Abstract
Staphylococcus aureus pathogenicity islands (SaPIs) are mobile elements that are induced by a helper bacteriophage to excise and replicate and to be encapsidated in phage-like particles smaller than those of the helper, leading to high-frequency transfer. SaPI mobilization is helper phage specific; only certain SaPIs can be mobilized by a particular helper phage. Staphylococcal phage 80α can mobilize every SaPI tested thus far, including SaPI1, SaPI2 and SaPIbov1. Phage 80, on the other hand, cannot mobilize SaPI1, and φ11 mobilizes only SaPIbov1. In order to better understand the relationship between SaPIs and their helper phages, the genomes of phages 80 and 80α were sequenced, compared with other staphylococcal phage genomes, and analyzed for unique features that may be involved in SaPI mobilization.
Introduction
Remarkably, most or all of the toxinosis-causing bacterial toxins are encoded by mobile genetic elements, including prophages, plasmids, transposons, and pathogenicity islands (R. P. Novick, 2003), and horizontal gene transfer plays a major role in the dissemination of these virulence determinants. In the staphylococci, temperate phages carry a variety of known virulence factors (reviewed in Christie et al., 2010). In addition, certain staphylococcal phages have been implicated in the high frequency mobilization of a family of phage-related chromosomal islands (reviewed in Novick et al., 2010). The superantigen-encoding pathogenicity islands of Staphylococcus aureus (SaPIs) are generally 15–18 kb in length and reside stably in their host chromosomes under the control of a master repressor (Ubeda et al., 2008). Following infection by particular helper bacteriophages, they are induced to excise and replicate autonomously, using a phage-like mode of replication (Ubeda et al., 2007). They are then encapsidated in phage-like particles composed entirely of phage virion proteins (Tallent et al., 2007; Tormo et al., 2008) but with smaller capsids that accommodate the SaPI genome while excluding that of the phage (Ruzin et al., 2001). These SaPI-containing particles are capable of very high transfer frequencies not only among strains of Staphylococcus aureus (Lindsay et al., 1998), but to other Staphylococcus sp. (Maiques et al., 2007) and also trans-generically to L. monocytogenes (Chen and Novick, 2009). One staphylococcal phage in particular, 80α, is capable of mobilizing a variety of SaPIs as well as enabling their transgeneric transfer. 80α was reportedly derived from one of the staphylococcal typing phages, 80, by selection for the ability to plate on strains of the NCTC 8325 lineage (R. Novick, 1967), and is in wide use as a generalized staphylococcal transducing phage. Phage 80, however, was originally reported to mobilize only one of the SaPIs thus far tested, SaPI2, which was not mobilized by 80α in initial studies (Lindsay et al., 1998).
We undertook the study of these two phages in an attempt to determine the genetic basis of their SaPI mobilization capacity, their SaPI mobilization specificities, and to clarify the origin of 80α. In this report, we present the sequences of the two phages, showing that 80α is very closely related to a different phage, staphylococcal typing phage 53, but is not closely related to 80. These results suggest that 80α is very unlikely to have originated as a mutant or restriction variant of 80, and is much more likely to represent a variant of 53, which was likely picked up as a contaminant in the initial study.
Studies with SaPIbov1 repressor mutants suggest that a primary determinant of specificity for SaPI mobilization is the ability to derepress the pathogenicity island (Ubeda et al., 2008), and recent work has identified different 80α proteins that interact specifically with the repressors of SaPIbov1, SaPI1 and SaPIbov2 (Tormo-Mas et al., 2010; M.D. Harwich, SMT, A. Shrestha, KDL, P.K. Damle, A. Poliakov, J. A. Mobley and GEC, in prep). Consistent with this model, the comparison presented here of 80 and 80α indicates that there is no correlation between the morphogenetic functions provided by the helper phage and the specificity for SaPI mobilization. The ability of both 80 and 80α to mobilize SaPI2 and SaPIbov1, however, raises some interesting questions about SaPI packaging determinants.
Results
General features of the 80α and 80 genomes
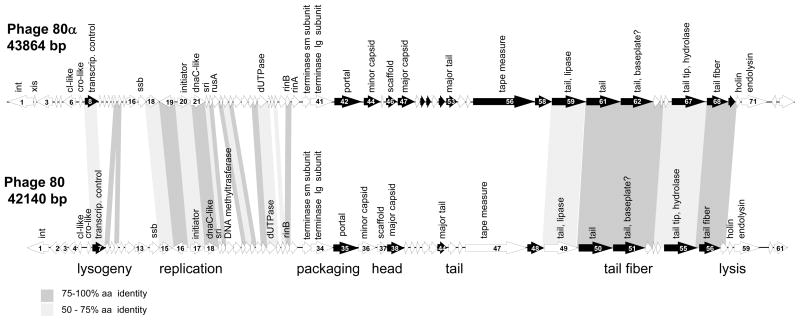
The genome length of 80α is 43,864 bp, containing the information for approximately 73 ORFs of 50 or more codons (Supplementary Table 1), and is deposited in Genbank under accession number DQ517338. The genome length of 80 is 42,140 bp, with approximately 61 ORFs (Supplementary Table 2), and is deposited in Genbank under accession number DQ908929. The assembled sequences of both phages were topologically circular, consistent with the circular permutation expected from the proposed headful packaging of these phages. The genomes are displayed in Figure 1, shown in the prophage orientation with the integrase gene at the left end. Both 80α and 80 belong to a class of related staphylococcal Siphoviridae that are highly mosaic but maintain a conserved organization of genes in functional modules (Kwan et al., 2005). While certain staphylococcal Siphoviridae encode virulence factors, such as staphylokinase, exfoliative toxin A, enterotoxin A, Panton-Valentine leukocidin, or the innate immune modulators SCIN and CHIPS (Winkler et al., 1965; Narita et al., 2001; Yamaguchi et al., 2000; Betley and Mekalanos, 1985; van Wamel et al., 2006), neither 80 nor 80α carries any known virulence factors.
Figure 1. Genomic maps of phages 80α and 80.
Predicted open reading frames of at least 50 codons are indicated. Open reading frames shown in black are those for which gene products have been identified in phage virions by mass spectrometry. Genes encoding proteins with at least 50% amino acid identity are indicated by shaded regions between the two genomes; darker shading indicates amino acid identity of at least 75%.
80α is a variant of phage 53
Although 80α was isolated as a single plaque arising during an attempt to adapt phage 80 for growth on NCTC 8325 (R. P. Novick, 1963), it now appears that typing phage 53 was the most likely source of that plaque. Nearly 90% of the 80α genome is identical to the published sequence of 53, a phage that forms plaques on NCTC 8325 and may have been a contaminant of the 80 lysate used in that experiment (see Figure S1). Most of the areas of divergence between 80α and 53 appear to be the result of recombination with φ11 and φ13, two of the three prophages in NCTC 8325 (Iandolo et al., 2002; R. Novick, 1967). The first 3000 bp of the 80α prophage would be exactly the same as the corresponding region of 53 except for a 1376 bp replacement that is identical to φ11 (Table 1). Two small blocks of sequence that are nearly identical (98%) to φ13 are found in the replication module. In the tail module, there is a 304 bp region that differs from the most recently published 53 sequence (Kwan et al., 2005; AY954952), yet its closest relative in Genbank is a different entry for 53 (Pantucek et al., 2004; AF513856). One of many plausible explanations for the differences between the two published 53 sequences is that recombination occurred between 53 and a prophage residing in the 53 propagating strain NCTC 8511. Two areas of divergence between 80α and 53 cannot be accounted for by simple recombination with known prophages in the propagating strain. A highly mosaic block from 3608 – 5271, affecting the immunity module, includes sequences unrelated to 53 or the NCTC 8325 prophages but highly conserved among other staphylococcal siphoviridae as well as a central region with similarity to both 53 and φ11, in which all but two nucleotides match one or the other genome and thus might have arisen by multiple recombination events between the two phages. The other divergent block, from 12121–12473, spans the C-terminus of ORF26 and the N-terminus of ORF27, two conserved genes of unknown function. The closest match in the database is φ11, but the sequence identity is only 91%.
Table 1.
Apparent origin of 80α genome segments
| Boundary in 80α genome | Putative Donor Phage (% identity) | ||
|---|---|---|---|
| Left | Right | ||
| 1 | 1470 | 53 | (100%) |
| 1470 | 2846 | φ11 | (100%) |
| 2846 | 3608 | 53 | (100% except 1 bp) |
| 3608 | 4295 | Unknown | closest match Mu3, Mu50 (99%) |
| 4295 | 5118 | Unknown | closest match 53 (97%); φ11 (97%); mosaic |
| 5118 | 5188 | Unknown | highly conserved in other related phages |
| 5188 | 5271 | Unknown | closest match φ69 (94%) |
| 5271 | 9399 | 53 | (100%) |
| 9399 | 9906 | φ13 | (98%) |
| 9906 | 10744 | 53 | (100%) |
| 10744 | 11357 | φ13 | (98%) |
| 11357 | 12121 | 53 | (100% except 1 bp) |
| 12121 | 12473 | Unknown | closest match φ11 (91%) |
| 12473 | 36384 | 53 | (100% except 4 bp) |
| 36384 | 36688 | 53* | (100% except 2 bp) |
| 36688 | 43864 | 53 | (100% except 1 bp) |
Does not match the whole genome 53 sequence (Accession no AY954952) used in all other comparisons. Does match a partial sequence entry for 53 (Accession no AF513856). Differences in the published sequences of 53 may have resulted from recombination between 53 and a prophage in its propagating strain.
Specificity of SaPI mobilization by helper phages
Because typing phage 53 is so closely related to 80α, we tested it for mobilization of SaPI1. We found that 53 could induce excision, replication, and transduction of SaPI1 tst::tetM at high frequency (Figure 2 and Table 2). When we attempted to confirm that 53 would mobilize SaPI1 but not SaPI2, as had been reported for 80α, we observed unexpectedly that both 80α and 53 mobilized SaPI2 tst::tetM in the RN4220 background (Table 2). Previous studies, in which 80α did not mobilize SaPI2 in its natural host strain, were likely complicated by the presence of 80α-like prophages in that strain that might have both interfered with 80α replication and assisted SaPI mobilization by 80. A comparison of mobilization by different helper phages in the RN4220 background, which has been cured of endogenous prophages, revealed that phage 80 was able to mobilize not only SaPI2, as had been originally reported, but also SaPIbov1 (Table 2). φ11 mobilized only SaPIbov1.
Figure 2. Mobilization of SaPI1 by phage 53.
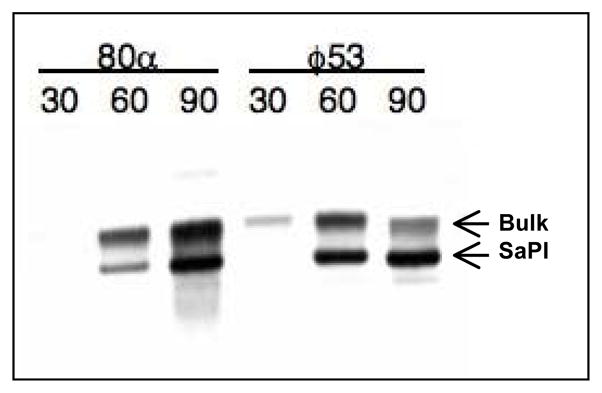
RN10822, a SaPI1-containing derivative of RN4220, was infected with either 80α or 53 as described in Materials and Methods. Standard minilysates were prepared at indicated times (minutes) after infection, separated on agarose, and probed for SaPI1 DNA by Southern blotting. The upper ‘bulk’ DNA band includes chromosomal DNA, replicating and linear phage-sized DNA, and replicating SaPI1 DNA; the lower band is SaPI1 linear monomers released from phage heads.
Table 2.
Relationships between helper phages and SaPIs.a
| SaPI::tetM | Phage | |||||||
|---|---|---|---|---|---|---|---|---|
| φ11 | 80α | 53 | 80 | |||||
| PFU | HFT | PFU | HFT | PFU | HFT | PFU | HFT | |
| SaPI1 | + | − | − | + | − | + | + | − |
| SaPI2 | + | − | − | + | − | + | Impaired | + |
| SaPIbov1 | − | + | − | + | − | + | Impaired | + |
Phages (top row) were titered by plating on indicator strain RN4220 or an RN4220 derivative (RN10822, RN10823, JP45) containing the indicated SaPI (left column). Infections of SaPI-containing strains that yielded similar titers as the control 4220 strain are marked as “+” for plaque forming units (PFU). Those that gave no plaques are marked “−” and had titers reduced by at least 105 as compared to the control strain. “Impaired” infections resulted in a 10- to 100-fold reduction in titer and smaller plaque size compared to the control strain. HFT indicates the presence of a high frequency of SaPI transducing particles in the lysate, measured by transduction of the tetM marker.
SaPI inhibition of helper phage plaque formation
Using the highly phage-sensitive, non-lysogenic strain RN4220, we confirmed that SaPIs block helper phage reproduction (Ruzin et al., 2001) and that this interference, which is sufficient to block plaque formation, is seen only with phages that induce the island. For example, φ11 does not mobilize SaPI1 and φ11 is not inhibited in its growth on a SaPI1 strain. At the same time, φ11 is a helper phage for high frequency SaPIbov1 transduction and φ11 cannot form plaques on a SaPIbov1 strain (Table 2). In SaPIbov1, gene 12 (pif) has been found to be responsible for this interference (Ubeda et al., 2009), and it is likely that non-inducing phages are not inhibited by SaPIbov1 because pif is not expressed unless the SaPI is induced.
Comparative genomics of phages 80 and 80α
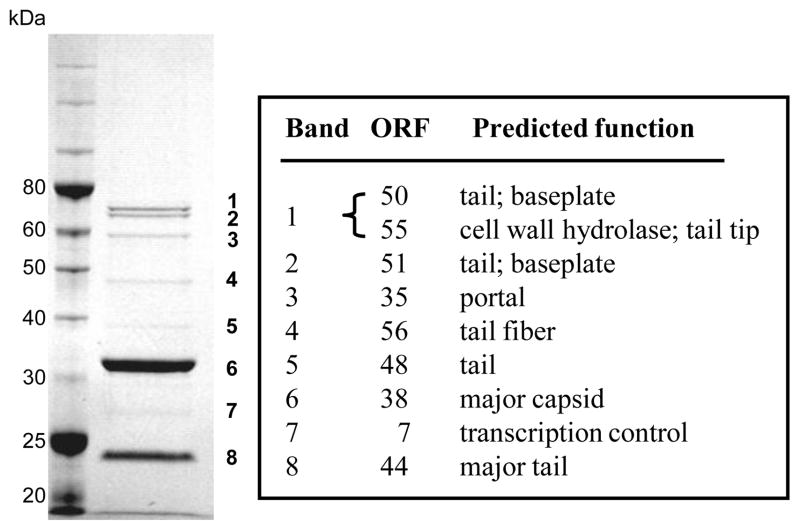
In order to better understand the biology of these staphylococcal Siphoviridae and to identify helper phage genes that may have a role in SaPI mobilization, we have analyzed the genomes of 80 and 80α and compared them with the other well-studied SaPI transducing phage, φ11(see Figure S1), as well as related staphylococcal phages. The genes and proteins described below are putative, with noted exceptions. Virion proteins of phage 80α have been identified previously (Tallent et al., 2007; Tormo et al., 2008), and are highly homologous to the virion proteins of φ11 (Tormo et al., 2008). We have also identified the most abundant proteins present in phage 80 virions, including the major capsid and tail proteins (Figure 3 and Supplementary Table 3). The confirmed virion proteins are indicated as shaded arrows in Figure 1. Despite the fact that both phages can serve as helpers for SaPI2 and SaPIbov1, and have a common genome organization similar to that of other staphylococcal siphoviridae, they share only 26 genes; 21 of these are common to φ11 as well (Table 3). Most strikingly, the capsid and major tail genes of 80, which encode essential components of SaPI transducing particles, are not homologous to those of 80α (or φ11) (Figure 1, Figure S1). Since relatively little is known about the biology of the large family of staphylococcal Siphoviridae to which these SaPI-mobilizing phages belong, an in-depth discussion of the 80 and 80α genomes is presented below.
Figure 3. Identification of 80 virion proteins.
Numbers indicate bands excised from the Coomassie blue-stained 10% SDS polyacrylamide gel; size markers are on the left. The protein(s) present in each band were identified by MS/MS and are indicated in the table.
Table 3.
Homologous genes in 80, 80α and φ11
| ORF |
AA identity |
Function | |||
|---|---|---|---|---|---|
| 80 | 80α | φ11 | 80/80α | 80α/φ11 | |
| 7 | 8 | 7 | 57% | 98% | putative transcriptional regulator |
| 9 | 13 | na1 | 83% | 86% | unknown |
| 11 | 14 | 11 | 95% | 59% | unknown |
| 15 | 18 | 14 | 61% | 60% | unknown |
| 16 | 19 | 100% | unknown | ||
| 17 | 20 | 75% | putative replisome organizer | ||
| 18 | 21 | 99% | DnaC-like protein | ||
| 19 | 22 | 53% | DnaI-binding protein; SaPI1 derepression (80α) | ||
| 20 | 23 | 17 | 98% | 95% | unknown |
| 23 | 25 | 19 | 86% | 73% | unknown |
| 24 | 26 | 21 | 65% | 91% | unknown |
| 25 | 27 | 22 | 89% | 91% | unknown |
| 27 | 31 | 23 | 85% | 96% | unknown |
| 28 | 32 | 25 | 74% | 78% | dUTPase; SaPIbov1 derepression |
| 29 | 33 | na1 | 65%* | 66%* | unknown; *similarity limited to N-term 32aa |
| 30 | 35 | 64% | unknown | ||
| 31 | 37 | 27 | 91% | 91% | RinB transcriptional regulator |
| 49 | 59 | 44 | 58% | 99% | minor tail protein |
| 50 | 61 | 45 | 90% | 97% | minor tail protein |
| 51 | 62 | 541 | 98% | 96% | minor tail protein |
| 52 | 64 | 46 | 96% | 92% | unknown |
| 53 | 65 | 47 | 98% | 91% | unknown |
| 54 | 66 | 48 | 63% | 62% | unknown |
| 55 | 67 | 49 | 58% | 98% | minor tail protein (tip?); cell wall hydrolase |
| 56 | 68 | 50 | 87% | 98% | putative tail fiber |
| 57 | 69 | 51 | 100% | 98% | minor tail protein |
na = not annotated in Genbank entry NC_004615; ORF54 described in Tormo et al., 2008.
Integration modules
Phage integration modules include the phage integrase gene (int) and the phage attachment site (attP). The excisionase gene (xis) is most often found in this module, upstream of the integrase start site, but may also be located in the switch module (Lucchini et al., 1999). The integrase catalyzes site-specific recombination between the core of attP and an identical sequence found in the bacterial chromosome (attB). The recombination reaction mediated by the integrase is driven toward excision by Xis. Xis binds specific prophage sequences adjacent to the attachment site and bends the DNA in such a way to favor the excisive recombination (Mumm et al., 2006).
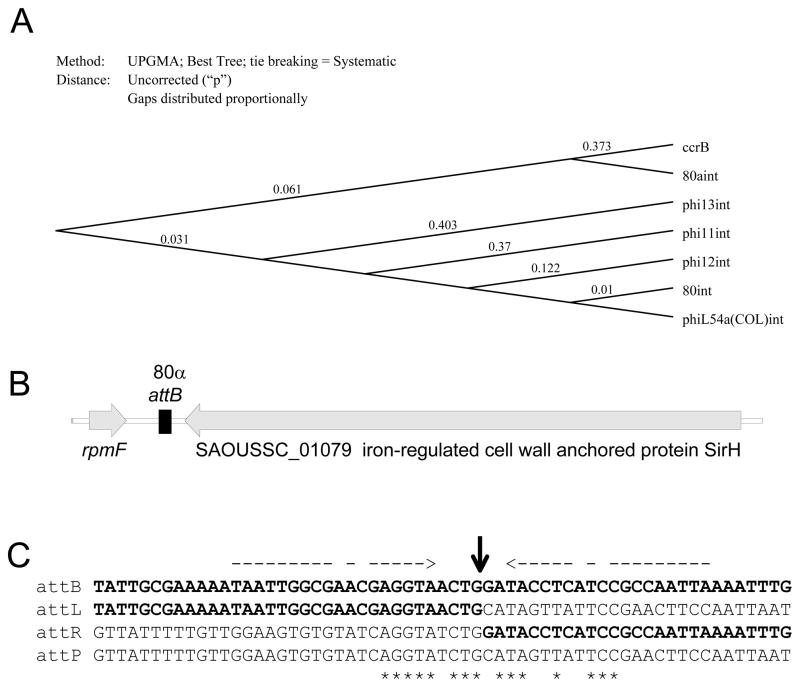
The integration modules of 80 and 80α are non-homologous. All SaPIs and the majority of the S. aureus siphoviruses sequenced thus far, including 80 and φ11, encode an integrase belonging to the tyrosine recombinase (integrase) family (see Grindley et al., 2006 for a review of integrases). The 80α integrase, on the other hand, belongs to the serine recombinase family, which also includes CcrB of staphylococcal SSC elements (see Figure 4A) as well as the integrases of the S. aureus phages 53 (AY954952), 85 (AY954953), 92 (AY954967), X2 (AY954968), 6390 (FM877489), and NM2 (AP009351).
Figure 4. 80α integrase and the 80α attachment site.
(A) 80α int is closely related to ccrB, and not to the tyrosine integrase of 80 and others commonly found in staphylococcal Siphoviridae. (B) Map illustrating the location of attB in an intergenic region between the coding regions of rmpF and sirH. (C) Sequences of the phage (attP) and bacterial (attB) attachment sites, as well as the left (attL) and right (attR) prophage junctions. Nucleotides corresponding to the bacterial sequence are shown in bold letters. Conserved nucleotides in the short, imperfect core sequence are indicated by asterisks. Broken arrows above the sequence mark the imperfect inverse repeat flanking the crossover site. The location of the crossover, which is inferred from the phage-bacterial junction revealed by alignment of the prophage att sites with attB and attP, is shown by a bold arrow.
The 80α integrase is identical to that of phage phiNM2, originally identified as a prophage in the chromosome of S. aureus substrain Newman (AP009351). The phiNM2 integration site lies in an intergenic region located between putative genes rmpF, encoding ribosomal protein L32, and sirH, encoding iron-regulated cell wall-anchored protein SirH. Based on the assumption that the 80α integrase would target an attB sequence similar or identical to that used by phiNM2, primers flanking this site and primers near the predicted ends of the 80α prophage genome were used to amplify and sequence the attL and attR prophage junctions in a lysogenic derivative of RN4220; attB and attP were sequenced as well. This analysis confirmed 80α integration between rmpF and sirH (Fig 4B), at a position corresponding to nucleotide 1,042,159 in the NCTC 8325 genome. Although the 80α attB is conserved among 18 of the S. aureus strains sequenced thus far, only S. aureus substrain Newman contains a prophage at this site. Serine recombinase family members recognize short core sequences that are often flanked by an imperfect direct repeat (Grindley et al., 2006). Consistent with this observation, the repeats flanking the 80α attP are short and imperfect, as is the conserved core of the attachment site (Figure 4C).
Directional control of the large serine recombinases is poorly understood (Groth and Calos, 2004). Excisionase genes are diverse, and the activity of xis genes has been experimentally demonstrated in only two of the staphylococcal siphoviridae, L54a and φ11, which both encode tyrosine integrases (Ye et al., 1990). The L54a family of integrases has been noted previously as an exception to the general rule that integrases and their cognate excisionases have co-evolved (Lewis and Hatfull, 2001). Our results are consistent with this exception; while 80α and 53 encode identical serine integrases, they carry different xis genes. Furthermore, both the 53 and 80α xis genes are found paired with a tyrosine integrase gene in another phage. Specifically, the 53 xis is identical to that of phage L54a, while the xis gene of 80α is also found in phage φ11. Since Xis proteins function in a primarily architectural role to bend the DNA and bring the att sites into the appropriate geometry for the excisive recombination reaction, it may not be so hard to understand how the same excisionase might function with two different integrases, especially since recognition by Xis proteins does not involve high DNA sequence specificity.
The 80 integrase, a member of the tyrosine recombinase family, belongs to integrase group Sa6, which includes phages 52A, L54a, and several others (Goerke et al., 2009) that are known to integrate into the geh gene, inactivating lipase (Ye and Lee, 1989; Bae et al., 2006). However, a comparison of phage 80 to these phages reveals a lack of sequence conservation downstream of the int genes, in a region containing the known L54a attP site. It appears that phage 80 is deleted for the att region, which is consistent with the observed inability of 80 to form a stable lysogen (unpublished data). There is also no homolog of L54a xis in the 80 genome, and there is no open reading frame that encodes an identifiable xis. Of the phages in this integrase group, four (NM4, ROSA, Sa6JH1 and Sa6JH9) carry an xis homologous to that of L54a (AAA98160; incorrectly annotated as int) (Ye and Lee, 1989). In the remainder, including prophages φtp310-2 and φCOL, an excisionase has not yet been identified. This is again consistent with the apparent lack of co-evolution of integrase and excisionase in this group of phages.
Leftward accessory regions
In all described cases of positive lysogenic conversion by a staphylococcal siphovirus, the converting gene is located either near the left end of the prophage genome between the integration module and the lysogeny module, or at the right end of the prophage genome, between the lysis module and attR or just upstream of the lysis module (Dempsey et al., 2005; Sumby and Waldor, 2003; van Wamel et al., 2006). We refer to these two regions as the leftward and rightward accessory regions. In phages 80α and 80, several different genes of unknown function are found in the leftward accessory region. There are homologues of each of these genes in the corresponding region in other staphylococcal siphoviruses, but none are shared between 80α and 80 (Figure 1). Some virulence genes in this region can be expressed in the prophage state of related phages (Sumby and Waldor, 2003); however, it is not known whether these 80 and 80α genes are similarly expressed, or whether they encode any product that might be involved in virulence.
Lysogeny modules
80 and 80α each contain a bidirectional switch region, characteristic of temperate phages, encoding a putative immunity repressor and a divergently transcribed gene that may correspond to λ cro. The 80α repressor is homologous to that of φPVL108 (BAF41155) and is predicted to be a 238 aa protein containing an N-terminal helix-turn-helix domain of the XRE family and a C-terminal RecA-mediated autopeptidase domain, consistent with SOS induction of the 80α prophage. The N-terminal domain is similar to that of the repressor of φETA (BAA97592) and prophage repressor homologs in several genomes, such as strain Mu50 (BAB58160), whose C-terminal domains do not match that of 80α. The C-terminal domain of 80α repressor is identical to the C-terminal domain found in a large number of phage repressors, including those of φ11, φ13 and 53. Thus, the 80α immunity repressor appears to be a mosaic of two independently assorting modules: a C-terminal one that confers SOS sensitivity and an N-terminal one that controls DNA binding specificity. This N-terminal domain is one of the places where 80α and its presumptive parent φ53 differ. A plasmid expressing the 80 repressor gene conferred immunity to 80α but not to φ11 (data not shown), even though it has long been known that φ11 cannot plate on an 80α lysogen. This suggests that 80α encodes a lysogenic exclusion function that blocks φ11, which remains to be identified.
The putative phage 80 repressor is a small protein of 92 aa and, like the 80α repressor, it contains a helix-turn-helix domain of the XRE family. However, it does not contain the C-terminal SOS-responsive domain. The 80 repressor is identical to the putative repressors of staphylococcal phages 52A, 85 and 96. Since phage 85 is SOS-inducible (Ubeda et al., 2007), this suggests an alternative mechanism for derepressing phages with this type of repressor. One attractive hypothesis is that these phages encode an SOS-regulated antirepressor, similar to what has been described for coliphage 186 (Shearwin et al., 1998).
The Cro-like proteins are also members of the XRE family of helix-turn-helix DNA binding proteins. The phage 80 cro-like gene is identical to the corresponding genes of 52A and 96 and nearly identical to that of phage 85. For these phages the immunity repressor, the cro-like gene and the intergenic putative operator region appear to reside in a conserved module. For 80α, the N-terminal domain of the cI-like repressor, intergenic region, and cro-like gene comprise a conserved module that can recombine with the C-terminal domain of a different repressor, as is seen in φETA.
The genes immediately downstream of the cro-like gene in 80 and 80α share a common N-terminal domain but have different C-terminal domains, a feature typical of genes located in the lysogeny modules of a variety of siphoviridae that infect low GC content Gram-positive bacteria (Lucchini et al., 1999). These genes are members of a large family of putative viral transcription regulators that contain distinct N-terminal DNA binding domains (the baculovirus Bro-N domain, in both 80 and 80α) paired with various C-terminal domains (Iyer et al., 2002). The C-terminal domain of 80α gp8 belongs to the phage P1 KilA-C family, while the C-terminal domain of 80 gp7 belongs to the ORF6C superfamily. The P1 KilA-C domain is found at the C-terminus of the P1 KilA protein, a nonessential protein in the phage P1 replication region (Hansen, 1989); it is also present at the C terminus of the P1 antirepressor Ant1 (YP_006515). This has led to the widespread annotation of genes in this large and diverse family as “putative antirepressor” despite the fact that many of these genes do not contain the KilA-C domain and the P1 antirepressor is the only member of this family with an established function (Riedel et al., 1993). Surprisingly, 80 gp7 was identified as one of the major bands present in CsCl-banded phage virions (Fig 3). The corresponding protein from 80α, gp8, was not found in the initial analyses of virion proteins (Tallent et al., 2007; Tormo et al., 2008); however, this protein did appear to be at least loosely associated with a CsCl-purified 80α procapsid fraction (Poliakov et al., 2008). Thus, it may play a dual role in regulation and virion structure, in a manner similar to that of the phage P4 Psu protein, which serves as a capsid decoration protein in addition to regulating Rho-dependent transcription termination (Dokland et al., 1993; Pani et al., 2006).
The area between the lysogeny module and the DNA replication module is highly mosaic. The functions of the assorted genes found in this region are largely unknown but they may play a role in the lysis-lysogeny decision or the ability of the phage to infect certain hosts. Most of these genes are not shared between 80 and 80α (Figure 1). The two common genes in this region (80α ORFs 13 and 14; 80 ORFs 9 and 11; see Table 3) encode proteins of unknown function that are highly conserved among staphylococcal siphoviruses. 80α ORF15 has recently been show to be required for derepression of SaPIbov2 (Tormo-Más et al., 2010). This protein contains a conserved domain of unknown function (DUF2483) and is found in several other sequenced staphylococcal siphoviruses but not phage 80.
Replication modules
The precise boundaries of staphylococcal siphovirus DNA replication modules are unknown. For present purposes, we will start at the genes predicted to encode single-stranded DNA binding proteins (80α ORF17 and 80 ORF14, respectively). These differ between 80α and 80 and represent examples of two different SSBs that are both widespread among staphylococcal siphoviridae. The putative proteins encoded immediately downstream of the ssb-like genes in each genome have divergent N-termini but highly conserved C-termini; they belong to a conserved family of proteins of unknown function (DUF968).
Phages 80 and 80α both encode a replication module of the initiator-helicase loader type, preceded by a large shared ORF of unknown function on the complementary strand. While the putative helicase loader, a homolog of DnaC, is highly conserved in these phages, the putative initiators show diversity in their N-terminal domains. Within the DNA sequence coding for the nonconserved N-terminal region of the putative initiator proteins there are clusters of direct and inverted repeats characteristic of a phage origin of replication, but their putative role has yet to be demonstrated.
Downstream of the initiator-origin region in both phages is a gene (80α ORF 22; 80 ORF 19) homologous to φ77 ORF104 (AAR87935), whose product has been shown to inhibit S. aureus DNA synthesis and to bind to the putative host helicase loader, DnaI (Liu et al., 2004). This gene is present in a number of other staphylococcal siphoviridae but is absent from the φ11 genome. It has recently been shown to be required for SaPI1 derepression by 80α and has been named sri (Tormo-Más et al., 2010; Harwich et al., unpublished); its absence from the φ11 genome likely accounts for the inability of φ11 to induce SaPI1. The protein encoded by phage 80 shares only 53% identity with that of 80α. 80 does not induce SaPI1 (see Figure 4), and the DnaI binding function of this allelic variant is unknown. The sri gene is followed by a highly conserved gene of unknown function (DUF3269). The next gene in each phage is different, but each can be assigned a tentative role based on sequence homology. 80α ORF 24 encodes a RusA-type Holliday junction resolvase while 80 ORF 21 encodes a DNA adenine methylase.
Between the replication module and the morphogenetic genes is another highly mosaic region containing assorted phage genes (Figure 1). The only gene in this region that can be assigned a function based upon sequence homology is the one encoding dUTPase. This gene product, which is shared by 80 and 80α, has been shown not only to have dUTPase activity but also to be required for derepression of SaPIbov1 (Tormo-Más et al., 2010). dUTPase is conserved among a wide variety of bacteriophages, and the gene preceding the dUTPase is highly conserved among staphylococcal siphoviruses. Upstream of these two genes, 80 and 80α share a cluster of three contiguous conserved genes of unknown function (see Table 3). Distal to dUTPase are two additional shared genes of unknown function that are also widespread in staphylococcal siphoviruses.
The only other genes in this region to be assigned a function based on published experimental evidence are the rin genes, which activate an int promoter-reporter fusion (Ye and Lee, 1993a). 80α contains both rinA and rinB, as well as a small intervening ORF, which we have labeled rinM. RinA has recently been implicated as the positive regulator of morphogenetic gene transcription in 80α and 11φ (M. Ferrer Garcia, N. Quiles, M.D. Harwich, M.A. Tormo-Mas,, GEC and J. R. Penades, in prep). Phage 80 and several other phages have only the rinB gene, without a homolog of the rinA gene. In these phages, a different conserved gene of unknown function replaces rinA (Kwan et al., 2005); this gene presumably serves as the functional equivalent of rinA for 80 late gene transcription, but this remains to be investigated.
Packaging and capsid morphogenesis modules
The organization of the packaging and capsid gene modules of both 80 and 80α is typical of that found in Gram-positive siphoviruses. The 80 and 80α terminases are nonhomologous, but both the small and large subunits belong to conserved classes of phage terminases. The portal proteins are members of a family exemplified by gp6 of B. subtilis phage SPP1, but share little amino acid similarity. Both phages also share a capsid protein gene related to SPP1 gp7; in 80α this protein (gp44) has been shown to be a minor component of the phage capsid (Poliakov et al., 2008; Tallent et al., 2007). Despite the fact that both the 80 and 80α proteins appear related to SPP1 gp7, they show little amino acid similarity to each other. In SPP1, gp7 appears to play a role in DNA exit from the virion (Vinga et al., 2006), and we predict a similar function in these staphylococcal phages. The scaffold and major capsid proteins are also nonhomologous. The 80α scaffold and capsid proteins are proteolytically processed at a conserved sequence in their N-termini (Poliakov et al., 2008); a similar sequence is found at the N-terminus of the phage 80 capsid protein but not the 80 scaffold.
Capsid morphogenesis is especially important for the production of SaPI transducing particles, since these are composed entirely of phage proteins (Tallent et al., 2007; Tormo et al., 2008). All SaPIs encode a terminase small subunit that is required for specific packaging of SaPI DNA (Ubeda et al., 2009), presumably by interacting with the large subunit of the helper phage terminase. An interesting question arising from the lack of similarity between the 80 and 80α packaging genes is how (or whether) the SaPI small terminase subunit is able to interact with the nonhomologous large terminase subunits of these two helper phages to effect SaPI-specific packaging. Additionally, most SaPIs encode functions that remodel the phage capsid to accommodate their smaller genomes, in a manner reminiscent of satellite coliphage P4 and its helper P2 (Lindqvist et al., 1993). There does not, however, appear to be a great deal of specificity with respect to the requirements for a particular capsid. The capsid morphogenesis modules of 80 and 80α differ considerably, but each phage can mobilize multiple SaPIs, including SaPI2 and SaPIbov1. Phage φ11, on the other hand, has a morphogenetic gene cluster virtually identical to that of 80α, but cannot mobilize SaPI2 or SaPI1, both of which are mobilized by 80α (see Table 2). Thus, the capsid is apparently not a key determinant of SaPI-helper phage specificity. Furthermore, although capsid size determination is a striking feature of SaPI mobilization, it appears to be nonessential. While 80 can serve as a helper for the high frequency transfer of both SaPI2 and SaPIbov1, no smaller SaPI-sized band is observed in lysates of 80-infected cells (Figure 5). This indicates that the SaPI DNA transduced by 80 must be packaged, presumably as multimers, into normal 80-sized heads. A similar observation has been described in the case of high frequency transfer by 80α of SaPIbov2, which lacks one of the genes required for small capsid formation (Maiques et al., 2007) and of SaPIbov1 carrying a mutation in the corresponding gene (Ubeda et al., 2007). What, then, is the role of the highly conserved SaPI small capsid size determination function? One attractive explanation is that it excludes the packaging of intact helper phage DNA, and therefore decreases the probability that a recipient bacterium would simultaneously be infected by the helper phage. This would be expected to increase the survival frequency of SaPI transductants.
Fig. 5. Specificity of SaPI mobilization by different helper phages.
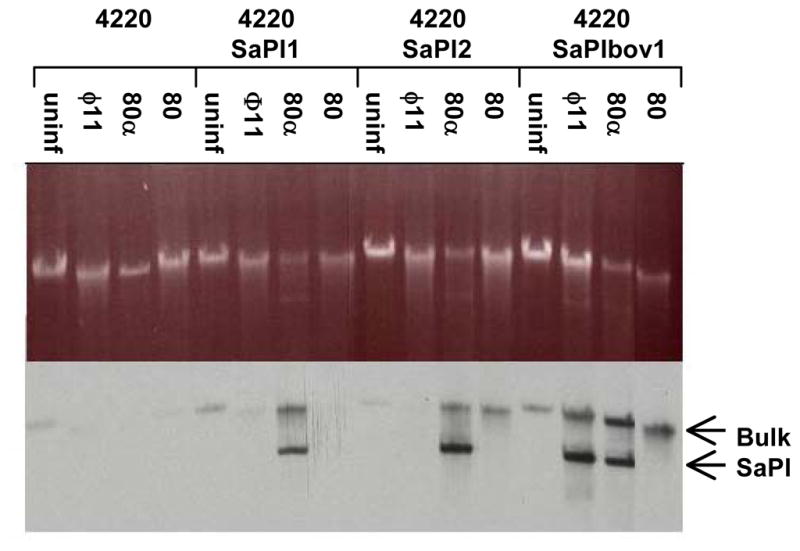
RN4220 and derivatives RN10822, RN10823 and JP45, carrying the indicated SaPIs, were infected with each of the indicated phages as described in Materials and Methods. Standard minilysates were prepared 60 minutes after infection, separated on agarose, and probed for SaPI DNA by Southern blotting with a ter probe, which is common to all three SaPIs. The upper ‘bulk’ DNA band includes chromosomal DNA, replicating and linear phage-sized DNA, and replicating SaPI1 DNA; the lower band is SaPI1 linear monomers released from phage heads.
Tail modules
Between the major capsid protein gene and the major tail protein gene are five open reading frames in each phage. Again, the predicted proteins show little amino acid similarity. One gene product from this region has been identified as a minor component in 80α virions (Tallent et al., 2007) and belongs to a family of putative head-tail connector proteins. Proteins encoded by the proximal part of the tail module also show little amino acid similarity, but the gene arrangement is highly conserved. The major tail protein gene lies upstream of the tape measure gene, and there are two intervening genes characterized by overlapping open reading frames related by a predicted programmed -1 translational frameshift. This arrangement of genes is preserved in a diverse collection of phages with both contractile and noncontractile tails (Christie et al., 2002; Levin et al., 1993; Xu et al., 2004). In both 80α and 80, clearly identifiable candidate heptanucleotide “slippery sequences” can be found in the region of overlap. 80α has the canonical 5′-GGGAAAG-3′, first identified in the bacteriophage λ G-T frameshift, which encodes a Gly Lys in both reading frames between 25268-25274. Phage 80 has a TTTTTTC at 23569-23575, encoding Phe Phe in both reading frames; this is identical to the putative frameshifting sequences identified in B. subtilis phage SPO1, S. flexneri phage SfV and B. halodurans phage f-halo1-pro (Xu et al., 2004)
The distal part of the tail gene module presumably encodes the proteins that make up the baseplate structure and the tail appendages involved in host cell attachment and penetration. Unlike the rest of the morphogenetic genes, those in this region are shared between 80α and 80 (Table 3). Further data are available for three of these genes. 80α ORF62 (80 ORF 51) encodes a minor tail protein. Like all tail proteins, it is present in both phage and SaPI transducing particles (Tallent et al., 2007; Tormo et al., 2008). However, while essential for phage plaque formation, 80α mutants lacking this gene are still capable of high frequency SaPI transfer (Tormo et al., 2008). Deletion of the homologous gene from φ11 yields phage particles lacking the knob-like complex structure at the end of the phage tail in both phage and SaPI particles (Tormo et al., 2008). Why the SaPI particles are competent for transduction is not yet understood. 80α ORF68 (80 ORF 56) encodes a protein containing a central collagen triple helix repeat motif and has tentatively been identified as a tail fiber. This gene is nonessential for phage growth or SaPI mobilization, at least in the laboratory strain RN4220 (Tormo et al., 2008). 80α ORF67 (80 ORF 55) encodes a low-abundance tail protein with an N-terminal CHAP domain and a C-terminal lysozyme domain, suggesting that this gene product has peptidoglycan hydrolytic activity. Such activity has recently been reported for a homologous protein from S. aureus phage φMR11, and this protein appears to reside at the tail tip (Rashel et al., 2008). It presumably plays a role in localized degradation of the cell wall during phage adsorption.
Lysis regions
The predicted 80 α holin and endolysin belong to a different family than those of 80. The previously published partial sequence for the 80 α lytic genes (U72397) most closely matches the sequence of the φ11 genome (AF424781) and the prophage sequence in the φ11 source strain NCTC 8325 (CP00253), rather than the 80α genomic sequence reported in this study. Presumably, propagation of phage 80α on NCTC 8325 allowed it the opportunity to recombine with φ11. Table 1 shows that the isolate of 80α used in this study, which was propagated recently on phage-free strains, also contains a DNA segment derived from φ11, but contains the lysis module of its presumptive parent phage, 53.
Comparison of the rightward accessory region
80α and 80 each have two open reading frames of unknown function downstream of the lysis module. This region is also highly mosaic. 80α ORF72 encodes a small 75 aa putative protein that is not annotated in any other genomes, but a TBLASTN search reveals this putative gene in several other staphylococcal Siphoviridae, including NM1, NM4, φCOL, ROSA, 53, Sa6JH1 and Sa6JH9. The larger open reading frame, ORF73, is also found in several other phages, including 53, 47 and φ12. Overlapping this ORF is a DNA segment of 161 nt that is also found, with 94% identity, near the lefthand attachment site of SaPI1. This shared area may be more appropriately grouped in the integration module, as it may contain binding sites for factors involved in integration and excision. However, the biological significance of this 161 nt non-coding sequenced shared by helper phage 80α and SaPI1 has not yet been determined. Phage 80 ORF60 appears to be a truncated open reading frame corresponding to the 3′ end of a homologous gene, ORF13, in the closely related phage 52A, and the downstream 80 gene, ORF61, is also homologous to the next gene in the 52A genome, ORF19. These two genes are not present in other sequenced staphylococcal phages.
Discussion
The sequencing of phages 80 and 80α accomplishes an important step in our understanding of the unique relationship between S. aureus pathogenicity islands and their helper phages. Recent evidence suggests that SaPI derepression is a key determinant of helper phage specificity (Tormo-Mas et al, 2010). The dut gene product, which is the phage-encoded antirepressor for SaPIbov1 (Tormo-Más et al, 2010), is only 74% identical between 80 and 80α We show in this study that both phages can mobilize SaPIbov1, indicating that these allelic variants presumably both have derepression activity. Derepression of SaPI1 requires the sri gene (Tormo-Más et al, 2010; Harwich et al, in prep). This gene, too, is present in both phages but the similarity is lower; the two proteins are only 53% identical. 80 does not mobilize SaPI1 and the lack of any amplification of SaPI1 signal in the bulk DNA following 80 infection (Fig 5) indicates that the 80 sri gene product does not derepress SaPI1. The sri gene is absent in φ11, which presumably accounts for the inability of this phage to mobilize SaPI1 since relief of SaPI1 repression by mutation of stl now allows SaPI1 mobilization by φ11 (Ubeda et al., 2008). We have also shown that 80 and 80α both mobilize SaPI2, but the gene required for SaPI2 derepression has not yet been identified.
DNA packaging can provide another level of discrimination in mobilization specificity. Phage φ13 does not mobilize SaPI1, even though it derepresses the pathogenicity island and is sensitive to SaPI1 interference (Lindsay et al., 1998). φ13 is not a generalized transducing phage and is presumed to utilize a cos site for packaging. Thus, the inability of φ13 to mobilize SaPI1 is likely due to an inability to recognize SaPI1 DNA for packaging. φ13 can complement φ11 for SaPI1 mobilization (data not shown), consistent with a model in which φ13 provides the derepression function while the packaging functions are provided by φ11.
A striking result of the comparison of 80 and 80α is the lack of homology between the morphogenetic gene clusters of the two phages, even though they can mobilize some of the same SaPIs. Unrelated genes include the phage terminase and the capsid gene cluster, which are the targets of the SaPI functions that redirect DNA packaging and alter capsid size determination, respectively. Thus, as discussed above, the capsid per se does not play a major role in determining SaPI-helper phage specificity. Furthermore, our results suggest that 80 is not subject to SaPI-dependent capsid size redirection. These observations raise interesting questions concerning the relationship between size determination, DNA packaging, and the SaPI-encoded phage interference function, pif. SaPIbov1 and SaPI1 carry different alleles of pif, and SaPIbov1 interferes with packaging of phage DNA while SaPI1 does not (Ubeda et al., 2009). Thus, SaPI1 must use a different mechanism to interfere with phage maturation. The allele of pif carried by SaPIbov1 is shared by SaPIbov2 and by SaPI2, all of which appear able to exploit helper phages in the absence of the ability to direct formation of small capsids. We speculate that direct interference with helper phage packaging by pif is more important for SaPIs under conditions where small capsids are not available to diminish the formation of viable phage particles. Such conditions would include not only SaPIs such as SaPIbov2, which have lost functions needed to direct small capsid formation, but also the exploitation of helper phages such as 80 which are apparently unaffected by the SaPI size determination functions. The effect of loss of capsid size determination on SaPIs that carry the SaPI1-like allele of pif has not yet been determined.
Another question raised by the lack of similarity between the 80 and 80α terminase subunits is how (or whether) the SaPI-encoded small terminase subunit is able to interact with the 80-encoded large subunit to redirect packaging specificity. While such redirection of packaging has been demonstrated in the case of 80α (Ubeda et al., 2009), a requirement for the SaPI-encoded small terminase subunit in mobilization by 80 has not been determined. It is formally possible that the two different large terminase subunits encoded by these two phages share a structural motif recognized by the SaPI small terminase (which is highly conserved among SaPIs). Alternatively, there may be a sequence in SaPI2 and SaPIbov1 that fortuitously resembles the packaging signal for the phage 80 terminase complex. A third possibility is that phage 80, as a generalized transducing phage, packages SaPI DNA nonspecifically – in this case, the SaPI pif function would be expected to play an especially critical role in ensuring preferential packaging of SaPI DNA by blocking phage DNA packaging. Studies are currently underway to address these questions and to clarify further the complex interplay between SaPIs and their helper phages that leads to the preferential high efficiency production of SaPI transducing particles.
Materials and Methods
Phage Sources, Propagation, and Preparation
The strains used in this study are summarized in Table 4. Standard methods and growth conditions were used as previously described (R. P. Novick, 1991). Strain RN450, which is considered to be free of prophages, was lysogenized by plaque-purified 80α to create strain RN10359. The phage particles produced by mitomycin-C induction of RN10359 were precipitated by ultracentrifugation, and subjected to phenol chloroform isoamyl alcohol (PCIA) DNA extraction. Phage 80 was grown lytically on strain NCTC 9789 and the resulting phage particles were prepared for DNA sequencing by polyethylene glycol precipitation, cesium chloride density gradient centrifugation, and then PCIA DNA extraction. Strain RN451 was induced with mitomycin C to produce φ11. Phage 53 was purchased from the American Type Culture Collection (Manassas, VA) and grown lytically on its propagating strain, NCTC 8511. Once it was discovered that all phages used in this study formed plaques on RN4220, a restriction defective derivative of RN450, lysogens of 80α, 53, and φ11 and SaPI tst::tetM transductants were made in this background and phage 80 was propagated lytically on RN4220 thereafter.
Table 4.
Strains used in this study.
| Strain | Description | Reference or source |
|---|---|---|
| RN450 | NCTC8325 cured of φ11, φ12, and φ13 | R. Novick, 1967 |
| RN451 | RN450 lysogenized by φ11. | R. Novick, 1967 |
| RN4220 | Restriction defective derivative of RN450 | de Azavedo et al., 1985 |
| RN8652 | RN3984 (SaPI2 tst::tetM) | Lindsay et al., 1998 |
| RN8685 | RN450 (SaPI1 tst::tetM) | Lindsay et al., 1998 |
| RN10359 | RN450 lysogenized by phage 53 | This study |
| RN10360 | RN450 lysogenized by plaque purified 80α | This study |
| RN10614 | RN4220 lysogenized by φ11 | This study |
| RN10616 | RN4220 lysogenized by 80α | Ubeda, et al., 2009 |
| RN10822 | RN4220 (SaPI1tst:tetM) | This study |
| RN10823 | RN4220 (SaPI2 tst:tetM) | This study |
| JP45 | RN4220 (SaPIbov1tst:tetM) | Maiques et al., 2006 |
| NCTC 9789 | Propagating strain for phage 80 | National Collection of Type Cultures |
| NCTC 8511 | Propagating strain for phage 53 | National Collection of Type Cultures |
Phage Genome Sequencing and Analysis
Shotgun sequencing of phage subclone libraries was supplemented by primer walking to close gaps and span low coverage regions and to minimize misassembly caused by background bacterial DNA. Both phages 80 and 80α are generalized transducing phages that occasionally encapsidate random fragments of host DNA, and about 5% of the sequenced clones contained S. aureus chromosomal DNA. Mechanically sheared phage DNA (1.5–3 kb for 80, 2–3 kb for 80α) was used to generate subclone libraries in high copy pUC18 derivatives. Libraries were sequenced to ten- and eight-fold coverage for 80 and 80α, respectively. The sequence of 80 was assembled using SeqMan II software (DNASTAR Inc, Madison, WI) and phredPhrap/Consed (University of Washington Genome Sciences Department; www.phrap.org) to confirm results. The sequence of 80α was assembled using Celera Assembler (Huson et al., 2001). ORF prediction and genome annotation were performed using MacVector software (Accelrys, San Diego, CA), Seqbuilder (DNASTAR Inc, Madison, WI) and GLIMMER (Delcher et al., 2007). The ORF prediction and gene family identifications were completed by previously described methods, including the use of hidden Markov models (HMMs) to determine ORF membership in families and superfamilies (Gill et al., 2005).
Identification of 80 structural proteins
Purified 80 virions were denatured in XT loading buffer provided by the manufacturer and proteins were resolved on a 10% Criterion XT SDS-PAGE bis-Tris polyacrylamide gel (Bio-Rad, Hercules, CA), and stained with Coomassie blue. Individual protein bands were excised, destained in 50% methanol, reduced with DTT, alkylated with iodoacetamide, digested overnight with trypsin, and analyzed by MS and MS/MS on a Thermo Electron Deca XP Plus mass spectrometer. The data were analyzed using the Sequest search algorithm (Thermo Fisher Scientific, San Jose, CA, USA; version SRF v. 2) against a phage 80 database, as well as against the NCBI non-redundant database. Scaffold (version Scaffold_3_00_02, Proteome Software Inc., Portland, OR) was used to validate MS/MS based peptide and protein identifications. Detailed methods are available upon request. Peptides found for each protein are summarized in Table S3.
Identification of the 80α attachment site
The prophage junctions were amplified and sequenced from genomic DNA isolated from strain RN10616, using primers flanking the predicted attL and attR sites. Primers SaRpmF: 5′-GACTGAATGCCCAAACTGTG-3′ and SMT178: 5-GGCTGGGAATTAATGGAAGATG-3′ (in 80α int) were used to amplify attL and primers SMT179 5′-GAGTCCTGTTTGCGAATTAGG-3′ (in 80 ORF73) and SaSirH: 5′-TTAAGTAGCATCGTTGCATTCG-3′ were used to amplify attR. Primers SaRpmF and SaSirH attB were also used to amplify and sequence attB, using RN4220 genomic DNA, and attP was amplified and sequenced from 80α virion DNA with SMT 178 and SMT 179.
SaPI Mobilization
SaPI1 tst::tetM was transduced by 80α from strain RN8685 to RN4220 and SaP2 tst::tetM was transduced by 80 from RN8652 to RN4220 to create strains RN10822 and RN10823, respectively. Strain JP45, SaPIbov1 tst::tetM in a RN4220 background, was provided by J. Penades. SaPI mobilization was analyzed by visualization of SaPI DNA and by measurement of SaPI transduction. For DNA analysis, strains were grown to mid-log phase and infected with phage at a MOI of 3. Samples (1 ml) were withdrawn at various times after infection and standard minilysates were prepared by treatment with lysostaphin and RNase followed by treatment with proteinase K and SDS, as described previously (Ruzin et al., 2001). The appearance of a discrete genome-length SaPI band was visualized by agarose gel electrophoresis and all SaPI DNA species were detected by ECL Southern blotting using a commercial kit (Amersham ECL Direct Nucleic Acid Labeling and Detection System), and probed with a peroxidase-labeled SaPI ter probe as described previously (Ubeda et al., 2008) Helper phage plaque forming units (pfu) and SaPI-tetM transducing units (tu) were quantified by titration of filter-sterilized overnight SaPI-phage lysates on RN4220 in a soft agar overlay (for phage plaques) or on plates containing 5 g/ml tetracycline (for SaPI-tetM transductants), using standard procedures (R. P. Novick, 1991).
Supplementary Material
Acknowledgments
This research was made possible thanks to the National Institutes of Health grant R01AI22159 to RPN, and NIH grant R21AI067654 and an A.D. Williams Trust and Baruch Foundation Trust Grant-In-Aid to GEC. DEK was supported in part by grant EEC02341014 through the NSF and NIH Bioinformatics and Bioengineering Summer Institute Program at VCU. AMM was supported in part by the NIH MSTP 5T32 GM07308. GEC would like to thank Dominic Esposito, Protein Expression Laboratory, SAIC-Frederick, Inc., for phage 80 DNA sequencing. Mass spectrometry was performed by Dr. Kristina Nelson at the VCU Mass Spectrometry Resource Center with funding from the VCU College of Humanities and Science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bae T, Baba T, Hiramatsu K, Schneewind O. Prophages of Staphylococcus aureus Newman and their contribution to virulence. Mol microbiol. 2006;62:1035–1047. doi: 10.1111/j.1365-2958.2006.05441.x. [DOI] [PubMed] [Google Scholar]
- Betley MJ, Mekalanos JJ. Staphylococcal enterotoxin A is encoded by phage. Science. 1985;229:185–187. doi: 10.1126/science.3160112. [DOI] [PubMed] [Google Scholar]
- Chen J, Novick RP. Phage-mediated intergeneric transfer of toxin genes. Science. 2009;323:139–141. doi: 10.1126/science.1164783. [DOI] [PubMed] [Google Scholar]
- Christie GE, Kropinski AM, Kuzio J, Łoś M, McConnell MR, Węgrzyn G. Lysogenic conversion in bacteria of importance to the food industry. In: Sabour MP, Griffiths M, editors. Bacteriophage in the Detection and Control of Foodborne Pathogens. ASM Press; Washington, D.C: 2010. pp. 157–198. [Google Scholar]
- Christie GE, Temple LM, Bartlett BA, Goodwin TS. Programmed translational frameshift in the bacteriophage P2 FETUD tail gene operon. J bacteriol. 2002;184:6522–6531. doi: 10.1128/JB.184.23.6522-6531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Azavedo JC, Foster TJ, Hartigan PJ, Arbuthnott JP, O’Reilly M, Kreiswirth BN, et al. Expression of the cloned toxic shock syndrome toxin 1 gene (tst) in vivo with a rabbit uterine model. Infect immun. 1985;50:304–309. doi: 10.1128/iai.50.1.304-309.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcher AL, Bratke KA, Powers EC, Salzberg SL. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics. 2007;23:673–679. doi: 10.1093/bioinformatics/btm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey RM, Carroll D, Kong H, Higgins L, Keane CT, Coleman DC. Sau42I, a BcgI-like restriction-modification system encoded by the staphylococcus aureus quadruple-converting phage Phi42. Microbiology. 2005;151:1301–1311. doi: 10.1099/mic.0.27646-0. [DOI] [PubMed] [Google Scholar]
- Dokland T, Isaksen ML, Fuller SD, Lindqvist BH. Capsid localization of the bacteriophage P4 psu protein. Virology. 1993;194:682–687. doi: 10.1006/viro.1993.1308. [DOI] [PubMed] [Google Scholar]
- Gill SR, Fouts DE, Archer GL, Mongodin EF, Deboy RT, et al. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol. 2005;187:2426–2438. doi: 10.1128/JB.187.7.2426-2438.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerke C, Pantucek R, Holtfreter S, Schulte B, Zink M, Grumann D, et al. Diversity of prophages in dominant staphylococcus aureus clonal lineages. J bacteriol. 2009;191:3462–3468. doi: 10.1128/JB.01804-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindley ND, Whiteson KL, Rice PA. Mechanisms of site-specific recombination. Annu rev biochem. 2006;75:567–605. doi: 10.1146/annurev.biochem.73.011303.073908. [DOI] [PubMed] [Google Scholar]
- Groth AC, Calos MP. Phage integrases: Biology and applications. J mol biol. 2004;335:667–678. doi: 10.1016/j.jmb.2003.09.082. [DOI] [PubMed] [Google Scholar]
- Hansen EB. Structure and regulation of the lytic replicon of phage P1. J mol biol. 1989;207:135–149. doi: 10.1016/0022-2836(89)90445-2. [DOI] [PubMed] [Google Scholar]
- Huson DH, Reinert K, Kravitz SA, Remington KA, Delcher AL, et al. Design of a compartmentalized shotgun assembler for the human genome. Bioinformatics. 2001;17(Suppl 1):S132–139. doi: 10.1093/bioinformatics/17.suppl_1.s132. [DOI] [PubMed] [Google Scholar]
- Iandolo JJ, Worrell V, Groicher KH, Qian Y, Tian R, Kenton S, et al. Comparative analysis of the genomes of the temperate bacteriophages phi 11, phi 12 and phi 13 of staphylococcus aureus 8325. Gene. 2002;289:109–118. doi: 10.1016/s0378-1119(02)00481-x. [DOI] [PubMed] [Google Scholar]
- Iyer LM, Koonin EV, Aravind L. Extensive domain shuffling in transcription regulators of DNA viruses and implications for the origin of fungal APSES transcription factors. Genome biol. 2002;3:RESEARCH0012. doi: 10.1186/gb-2002-3-3-research0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan T, Liu J, DuBow M, Gros P, Pelletier J. The complete genomes and proteomes of 27 staphylococcus aureus bacteriophages. Proc natl acad sci USA. 2005;102:5174–5179. doi: 10.1073/pnas.0501140102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ME, Hendrix RW, Casjens SR. A programmed translational frameshift is required for the synthesis of a bacteriophage lambda tail assembly protein. J mol biol. 1993;234:124–139. doi: 10.1006/jmbi.1993.1568. [DOI] [PubMed] [Google Scholar]
- Lewis JA, Hatfull GF. Control of directionality in integrase-mediated recombination: Examination of recombination directionality factors (RDFs) including xis and cox proteins. Nucleic acids res. 2001;29:2205–2216. doi: 10.1093/nar/29.11.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay JA, Ruzin A, Ross HF, Kurepina N, Novick RP. The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in staphylococcus aureus. Mol microbiol. 1998;29:527–543. doi: 10.1046/j.1365-2958.1998.00947.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Dehbi M, Moeck G, Arhin F, Bauda P, Bergeron D, et al. Antimicrobial drug discovery through bacteriophage genomics. Nat biotechnol. 2004;22:185–191. doi: 10.1038/nbt932. [DOI] [PubMed] [Google Scholar]
- Lucchini S, Desiere F, Brussow H. Similarly organized lysogeny modules in temperate siphoviridae from low GC content gram-positive bacteria. Virology. 1999;263:427–435. doi: 10.1006/viro.1999.9959. [DOI] [PubMed] [Google Scholar]
- Maiques E, Ubeda C, Campoy S, Salvador N, Lasa I, Novick RP, et al. Beta-lactam antibiotics induce the SOS response and horizontal transfer of virulence factors in staphylococcus aureus. J bacteriol. 2006;188:2726–2729. doi: 10.1128/JB.188.7.2726-2729.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiques E, Ubeda C, Tormo MA, Ferrer MD, Lasa I, Novick RP, et al. Role of staphylococcal phage and SaPI integrase in intra- and interspecies SaPI transfer. J bacteriol. 2007;189:5608–5616. doi: 10.1128/JB.00619-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm JP, Landy A, Gelles J. Viewing single lambda site-specific recombination events from start to finish. EMBO J. 2006;25:4586–4595. doi: 10.1038/sj.emboj.7601325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita S, Kaneko J, Chiba J, Piemont Y, Jarraud S, Etienne J, et al. Phage conversion of panton-valentine leukocidin in staphylococcus aureus: Molecular analysis of a PVL-converting phage, phiSLT. Gene. 2001;268:195–206. doi: 10.1016/s0378-1119(01)00390-0. [DOI] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in staphylococcus aureus. Virology. 1967;33:155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Novick RP. Analysis by transduction of mutations affecting penicillinase formation in staphylococcus aureus. J gen microbiol. 1963;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- Novick RP. Genetic systems in staphylococci. Methods enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- Novick RP. Mobile genetic elements and bacterial toxinoses: The superantigen-encoding pathogenicity islands of staphylococcus aureus. Plasmid. 2003;49:93–105. doi: 10.1016/s0147-619x(02)00157-9. [DOI] [PubMed] [Google Scholar]
- Novick RP, Christie GE, Penadés JR. The phage-related chromosomal islands of Gram-positive bacteria. Nat Rev Microbiol. 2010;8:541–551. doi: 10.1038/nrmicro2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani B, Banerjee S, Chalissery J, Muralimohan A, Loganathan RM, Suganthan RB, et al. Mechanism of inhibition of rho-dependent transcription termination by bacteriophage P4 protein psu. J biol chem. 2006;281:26491–26500. doi: 10.1074/jbc.M603982200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantucek R, Doskar J, Ruzickova V, Kasparek P, Oracova E, Kvardova V, et al. Identification of bacteriophage types and their carriage in staphylococcus aureus. Arch virol. 2004;149:1689–1703. doi: 10.1007/s00705-004-0335-6. [DOI] [PubMed] [Google Scholar]
- Poliakov A, Chang JR, Spilman MS, Damle PK, Christie GE, Mobley J, et al. Capsid size determination by staphylococcus aureus pathogenicity island SaPI1 involves specific incorporation of SaPI1 proteins into procapsids. Journal of molecular biology. 2008;380:465–475. doi: 10.1016/j.jmb.2008.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashel M, Uchiyama J, Takemura I, Hoshiba H, Ujihara T, Takatsuji H, et al. Tail-associated structural protein gp61 of staphylococcus aureus phage phi MR11 has bifunctional lytic activity. FEMS microbiol lett. 2008;284:9–16. doi: 10.1111/j.1574-6968.2008.01152.x. [DOI] [PubMed] [Google Scholar]
- Riedel HD, Heinrich J, Heisig A, Choli T, Schuster H. The antirepressor of phage P1. isolation and interaction with the C1 repressor of P1 and P7. FEBS lett. 1993;334:165–169. doi: 10.1016/0014-5793(93)81705-5. [DOI] [PubMed] [Google Scholar]
- Ruzin A, Lindsay J, Novick RP. Molecular genetics of SaPI1--a mobile pathogenicity island in staphylococcus aureus. Mol microbiol. 2001;41:365–377. doi: 10.1046/j.1365-2958.2001.02488.x. [DOI] [PubMed] [Google Scholar]
- Shearwin KE, Brumby AM, Egan JB. The tum protein of coliphage 186 is an antirepressor. J biol chem. 1998;273:5708–5715. doi: 10.1074/jbc.273.10.5708. [DOI] [PubMed] [Google Scholar]
- Sumby P, Waldor MK. Transcription of the toxin genes present within the staphylococcal phage phiSa3ms is intimately linked with the phage’s life cycle. J bacteriol. 2003;185:6841–6851. doi: 10.1128/JB.185.23.6841-6851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallent SM, Langston TB, Moran RG, Christie GE. Transducing particles of staphylococcus aureus pathogenicity island SaPI1 are comprised of helper phage-encoded proteins. J bacteriol. 2007;189:7520–7524. doi: 10.1128/JB.00738-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo MA, Ferrer MD, Maiques E, Ubeda C, Selva L, Lasa I, et al. Staphylococcus aureus pathogenicity island DNA is packaged in particles composed of phage proteins. J bacteriol. 2008;190:2434–2440. doi: 10.1128/JB.01349-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo-Más MÁ, Mir I, Shrestha A, Tallent SM, Campoy S, Lasa Í, Barbé J, Novick RP, Christie GE, Penadés JR. Moonlighting phage proteins de-repress staphylococcal pathogenicity islands. Nature. 2010;465:779–782. doi: 10.1038/nature09065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda C, Barry P, Penades JR, Novick RP. A pathogenicity island replicon in staphylococcus aureus replicates as an unstable plasmid. Proc natl acad sci USA. 2007;104:14182–14188. doi: 10.1073/pnas.0705994104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda C, Maiques E, Barry P, Matthews A, Tormo MA, Lasa I, et al. SaPI mutations affecting replication and transfer and enabling autonomous replication in the absence of helper phage. Mol microbiol. 2008;67:493–503. doi: 10.1111/j.1365-2958.2007.06027.x. [DOI] [PubMed] [Google Scholar]
- Ubeda C, Maiques E, Tormo M, Campoy S, Lasa I, Barbe J, et al. SaPI operon I is required for SaPI packaging and is controlled by LexA. Mol microbiol. 2007;65:41–50. doi: 10.1111/j.1365-2958.2007.05758.x. [DOI] [PubMed] [Google Scholar]
- Ubeda C, Olivarez NP, Barry P, Wang H, Kong X, Matthews A, et al. Specificity of staphylococcal phage and SaPI DNA packaging as revealed by integrase and terminase mutations. Mol microbiol. 2009;72:98–108. doi: 10.1111/j.1365-2958.2009.06634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wamel WJ, Rooijakkers SH, Ruyken M, van Kessel KP, van Strijp JA. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of staphylococcus aureus are located on beta-hemolysin-converting bacteriophages. J bacteriol. 2006;188:1310–1315. doi: 10.1128/JB.188.4.1310-1315.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinga I, Droge A, Stiege AC, Lurz R, Santos MA, Daugelavicius R, et al. The minor capsid protein gp7 of bacteriophage SPP1 is required for efficient infection of bacillus subtilis. Mol microbiol. 2006;61:1609–1621. doi: 10.1111/j.1365-2958.2006.05327.x. [DOI] [PubMed] [Google Scholar]
- Winkler KC, de Waart J, Grootsen C. Lysogenic conversion of staphylococci to loss of beta-toxin. J gen microbiol. 1965;39:321–333. doi: 10.1099/00221287-39-3-321. [DOI] [PubMed] [Google Scholar]
- Xu J, Hendrix RW, Duda RL. Conserved translational frameshift in dsDNA bacteriophage tail assembly genes. Mol cell. 2004;16:11–21. doi: 10.1016/j.molcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Hayashi T, Takami H, Nakasone K, Ohnishi M, Nakayama K, et al. Phage conversion of exfoliative toxin A production in staphylococcus aureus. Mol microbiol. 2000;38:694–705. doi: 10.1046/j.1365-2958.2000.02169.x. [DOI] [PubMed] [Google Scholar]
- Ye ZH, Buranen SL, Lee CY. Sequence analysis and comparison of int and xis genes from staphylococcal bacteriophages L54a and phi 11. J bacteriol. 1990;172:2568–2575. doi: 10.1128/jb.172.5.2568-2575.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZH, Lee CY. Nucleotide sequence and genetic characterization of staphylococcal bacteriophage L54a int and xis genes. J bacteriol. 1989;171:4146–4153. doi: 10.1128/jb.171.8.4146-4153.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.