Abstract
Human papillomaviruses (HPV) are common sexually transmitted pathogens that in women predispose them to cervical and other anogenital cancers. HPV vaccines can prevent infection by some but not other sexually transmitted HPVs, but are too costly for use in much of the world at greatest risk to HPV-associated cancers. Microbicides provide an inexpensive alternative to vaccines. In a high throughput screen, drugs that inhibit the cellular protein complex known as gamma secretase were identified as potential HPV microbicides. Gamma secretase inhibitors (GSIs) inhibited the infectivity of HPV pseudoviruses both in human keratinocytes and in mouse cells, with IC50s in the picomolar to nanomolar range. Using a mouse model, we observed that a GSI could inhibit HPV infection to the same degree as its effectiveness in inhibiting gamma secretase activity in vivo. We conclude that gamma secretase activity is required for HPV infection, and that GSIs are effective microbicides against anogenital HPVs.
Keywords: human papillomavirus (HPV), gamma secretase inhibitor, gamma secretase, infection, microbicide
Introduction
Papillomaviruses (PV) are a diverse group of small, nonenveloped double stranded DNA tumor viruses that infect the skin and mucosal tissues and cause benign lesions called papillomas or warts in a wide variety of animals, such as rabbit, bovine, and human. An etiological association of human papillomarviruses (HPVs) with cervical cancer was first identified in the laboratory of Dr. Harald zur Hausen (Durst et al., 1983). A subset of about a dozen sexually-transmitted HPV genotypes, so-called 'high risk' HPVs, collectively cause nearly all cases of cervical cancer. A single genotype, HPV16, causes approximately half of all cervical cancers, as well as a substantial fraction of other anogenital cancers and head-and-neck cancers (zur Hausen, 2009) (Smith et al., 2007). The different, relatively non-carcinogenic pair of HPV genotypes, HPV6 and HPV11, cause genital warts, known as condylomata acuminata (Lacey et al., 2006). Although approximately 75% of sexually active adults become infected with one or more anogenital HPV types (Koutsky, 1997), most HPV infections are transient and asymptomatic, and about 90% of HPV infected women become HPV DNA negative within two years (Ho et al., 1998). However, a minority of high risk HPV-infected individuals develop persistent HPV infection that can lead to the development of cervical cancer, other anogenital cancers, and a subset of head and neck cancers.
Two highly effective prophylactic HPV vaccines, Cervarix and Gardasil, are currently available. These vaccines prevent infection by HPV genotypes 16 and 18, and. in the case of Gardasil, also by HPVs 6 and 11 (Garland et al., 2007; Paavonen et al., 2007). One drawback to these vaccines is that they do not protect against the full range of cancer-causing HPV serotypes. The vaccines are also relatively expensive, which limits their availability in developing countries wherein there is the highest risk of developing cervical cancer because of inadequate screening using the PAP smear. Thus, the development of inexpensive and broad-spectrum topical microbicides active against sexually-transmitted HPVs could provide additional protection against HPV serotypes not covered by the vaccines and serve as useful, inexpensive adjuncts to vaccination programs.
Results
Gamma Secretase inhibitors block papillomavirus infection in a dose dependent manner
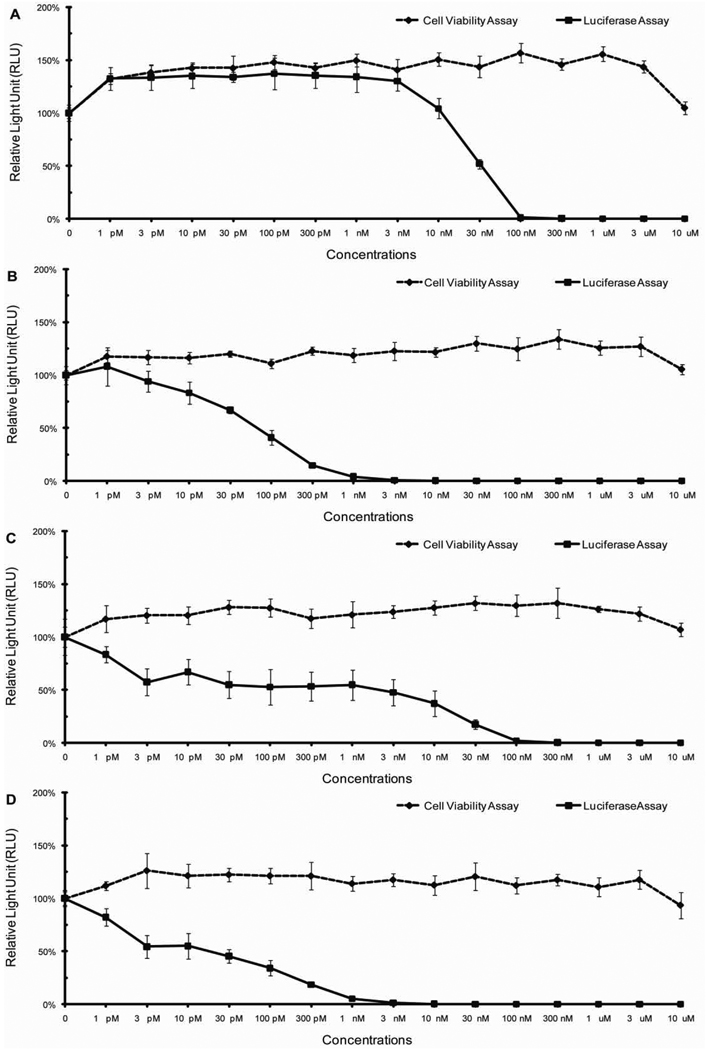
In a directed, HPV16 reporter pseudovirus-based screen of various commercially-available drugs, we discovered that inhibitors of the cellular protein complex known as gamma secretase efficiently blocked the infectivity of the pseudovirions at non-cytotoxic doses. In secondary screens, we confirmed the ability of two gamma secretase inhibitors, numbers IX and X to inhibit HPV infection in immortalized human keratinocytes (HaCat cells), with IC50s in the picomloar to nanomolar range (Figure 1A, 1B). Similar results were observed when HPV16-GFP pseudovirions matured under neutral buffered conditions were tested against gamma secretase inhibitor X (data not shown). To test whether the inhibitory effects of GSI-IX and GSI-X are HPV genotype or human cell type-specific, we repeated the luciferase and cell viability assays in mouse keratinocytes C127 cells with HPV16:LucF pseudovirus (Fig. 1C, 1D), and in HaCat cells with HPV11:LucF or HPV31:LucF pseudoviruses (Fig. 2A, 2B)(Fig. 3A, 3B). Regardless of the cell or virus types evaluated, the IC50s of gamma secretase inhibitors IX and X in blocking HPV infection were consistently in the picomolar to nanomolar range, respectively. We also conducted a focal transformation assay using mouse C127 cells and native bovine papillomavirus type 1 (BPV1) virions isolated from bovine warts to confirm the capacity of gamma secretase inhibitors to block infection by naturally sourced papillomavirus (Fig. 4). These data indicate that GSI-IX and GSI-X function as potential microbicides for a wide range of different papillomavirus species.
Fig. 1. Cell cytotoxicity (cell viability assays) and infectivity of HPV16 pseudovirus (luciferase assays) in cells treated with gamma secretase inhibitors.
Human keratinocytes, HaCat cells, were treated with serial dilutions of (A) GSI-IX or (B) GSI-X 4 hours prior HPV16 pseudovirus exposure. Mouse C127 cells were treated with serial dilutions of (C) GSI-IX or (D) GSI-X 4 hours prior HPV16 pseudovirus exposure. Bioluminescent signals of cells with mock treatment (HPV16 pseudovirus only) were set as 100% respectively. N = 6.
Fig. 2. Cell cytotoxicity (cell viability assays) and infectivity of HPV11 and HPV31 pseudovirus (luciferase assays) in HaCat cells treated with gamma secretase inhibitor IX.
HaCat cells, were treated with serial dilutions of GSI-IX 4 hours prior (A) HPV11 pseudovirus or (B) HPV31 pseudovirus exposure. N = 6.
Fig. 3. Cell cytotoxicity (cell viability assays) and infectivity of HPV11 and HPV31 pseudovirus (luciferase assaya) in HaCat cells treated with gamma secretase inhibitor X.
HaCat cells, were treated with serial dilutions of GSI-X 4 hours prior (A) HPV11 pseudovirus or (B) HPV31 pseudovirus exposure. N = 6.
Fig. 4. Infectivity of native bovine papillomavirus type 1 (BPV1) in mouse C127 cells treated with gamma secretase inhibitors.
Mouse C127 cells were treated with serial dilutions of (A) gamma secretase inhibitor IX or (B) gamma secretase inhibitor X 4 hours prior native bovine papillomavirus type 1 exposure. 24 hours after BPV1 exposure, the cells were washed to remove unbound BPV1 and gamma secretase inhibitors. 2 ~ 3 weeks later, infectivity was determined by the efficiency of focal transformations. Foci of mock treatment (BPV-1 only) was set as 100%.
Gamma secretase inhibitors block the infectivity of cell-bound virions
Previous studies have shown that papillomavirus infection of cultured cells is an unusually protracted process compared to the infection kinetics of many other viral families (Buck et al., 2006), reviewed in (Day and Schiller, 2009). Thus, drugs that target the papillomavirus infectious entry process may have a window of many hours during which to act. To assess at what stage of infection gamma secretase inhibitors acted to inhibit papillomavirus infection, we performed time of addition studies. We observed that, even at 12 hours post pseudovirus exposure, GSI-X treatment resulted in nearly complete blocking of the infectivity of the pseudovirus inoculum (Fig. 5). However, infection was inefficiently blocked when drug was added at later time points. These results suggest that gamma secretase activity is required for a relatively late step during the papillomavirus infectious entry process.
Fig. 5. Infectivity of HPV16 pseudovirus in HaCat cells treated with gamma secretase inhibitor X at different time points (time course luciferase assays).
HaCat cells were treated with 1 nM GSI-X at the time relative to HPV16 pseudovirus infection, which was set as zero hour. For the last three conditions, cells were washed to remove GSI-X at indicated time points. 48 hours post infection, infectivity was determining by performing luciferase assays. The X axis represents the time at which HaCat cells were treated with GSI-X. Each condition was normalized with individual internal controls (same condition without DMSO or GSI-X), and bioluminescent signal of cells with mock treatment (DMSO only) was set as 100%. N = 12.
Gamma secretase inhibitors reduce HPV infection significantly in mouse female reproductive tract
The ultimate goal of our studies is to identify potential HPV microbicides as an economical adjunct to HPV vaccines for preventing sexual transmission of HPVs. To this end, we tested whether topical treatment of GSI-IX and GSI-X inhibit the infectivity of HPV pseudoviruses in the female murine reproductive tract, using a modified version of the in vivo infection assay recently developed by the laboratory of Dr. John Schiller (Roberts et al., 2007). This mouse model provides a novel mean to recapitulate the initial phases of papillomavirus infection in cervicovaginal epithelia. Specifically it allows one to monitor binding, entry and delivery of encapsidated DNA to the nucleus, which represent earliest steps in the natural infectious life cycle. The efficiency of process is greatly increased by mechanical or chemical disruption of the cervicovaginal epithelium. In the case of our studies, we used pretreatment with nanoxynol-9, a detergent and spermicidal agent. HPV16:LucF was instilled into the female genital tract in the presence or absence gamma secretase inhibitors. Tissue lysates of reproductive tracts were harvested for luciferase assays after an infection period of two days. We observed an approximately 50% reduction in luciferase activity in mice that were administered GSI-X prior to HPV16 pseudovirion inoculation (Fig. 6). Given the modest reduction in HPV pseudovirus infection observed in vivo upon topical treatment with GSI-IX (data not shown) and GSI-X, we pursued additional studies in which we examined the effectiveness of GSI-IX as a microbicide when delivered systemically (due to toxicity, systemic delivery of GSI-X is not possible). Using a fluorogenic substrate, we found that gamma secretase activity was reduced by 50% in the presence of sera from mice treated systemically with GSI-IX (data not shown), consistent with prior studies (Comery et al., 2005). There was likewise a 50% reduction in HPV16 pseudovirus infectivity in mice systemically treated with GSI-IX (Fig 7). The results support the hypothesis that inhibition of gamma secretase inhibits HPV infection.
Fig. 6. Infectivity of HPV16 pseudovirus in mouse reproductive tracts treated with gamma secretase inhibitor X topically.
DMSO or GSI-10 was delivered with or without HPV16 pseudoviruses in mouse reproductive tracts. The time point for exposure to HPV16 pseudoviruses was set as zero hour. 48 hours later, mouse female reproductive tracts were harvested, protein lysates made, and luciferase assays were carried out to quantify the infectivity. Tissue lysates from mice receiving DMSO with or without HPV16 pseudovirus was used as positive and negative controls respectively. The infectivity level (luciferase assay) of positive control was set as 100%. N = 7, * / ** P < 0.05 (Wilcoxon rank sum test, 2 sided).
Fig. 7. Infectivity of HPV16 pseudovirus in mouse reproductive tracts treated with gamma secretase inhibitor IX systemically.
The time point for exposure to HPV16 pseudoviruses was set as zero hour. At -4 and zero hours mice were fed with DMSO or GSI-IX. 48 hours later, mouse female reproductive tracts were harvested, protein lysates made, and luciferase assays were carried out to quantify the infectivity. Tissue lysates from mice receiving DMSO with or without HPV16 pseudovirus was used as positive and negative controls respectively. The infectivity level (luciferase assay) of positive control was set as 100%. N = 6, * / ** P = 0.07 (Wilcoxon rank sum test, 2 sided).
Discussion
Our data indicate that gamma secretase inhibitors IX and X inhibit the infectivity of papillomaviruses. In tissue culture, their IC50s are amongst the best of any HPV microbicide identified to date, and their inhibitory effects are active against a range of papillomavirus species. GSI-IX inhibited HPV pseudovirus infection of the female reproductive tract in live mice to the same extent as its effectiveness in inhibiting gamma secretase activity in vivo. These studies indicate that gamma secretase activity is essential for HPV infection and the inhibitors of gamma secretase may be effective microbicides for HPV. Our findings are corroborated by a coincident study performed by the lab of Richard Roden that is in press.
Since these gamma secretase inhibitors can inhibit infectivity for up to 12 hours after pseudovirion exposure in vitro, it seems likely that the gamma secretase inhibitors inhibit a relatively late step in the infectious entry process. Gamma secretase catalyzes the proteolytic cleavage of the transmembrane domains of a variety of protein substrates. Thus, one hypothesis would be that gamma secretase’s proteolytic function is required for cleavage of a cellular protein involved in infectious entry. Another possibility would be that gamma secretase must cleave one of the viral capsid proteins after their insertion into a cellular membrane. To examine this latter hypothesis, we performed a series of experiments in which pseudovirions were applied to cells for varying amounts of time in the presence or absence of gamma secretase inhibitors and hypothetical cleavage of the capsid proteins was monitored by Western blotting. In these experiments, no gamma secretase-specific cleavage of L1 or L2 was observed (unpublished result). However, it remains conceivable that too small fraction of either protein is cleaved by gamma secretase to be detected by Western blotting. Further study is needed to define the relevant target(s) of gamma secretase that are critical for efficient HPV infection.
Although gamma secretase inhibitors are exceptionally potent for inhibiting papillomavirus infectivity in vitro, it appears that their use as topically-applied microbicides in vivo may be limited by their bioavailability in the genital tract. The result highlights the importance of validating candidate microbicides in live animal models, such as the modified mouse challenge model reported here.
Material and methods
Cell cultures
293FT cells (human kidney 293 cells expressing SV40 large T antigen) (Invitrogen) and HeLa cells were maintained in DMEM medium (high glucose) (Invitrogen) containing 10% fetal bovine serum (Harlan) supplemented with 0.1 mM MEM non-essential amino acids (Invitrogen), 1 mM sodium pyruvate (Invitrogen), 2 mM L-glutamine (Invitrogen) and 500 µg/mL Geneticin. HaCat cells (spontaneously immortalized human keratinocytes) were cultured in F12/DMEM (1:1) medium (Invitrogen) containing 10% fetal bovine serum. C127 mouse keratinocytes were cultured in DMEM medium containing 10% fetal bovine serum.
Gamma secretase inhibitors and substrate
Gamma secretase inhibitors, numbers IX (GSI-IX, N-[N-(3,5-Difluorophenacetyl-L-alanyl)]-S-phenylglycine t-Butyl Ester, DAPT) (Calbiochem, Cat. # 565784 and # 565770) and numbers X (GSI-X, 1S-Benzyl-4R-[1-(1S-carbamoyl-2-phenethylcarbamoyl)-1S-3-methylbutylcarbamoyl]-2R-hydroxy-5-phenylpentyl}carbamic Acid tert-butyl Ester) (Calbiochem, Cat. # 565771), were dissolved in 100% DMSO. Gamma secretase substrate, fluorogenic (NIMA-GGVVIATVK(DNP)-DRDRDR-NH2) (Calbiochem, Cat.# 565764).
Generation of HPV16:LucF pseudoviruses (PVs) and directed drug screens
HPV virus-like particles (VLPs) carrying encapsidated reporter plasmids are referred in the papilomavirus field as HPV pseudoviruses. Reporter pseudovirions were produced using previously-described methods (Buck et al., 2004; Buck and Thompson, 2007). Briefly, two plasmids were cotransfected into 293FT. One plasmid is p16SheLL, a 10.8 kilo base pair (Kbp) plasmid (too large to be packaged in HPV VLPs) that expresses the HPV16 major capsid protein, L1, and minor capsid protein, L2. The second plasmid is pLucF that encodes both firefly luciferase (Luc) and green fluorescent protein (GFP) and is encapsidated efficiently. Pseudovirions were harvested by detergent lysis of the cells 48 hours after transfection. Particles were allowed to mature overnight (Buck et al., 2005) and were purified by ultracentrifugation through Optiprep gradients. The titers of HPV16:LucF pseudoviruses were determined by flow cytometric analysis of 293FT cells treated with various dilutions of purified pseudovirion stock.
For initial drug screening experiments, an HPV16-based pseudovirus encoding GFP was used to infect HeLa cells pre-plated a day in advance at 7500 cells/well in 96-well plates. A variety of drugs were selected from the EMD Biosciences or Sigma-Aldrich catalogs. Drug selection was essentially based on guesswork informed by reviews of relevant literature. Each candidate entry inhibitor drug was subjected to serial dilution and applied to the cell cultures for 30 minutes prior to addition of 6 × 104 GFP transducing units of HPV16 pseudovirus stock. Inhibition of pseudovirus infectivity was monitored by flow cytometric analysis of the cell cultures 48 hours after addition of the pseudovirions.
Dose-dependent luciferase assays and cell viability assays
1×104 HaCat or C127 cells/well in 96-well plates were pretreated with serial dilutions of gamma secretase inhibitor IX or X (Calbiochem) (from 0 to 10 µM) 4 hours prior to addition of 1×105 GFP transducing units of HPV16:LucF pseudovirus (10 M.O.I.). 72 hours post pseudovirus treatment, cells were lysed, and the infectivity was measured by quantitative luciferase assays (Promega, Luciferase Assay System Cat. #1501 and #1531). Parallel cell viability assays (Promega, Luminescent Cell Viability Assay Cat. #G7571) were performed to monitor cytotoxicity of the gamma secretase inhibitors IX and X. Alternatively, experiments above were repeated by using HPV11:LucF and HPV31:LucF instead of HPV16:LucF in HaCat cells.
Focus formation assay
The ability of gamma secretase inhibitors to block the infectivity of authentic papillomavirus virions was examined using a previously-describe assay for cellular transformation by bovine papillomavirus type 1(Dvoretzky et al., 1980). Briefly, 1×15 C127 cells (immortalized mouse keratinocytes) were pre-plated in 60 mm culture dishes overnight then treated with serial dilutions of gamma secretase inhibitors IX or X (from 0 to 1 µM) for 4 hours. After drug treatment, the cell monolayer was exposed to native BPV1 virions isolated from bovine skin warts. 24 hours after BPV1 exposure, the cells were washed to remove unbound BPV1 and gamma secretase inhibitors. 2 ~ 3 weeks later, focal transformations were detected by staining the cell cultures with 1 % methylene blue.
Time course luciferase assays
We set the time at which HaCat cells were exposed to HPV16 pseudovirus as the zero hour. We treated or mock-treated 1×104 HaCat cells with 1×105 HPV16:LucF (10 M.O.I.) and added 1 nM gamma secretase inhibitor X or 2% DMSO (vehicles) at different time points (relative to the time of infection). Parallel, experiments without DMSO or GSI-X were performed to serve as controls. 48 hours post infection, cells were lysed, and luciferase assays were performed to measure the efficiency of infection.
Mouse model for quantifying the in vivo efficiency of HPV infection
HPV16:LucF pseudoviruses were used to establish a quanititative mouse infection model for HPV in the female reproductive tract. The method is a modified version of a previously published model (Roberts et al., 2007). Indicated numbers of HPV16 pseudovirus were delivered intravaginally in 4% carboxyl methyl cellulose (Sigama Cat. #C4888) using six or seven 6 ~ 8 weeks old virgin female mice per condition. The mice were prepared by subcutaneous injection with 3 mg medroxyprogesterone acetate (Sicor, Depo-Provera) in 1X PBS 4 days prior pseudovirus challenge to induce diestrus. 6 hours prior to pseudovirus instillation, the mice were pre-treated vaginally with with Conceptrol (Caldwell Consumer Health), an over the counter spermicide product containing 4% nonoxynol-9. Administration of nonoxynol-9 has previously been shown to potentiate HPV pseudovirus infection due to chemical injury of the the vaginal/cervical epithelium (Roberts et al., 2007). 48 hours after delivery of the pseudovirions, mouse female reproductive tracts were harvested, protein lysates made, and luciferase assays were carried out to quantify the efficiency of infection in a dose dependent manner.
Assessment of GSI microbicidal activity in the mouse challenge model
Topical treatment of gamma secretase inhibitors. The time point for exposure to 3 × 106 HPV16:LucF pseudoviruses was set as zero hour. To ensure gamma secretase inhibitor was presented during HPV16 pseudovirus infection, mice were treated topically with 2 µM or 20 µM gamma secretase inhibitor X at -2 / zero / +4 / +6 / +8 hours. 48 hours after HPV16:LucF pseudovirus exposure, infectivity was determined by quantitative luciferase assays as described as above.
Systemic treatment of gamma secretase inhibitors. At -4 and zero hours we fed mice with 2% DMSO or 2.5 mg gamma secretase inhibitor IX, and 3 × 106 HPV16:LucF pseudoviruses were delivered to the mouse reproductive tract at time zero. 4 hours post delivery of the pseudovirus, female reproductive tracts was harvested, tissue lysates made, and gamma secretase activity was measured as described previously. 48 hours after pseudovirus treatment, another set of mouse female reproductive tracts was lysed and luciferase assays were carried out as described as above.
Acknowledgements
This work was supported by grants from the NIH (CA022443 and AI071947).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Buck CB, Pastrana DV, Lowy DR, Schiller JT. Efficient intracellular assembly of papillomaviral vectors. J Virol. 2004;78:751–757. doi: 10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CB, Thompson CD. Production of papillomavirus-based gene transfer vectors. Chapter 26, Unit 26 21. Curr Protoc Cell Biol. 2007 doi: 10.1002/0471143030.cb2601s37. [DOI] [PubMed] [Google Scholar]
- Buck CB, Thompson CD, Pang YY, Lowy DR, Schiller JT. Maturation of papillomavirus capsids. J Virol. 2005;79:2839–2846. doi: 10.1128/JVI.79.5.2839-2846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CB, Thompson CD, Roberts JN, Muller M, Lowy DR, Schiller JT. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006;2:e69. doi: 10.1371/journal.ppat.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comery TA, Martone RL, Aschmies S, Atchison KP, Diamantidis G, Gong X, Zhou H, Kreft AF, Pangalos MN, Sonnenberg-Reines J, Jacobsen JS, Marquis KL. Acute gamma-secretase inhibition improves contextual fear conditioning in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2005;25:8898–8902. doi: 10.1523/JNEUROSCI.2693-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day PM, Schiller JT. The role of furin in papillomavirus infection. Future Microbiol. 2009;4:1255–1262. doi: 10.2217/fmb.09.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durst M, Gissmann L, Ikenberg H, zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoretzky I, Shober R, Chattopadhyay SK, Lowy DR. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980;103:369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, Tang GWK, Ferris DG, Steben M, Bryan J, Taddeo FJ, Railkar R, Esser MT, Sings HL, Nelson M, Boslego J, Sattler C, Barr E, Koutsky LA. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med. 1997;102:3–8. doi: 10.1016/s0002-9343(97)00177-0. [DOI] [PubMed] [Google Scholar]
- Lacey CJN, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006;24 Suppl 3:S3/35–S3/41. doi: 10.1016/j.vaccine.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, Chow S-N, Apter DL, Kitchener HC, Castellsague X, de Carvalho NS, Skinner SR, Harper DM, Hedrick JA, Jaisamrarn U, Limson GAM, Dionne M, Quint W, Spiessens B, Peeters P, Struyf F, Wieting SL, Lehtinen MO, Dubin G. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13:857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, Clifford GM. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121:621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384:260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]