Abstract
We report two new MPZ mutations causing congenital hypomyelinating neuropathies; c.368_382delGCACGTTCACTTGTG (in-frame deletion of five amino acids) and c.392A>G, Asn131Ser. Each child had clinical and electrodiagnostic features consistent with an inherited neuropathy, confirmed by sural nerve biopsy. The cases illustrate the clinically heterogeneity that exists even within early-onset forms of this disease. They also lend additional support to the emerging clinical and laboratory evidence that impaired intracellular protein trafficking may represent the cause of some congenital hypomyelinating neuropathies.
Keywords: Charcot-Marie-Tooth, type 1B; genetic variation; sequence homology; myelin protein zero; hereditary motor and sensory neuropathies
INTRODUCTION
Myelin protein zero (MPZ) or protein zero (P0) is an essential, structural protein required for normal peripheral nerve myelination. It is comprised of a single extracellular, transmembrane and cytosolic domain [1]. Extracellular domains bind to adjacent molecules forming tetramers which then link to opposing tetramers on adjacent wraps of myelin [2].
MPZ mutations exert a dominant-negative effect over the normal allele thus demonstrating a dominant inheritance pattern [3]. Over 120 MPZ mutations have now been reported [4] which can give rise to highly variable clinical phenotypes. Patients have been classified by age of disease onset (i.e. early-childhood or late-adult onset) and by the predominant electrodiagnostic and pathologic features (i.e. congenital hypomyelinating, demyelinating Charcot-Marie-Tooth, type 1B (CMT1B) or axonal (CMT2I / 2J) forms.
Careful evaluation of MPZ mutations (i.e. frameshift, nonsense or missense) and its effect upon the MPZ protein (i.e. alteration of charge, polarity, size and structure) has permitted the prediction of many disease phenotypes from MPZ genotypes [5,6]. Molecular modeling of MPZ tertiary protein structure has also proven useful in this regard [7]. However, the greatest breakthrough in our understanding of this clinically heterogeneous disease has come from recent clinical and laboratory research of cellular MPZ protein trafficking [3].
We report two children with congenital hypomyelinating neuropathy due to novel MPZ mutations. Each child had clinical and electrophysiological features consistent with an inherited neuropathy with nerve biopsy confirming the diagnosis of congenital hypomyelinating neuropathy. The clinical phenotype differed between the two children, with the more severely affected child showing; clinical features evident at birth, nerve biopsy demonstrating a dramatic absence of nearly all myelination, and genetic evidence for a deletion within the MPZ gene. The cases illustrate clinically heterogeneity even within early-onset forms of this disease. They also provide additional support that defective intracellular transport of mutant MPZ may be associated with some forms of congenital hypomyelinating neuropathies.
Case 1
A 5 month old boy was evaluated for congenital hypotonia and weakness. He was born at 38 weeks after an uneventful gestation. He was the first child to non-consanguineous parents. Birth weight was 2.984 kg. Due to difficulty breast feeding, he required bottle feeds which were also difficult for him to complete. He had failure to thrive, poor head control, and a weak palmar grasp. He had some anti-gravity movements in his arms. Early social and language milestones were normal. His vision and hearing were intact. Family history was unremarkable. On physical examination his cranial nerves were intact. He was hypotonic and lay in a frog-leg posture. He was diffusely weak, showed near-complete head-lag and areflexia. He could bring his hands to his chest, but was unable to extend his arms against gravity. His examination was otherwise unremarkable.
Nerve conduction studies revealed absent sensory and motor nerve responses in his upper and lower extremities. Concentric needle EMG showed fibrillation potentials in the left tibialis anterior and medial gastrocnemius. Decreased recruitment was seen in the left tibialis anterior, medial gastrocnemius, vastus lateralis and iliopsoas. Serum CK was 94 U/L [normal 4-175 U/L]. CSF analysis revealed; protein 95 mg/dL [normal: 15–45 mg/dL] and WBC 0. Sural nerve biopsy at the lateral ankle (Figure 1) revealed a near-complete absence of myelination of both large or small fibers, despite intact axons seen on immunohistochemical stain for neurofilament protein. Clinical MPZ gene sequencing revealed one allele to possess a 15 bp deletion in exon 3 (c.368_382delGCACGTTCACTTGTG; May 2004 MPZ assembly [4,8]) causing an in-frame deletion of five amino acids (Gly/Thr/Phe/Thr/Cys; Figure 2). His mother and father’s MPZ gene sequences were normal. Sequencing of PMP22, EGR2, GDAP1 and SH3TC2 genes were normal. We evaluated the degree of sequence homology of the MPZ gene using a text-based computer program (FASTA [9]). The amino acids in this region were highly conserved, with two of the five deleted amino acids common among 16 species. There are two reports of congenital hypomyelinating neuropathy associated with single amino-acid substitutions in this region [10, 11] as well as another report of disease resulting from the deletion of two amino acids [12]. MRI of his brain (7-months old) revealed age-appropriate central-myelination, and no evidence of structural abnormality. Leukocyte galactocerbrosidase and arylsulfatase A activity was normal. Plasma carnitine and carbohydrate deficient transferritin testing was normal.
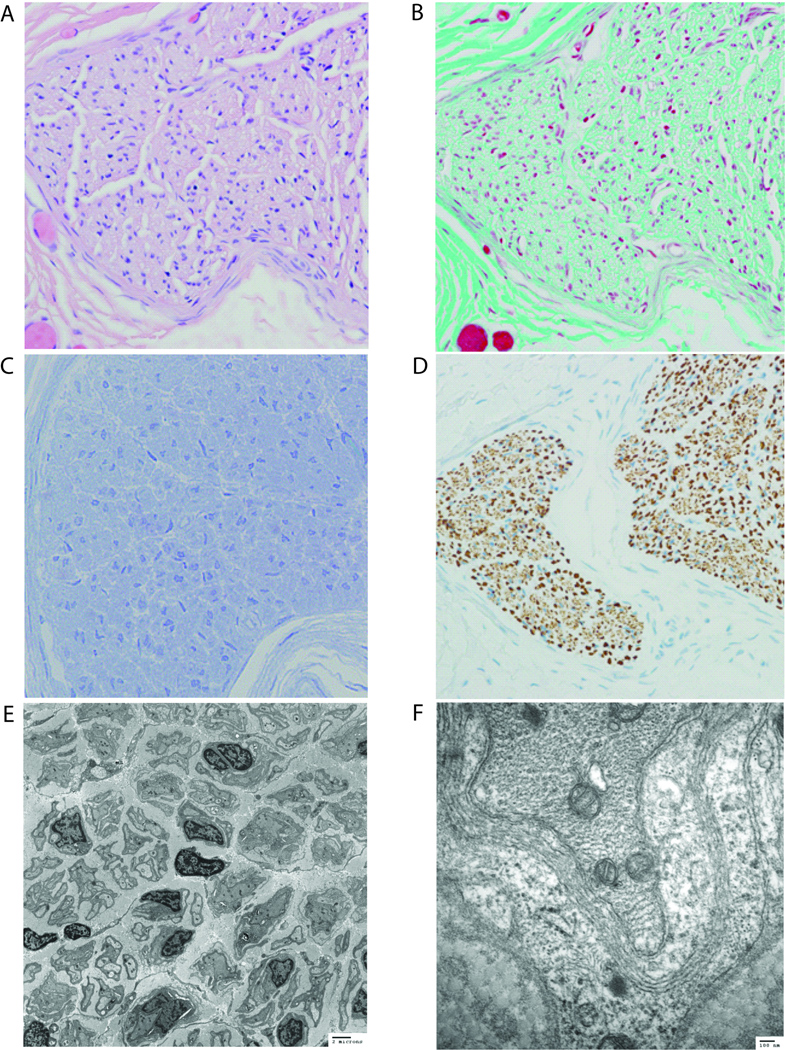
Figure 1.
Sural nerve biopsy (Case 1) stained with (A) H&E (400× magnification), (B) trichrome (400×) and (C) toluidine blue (1000×) demonstrates essentially undetectable myelination. (D) Immunohistochemistry for neurofilament protein (400×) reveals intact axons. (E, F) Transmission electron microscopy reveals a near total absence of myelin with a rare fiber demonstrating extremely thin, uncompacted myelin. Scattered, degenerating, myelin debris and very early abortive onion-bulb formation was seen.
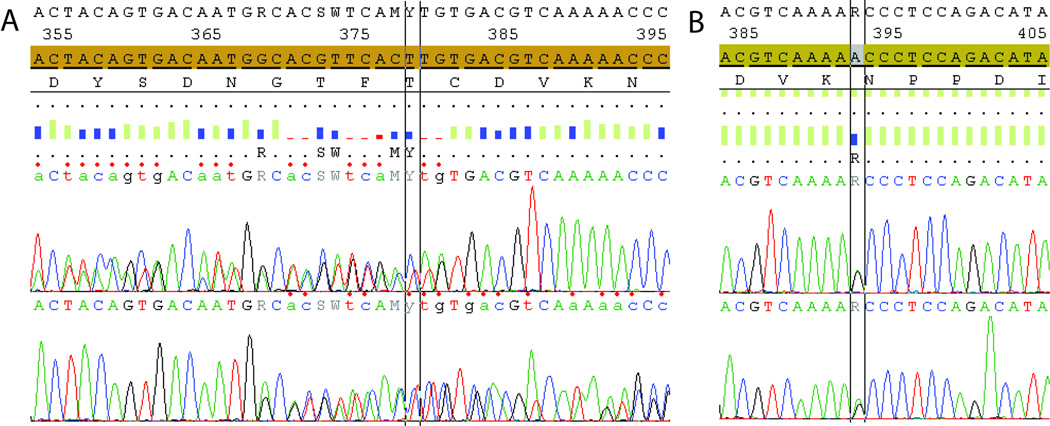
Figure 2.
DNA sequence chromatograms of Case 1 (top) showing c.368_382delGCACGTTCACTTGTG (in-frame deletion of five amino acids) and Case 2 (bottom) c.392A>G, Asn131Ser. Each chromatogram shows the normal MPZ nucleotide sequence with patients’ MPZ mutations below.
Case 2
A 3 year old boy was evaluated for hypotonia and gross motor delay. He was born at 39 weeks, after an uneventful pregnancy. His birth weight was 2.975 kg. He sat independently at 7–8 months, began crawling at 13–14 months and walked independently at 2½ years of age. He was unable to run. His language and fine motor development was normal. He was otherwise healthy with no prior hospitalizations or other medical problems. His family history was limited, as he was adopted immediately after birth. The biological mother was not known to have any symptoms of neuromuscular disease.
Physical examination revealed an alert and bright boy. His general medical examination revealed pes planus, but no heel cord contractures, no high mid-foot arch nor hammertoes. Cranial nerves were intact. Strength testing revealed decreased distal upper-extremity strength. Appendicular hypotonia was evident. Deep tendon reflexes were depressed at brachioradialis (1+) and absent at the biceps, triceps, patella and ankle. Plantar responses were flexor. His gait was broad-based and slow. He rose from a seated position using a modified Gowers maneuver.
Serum CK was 109 U/L. MR of the brain was unremarkable. Clinical MPZ gene sequencing revealed a sequence variant in exon 3 (c.392A>G; Asn131Ser; May 2004 MPZ assembly; Figure 2). The degree of sequence homology was evaluated with all 16 species found to possess an asparagine at the equivalent codon indicating the amino acid to be highly conserved. Alteration of asparagine at this codon has been previously linked to an early-onset disease [13,14], although the prior reports involved an amino acid substitution (c.393C>A; Asn131Lys) where a neutral asparagine was substituted for a basic lysine [13]. Clinical genetic testing revealed no PMP22 deletion/duplication. Sequencing of PMP22, Cx32, EGR2, GDAP1 and SH3TC2 genes were normal. Nerve conduction studies on two occasions revealed absent sensory and motor nerve responses from his upper and lower extremities. Concentric needle electromyography of the left tibialis anterior and medial gastrocnemius did not identify any fibrillation potentials.
Sural nerve biopsy at the lateral ankle (Figure 3) revealed a severe decrease in the number of large myelinated fibers with Gomori trichrome stain showing evidence of ongoing demyelination. Occasional “onion-bulb-like” formations were seen, comprised of Schwann cell basal lamina. No inflammatory infiltrates, nor abnormal inclusions or storage material was seen.
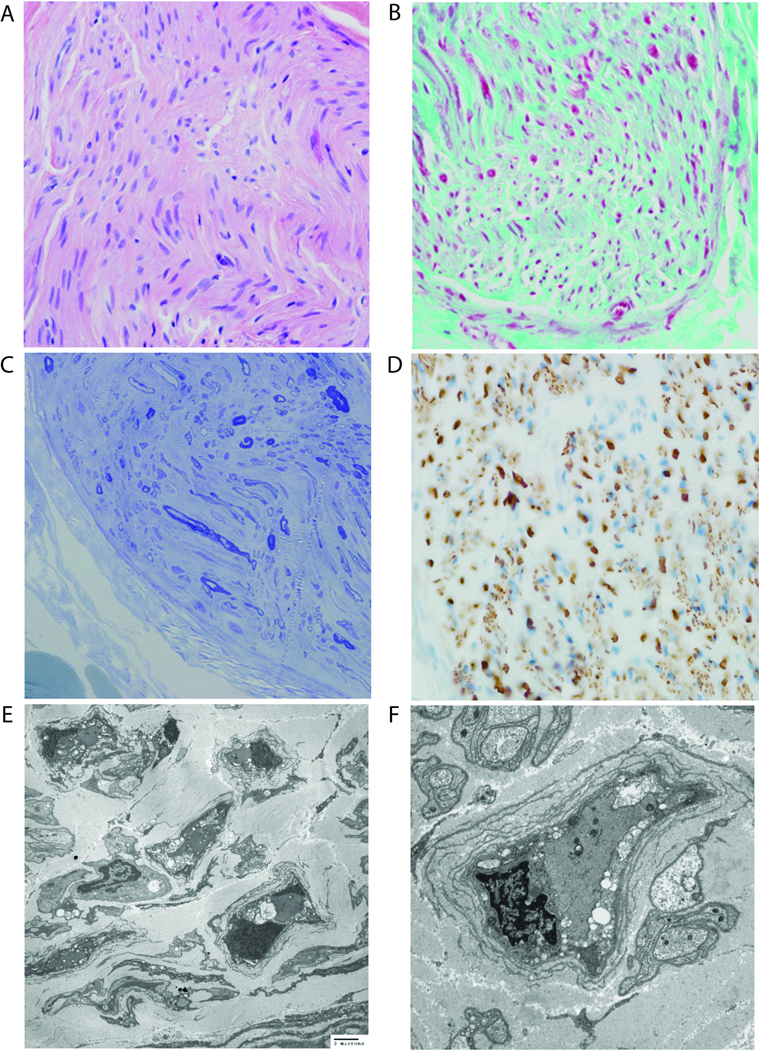
Figure 3.
Sural nerve biopsy (Case 2) stained with (A) H&E (400×), (B) trichrome (400×), and (C) toluidine blue (400×) and (D) immunohistochemistry for neurofilament protein (400×) show severe hypomyelination with intact axons. (E, F) Transmission electron microscopy reveals a dramatic reduction in the myelination of fibers and very thin myelin sheaths around infrequent fibers. Acute myelin breakdown and well-developed onion-bulb formation are focally identified.
DISCUSSION
MPZ mutations give rise to a spectrum of clinical phenotypes. Our patients, with previously unreported MPZ mutations, showed clinical symptoms characteristic of early-onset or congenital hypomyelinating neuropathy. They illustrate the clinically heterogeneity that exists even within the early-onset forms of this disease.
Neuropathies associated with MPZ mutations can be categorized within three large groups. The early-onset form typically presents in infancy with hypotonia and delayed motor milestones. Most children do not walk independently until 18 mos – 5yrs old [6]. Nerve conduction studies demonstrate severe slowing (<15 m/s) or absent responses [6]. Cerebral spinal fluid protein levels may be elevated [15]. Some patients may eventually require a wheelchair for mobilization however data pertaining to long-term outcome is limited. MPZ mutations account for a large proportion of all inherited peripheral neuropathies that present during infancy [16]. The “classic” demyelinating phenotype (CMT1B) typically presents in the first decade with gait impairment, frequent falling and/or foot deformity. The late-onset form (CMT2I, CMT2J) presents in adulthood (18 – 50yr old) [17] with clinical and electrodiagnostic evidence of an axonal polyneuropathy. Nerve biopsy reveals length-dependent, axonal loss without segmental demyelination [18]. CMT2J is also associated with pupillary abnormalities and deafness, not seen with CMT2I [17,19,20].
Recent clinical and laboratory research has provided insight into the pathogenesis of MPZ neuropathies and has aided our understanding of the clinical heterogeneity that exists. MPZ mutation may give rise to neuropathy by at least three distinct mechanisms. First, MPZ mutation may result in a protein product that completely fails to reach the plasma membrane instead accumulating within the cytoplasm or endoplasmic reticulum [3]. Such abnormal protein aggregates have been shown to attenuate protein translation, activate protein degradation and even induce apoptosis [21,22]. Second, MPZ mutation may cause impaired myelin formation where protein reachs the plasma membrane but fails to adhere normally to adjacent MPZ molecules [23]. Finally, MPZ mutation may give rise to protein that reaches the plasma membrane and adheres to adjacent molecules but fail to maintain normal Schwann cell-axon interactions required for long-term cell viability [3,6].
These two cases of congenital hypomyelinating neuropathy demonstrate the different clinical and pathological features that are seen even among early-onset forms of this disease. The first patient with the more severe congenital hypomyelinating phenotype demonstrated no visible myelin on nerve biopsy (Figure 1). This stands in contrast to the second child with the less a severe clinical phenotype and biopsy evidence of incomplete myelination, ongoing myelin breakdown and rare “onion bulb” formation (Figure 3). The discrepant biopsy findings confirm that less myelin is associated with a more severe phenotype and provide further data with which to explore the pathophysiology of neuropathies related to MPZ mutations.
ACKNOWLEDGEMENTS
The authors thank Rebecca D. Folkerth, MD for her interpretation of the nerve biopsy results in Case 2. PBK is supported by: NINDS K08 NS048180.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Roomi MW, Ishaque A, Khan NR, Eylar EH. The P0 protein. The major glycoprotein of peripheral nerve myelin. Biochim Biophys Acta. 1978;536:112–121. doi: 10.1016/0005-2795(78)90057-0. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro L, Doyle JP, Hensley P, Colman DR, Hendrickson WA. Crystal structure of the extracellular domain from P0 the major structural protein of peripheral nerve myelin. Neuron. 1996;17:435–449. doi: 10.1016/s0896-6273(00)80176-2. [DOI] [PubMed] [Google Scholar]
- 3.Grandis M, Vigo T, Passalacqua M, Jain M, Scazzola S, La Padula V, Brucal M, Benvenuto F, Nobbio L, Cadoni A, Mancardi GL, Kamholz J, Shy ME, Schenone A. Different cellular and molecular mechanisms for early and late-onset myelin protein zero mutations. Hum Molecular Genet. 2008;17:1877–1889. doi: 10.1093/hmg/ddn083. [DOI] [PubMed] [Google Scholar]
- 4. [cited 2010-Mar-19];Inherited peripheral neuropathies mutation database. [homepage on the internet]. Belgium. Available from: http://www.molgen.ua.ac.be/CMTMutations/
- 5.Shy ME, Jani A, Krajewski K, et al. Phenotypic clustering in MPZ mutations. Brain. 2004;127:371–384. doi: 10.1093/brain/awh048. [DOI] [PubMed] [Google Scholar]
- 6.Shy ME. Peripheral neuropathies caused by mutations in myelin protein zero. J Neur Sci. 2006;242:55–56. doi: 10.1016/j.jns.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Mandich P, Fossa P, Capponi S, Geroldi A, Acquaviva M, Gulli R, Ciotti P, Manganelli F, Grandis M, Bellone E. Clinical features and molecular modeling of novel MPZ mutations in demyelinating and axonal neuropathies. Eur J Hum Genet. 2009;17:1129–1134. doi: 10.1038/ejhg.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. [cited 2010-Mar-19];UCSC Biogenome Informatics. [homepage on the internet]. U.S. Available from: http://genome.ucsc.edu.
- 9. [cited 2010-Mar-19];European Bioinformatics Institute [homepage on the internet]. U.K. http://www.ebi.ac.uk.
- 10.Fabrizi GM, Cavallaro T, Morbin M, et al. Novel mutation of the P0 extracellular domain causes a Dejerine-Sottas syndrome. J Neurol Neurosurg Psychiatry. 1999;66:386–389. doi: 10.1136/jnnp.66.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warner LE, Shohat M, Shorer Z, Lupski JR. Multiple de novo MPZ (P0) point mutations in a sporadic Dejerine-Sottas case. Hum Mutat. 1997;10:21–24. doi: 10.1002/(SICI)1098-1004(1997)10:1<21::AID-HUMU3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 12.Schiavon F, Rampazzo A, Merlini L, Angelini C, Mostacciuolo ML. Mutations of the same sequence of the myelin P0 gene causing two different phenotypes. Hum Mutat. 1998 Suppl 1:S217–S219. doi: 10.1002/humu.1380110170. [DOI] [PubMed] [Google Scholar]
- 13.Planté-Bordeneuve V, Guiochon-Mantel A, Lacroix C, Lapresle J, Said G. The Roussy-Lévy family: from the original description to the gene. Ann Neurol. 1999;46:770–773. doi: 10.1002/1531-8249(199911)46:5<770::aid-ana13>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 14.Kochanski A, Drac H, Jedrzejowska H, Hausmanowa-Petrusewicz I. Focally folded myelin in Charcot-Marie-Tooth type 1B disease is associated with Asn131Lys mutation in myelin protein zero gene: short report. Eur J Neurol. 2003;10:547–549. doi: 10.1046/j.1468-1331.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 15.Tachi N, Kozuka N, Ohya K, Chiba S, Yamashita S. A small direct tandem duplication of the myelin protein zero gene in a patient with Dejerine-Sottas disease phenotype. J Neurol Sci. 1998;156:167–171. doi: 10.1016/s0022-510x(98)00028-8. [DOI] [PubMed] [Google Scholar]
- 16.Wilmshurst JM, Pollard JD, Nicholson G, Antony J, Ouvrier R. Peripheral neuropathies of infancy. Dev Med Child Neurol. 2003;45:408–414. doi: 10.1017/s0012162203000768. [DOI] [PubMed] [Google Scholar]
- 17.Chapon F, Latour P, Diraison P, Schaeffer S, Vandenberghe A. Axonal phenotype of Charcot-Marie-Tooth disease associated with a mutation in the myelin protein zero gene. J Neurol Neurosurg Psychiatry. 1999;66:779–782. doi: 10.1136/jnnp.66.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Bai Y, Ianakova E, et al. Major myelin protein gene (P0) mutation causes a novel form of axonal degeneration. J Comp Neurol. 2006;498:252–265. doi: 10.1002/cne.21051. [DOI] [PubMed] [Google Scholar]
- 19.De Jonghe P, Timmerman V, Ceuterick C, et al. The Thr124Met mutation in the peripheral myelin protein zero (MPZ) gene is associated with a clinically distinct Charcot-Marie-Tooth phenotype. Brain. 1999;122:281–290. doi: 10.1093/brain/122.2.281. [DOI] [PubMed] [Google Scholar]
- 20.Misu K, Yoshihara T, Shikama Y, et al. An axonal form of Charcot-Marie-Tooth disease showing distinctive features in association with mutations in the peripheral myelin protein zero gene (Thr124Met or Asp75Val) J Neurol Neurosurg Psychiatry. 2000;69:806–811. doi: 10.1136/jnnp.69.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pennuto M, Tinelli E, Malaguti M, Del Carrro U, D’Antonio M, Ron D, Quattrini A, Feltri M, Wrabetz L. Ablation of the UPR-mediator CHOP restores motor function and reduces demyelination in Charcot-Marie-Tooth 1B mice. Neuron. 2008;57:393–405. doi: 10.1016/j.neuron.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell. Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 23.Filbin MT, Zhang K, Li W, Gao Y. Characterization of the effect on adhesion of different mutations in myelin P0 protein. Ann NY Acad Sci. 1999;883:160–167. [PubMed] [Google Scholar]