Abstract
We present here a gel-based method for rapid purification of apolipoprotein A-I (apoA-I) from small volumes of human plasma. After isolation of high density lipoprotein from plasma, the apoA-I protein was separated by electrophoresis and the apoA-I band excised from the gel. The apoA-I was then eluted from the gel strip, concentrated, and delipidated ready for use. The structure and function of the gel-purified apoA-I protein was compared against apoA-I purified by the traditional size-exclusion chromatography method. The α-helical content of the gel-purified apoA-I as determined by circular dichroism was similar to chromatography-purified apoA-I. The functional activity of gel-purified apoA-I, as determined by cholesterol efflux assays in primary human fibroblasts and RAW264.7 macrophages, was also comparable with chromatography-purified apoA-I. This method is a valid alternative for apoA-I purification with some advantages over traditional chromatography purification including a much reduced plasma volume requirement, less time and cost, and a higher percentage protein recovery. The method is particularly suitable for applications requiring the purification of apoA-I from multiple human or animal samples of interest.
Keywords: high density lipoproteins, gel purification, size-exclusion chromatography, cholesterol efflux
Apolipoprotein A-I (apoA-I) is the major protein component of high density lipoprotein (HDL) comprising approximately 70% of the apolipoprotein content of the particle (1). ApoA-I is synthesized in the liver and intestine as the 267 amino acid precursor, preproapoA-I (2). Cleavage of a signal peptide from the preproapoA-I N-terminus leads to the formation of proapoA-I, which is secreted into circulation. A further cleavage by bone morphogenic protein-1 forms the mature 243 amino acid, apoA-I protein (3). The lipidation of mature apoA-I in circulation by the ATP-binding cassette A1 (ABCA1) transporter to form the preβ HDL particle is the initiating step in the pathway of HDL formation (4). Subsequent lipidation by the lipid transporters, ATP-binding cassette G1 and scavenger receptor B1, leads to the formation of the mature HDL particle (4).
Purification of apoA-I is often required for studies of HDL structure and function. Recombinant apoA-I protein production systems have been reported in bacteria (5–7), mammalian cell lines (8), yeast (9), and baculovirus-insect cell systems (10) to varying degrees of success. In general, protein yields are low and there have been inherent problems with the cleavage events required for production of the mature apoA-I protein. Modifications of the apoA-I sequence, such as codon optimization to suit the expression system, have helped to improve the overall yield of protein (7). The establishment of a reliable and high-yield system for recombinant apoA-I production remains an important goal for the pharmaceutical industry to allow for the large-scale production of apoA-I variants such as apoA-IMilano, which show much promise in clinical trials (11).
Despite advances in recombinant apoA-I production, many research laboratories using apoA-I protein prefer to purify mature apoA-I directly from normolipidemic donor plasma. Traditional methods of apoA-I isolation include size-exclusion chromatography of isolated HDL to separate the apoA-I from other HDL-associated proteins (12). This method can be labor intensive and time consuming and requires large amounts of donor plasma (>100 ml). Additionally, the resulting apoA-I protein may not be pure and may require repeat chromatography (13, 14). Alternative methods of apoA-I purification are available such as denaturation of HDL with guanidine-HCl (15). However, this method requires extensive dialysis and an additional ultracentrifugation step (15).
We have developed an alternative isolation method that is rapid, has a high percentage yield of protein recovery, and can be used with relatively small amounts of plasma. Additionally, this method can be performed using reagents and equipment present in virtually any biochemistry laboratory.
MATERIALS AND METHODS
Materials
Plasma for HDL isolation was obtained from blood donated by a normolipidemic healthy subject after written informed consent. Fibroblasts were cultured from 2 mm skin punch biopsies obtained from members of a Tangier disease family (16) after written informed consent in a study approved by the regional ethics committee. Precision Plus Dual prestained protein marker was obtained from Bio-Rad Laboratories (Hercules, CA). Vivaspin concentrating columns were from Sartorius Stedim Biotech (Aubagne, France). Sephacryl S-300 was from Pharmacia-Biotech (Piscataway, NJ). Nitrocellulose membrane was purchased from Whatman (Maidstone, UK). The polyclonal antibody to apoA-I was purchased from Abcam (Cambridge, MA) and the HRP-conjugated detection antibody from Santa Cruz (Santa Cruz, CA). The ECL detection system was from Amersham (Piscataway, NJ). Dimyristoylphosphatidylcholine (DMPC) was from Avanti Polar Lipids (Alabaster, AL). Advanced-DMEM, DMEM, L-glutamine, penicillin/streptomycin, and amphotericin B were obtained from Invitrogen (Carlsbad, CA). Fetal bovine serum was from Bio International (Auckland, New Zealand). [1,2-3H(N)] cholesterol was from Perkin Elmer (Boston, MA). RAW264.7 murine macrophage cells were from American Type Tissue Collection (Manassas, VA). TO901317 and sodium cholate was from Sigma (St. Louis, MO). All other reagents were analytical grade.
Methods
Isolation of HDL.
HDL (1.063 < d < 1.21 g/ml) was isolated from 4 ml of donor plasma by sequential ultracentrifugation of KBr-density adjusted plasma using a Beckman TLA-100 tabletop ultracentrifuge and TLA-100.3 rotor (Beckman Coulter Inc., Brea, CA) and the method of Havel (17). Alternatively, HDL was isolated from a smaller volume (1–2 ml) using a TLA-100.2 rotor. Isolated HDL was dialyzed against PBS at 4°C for 48 h. Total HDL protein was quantified by the Bradford assay (18).
Separation of apoA-I by electrophoresis.
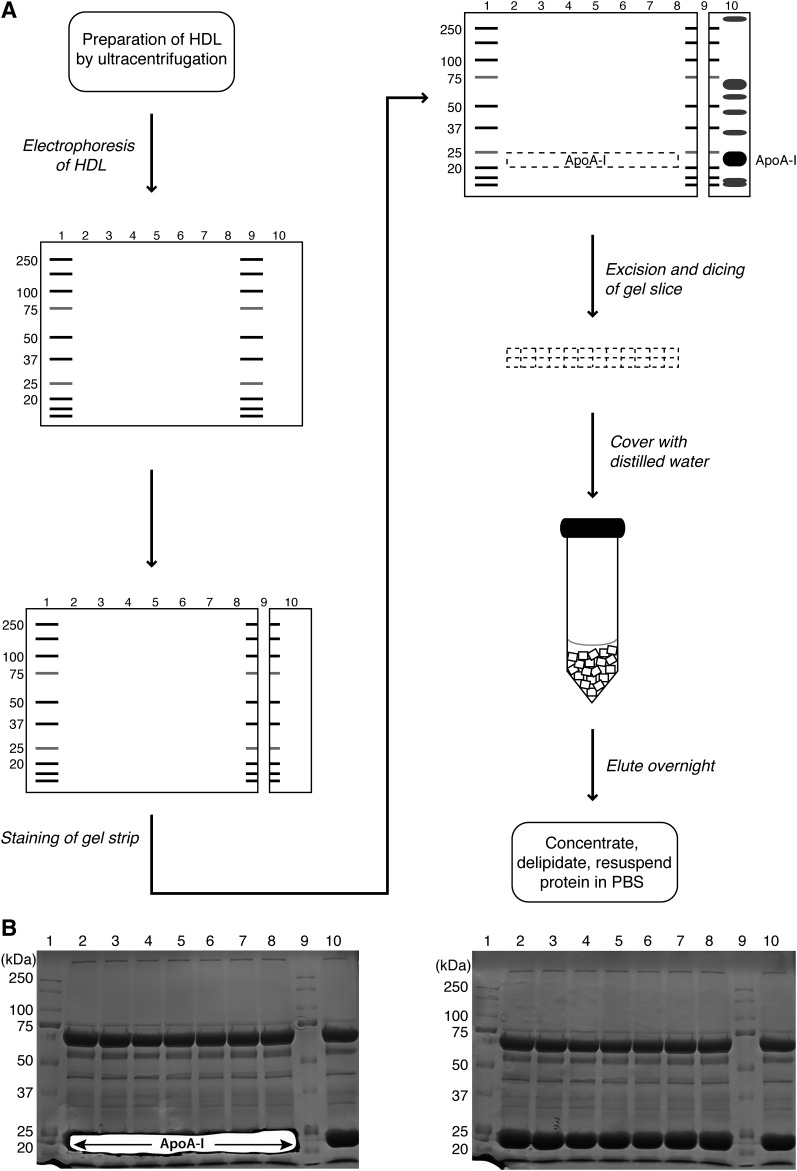
The proteins in HDL were separated by SDS-PAGE on 1.5 mm 10%-polyacrylamide mini-gels under reducing conditions using a 10-well comb and the Protean III Mini system from Bio-Rad Laboratories. The general layout of the method is given in Fig. 1A. Typically, four gels were run simultaneously. Prestained protein marker was loaded in lanes 1 and 9 and 30 μg of HDL protein loaded in each of lanes 2–8 and in lane 10 (i.e., 240 μg total HDL protein per gel). Electrophoresis was at 80 V for 30 min, then 40 mA per gel for 80 min. After electrophoresis, the gels were disassembled and sliced vertically midway through lane 9 containing the protein marker. The larger section of the gel, comprising lanes 1–8 and half of lane 9, was placed in deionized water. The smaller section of the gel was rinsed twice in deionized water then rapidly stained in Coomassie stain (0.1% Coomassie Brilliant Blue, 40% ethanol, 10% acetic acid) by microwaving for 1 min and shaking gently for 2–3 min. Destain solution (40% ethanol, 10% acetic acid) was added and the gel piece microwaved for 1 min then shaken for 2–3 min.
Fig. 1.
Gel purification of apoA-I. A: Layout of purification method. Human HDL (30 μg per well) was loaded into lanes 2–8 and 10 of a 10% SDS- polyacrylamide gel and electrophoresed under reducing conditions alongside prestained protein standards (lanes 1 and 9). Following electrophoresis, the gel was sliced vertically midway through lane 9 and the small gel strip stained with Coomassie Blue to detect HDL proteins. The stained strip was then aligned with the unstained gel and the stained apoA-I band used as a guide for excision of the unstained apoA-I band in lanes 2–8. The band was chopped into small 2–3 mm pieces, covered in deionized water, and the apoA-I protein eluted overnight by gentle agitation. The eluted protein was then concentrated, delipidated, and resuspended in PBS. B: Coomassie Blue-stained gels showing electrophoresis of HDL proteins for apoA-I purification as depicted in A. The gel on the left was stained after excision of the apoA-I band. The gel on the right is an unprocessed gel for comparison.
Excision and recovery of apoA-I.
Once the apoA-I band was visible adjacent to the 25 kDa band (pre-stained in pink), the stained gel piece was aligned with the larger, unstained gel piece (Fig. 1A). A section across the larger gel piece was then excised from where the apoA-I band was inferred to run by comparison with the stained piece. Subsequent staining of the gel after excision revealed the accuracy of excision (Fig. 1B). The excised gel slice was cut into 2 to 3 mm sized fragments mm fragments and placed into 5 ml of deionized water overnight with gentle agitation. The following day, the eluted apoA-I protein was concentrated 10-fold using a Vivaspin column and left overnight at 4°C to maximize the amount of protein recovered from the concentrating column. The recovered protein was delipidated in 9 vols of 3:1 ethanol:diethyl ether for 6 h at −80°C, pelleted by centrifugation at 13,000 rpm for 15 min and washed in diethyl ether. The protein precipitate was pelleted again by centrifugation at 13,000 rpm for 5 min, the solvent evaporated under nitrogen gas, and the protein pellet resuspended in PBS.
Chromatography purification of apoA-I.
ApoA-I was alternatively isolated by size-exclusion chromatography. HDL was isolated from 250 ml of donor plasma using the method of Havel (17) in a Beckman Optima L-70 ultracentrifuge with a Ti60 rotor. HDL was lyophilized, resuspended, and delipidated with a 1:1:2 methanol:chloroform:ether mix followed by a 1:3 methanol:ether mix then washed with ether. The protein pellet was resuspended in urea buffer (6 M urea, 50 mM Tris, pH 8.0). The HDL proteins (50 mg) were fractionated by size-exclusion chromatography on a Sephacryl S-300 column (100 × 3 cm) at a flow rate of 20 ml/h in urea buffer. One hundred 5 ml fractions were collected and protein-containing fractions were analyzed for apoA-I content and purity by SDS-PAGE. Fractions containing pure apoA-I were pooled and dialyzed against PBS for several days.
Analysis of purity and yield.
The protein concentration of purified samples was measured by the Bradford assay (18). Samples were separated on 10% SDS-PAGE gels and either stained with silver stain (19), or transferred to nitrocellulose membrane for Western blot analysis with a polyclonal antibody to apoA-I. Antibody binding was detected with an HRP-conjugated antibody and bands were visualized using the ECL detection system on a Fuji Film LAS-3000 Imager (Tokyo, Japan). Protein samples were also run on 1% agarose gels using the Helena TITAN electrophoresis system (Helena Laboratories, Beaumont, TX) and stained with Amido Black.
The molecular weight of both the gel-purified and chromatography-purified apoA-I was determined by MALDI-time-of-flight (TOF) MS on an Applied Biosystems 4800 MALDI-TOF/TOF analyzer (Framingham, MA). For protein identification, mass spectra were acquired on a tryptic digest of the purified apoA-I samples by MALDI-TOF/TOF MS.
Preparation of rHDL complexes.
The interaction of the purified apoA-I protein with lipid was examined by preparation of rHDL using the cholate-lipid dispersion method (20). DMPC was used as the phospholipid in this case and complexes prepared with a 100:1:100 molar ratio of DMPC:apoA-I:sodium cholate. The rHDL complexes were analyzed on 1% agarose gels stained with Amido Black.
CD spectroscopy.
Purified apoA-I samples were dialyzed into a Tris buffer (10 mM Tris, 10 mM NaCl, pH 7.2) and diluted to 0.1 mg/ml. Circular dichroism (CD) spectra were recorded at 20°C on an Olis DSM 10 spectrophotometer (Olis, Bogart, GA) using a 1 mm path length quartz cuvette. Spectra were recorded between the wavelengths of 260 nm and 195 nm with a 1.0 nm step size and slit bandwidth of 1.5 nm. Signal averaging time was 3 s and ellipticites reported as mean residue ellipticity (θ) in degree cm2/dmol. Spectra are presented as an average of three separate scans with baseline correction. Percentage α-helix of each protein sample was calculated using the mean residue ellipticity at 222 nm according to Equation 2 by Aggerbeck et al. (21).
Cholesterol efflux assays.
Primary fibroblasts were maintained in Advanced-DMEM and RAW264.7 macrophages in DMEM supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 0.25 μg/ml amphotericin B, 100 units/ml of penicillin, and 100 μg/ml of streptomycin. Cells were seeded in 12-well plates and labeled with 0.5 μCi per well of [1,2-3H(N)] cholesterol in serum-containing media for 48 h until confluent. Cells were washed three times in PBS and incubated for 18 h in serum-free medium. In some cases, the LXR agonist TO901317 (2 μM) was added to the serum-free medium to increase ABCA1 expression. Cells were washed again four times in PBS and incubated with serum-free medium containing 10 μg/ml apoA-I for 8 h. The medium was collected and centrifuged for 2 min at 5,000 rpm to remove cellular debris and 3H radioactivity in the media counted. The 3H radioactivity remaining in the cells was counted after lysis in 0.1 M NaOH. Percentage cholesterol efflux was expressed as the ratio of 3H counts in the media to total 3H counts in both the medium and cell fraction for each treatment. The Students's t-test was used to determine any statistical differences between the various apoA-I preparations.
RESULTS
Analysis of purity and yield of gel-purified apoA-I
The gel-purified apoA-I protein was analyzed by both SDS-PAGE and Western blot analysis. Silver staining of the gel-purified protein revealed a single band (Fig. 2A, lane 2), which was confirmed by Western blotting to be apoA-I (Fig. 2B, lane 2). Chromatography purified apoA-I was included on the gels as a comparison (Figs. 2A and B, lane 3) and also showed a single band of apoA-I. The electrophoretic mobility of the purified apoA-I protein was examined by electrophoresis on an agarose gel (Fig. 2C) alongside HDL and chromatography purified apoA-I. The gel-purified apoA-I protein, like the chromatography purified apoA-I, showed the expected preβ migratory position (Fig. 2C, lanes 3 and 4). Also included on this gel was the gel-purified apoA-I before delipidation (Fig. 2C, lane 2). Note the highly negatively charged migration of the apoA-I in this case, likely due to the presence of residual SDS, which is removed on delipidation.
Fig. 2.
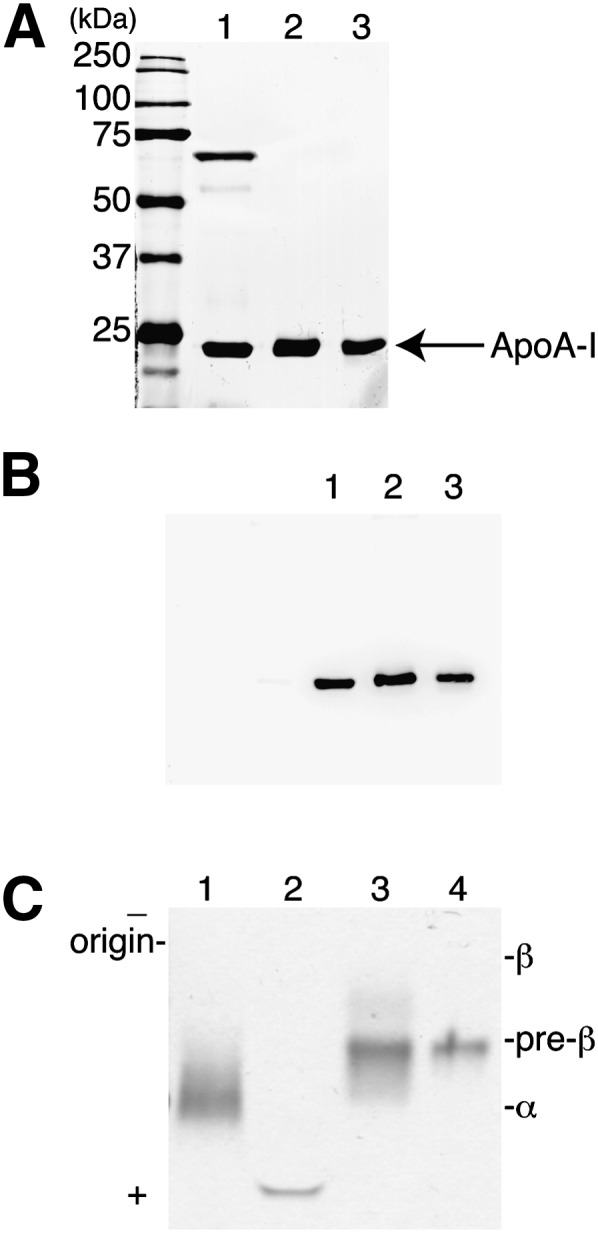
Electrophoresis of gel-purified apoA-I. A: SDS-PAGE analysis of gel-purified apoA-I. Lane 1, 2 μg of HDL protein; lane 2, 1 μg of gel-purified apoA-I; lane 3, 1 μg of chromatography-purified apoA-I. Samples were electrophoresed on 10% SDS-polyacrylamide gels and proteins were visualized with silver stain. B: Western blot analysis of purified apoA-I. An SDS-PAGE gel identical to that shown in A was subjected to Western blot analysis with a polyclonal antibody to apoA-I after transfer of electrophoresed proteins to nitrocellulose. C: Agarose gel electrophoresis of apoA-I preparations. Lane 1, 2 μg of HDL protein; lane 2, 1 μg of gel-purified apoA-I before delipidation; lane 3, 1 μg of gel-purified apoA-I after delipidation; lane 4, chromatography-purified apoA-I. Samples were run on a 1% agarose gel and proteins visualized with Amido Black.
Gel-purified and chromatography-purified apoA-I was tested for the ability to interact with DMPC to form rHDL complexes. Analysis of the complexes by agarose gel electrophoresis (Fig. 3) showed that both preparations bound DMPC to form more negatively charged rHDL complexes that migrated in the α position similar to native HDL.
Fig. 3.
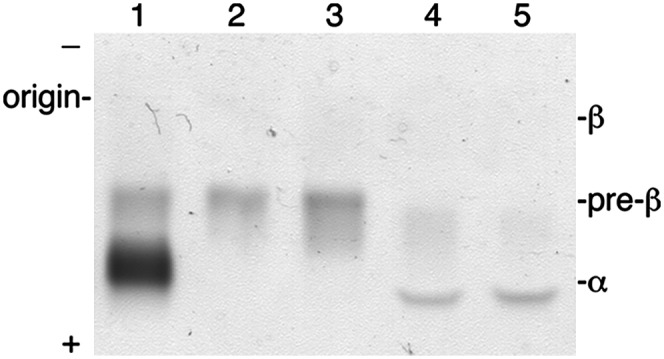
Agarose gel electrophoresis of apoA-I rHDL. rHDL complexes were prepared with DMPC and either gel-purified or chromatography-purified apoA-I. Lane 1, 2 μg HDL; lane 2, 1 μg chromatography-purified apoA-I; lane 3, 1 μg gel-purified apoA-I; lane 4, rHDL prepared with chromatography-purified apoA-I; lane 5, rHDL prepared with gel-purified apoA-I. Samples were run on a 1% agarose gel and proteins visualized with Amido Black.
The molecular weights of the gel-purified and chromatography-purified apoA-I as determined by mass spectrometry were 28,049 Da and 28,058 Da, respectively, which matches well with the theoretical value of 28,078 Da for mature human plasma apoA-I. The Mascot search engine identified >16 significant apoA-I peptide hits (Mascot score >58) from the mass spectra of both the gel-purified and chromatography-purified apoA-I preparations (data not shown). No other protein peptides were identified in either preparation indicating that both were free of contamination from other proteins.
Yields for the gel-purification method are given in Table 1 and compared with typical yields for chromatography-purified apoA-I. A typical ‘run’ of the gel-purification method consists of 16 mini-gels and yields just over 1.3 mg of pure apoA-I protein from 3.4 mg total HDL protein. Although the total amount of apoA-I protein obtained was much less than the chromatography method, due to the much smaller scale of purification, the percentage yield of protein was higher relative to starting material.
TABLE 1.
Comparative apoA-I yields using the chromatography or gel-purification isolation methods
| Method | Chromatography | Gel Purificationa |
|---|---|---|
| HDL as starting material (mg of protein) | 50 | 3.4 |
| Final apoA-I yield (mg of protein) | 12.5 | 1.3 |
| Final apoA-I yield (% of start material) | 25 | 38 |
Gel purification yields are given from a run of 16 mini-gels.
Structural analysis of gel-purified apoA-I
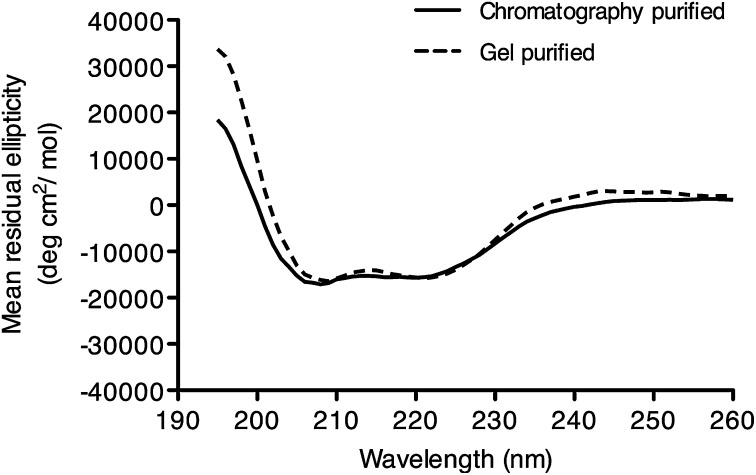
Gel-purified apoA-I was analyzed using CD to confirm that the structural integrity of the isolated protein was retained. The CD spectra of gel-purified apoA-I (Fig. 4) were similar to that of chromatography-purified A-I, displaying the two minima at 208 and 222 nm characteristic of proteins with significant α-helical content. The α-helical content was calculated as 46.7% ± 1.7 for chromatography-purified protein and 47.2% ± 2.0 for gel-purified protein. These values agree with experimentally determined values for the α-helical content of lipid-free apoA-I (22).
Fig. 4.
Circular dichroism spectra of gel-purified apoA-I. Circular dichroism spectroscopy was conducted on both gel-purifed and chromatography-purified apoA-I. Samples were analyzed in 50 mM Tris, 50 mM NaCl, pH 7.2, at a protein concentration of 100 μg/ml at 20°C on an Olis DSM 10 spectrophotometer. Spectra were recorded between the wavelengths of 260 nm and 195 nm with a 1.0 nm step size. The data represent the mean residual ellipticity from three independent measurements after baseline correction.
Functional analysis of gel-purified apoA-I
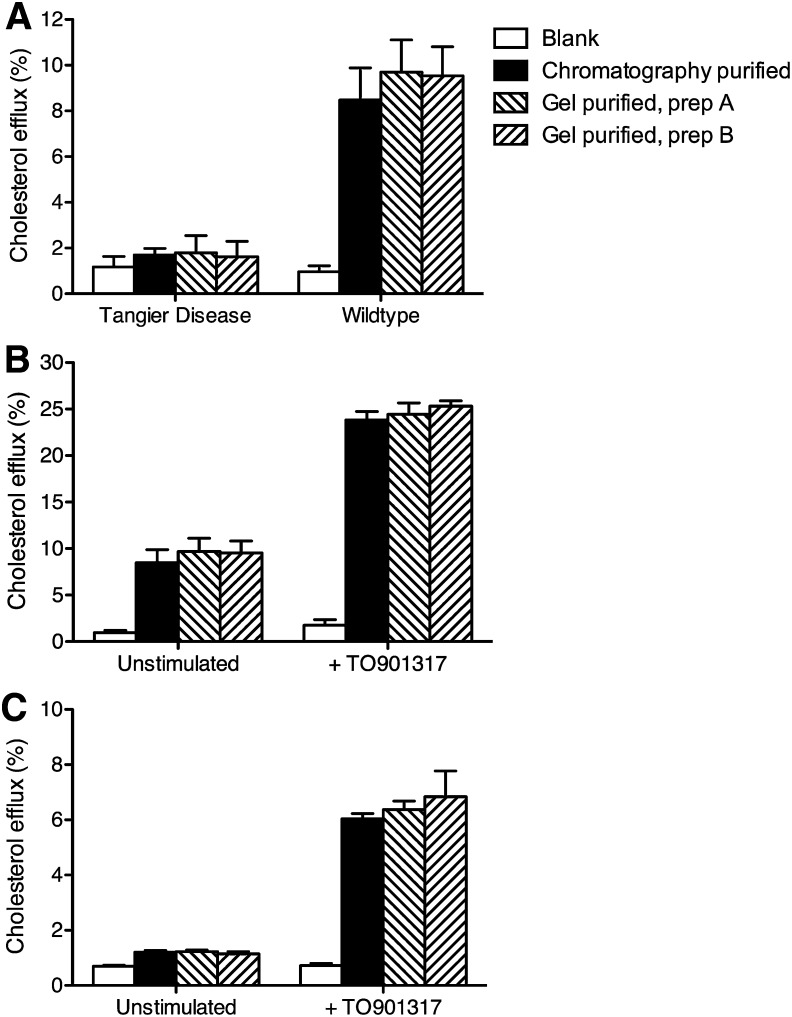
To confirm that the function of the purified apoA-I protein was retained, we tested the cholesterol efflux capability of the gel-purified apoA-I alongside the chromatography-purified apoA-I. Cholesterol efflux assays were conducted in human primary fibroblasts collected from an unaffected and an affected member of a Tangier disease pedigree (16) and in RAW264.7 macrophage cells. Cholesterol efflux was also measured to two independent gel-purified apoA-I preparations isolated one month apart.
Cholesterol efflux assays in Tangier disease fibroblasts showed no significant cholesterol efflux above the blank (i.e., the efflux when no acceptor was added to the medium) as expected (Fig. 5A). Cholesterol efflux from fibroblasts from the unaffected individual (wild-type) gave values of around 8–10% cholesterol efflux (Fig. 5A), with no significant difference in efflux between the chromatography or the two gel-purified apoA-I preparations, or between the two gel-purified apoA-I preparations. When the two fibroblast cell lines were preincubated with the LXR agonist TO901317 to upregulate ABCA1, cholesterol efflux increased by approximately 3-fold in the cells from the unaffected individual (Fig. 5B). Again, there was no significant difference in the ability of all three apoA-I preparations to promote cholesterol efflux under these conditions.
Fig. 5.
Cholesterol efflux ability of gel-purified apoA-I. A: Cholesterol efflux to gel-purified apoA-I from fibroblasts. Two independent preparations of gel-purified apoA-I and a chromatography-purified apoA-I preparation were tested as acceptors in cholesterol efflux assays performed in primary fibroblasts from a Tangier disease patient and an unaffected subject (wildtype). B: Cholesterol efflux to gel-purified apoA-I from stimulated fibroblasts. The same apoA-I preparations were tested as acceptors in cholesterol efflux assays in wild-type fibroblasts with or without incubation in 2 μM of the LXR agonist, TO901317. C: Cholesterol efflux to gel-purified apoA-I from RAW264.7 macrophage cells. ApoA-I preparations were also tested as acceptors in cholesterol efflux assays performed in RAW264.7 macrophages with or without incubation with 2 μM of the LXR agonist, TO901317. In all experiments, cells were loaded with 3H-cholesterol and incubated with 10 μg/ml apoA-I for 8 h. Experiments are presented as mean ± SD of quadruplicate measurements.
Cholesterol efflux assays were also conducted in RAW264.7 cells to confirm the results obtained in primary fibroblasts. Cholesterol efflux from unstimulated RAW264.7 macrophages was low (Fig. 5C) but increased to approximately 6% after preincubation with TO901317. As with the fibroblast cell lines, there was no significant difference in cholesterol efflux to apoA-I isolated by the chromatography or gel-purification method or between the two gel-purified apoA-I preparations.
DISCUSSION
ApoA-I is a multifunctional protein integral for the metabolism of HDL and its many anti-atherogenic properties (23). The production of purified apoA-I for research and therapeutic purposes relies largely on recombinant expression and affinity purification techniques of which there are many different systems available (5–10). Studies of endogenous apoA-I, however, require the isolation of apoA-I from individual plasma samples. This can be problematic due to lengthy costly procedures that use large amounts of plasma and give low yields (13). Established purification protocols use size-exclusion (12) or ion-exchange chromatography (24) of apoA-I from isolated HDL. These chromatography-based methods allow for large scale purification but they are not suitable for isolation of apoA-I from multiple individuals and/or small samples such as those available from routine blood tests or from experiments with small animals. In this report, we describe a gel-based method for the rapid isolation of apoA-I from small amounts of human plasma, which yields pure apoA-I with an electrophoretic mobility, phospholipid binding capability, CD spectra, and cholesterol efflux ability analogous to that of chromatography-purified apoA-I.
The major advantage the gel-purification method has over chromatography is that small volumes of plasma can be used with apoA-I being isolated from just 1–2 ml of plasma. This is a significant advantage in a common situation when only a limited amount of blood from an individual patient is available. It also avoids the need for a larger repeat blood sample thus reducing associated patient compliance and health and safety issues. This method would facilitate the screening of apoA-I from individual patients to look at structural or functional modifications to apoA-I as may be present in diseased states such as advanced atherosclerosis or diabetes (25, 26). Furthermore, it can potentially be adapted for isolation of apoA-I from animal blood for structural and functional studies. The gel-purification method has some other additional benefits over chromatography isolation such as a quicker isolation time, cheaper equipment requirements, and a higher percentage yield of protein. Both methods yield apoA-I with properties similar to that previously reported for native apoA-I.
With regards to yields, the recovery of apoA-I protein from the gel-purification method is higher than the recovery from chromatography, leading to a much improved percentage yield. The caveat here is that the total amount of apoA-I purified by the gel method is much less than with chromatography purification, making it unsuitable when large amounts of apoA-I are required for large scale experiments. In this case, the extra time and costs required to carry out the chromatography are offset by the much larger absolute amount of apoA-I purified. The gel method presented here was performed using a mini-gel system; however, the method could be potentially scaled up to use a larger electrophoresis system and is only limited by the number of gels that can be run on one day. Larger gel systems would require a longer electrophoresis time but would mean fewer gels to manipulate and a higher total yield.
In conclusion, this gel-based method of apoA-I purification reproducibly produces a pure protein preparation that is structurally and functionally equivalent to chromatography-purified apoA-I. It provides a quicker and cheaper alternative to chromatography-based methods for studies requiring multiple isolations of apoA-I from small amounts of plasma.
Acknowledgments
The authors thank Anne von Zychlinski and the Centre for Protein Research, University of Otago, for the mass spectrometry measurements of apoA-I and Ms. Anh Hoang for isolating apoA-I by the chromatography method.
Footnotes
Abbreviations:
- apoA-I
- apolipoprotein A-I
- CD
- circular dichroism
- DMPC
- dimyristoylphosphatidylcholine
- rHDL
- reconstituted HDL
This work was supported by a grant from the Health Research Council of New Zealand. R.J.B. and B.S. are funded by University of Otago Postgraduate Scholarships.
REFERENCES
- 1.Santos-Gallego C. G., Ibanez B., Badimon J. J. 2008. HDL-cholesterol: is it really good? Differences between apoA-I and HDL. Biochem. Pharmacol. 76: 443–452. [DOI] [PubMed] [Google Scholar]
- 2.Gordon J. I., Sims H. F., Lentz S. R., Edelstein C., Scanu A. M., Strauss A. W. 1983. Proteolytic processing of human preproapolipoprotein A-I. A proposed defect in the conversion of pro A-I to A-I in Tangier's disease. J. Biol. Chem. 258: 4037–4044. [PubMed] [Google Scholar]
- 3.Zhu J., Gardner J., Pullinger C. R., Kane J. P., Thompson J. F., Francone O. L. 2009. Regulation of apoAI processing by procollagen C-proteinase enhancer-2 and bone morphogenetic protein-1. J. Lipid Res. 50: 1330–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jessup W., Gelissen I. C., Gaus K., Kritharides L. 2006. Roles of ATP binding cassette transporters A1 and G1, scavenger receptor BI and membrane lipid domains in cholesterol export from macrophages. Curr. Opin. Lipidol. 17: 247–257. [DOI] [PubMed] [Google Scholar]
- 5.McGuire K. A., Davidson W. S., Jonas A. 1996. High yield overexpression and characterization of human recombinant proapolipoprotein A-I. J. Lipid Res. 37: 1519–1528. [PubMed] [Google Scholar]
- 6.Bergeron J., Frank P. G., Emmanuel F., Latta M., Zhao Y., Sparks D. L., Rassart E., Denefle P., Marcel Y. L. 1997. Characterization of human apolipoprotein A-I expressed in Escherichia coli. Biochim. Biophys. Acta. 1344: 139–152. [DOI] [PubMed] [Google Scholar]
- 7.Ryan R. O., Forte T. M., Oda M. N. 2003. Optimized bacterial expression of human apolipoprotein A-I. Protein Expr. Purif. 27: 98–103. [DOI] [PubMed] [Google Scholar]
- 8.Brissette L., Cahuzac-Bec N., Desforges M., Bec J. L., Marcel Y. L., Rassart E. 1991. Expression of recombinant human apolipoprotein A-I in Chinese hamster ovary cells and Escherichia coli. Protein Expr. Purif. 2: 296–303. [DOI] [PubMed] [Google Scholar]
- 9.Feng M. Q., Cai Q. S., Song D. X., Dong J. B., Zhou P. 2006. High yield and secretion of recombinant human apolipoprotein AI in Pichia pastoris. Protein Expr. Purif. 46: 337–342. [DOI] [PubMed] [Google Scholar]
- 10.Pyle L. E., Fidge N. H., Barton P. A., Luong A., Sviridov D. 1997. Production of mature human apolipoprotein A-I in a baculovirus-insect cell system: propeptide is not essential for intracellular processing but may assist rapid secretion. Anal. Biochem. 253: 253–258. [DOI] [PubMed] [Google Scholar]
- 11.Nissen S. E., Tsunoda T., Tuzcu E. M., Schoenhagen P., Cooper C. J., Yasin M., Eaton G. M., Lauer M. A., Sheldon W. S., Grines C. L., et al. 2003. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 290: 2292–2300. [DOI] [PubMed] [Google Scholar]
- 12.Scanu A., Toth J., Edelstein C., Koga S., Stiller E. 1969. Fractionation of human serum high density lipoprotein in urea solutions. Evidence for polypeptide heterogeneity. Biochemistry. 8: 3309–3316. [DOI] [PubMed] [Google Scholar]
- 13.Brewer H. B., Ronan R., Meng M., Bishop C. 1986. Isolation and characterisation of apolipoproteins A-I, A-II, and A-IV. Methods Enzymol. 128: 223–246. [DOI] [PubMed] [Google Scholar]
- 14.Schonfeld G., Pfleger B. 1974. The structure of human high density lipoprotein and the levels of apolipoprotein A-I in plasma as determined by radioimmunoassay. J. Clin. Invest. 54: 236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nichols A. V., Gong E. L., Blanche P. J., Forte T. M., Anderson D. W. 1976. Effects of guanidine hydrochloride on human plasma high density lipoproteins. Biochim. Biophys. Acta. 446: 226–239. [DOI] [PubMed] [Google Scholar]
- 16.Slatter T. L., Williams M. J. A., Frikke-Schmidt R., Tybjaerg-Hansen A., Morison I. M., McCormick S. P. A. 2006. Promoter haplotype of a new ABCA1 mutant influences expression of familial hypoalphalipoproteinemia. Atherosclerosis. 187: 393–400. [DOI] [PubMed] [Google Scholar]
- 17.Havel R. J., Eder H. A., Bragdon J. H. 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34: 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradford M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 19.Mortz E., Krogh T. N., Vorum H., Gorg A. 2001. Improved silver staining protocols for high sensitivity protein identification using matrix-assisted laser desorption/ionization-time of flight analysis. Proteomics. 1: 1359–1363. [DOI] [PubMed] [Google Scholar]
- 20.Matz C. E., Jonas A. 1982. Micellar complexes of human apolipoprotein A-I with phosphatidylcholines and cholesterol prepared from cholate-lipid dispersions. J. Biol. Chem. 257: 4535–4540. [PubMed] [Google Scholar]
- 21.Aggerbeck L. P., Wetterau J. R., Weisgraber K. H., Wu C. S., Lindgren F. T. 1988. Human apolipoprotein E3 in aqueous solution. II. Properties of the amino- and carboxyl-terminal domains. J. Biol. Chem. 263: 6249–6258. [PubMed] [Google Scholar]
- 22.Saito H., Dhanasekaran P., Nguyen D., Holvoet P., Lund-Katz S., Phillips M. C. 2003. Domain structure and lipid interaction in human apolipoproteins A-I and E, a general model. J. Biol. Chem. 278: 23227–23232. [DOI] [PubMed] [Google Scholar]
- 23.Scanu A. M., Edelstein C. 2008. HDL: bridging past and present with a look at the future. FASEB J. 22: 4044–4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison J. R., Fidge N. H., Grego B. 1990. Studies on the formation, separation, and characterization of cyanogen bromide fragments of human AI apolipoprotein. Anal. Biochem. 186: 145–152. [DOI] [PubMed] [Google Scholar]
- 25.Zheng L., Nukuna B., Brennan M. L., Sun M., Goormastic M., Settle M., Schmitt D., Fu X., Thomson L., Fox P. L., et al. 2004. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Invest. 114: 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoang A., Murphy A. J., Coughlan M. T., Thomas M. C., Forbes J. M., O'Brien R., Cooper M. E., Chin-Dusting J. P., Sviridov D. 2007. Advanced glycation of apolipoprotein A-I impairs its anti-atherogenic properties. Diabetologia. 50: 1770–1779. [DOI] [PubMed] [Google Scholar]