Abstract
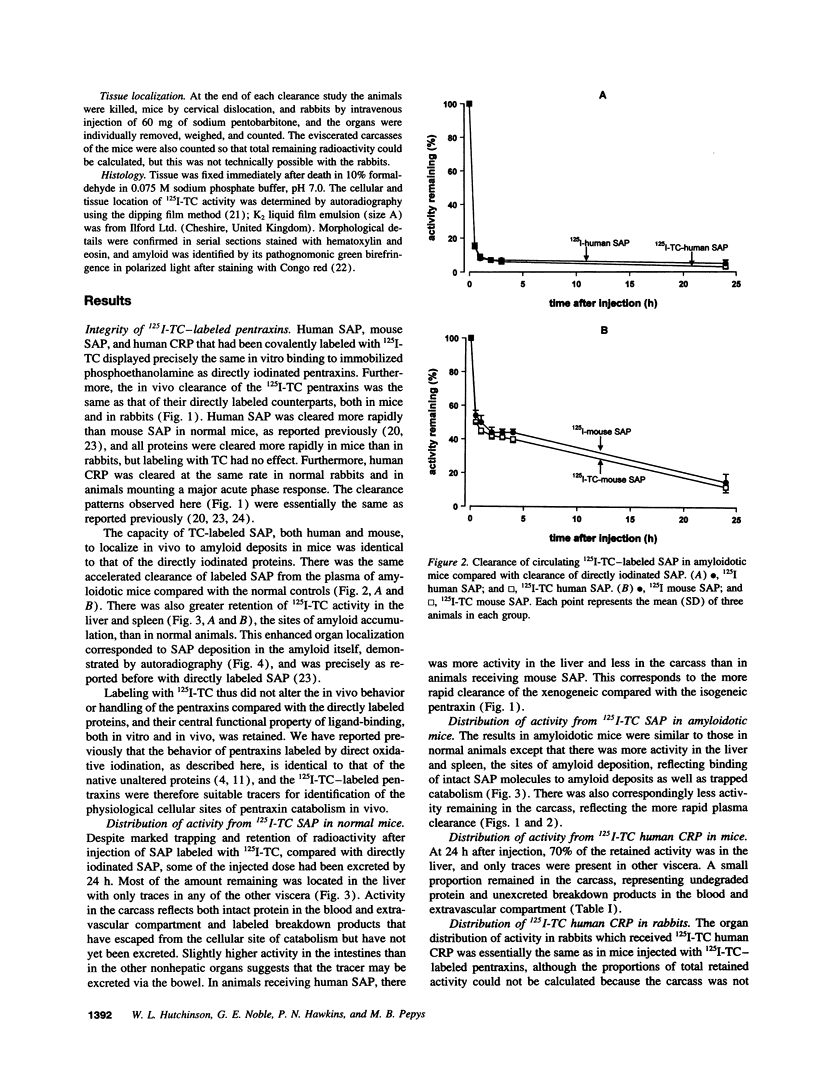
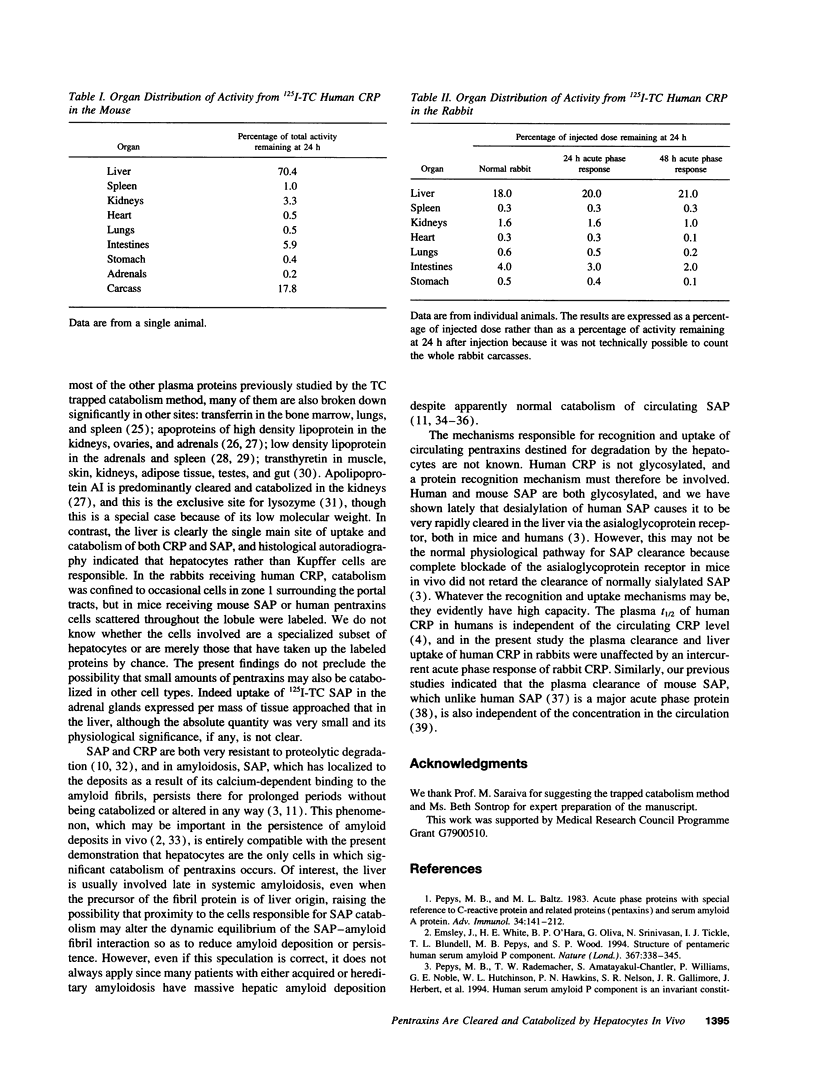
The cellular sites of clearance and degradation of the pentraxin plasma proteins, C-reactive protein, the classical acute phase reactant, and serum amyloid P component (SAP), a universal constituent of amyloid deposits, were sought using the ligand 125I-tyramine cellobiose (TC) which is substantially retained within the cells in which catabolism takes place. Pentraxins labeled with 125I-TC showed the same in vitro and in vivo ligand binding and the same in vivo plasma t1/2 as the directly iodinated proteins and the native unlabeled pentraxins, indicating that their mode of clearance was likely to be physiological. After intravenous injection into mice and rabbits of human C-reactive protein, human SAP, and mouse SAP, each labeled with 125I-TC, most of the radioactivity remaining in the body at 24 h was located in hepatocytes. None was detected in other liver cells, and only traces were present in other viscera; the rest was in the carcass, representing intact pentraxins in the blood and extravascular compartment, and escaped label which had not yet been excreted. Hepatocytes are thus the single major site of pentraxin clearance and catabolism in vivo. This is consistent with the observation that SAP that has localized to amyloid deposits persists there and is not degraded.
Full text
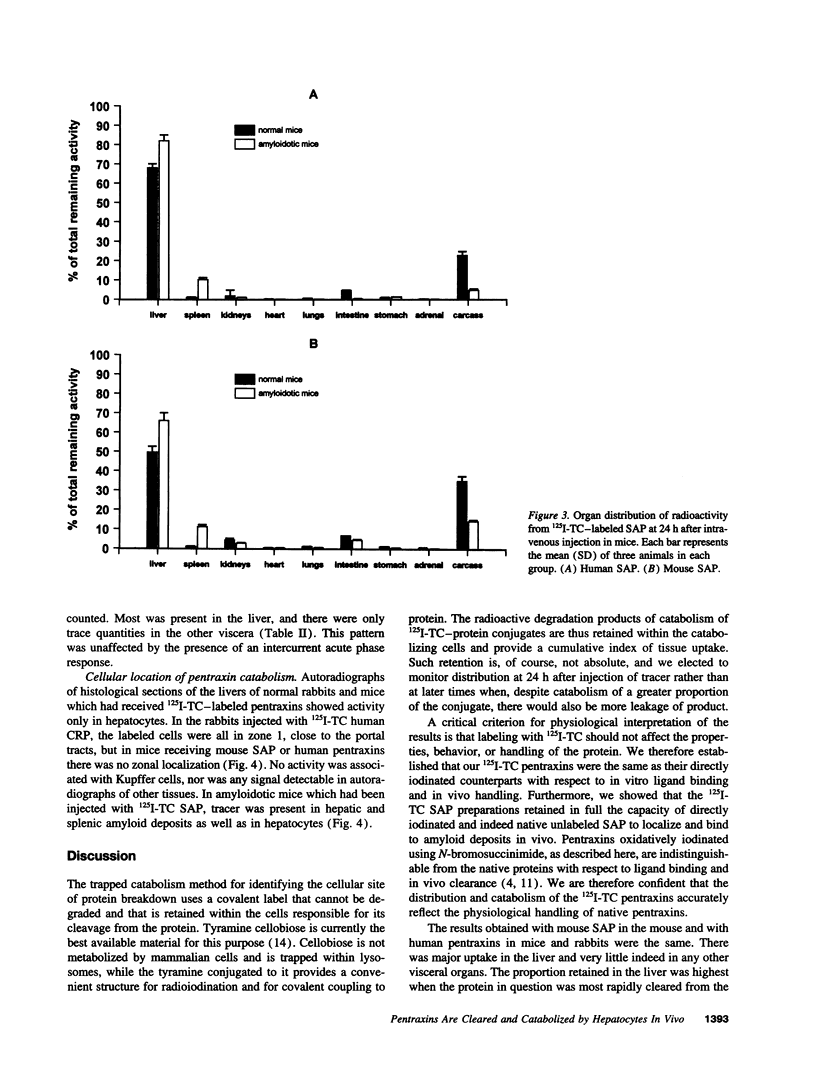
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltz M. L., Caspi D., Evans D. J., Rowe I. F., Hind C. R., Pepys M. B. Circulating serum amyloid P component is the precursor of amyloid P component in tissue amyloid deposits. Clin Exp Immunol. 1986 Dec;66(3):691–700. [PMC free article] [PubMed] [Google Scholar]
- Baltz M. L., Dyck R. F., Pepys M. B. Studies of the in vivo synthesis and catabolism of serum amyloid P component (SAP) in the mouse. Clin Exp Immunol. 1985 Jan;59(1):235–242. [PMC free article] [PubMed] [Google Scholar]
- Baltz M. L., Gomer K., Davies A. J., Evans D. J., Klaus G. G., Pepys M. B. Differences in the acute phase responses of serum amyloid P-component (SAP) and C3 to injections of casein or bovine serum albumin in amyloid-susceptible and -resistant mouse strains. Clin Exp Immunol. 1980 Feb;39(2):355–360. [PMC free article] [PubMed] [Google Scholar]
- Coe J. E., Ross M. J. Amyloidosis and female protein in the Syrian hamster. Concurrent regulation by sex hormones. J Exp Med. 1990 Apr 1;171(4):1257–1267. doi: 10.1084/jem.171.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beer F. C., Pepys M. B. Isolation of human C-reactive protein and serum amyloid P component. J Immunol Methods. 1982;50(1):17–31. doi: 10.1016/0022-1759(82)90300-3. [DOI] [PubMed] [Google Scholar]
- Emsley J., White H. E., O'Hara B. P., Oliva G., Srinivasan N., Tickle I. J., Blundell T. L., Pepys M. B., Wood S. P. Structure of pentameric human serum amyloid P component. Nature. 1994 Jan 27;367(6461):338–345. doi: 10.1038/367338a0. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Pittman R. C., Keller G. A., Steinberg D. Tissue sites of degradation of apoprotein A-I in the rat. J Biol Chem. 1983 Jun 10;258(11):7161–7167. [PubMed] [Google Scholar]
- Goldberg I. J., Blaner W. S., Vanni T. M., Moukides M., Ramakrishnan R. Role of lipoprotein lipase in the regulation of high density lipoprotein apolipoprotein metabolism. Studies in normal and lipoprotein lipase-inhibited monkeys. J Clin Invest. 1990 Aug;86(2):463–473. doi: 10.1172/JCI114732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson S., Vahlquist C., Sjöblom L., Eklund A., Vahlquist A. Metabolism of very low density lipoproteins in rats with isotretinoin (13-cis retinoic acid)-induced hyperlipidemia. J Lipid Res. 1990 Feb;31(2):183–190. [PubMed] [Google Scholar]
- Hawkins P. N., Lavender J. P., Pepys M. B. Evaluation of systemic amyloidosis by scintigraphy with 123I-labeled serum amyloid P component. N Engl J Med. 1990 Aug 23;323(8):508–513. doi: 10.1056/NEJM199008233230803. [DOI] [PubMed] [Google Scholar]
- Hawkins P. N., Myers M. J., Epenetos A. A., Caspi D., Pepys M. B. Specific localization and imaging of amyloid deposits in vivo using 123I-labeled serum amyloid P component. J Exp Med. 1988 Mar 1;167(3):903–913. doi: 10.1084/jem.167.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins P. N., Richardson S., MacSweeney J. E., King A. D., Vigushin D. M., Lavender J. P., Pepys M. B. Scintigraphic quantification and serial monitoring of human visceral amyloid deposits provide evidence for turnover and regression. Q J Med. 1993 Jun;86(6):365–374. [PubMed] [Google Scholar]
- Hawkins P. N., Richardson S., Vigushin D. M., David J., Kelsey C. R., Gray R. E., Hall M. A., Woo P., Lavender J. P., Pepys M. B. Serum amyloid P component scintigraphy and turnover studies for diagnosis and quantitative monitoring of AA amyloidosis in juvenile rheumatoid arthritis. Arthritis Rheum. 1993 Jun;36(6):842–851. doi: 10.1002/art.1780360616. [DOI] [PubMed] [Google Scholar]
- Hawkins P. N., Tennent G. A., Woo P., Pepys M. B. Studies in vivo and in vitro of serum amyloid P component in normals and in a patient with AA amyloidosis. Clin Exp Immunol. 1991 May;84(2):308–316. doi: 10.1111/j.1365-2249.1991.tb08166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins P. N., Wootton R., Pepys M. B. Metabolic studies of radioiodinated serum amyloid P component in normal subjects and patients with systemic amyloidosis. J Clin Invest. 1990 Dec;86(6):1862–1869. doi: 10.1172/JCI114917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hind C. R., Collins P. M., Caspi D., Baltz M. L., Pepys M. B. Specific chemical dissociation of fibrillar and non-fibrillar components of amyloid deposits. Lancet. 1984 Aug 18;2(8399):376–378. doi: 10.1016/s0140-6736(84)90544-0. [DOI] [PubMed] [Google Scholar]
- Hu W. L., Chindemi P. A., Regoeczi E. In vivo behaviour of rat transferrin bearing a hybrid glycan and its interaction with macrophages. Biochem Cell Biol. 1992 Aug;70(8):636–642. doi: 10.1139/o92-098. [DOI] [PubMed] [Google Scholar]
- Hysing J., Tolleshaug H. Quantitative aspects of the uptake and degradation of lysozyme in the rat kidney in vivo. Biochim Biophys Acta. 1986 Jun 16;887(1):42–50. doi: 10.1016/0167-4889(86)90120-5. [DOI] [PubMed] [Google Scholar]
- JANIGAN D. T. EXPERIMENTAL AMYLOIDOSIS: STUDIES WITH A MODIFIED CASEIN METHOD, CASEIN HYDROLYSATE AND GELATIN. Am J Pathol. 1965 Jul;47:159–171. [PMC free article] [PubMed] [Google Scholar]
- Kinoshita C. M., Gewurz A. T., Siegel J. N., Ying S. C., Hugli T. E., Coe J. E., Gupta R. K., Huckman R., Gewurz H. A protease-sensitive site in the proposed Ca(2+)-binding region of human serum amyloid P component and other pentraxins. Protein Sci. 1992 Jun;1(6):700–709. doi: 10.1002/pro.5560010602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita C. M., Ying S. C., Hugli T. E., Siegel J. N., Potempa L. A., Jiang H., Houghten R. A., Gewurz H. Elucidation of a protease-sensitive site involved in the binding of calcium to C-reactive protein. Biochemistry. 1989 Dec 12;28(25):9840–9848. doi: 10.1021/bi00451a044. [DOI] [PubMed] [Google Scholar]
- Makover A., Moriwaki H., Ramakrishnan R., Saraiva M. J., Blaner W. S., Goodman D. S. Plasma transthyretin. Tissue sites of degradation and turnover in the rat. J Biol Chem. 1988 Jun 25;263(18):8598–8603. [PubMed] [Google Scholar]
- Moerlein S. M., Dalal K. B., Ebbe S. N., Yano Y., Budinger T. F. Residualizing and non-residualizing analogues of low-density lipoprotein as iodine-123 radiopharmaceuticals for imaging LDL catabolism. Int J Rad Appl Instrum B. 1988;15(2):141–149. doi: 10.1016/0883-2897(88)90080-3. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Baltz M. L. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Baltz M., Gomer K., Davies A. J., Doenhoff M. Serum amyloid P-component is an acute-phase reactant in the mouse. Nature. 1979 Mar 15;278(5701):259–261. doi: 10.1038/278259a0. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Dash A. C., Markham R. E., Thomas H. C., Williams B. D., Petrie A. Comparative clinical study of protein SAP (amyloid P component) and C-reactive protein in serum. Clin Exp Immunol. 1978 Apr;32(1):119–124. [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B., Hawkins P. N., Booth D. R., Vigushin D. M., Tennent G. A., Soutar A. K., Totty N., Nguyen O., Blake C. C., Terry C. J. Human lysozyme gene mutations cause hereditary systemic amyloidosis. Nature. 1993 Apr 8;362(6420):553–557. doi: 10.1038/362553a0. [DOI] [PubMed] [Google Scholar]
- Pepys M. B. Isolation of serum amyloid P-component (protein SAP) in the mouse. Immunology. 1979 Jul;37(3):637–641. [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B., Rademacher T. W., Amatayakul-Chantler S., Williams P., Noble G. E., Hutchinson W. L., Hawkins P. N., Nelson S. R., Gallimore J. R., Herbert J. Human serum amyloid P component is an invariant constituent of amyloid deposits and has a uniquely homogeneous glycostructure. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5602–5606. doi: 10.1073/pnas.91.12.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman R. C., Carew T. E., Glass C. K., Green S. R., Taylor C. A., Jr, Attie A. D. A radioiodinated, intracellularly trapped ligand for determining the sites of plasma protein degradation in vivo. Biochem J. 1983 Jun 15;212(3):791–800. doi: 10.1042/bj2120791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reay P. Use of N-bromosuccinimide for the iodination of proteins for radioimmunoassay. Ann Clin Biochem. 1982 Mar;19(Pt 2):129–133. doi: 10.1177/000456328201900214. [DOI] [PubMed] [Google Scholar]
- Rowe I. F., Baltz M. L., Soutar A. K., Pepys M. B. In vivo turnover studies of C-reactive protein and lipoproteins in the rabbit. Clin Exp Immunol. 1984 Oct;58(1):245–252. [PMC free article] [PubMed] [Google Scholar]
- Snel F. W., Niewold T. A., Baltz M. L., Hol P. R., Van Ederen A. M., Pepys M. B., Gruys E. Experimental amyloidosis in the hamster: correlation between hamster female protein levels and amyloid deposition. Clin Exp Immunol. 1989 May;76(2):296–300. [PMC free article] [PubMed] [Google Scholar]
- Soutar A. K., Hawkins P. N., Vigushin D. M., Tennent G. A., Booth S. E., Hutton T., Nguyen O., Totty N. F., Feest T. G., Hsuan J. J. Apolipoprotein AI mutation Arg-60 causes autosomal dominant amyloidosis. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7389–7393. doi: 10.1073/pnas.89.16.7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigushin D. M., Pepys M. B., Hawkins P. N. Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J Clin Invest. 1993 Apr;91(4):1351–1357. doi: 10.1172/JCI116336. [DOI] [PMC free article] [PubMed] [Google Scholar]