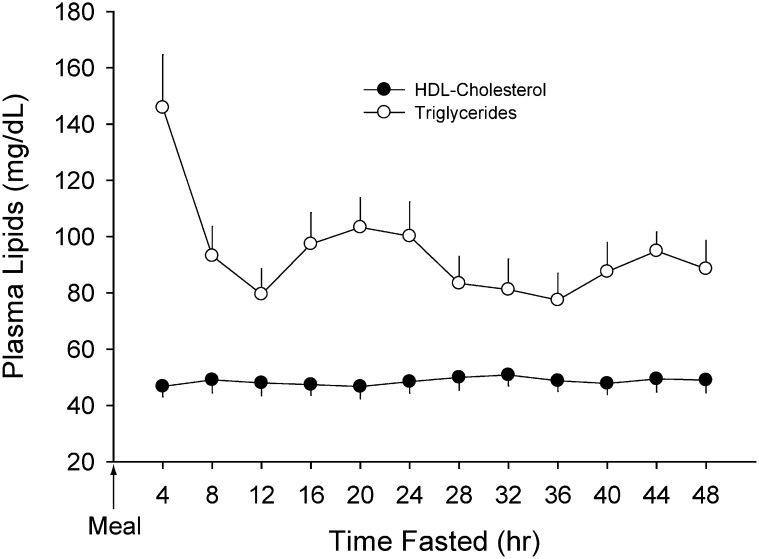
The effect of a 48 h fast on plasma TG and HDL-c are presented in Fig. 2. Plasma TG was elevated at 4 h postprandial (146 ± 77 mg/dl) and rapidly declined by 12 h postprandial (80 ± 39 mg/dl; P < 0.001). Following this decline, a more modest decline was apparent over the remainder of the fast (P = 0.031) with notable diurnal variation in plasma TG levels. Concentrations of plasma HDL-c were not altered over the 48 h fast (49 ± 1 mg/dl; P = 0.610).
Fig. 2.
Plasma TG and HDL-c concentrations during a 48 h fast. Plasma TG peaked in the immediate postprandial period (0–4 h) and then declined rapidly by 8 h fasted (P < 0.001). Over the remaining fasting period, diurnal variation was apparent and TG continued to decline modestly (P = 0.031). There was no significant change in plasma levels of HDL-c over the 48 h fast. Data (mean ± SEM) were derived from 18 healthy subjects (described in Table 1) who underwent an observed 48 h fast. HDL-c, high-density lipoprotein cholesterol; TG, triglyceride.
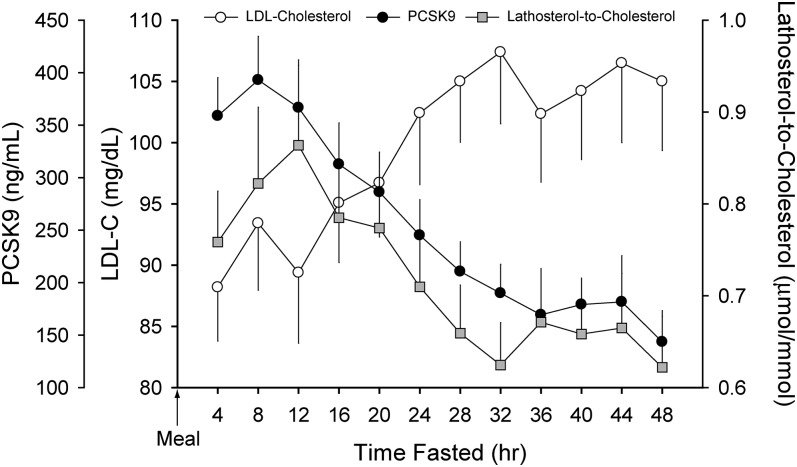
We next examined the effect of prolonged fasting on plasma LDL-c and PCSK9 levels (Fig. 3). Values of LDL-c were initially low (88 ± 18 mg/dl) and increased steadily to a peak of 107 ± 25 mg/dl after 32 h of fasting (P = 0.004). Further increases beyond this time point were not apparent (P = 0.816). Plasma levels of PCSK9 declined during the fast, reaching a nadir by 36 h (169 ± 98 ng/ml) that was ∼58% lower than that measured in the fed state.
Fig. 3.
Trends in plasma LDL-c, PCSK9, and lathosterol-to-cholesterol ratio during a 48 h fast. Plasma levels of LDL-c increased modestly over the initial 32 h of the two-day fast (P = 0.004) and then stabilized. Plasma PCSK9 levels declined steadily after 8 h of fasting and reached a nadir by 36 h (P = 0.024). The decline in plasma PCSK9 occurred in tandem with a decrease in the lathosterol-to-cholesterol ratio (P = 0.091). Data (mean ± SEM) were derived from 18 healthy subjects (described in Table 1) who underwent an observed 48 h fast. PCSK9, proprotein convertase, subtilisin/kexin type 9; LDL-c, low-density lipoprotein cholesterol.
To further investigate the rise in LDL-c observed during fasting as well as the inverse relationship between LDL-c and PCSK9 in these subjects, we determined how cholesterol synthesis, which is a marker of SREBP-2 activity, changed during the fast. The lathosterol-to-cholesterol ratio was used as a surrogate for cholesterol synthesis (18). The data are presented in Fig. 3. The ratio peaked at 12 h postprandial (0.86 ± 0.36 μmol/mmol) and then steadily declined, reaching a nadir by 32 h fasted (0.62 ± 0.19 μmol/mmol; P = 0.024). Thus, despite the rise in LDL-c, SREBP-2 activity and cholesterol synthesis decreased with fasting, and plasma PCSK9 levels paralleled these changes.
We next examined the correlation between plasma PCSK9 concentrations and plasma metabolites. In the population as a whole (between-subject correlation), there was a significant inverse relationship between levels of plasma insulin and PCSK9 (r = −0.545; P = 0.019), indicating that individuals in this population with higher insulin levels tended to have lower PCSK9 levels. However, plasma PCSK9 levels fell in parallel with insulin over the fasting period in each individual, as would be anticipated based upon the insulin-dependent regulation of SREBP-2 (r = 0.430, P < 0.001 by ANCOVA).
There was no significant relationship between levels of plasma HDL-c and PCSK9 over the duration of fasting. There were no between-subject correlations for levels of plasma TG and PCSK9; however, there was a tendency for changes in concentrations of plasma TG to parallel PCSK9 within individuals over the fasting period (r = 0.235, P < 0.001 by ANCOVA).
Prior studies demonstrated a modest but significant positive correlation between levels of LDL-c and plasma PCSK9 concentrations (8, 19). Initially, we determined the correlation between these two metabolites at fasting intervals most likely to be encountered in overnight-fasted individuals (8, 12, and 16 h fasted); no significant correlations were observed at any time point (r = −0.053–0.106; P = 0.668–0.895). Likewise, the between-subject correlation for plasma LDL-c and PCSK9 over the entire fasting period was not significant (r = −0.064; P = 0.800), indicating that individuals with similar levels of PCSK9 in this population had markedly different levels of LDL-c. However, within each subject, levels of LDL-c and PCSK9 over the fasting period were modestly, but inversely, correlated (r = −0.336; P < 0.001).
A positive intra-individual correlation of cholesterol synthesis and PCSK9 was found over the period of observation (r = 0.388, P < 0.001), suggesting that plasma levels of these two variables are commonly regulated (i.e., via SREBP-2). However, the between-subject correlation of cholesterol synthesis, as measured by lathosterol-to-cholesterol ratio, and PCSK9 over the 48 h fast was not significant (r = −0.394; P = 0.117). This finding suggests that a given concentration of PCSK9 in plasma cannot predict the absolute rate of cholesterol synthesis. Additionally, an inverse relationship was noted between cholesterol synthesis and levels of plasma LDL-c, with both between-subject (r = −0.662; P = 0.004) and within-subject (r = −0.757; P < 0.001) correlations being highly significant. This finding supports the generalization that lower rates of cholesterol synthesis during fasting are associated with higher LDL-c levels.
DISCUSSION
In the current study, plasma PCSK9 concentrations were measured in 18 healthy individuals to investigate the effects of fasting on plasma PCSK9 levels and its relationship to cholesterol synthesis. This is the first study to characterize the effects of a physiologic, nonpharmaceutical intervention on plasma PCSK9 in human subjects. The primary finding of this study was that plasma levels of PCSK9 declined by ∼58% with fasting, reaching a nadir by 36 h of fasting. This occurred in tandem with an ∼28% decline in the lathosterol-to-cholesterol ratio, a marker of cholesterol biosynthesis and SREBP-2 activity. Despite the fall in both PCSK9 and cholesterol synthesis during fasting, levels of LDL-c rose modestly, resulting in a significant, but inverse, relationship between LDL-c and both plasma PCSK9 levels and rates of cholesterol synthesis (Fig. 3).
Our previous studies in rodents have shown that fasting results in the reduction of SREBP-2 levels in liver. This reduction leads to lower mRNA levels for genes required for cholesterol biosynthesis and, hence, to lower rates of de novo cholesterol synthesis (20). Thus, we hypothesized that fasting in humans would also result in reduced hepatic SREBP-2 levels, lower rates of cholesterol synthesis, and as SREBP-2 is the major transcriptional activator of PCSK9 (21, 22), lower plasma PCSK9 levels. Inasmuch as the metabolic rates of rodents and humans are significantly different, we did not know the duration of fasting required to reach a nadir of cholesterol synthesis and plasma PCSK9 levels. Consequently, we collected blood at 4 h intervals during the 48 h fast, with the data demonstrating that a prolonged fast (36 h) was required to reach the lowest plasma concentrations of PCSK9. The reduction in cholesterol synthesis as measured by the lathosterol-to-cholesterol ratio also reached its lowest level at 36 h fasted, further supporting the hypothesis derived from rodent studies that PCSK9 and cholesterol biosynthetic genes are coregulated.
Somewhat unexpected was the increase in plasma LDL-c that was measured during the fast. Prior human studies have demonstrated a variable effect of fasting on LDL-c levels, with the effect being dependent upon whether the study population was lean or obese (increasing and decreasing, respectively) (23, 24). The increase in LDL-c in this population occurred despite a reduction in measures of cholesterol synthesis and plasma PCSK9 concentrations, both of which might be expected to reduce plasma LDL-c levels. The most likely explanation for the increase in LDL-c levels with fasting is a reduction in SREBP-2 activity, which would also be predicted to reduce the transcriptional activation of the LDLR (6) and, thus, reduce LDL-c clearance and increase plasma LDL-c concentrations.
The results of this study provide an important framework for future studies that focus on the measurement of plasma PCSK9 concentrations in humans, as plasma levels of PCSK9 declined markedly with fasting, demonstrating that the fed state of the individual will have a marked effect on plasma concentrations. The decrease in plasma PCSK9 concentrations induced by fasting occurs in tandem with reduced cholesterol biosynthesis, with both reaching a nadir by 36 h. Inasmuch as both cholesterol synthesis and PCSK9 are regulated by SREBP-2, these results suggest that plasma PCSK9 levels may serve as a surrogate marker for SREBP-2 activity in liver.
Acknowledgments
The authors thank Dr. Jonathan Cohen for helpful scientific discussion; Dr. Fong Xu for the plasma lathosterol and cholesterol measurements; Norma Anderson and Tuyet Dang for excellent technical assistance; Jeannie Davis, Sonya Rios, and Carol Parcel for aid in study execution; and Janet Jerrow for data management.
Footnotes
Abbreviations:
- ANCOVA
- analysis of covariance
- CTRC
- Clinical Trials Research Center
- HDL-c
- high-density lipoprotein cholesterol
- PCSK9
- proprotein convertase, subtilisin/kexin type 9
- LDL-c
- low-density lipoprotein cholesterol
- HMG
- hydroxylmethylglutaryl
- LDLR
- LDL receptor
- SREBP
- sterol response element-binding protein
- TG
- triglyceride
This work was supported by the Clinical and Translational Research Award (CTSA) at University of Texas Southwestern (UL1RR024982), the Taskforce for Obesity Research (TORS) at University of Texas Southwestern (5UL1DE019584), the TORS Human Biology Core (5PL1DK081183), and the TORS Molecular and Metabolic Mouse Phenotyping Core (5PL1DK081182); Perot Family Foundation (J.D.H.); and National Institutes of Health Grants 5RL1DK-081187 (J.D.B.), 1K23DK-074396 (J.D.B.), and HL-20948 (J.D.H.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Horton J. D., Cohen J. C., Hobbs H. H. 2009. PCSK9: a convertase that coordinates LDL catabolism. J. Lipid Res. 50(Suppl): S172–S177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abifadel M., Varret M., Rabes J. P., Allard D., Ouguerram K., Devillers M., Cruaud C., Benjannet S., Wickham L., Erlich D., et al. 2003. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 34: 154–156. [DOI] [PubMed] [Google Scholar]
- 3.Cohen J. C., Boerwinkle E., Mosley T. H., Hobbs H. H. 2006. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 354: 1264–1272. [DOI] [PubMed] [Google Scholar]
- 4.Rashid S., Curtis D. E., Garuti R., Anderson N. N., Bashmakov Y., Ho Y. K., Hammer R. E., Moon Y-A., Horton J. D. 2005. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc. Natl. Acad. Sci. USA. 102: 5374–5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berge K. E., Ose L., Leren T. P. 2006. Missense mutations in the PCSK9 gene are associated with hypocholesterolemia and possibly increased response to statin therapy. Arterioscler. Thromb. Vasc. Biol. 26: 1094–1100. [DOI] [PubMed] [Google Scholar]
- 6.Horton J. D., Shimomura I., Brown M. S., Hammer R. E., Goldstein J. L., Shimano H. 1998. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. J. Clin. Invest. 101: 2331–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Careskey H. E., Davis R. A., Alborn W. E., Troutt J. S., Cao G., Konrad R. J. 2008. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J. Lipid Res. 49: 394–398. [DOI] [PubMed] [Google Scholar]
- 8.Lakoski S. G., Lagace T. A., Cohen J. C., Horton J. D., Hobbs H. H. 2009. Genetic and metabolic determinants of plasma PCSK9 levels. J. Clin. Endocrinol. Metab. 94: 2537–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubuc G., Tremblay M., Pare G., Jacques H., Hamelin J., Benjannet S., Boulet L., Genest J., Bernier L., Seidah N. G., et al. 2010. A new method for measurement of total plasma PCSK9: clinical applications. J. Lipid Res. 51: 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welder G., Zineh I., Pacanowski M. A., Troutt J. S., Cao G., Konrad R. J. 2010. High-dose atorvastatin causes a rapid sustained increase in human serum PCSK9 and disrupts its correlation with LDL cholesterol. J. Lipid Res. 51: 2714–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lakoski S. G., Xu F., Vega G. L., Grundy S. M., Chandalia M., Lam C., Lowe R. S., Stepanavage M. E., Musliner T. A., Cohen J. C., et al. 2010. Indices of cholesterol metabolism and relative responsiveness to ezetimibe and simvastatin. J. Clin. Endocrinol. Metab. 95: 800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davignon J., Dubuc G. 2009. Statins and ezetimibe modulate plasma proprotein convertase subtilisin kexin-9 (PCSK9) levels. Trans. Am. Clin. Climatol. Assoc. 120: 163–173. [PMC free article] [PubMed] [Google Scholar]
- 13.Mayne J., Dewpura T., Raymond A., Cousins M., Chaplin A., Lahey K. A., Lahaye S. A., Mbikay M., Ooi T. C., Chretien M. 2008. Plasma PCSK9 levels are significantly modified by statins and fibrates in humans. Lipids Health Dis. 7: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Troutt J. S., Alborn W. E., Cao G., Konrad R. J. 2010. Fenofibrate treatment increases human serum proprotein convertase subtilisin kexin type 9 levels. J. Lipid Res. 51: 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilund K. R., Yu L., Xu F., Vega G. L., Grundy S. M., Cohen J. C., Hobbs H. H. 2004. No association between plasma levels of plant sterols and atherosclerosis in mice and men. Arterioscler. Thromb. Vasc. Biol. 24: 2326–2332. [DOI] [PubMed] [Google Scholar]
- 16.Bland J. M., Altman D. G. 1995. Calculating correlation coefficients with repeated observations: Part 2–correlation between subjects. BMJ. 310: 633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bland J. M., Altman D. G. 1995. Calculating correlation coefficients with repeated observations: Part 1–correlation within subjects. BMJ. 310: 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kempen H. J., Glatz J. F., Gevers Leuven J. A., van der Voort H. A., Katan M. B. 1988. Serum lathosterol concentration is an indicator of whole-body cholesterol synthesis in humans. J. Lipid Res. 29: 1149–1155. [PubMed] [Google Scholar]
- 19.Alborn W. E., Cao G., Careskey H. E., Qian Y-W., Subramaniam D. R., Davies J., Conner E. M., Konrad R. J. 2007. Serum proprotein convertase subtilisin kexin type 9 is correlated directly with serum LDL cholesterol. Clin. Chem. 53: 1814–1819. [DOI] [PubMed] [Google Scholar]
- 20.Horton J. D., Bashmakov Y., Shimomura I., Shimano H. 1998. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc. Natl. Acad. Sci. USA. 95: 5987–5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horton J. D., Shah N. A., Warrington J. A., Anderson N. N., Park S. W., Brown M. S., Goldstein J. L. 2003. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. USA. 100: 12027–12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeong H. J., Lee H-S., Kim K-S., Kim Y-K., Yoon D., Park S. W. 2008. Sterol-dependent regulation of proprotein convertase subtilisin/kexin type 9 expression by sterol-regulatory element binding protein-2. J. Lipid Res. 49: 399–409. [DOI] [PubMed] [Google Scholar]
- 23.Jackson I. M. 1969. Effect of prolonged starvation on blood lipid levels of obese subjects. Metabolism. 18: 13–17. [DOI] [PubMed] [Google Scholar]
- 24.Ende N. 1960. Serum cholesterol in acute starvation: a report of 20 cases. J. Nutr. 71: 85–90. [DOI] [PubMed] [Google Scholar]