Abstract
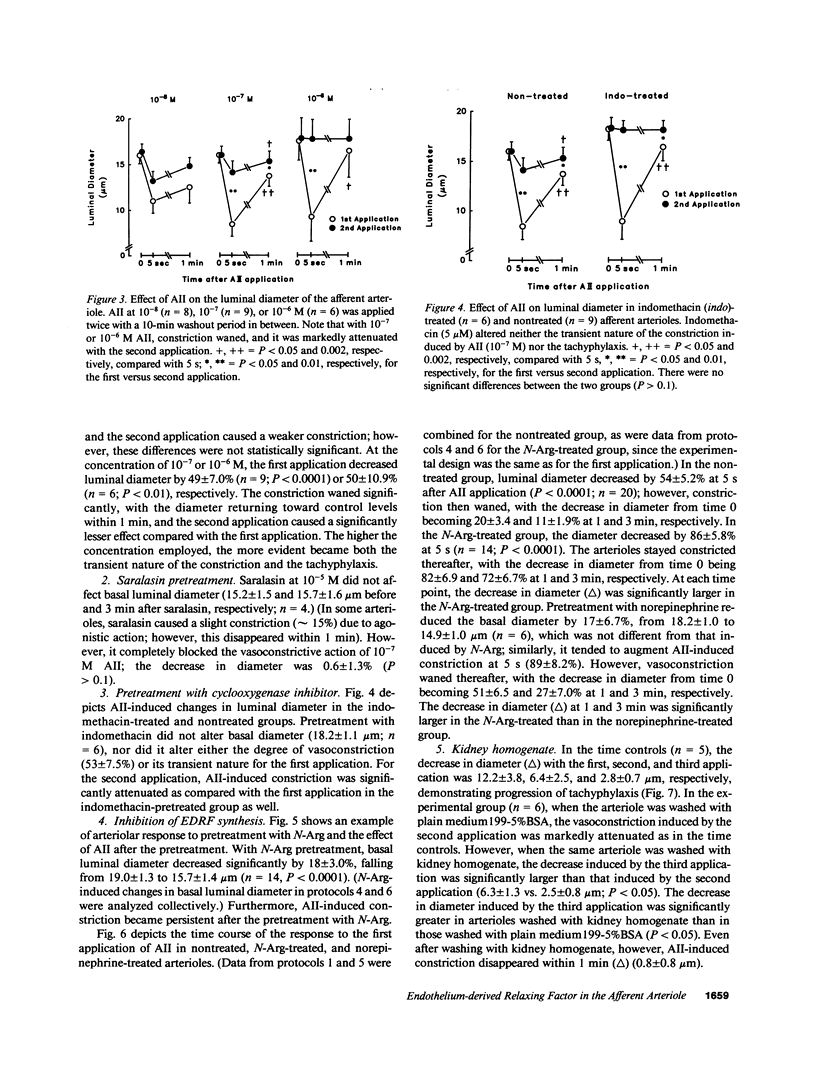
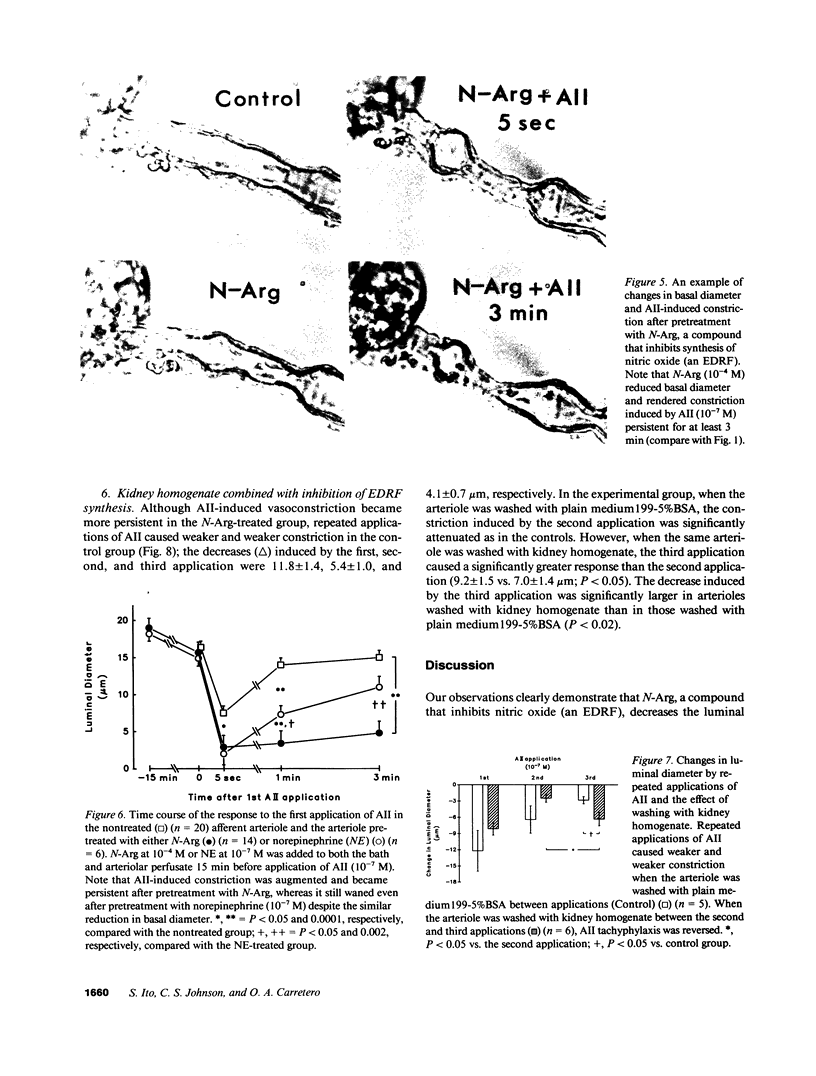
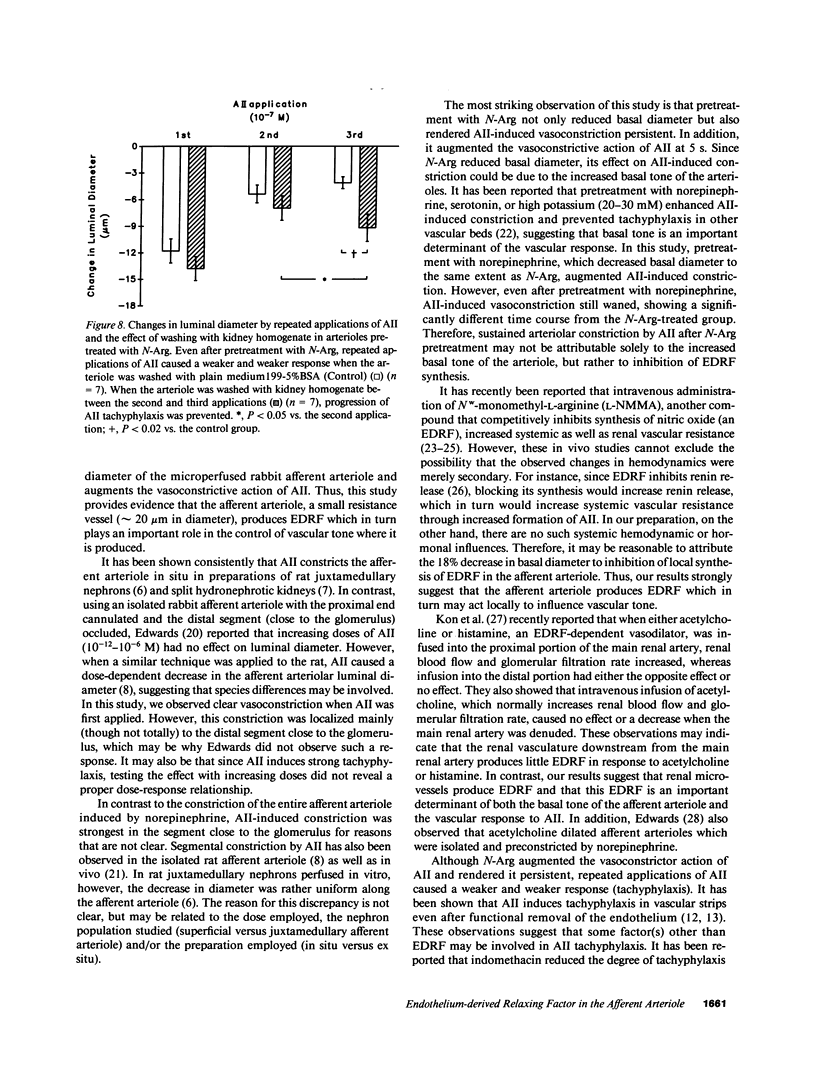
Although endothelium-derived relaxing factor (EDRF) has been studied extensively in large vessels, little is known about its role in the preglomerular afferent arteriole (Af-Art). We tested the hypothesis that EDRF, which is produced locally in the Af-Art, modulates arteriolar responses to angiotensin II (AII). A single rabbit Af-Art with its glomerulus intact was microperfused in vitro at 60 mmHg. When 0.1 microM AII was first applied, luminal diameter decreased by 49 +/- 7.0% (n = 9; P less than 0.0001); however, constriction waned, with the decrease becoming 15 +/- 3.5% at 1 min. After washing the Af-Art, repeated AII caused less constriction (13 +/- 4.0%; P less than 0.0002 vs. first application), showing tachyphylaxis. Pretreatment with Nw-nitro-L-arginine (N-Arg), which inhibits synthesis of nitric oxide (an EDRF), decreased basal diameter by 18 +/- 3.0% (n = 14; P less than 0.0001). N-Arg also augmented AII-induced constriction (86 +/- 6.8%; P less than 0.02 vs. nontreated Af-Art) and rendered it persistent (82 +/- 6.9% at 1 min). Even after pretreatment with N-Arg, repeated AII caused a weaker response, which was restored by washing with kidney homogenate rich in angiotensinase. In conclusion, this study provides evidence that local production of EDRF is an important determinant of the tone of the Af-Art. Our results suggest that the transient nature of AII-induced constriction of the Af-Art may be due to production of EDRF, while tachyphylaxis may be the result of long lasting receptor occupancy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiken J. W. Effects of prostaglandin synthesis inhibitors on angiotensin tachyphylaxis in the isolated coeliac and mesenteric arteries of the rabbit. Pol J Pharmacol Pharm. 1974 Jan-Apr;26(1):217–227. [PubMed] [Google Scholar]
- Carmines P. K., Morrison T. K., Navar L. G. Angiotensin II effects on microvascular diameters of in vitro blood-perfused juxtamedullary nephrons. Am J Physiol. 1986 Oct;251(4 Pt 2):F610–F618. doi: 10.1152/ajprenal.1986.251.4.F610. [DOI] [PubMed] [Google Scholar]
- Casellas D., Moore L. C. Autoregulation and tubuloglomerular feedback in juxtamedullary glomerular arterioles. Am J Physiol. 1990 Mar;258(3 Pt 2):F660–F669. doi: 10.1152/ajprenal.1990.258.3.F660. [DOI] [PubMed] [Google Scholar]
- Chiba S., Tsukada M. Angiotensin II-induced tachyphylactic constrictions in isolated and perfused canine mesenteric arteries. Tohoku J Exp Med. 1986 Dec;150(4):417–426. doi: 10.1620/tjem.150.417. [DOI] [PubMed] [Google Scholar]
- Edwards R. M. Response of isolated renal arterioles to acetylcholine, dopamine, and bradykinin. Am J Physiol. 1985 Feb;248(2 Pt 2):F183–F189. doi: 10.1152/ajprenal.1985.248.2.F183. [DOI] [PubMed] [Google Scholar]
- Edwards R. M. Segmental effects of norepinephrine and angiotensin II on isolated renal microvessels. Am J Physiol. 1983 May;244(5):F526–F534. doi: 10.1152/ajprenal.1983.244.5.F526. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gerová M., Smiesko V., Gero J., Barta E. Dilatation of conduit coronary artery induced by high blood flow. Physiol Bohemoslov. 1983;32(1):55–63. [PubMed] [Google Scholar]
- Hayashi K., Epstein M., Loutzenhiser R. Pressure-induced vasoconstriction of renal microvessels in normotensive and hypertensive rats. Studies in the isolated perfused hydronephrotic kidney. Circ Res. 1989 Dec;65(6):1475–1484. doi: 10.1161/01.res.65.6.1475. [DOI] [PubMed] [Google Scholar]
- Ishii K., Chang B., Kerwin J. F., Jr, Huang Z. J., Murad F. N omega-nitro-L-arginine: a potent inhibitor of endothelium-derived relaxing factor formation. Eur J Pharmacol. 1990 Feb 6;176(2):219–223. doi: 10.1016/0014-2999(90)90531-a. [DOI] [PubMed] [Google Scholar]
- Ito S., Carretero O. A. An in vitro approach to the study of macula densa-mediated glomerular hemodynamics. Kidney Int. 1990 Dec;38(6):1206–1210. doi: 10.1038/ki.1990.335. [DOI] [PubMed] [Google Scholar]
- Itoh S., Carretero O. A., Murray R. D. Renin release from isolated afferent arterioles. Kidney Int. 1985 May;27(5):762–767. doi: 10.1038/ki.1985.77. [DOI] [PubMed] [Google Scholar]
- Juul B., Aalkjaer C., Mulvany M. J. Responses of femoral resistance vessels to angiotensin in vitro. Eur J Pharmacol. 1987 Mar 3;135(1):61–68. doi: 10.1016/0014-2999(87)90757-6. [DOI] [PubMed] [Google Scholar]
- Khairallah P. A., Page I. H., Bumpus F. M., Türker R. K. Angiotensin tachyphylaxis and its reversal. Circ Res. 1966 Aug;19(2):247–254. doi: 10.1161/01.res.19.2.247. [DOI] [PubMed] [Google Scholar]
- Kon V., Harris R. C., Ichikawa I. A regulatory role for large vessels in organ circulation. Endothelial cells of the main renal artery modulate intrarenal hemodynamics in the rat. J Clin Invest. 1990 Jun;85(6):1728–1733. doi: 10.1172/JCI114628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell K. D., Navar L. G. Superficial nephron responses to peritubular capillary infusions of angiotensins I and II. Am J Physiol. 1987 May;252(5 Pt 2):F818–F824. doi: 10.1152/ajprenal.1987.252.5.F818. [DOI] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osgood R. W., Patton M., Hanley M. J., Venkatachalam M., Reineck H. J., Stein J. H. In vitro perfusion of the isolated dog glomerulus. Am J Physiol. 1983 Mar;244(3):F349–F354. doi: 10.1152/ajprenal.1983.244.3.F349. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E. G., Ferreira A. T., Paiva A. C., Paiva T. B. Angiotensin tachyphylaxis in normal and everted rings of rabbit aorta. Eur J Pharmacol. 1988 Aug 24;153(2-3):185–190. doi: 10.1016/0014-2999(88)90605-x. [DOI] [PubMed] [Google Scholar]
- Steinhausen M., Sterzel R. B., Fleming J. T., Kühn R., Weis S. Acute and chronic effects of angiotensin II on the vessels of the split hydronephrotic kidney. Kidney Int Suppl. 1987 May;20:S64–S73. [PubMed] [Google Scholar]
- Tolins J. P., Palmer R. M., Moncada S., Raij L. Role of endothelium-derived relaxing factor in regulation of renal hemodynamic responses. Am J Physiol. 1990 Mar;258(3 Pt 2):H655–H662. doi: 10.1152/ajpheart.1990.258.3.H655. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Rubanyi G. M., Miller V. M., Houston D. S. Modulation of vascular smooth muscle contraction by the endothelium. Annu Rev Physiol. 1986;48:307–320. doi: 10.1146/annurev.ph.48.030186.001515. [DOI] [PubMed] [Google Scholar]
- Vidal M. J., Romero J. C., Vanhoutte P. M. Endothelium-derived relaxing factor inhibits renin release. Eur J Pharmacol. 1988 May 10;149(3):401–402. doi: 10.1016/0014-2999(88)90679-6. [DOI] [PubMed] [Google Scholar]
- Whittle B. J., Lopez-Belmonte J., Rees D. D. Modulation of the vasodepressor actions of acetylcholine, bradykinin, substance P and endothelin in the rat by a specific inhibitor of nitric oxide formation. Br J Pharmacol. 1989 Oct;98(2):646–652. doi: 10.1111/j.1476-5381.1989.tb12639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. K. A comparison of the effects of angiotensin II and norepinephrine on intrarenal arteries in the rat. Kidney Int Suppl. 1987 May;20:S193–S199. [PubMed] [Google Scholar]
- Yuan B. H., Robinette J. B., Conger J. D. Effect of angiotensin II and norepinephrine on isolated rat afferent and efferent arterioles. Am J Physiol. 1990 Mar;258(3 Pt 2):F741–F750. doi: 10.1152/ajprenal.1990.258.3.F741. [DOI] [PubMed] [Google Scholar]
- de Nucci G., Thomas R., D'Orleans-Juste P., Antunes E., Walder C., Warner T. D., Vane J. R. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]