Abstract
Objectives
The expression of FOS, a gene critical for monocyte and macrophage function, can be inhibited by statins through the disruption of a cholesterol independent signaling pathway. In this pilot study, we hypothesized that blood FOS mRNA levels will be sensitive to statin treatment independent of LDL cholesterol levels.
Methods
Three cohorts at increased risk of or with cardiovascular disease (CVD) were studied. Blood FOS mRNA levels were measured before and after statin treatment or in patients under stable treatment.
Results
Statin treatment for three months significantly reduced blood FOS mRNA and LDL cholesterol levels. However, in subjects with similar LDL levels achieved by different doses of long term statin treatment, there was an inverse relationship between statin dose and FOS expression.
Conclusions
FOS mRNA levels appear to be a sensitive marker of statin treatment that is dissociated from cholesterol levels.
Statins have been proposed to exert pleiotropic benefits that extend beyond that predicted by cholesterol reduction (1,2), even though some analyses argue against the existence of such non-cholesterol related effects in preventing adverse cardiovascular events (3). A meta-regression analysis of data from statin and non-statin clinical treatment trials has shown that the changes in LDL cholesterol levels alone are sufficient to account for the decrease in cardiovascular events (3). However, at the cellular level, statins have potent anti-inflammatory and other beneficial effects on immune, endothelial and smooth muscle cells (2). At the molecular level, these HMG CoA reductase inhibitors not only decrease cholesterol synthesis but also inhibit the isoprenylation and activation of important intracellular signaling proteins such as RhoA and Ras that can affect pathogenesis (1,2). These disparate basic and clinical observations indicate a need for further translational investigations to improve our understanding of statin treatment effects. In this regard, the identification of biomarkers that provide additional information about the therapeutic activities of statins may be helpful.
Various cellular elements and signaling pathways contribute to atherosclerosis, but circulating inflammatory cells play a pivotal role in the initiation and final manifestation of disease (4,5). Recent work examining the transcriptional profiles of blood cells in patients with coronary artery disease provide insights into cardiovascular pathogenesis supporting the potential clinical utility of such investigations (6-8). We previously reported that the mononuclear cell mRNA level of the Finkel-Biskis-Jinkins Osteosarcoma (FOS) gene, a transcription factor essential for monocyte differentiation into macrophages (9), was correlated with the severity of atherosclerosis (10). The localization of FOS to macrophages and smooth muscle cells in plaques and its importance in calcification suggest that it may also play a direct role in atherosclerosis (10-12). Based on the known transcriptional regulation of FOS through a cholesterol-independent pathway (13), we hypothesized that blood FOS levels will be sensitive to statin treatment independent of LDL cholesterol levels and in a dose dependent manner. The following translational study was designed to determine whether FOS expression in blood can serve as a statin treatment response marker that could then be evaluated further with regard to clinical application given, for example, the questions about the pleiotropic effects of statins.
Methods
Patient population and study design
In accordance with the guidelines of the National Institutes of Health and Emory University Institutional Review Board committees, subjects were enrolled into three separate clinical studies between 2005 and 2009.
Study 1 -- Prospective statin intervention study
Nine subjects at increased risk for cardiovascular disease (CVD) either with known diabetes or with at least three components of the metabolic syndrome were enrolled at Emory University School of Medicine (Supplementary Table 1). Blood samples were obtained at baseline and after 3 months of treatment at a 10 mg/day atorvastatin-equivalent dose by their LDL cholesterol lowering potency (5 subjects, atorvastatin 10 mg/day; 4 subjects, pravastatin 80 mg/day) (14).
Study 2 -- Statin dose effect on FOS expression in mononuclear cells
46 patients were enrolled in this cross-sectional study examining the effect of statin dose on mononuclear cell FOS expression. Most of these patients had known coronary artery disease and were being treated long term with various doses of statins to target LDL levels according to clinical guidelines (LDL < 100 mg/dL) at the time of the study (Supplementary Table 2) (14). Because of the small number of subjects on higher statin doses, subjects were pooled together into one “high dose” group (40-80 mg atorvastatin equivalent/day: 40 mg, n=7; 60 mg, n=1; 80 mg, n=6) and one “low dose” group (10-20 mg atorvastatin equivalent/day: 10 mg, n=15; 20 mg, n=17). Within this cross-sectional cohort, 78% of the patients (36 out of 46) were on atorvastatin and the remainder were on various other statins which were converted to atorvastatin equivalents (14). The clinical characteristics of the low and high statin dose groups were not significantly different (Supplemenary Table 2).
Study 3 -- Statin dose effect on FOS expression using whole blood samples
For examining FOS expression in whole blood, samples collected in PAXgene tubes from 32 subjects enrolled in the ClinSeq™ Project (15), a whole-genome sequencing NHGRI-NIH study initially targeting cardiovascular diseases, were obtained and analyzed. These subjects at high risk for coronary artery disease or with known disease were on long term statin treatment and their clinical characteristics were not significantly different (Supplementary Table 3). They were also pooled into a “low dose” group (10-20 mg atorvastatin equivalent dose/day: 10 mg, n = 7; 20 mg, n = 12) and a “high dose” group (40-80 mg atorvastatin equivalent dose/day: 40 mg, n = 4; 60 mg, n = 3; 80 mg, n = 6). In this cohort, 56% of the patients (18 out of 32) were on atorvastatin and the remainder were on various other statins.
Blood sample processing, FOS mRNA expression and hsCRP quantification
To purify mononuclear cells, blood samples were drawn into citrate-containing Vacutainer CPT tubes (Becton Dickinson), density gradient centrifuged, and frozen within 2 h for subsequent RNA purification (10). To obtain RNA from whole blood, the samples were drawn into PAXgene Blood RNA tubes (PreAnalytiX) and also frozen within 2 h prior to RNA purification. Although PAXgene blood samples have stable RNA levels up to at least 6 months at −20 °C according to manufacturer’s data, we stored all our samples at −80 °C to ensure longer term stability. Furthermore, mononuclear cells stored in RNA lysis buffer at −80 °C have continued to retain stable FOS expression levels over the course of years in tested samples. FOS mRNA levels were measured by real-time reverse transcriptase polymerase chain reaction (RT-PCR) and normalized to an internal control gene as previously described (10). Serum hs-CRP levels were determined by immunophelometry using the Siemens/Dade Behring or IMMAGE CRPH (Beckman Coulter) assay.
Statistical analysis
Statistical analyses were performed using GraphPad Instat (version 3.06) and Prism (version 4.00). Wilcoxon matched-pairs signed ranks test was used to compare variables before and after statin treatment in the prospective study group, and the Mann-Whitney test was used to analyze continuous variables between the different statin dose groups. The Fisher exact test was used to compare categorical variables. The Spearman’s rank correlation test was used to analyze for the linear relationship between FOS and LDL or hsCRP levels in each subject. p values were calculated by two-tailed tests and considered statistically significant if p<0.05.
Results
Effect of prospective statin treatment on FOS expression in blood
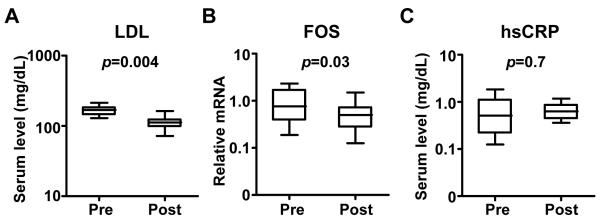
We first investigated whether statin treatment prospectively lowers FOS levels in subjects with diabetes or components of the metabolic syndrome at high risk for CVD (Supplementary Table 1). Circulating mononuclear cells were obtained at baseline and after three months of treatment. After statin therapy the median LDL cholesterol level decreased from 169 mg/dL [interquartile range (IQR) 144 to 184] to 112 mg/dL [IQR 98 to 124] and the mean value decreased by 33% (p=0.004, Fig. 1A). The median mononuclear cell FOS mRNA level decreased from 0.76 [IQR 0.38 to 1.7] to 0.50 [IQR 0.28 to 0.73] and the mean value decreased by 44% (p=0.03, Fig. 1B). Importantly, there was no significant correlation between the relative decreases in LDL cholesterol and FOS mRNA levels, demonstrating their differential responses to statin treatment (Spearman correlation coefficient= −0.2, p=0.7). Furthermore, hsCRP levels were not significantly affected by statin treatment during the time course of this study (pre-treatment median 5.1 [IQR 2.2 to 11] versus post-treatment median 6.3 [IQR 4.4 to 8.6], p=0.7, Fig. 1C).
Fig. 1.
The effect of prospective statin treatment on (A) LDL cholesterol, (B) mononuclear cell FOS mRNA, and (C) hsCRP in patients with diabetes or with features of the metabolic syndrome before (Pre) and after 3 months (Post) of statin treatment (Study 1: n = 9 patients). Wilcoxon matched-pairs signed ranks test was used to determine p value. Data shown as median, interquartile (box) and entire sample range (bars).
Effect of low versus high dose statin on FOS expression in blood
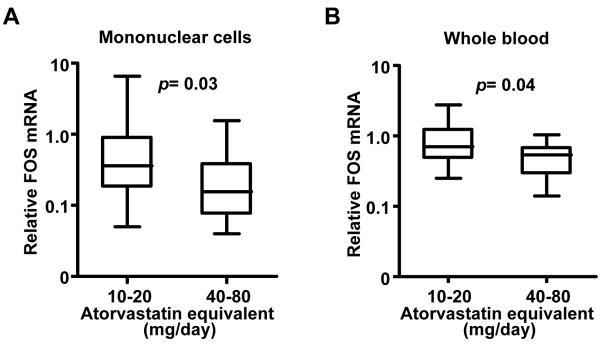
We next examined the relationship of statin dose to FOS mRNA levels. In this cohort of patients at high risk or with known coronary artery disease, the cardiovascular risk factor profiles were similar for the low and high statin dose groups (Supplementary Table 2). The mean LDL cholesterol levels were also similar for the two groups indicating that they had been successfully titrated to target levels according to clinical guidelines (p=0.5). In contrast, we observed an inverse relationship between atorvastatin equivalent dose and the median mononuclear cell FOS level in the high (0.16 [IQR 0.08 to 0.39]) versus low (0.36 [IQR 0.18 to 0.91]) statin dose group (p=0.03, Figure 2A). In further support of their independent modulation, there was no significant linear relationship between FOS mRNA and LDL cholesterol levels in the individual subjects (Spearman r= −0.09, p=0.5). Again, as in our prospective statin treatment study, the inflammatory marker hsCRP levels were not significantly different by statin dose in this cross-sectional cohort of subjects (Supplementary Table 2).
Fig. 2.
The relationship between statin dose and FOS mRNA levels in blood. (A) Dependence of mononuclear cell FOS mRNA levels on statin dose (Study 2: atorvastatin equivalent group is as follows: 10-20 mg/d, n=32; and 40-80 mg/d, n=14). (B) Dependence of whole blood FOS mRNA levels on statin dose (Study 3: atorvastatin equivalent group is as follows: 10-20 mg/d, n=19; and 40-80 mg/d, n=13). Mann-Whitney test was used to determine p values. Relative FOS mRNA levels shown as median, interquartile (box) and entire sample range (bars).
Because special blood sample handling and processing requirements can hinder the performance of large-scale clinical studies, we tried to replicate our findings in an independent population of patients using a simplified collection technique. To achieve this, we examined FOS expression in whole blood samples collected from subjects in the NHGRI-NIH ClinSeq™ Project (15) at high risk for coronary artery disease or with known disease (Supplementary Table 3). In these subjects who were being treated with statins to similar target LDL cholesterol levels, we again observed a significantly lower median FOS mRNA level in the higher dose statin treatment group (0.54 [IQR 0.29 to 0.68]) compared to the lower dose group (0.70 [IQR 0.48 to 1.24]) (p=0.04, Fig. 2B), a pattern that was not evident for LDL cholesterol or hsCRP (Supplementary Table 3). There was also no significant correlation between FOS mRNA and LDL cholesterol levels in the individual subjects from this cohort (Spearman r=0.2, p=0.2).
Discussion
Using samples obtained from patients in three different clinical studies, we have demonstrated that FOS mRNA levels in blood are reduced by statin therapy and that the reduction is reflective of statin dose. Furthermore, the inhibition of FOS expression by statin appears to be dissociated from its effects on LDL cholesterol and hsCRP. This observation is consistent with the known molecular mechanism by which non-cholesterol related pathways can modulate FOS expression (1,13). In summary, our current observations provide support for the idea that FOS, a transcription factor that responds to and integrates multiple signaling pathways, could serve as an in vivo readout of statin treatment response independent of LDL cholesterol.
There are some limitations to this study. It was not designed to determine the prognostic significance of FOS levels but, rather, to determine whether FOS levels in blood can serve as a marker of statin treatment independent of LDL cholesterol levels. Although our current study provides proof of concept, it will be important to further characterize our findings in a larger prospective treatment study using different doses and types of statins and to determine its clinical significance. In addition, it may be useful to serially examine both the mononuclear cell and whole blood levels of FOS in the same subjects. In the cross-sectional studies (Studies 2 and 3), we do not have the pre-statin treatment LDL cholesterol or hsCRP measurements, therefore, we cannot conclude whether the degree of reduction compared to their baseline levels correlate with FOS reduction. However, in our prospective statin treatment group (Study 1), there was no significant correlation between the reductions in LDL or hsCRP and FOS levels.
The demonstrated sensitivity of FOS to high dose statin using a simplified method of blood collection as with the ClinSeq™ Project samples shows the feasibility of conducting larger scale prospective studies or retrospective investigations of stored samples. Increased interest in genetic biomarkers has resulted in larger numbers of clinical studies storing whole blood samples for gene expression analysis (15), thus retrospective analysis of such samples from large statin treatment clinical trials, for example, may be useful in the future. Another possible application of measuring FOS levels may be for monitoring treatment compliance which is known to significantly impact the outcome of various patient populations with heart disease. This may be especially useful in patients whose pretreatment cholesterol levels may be low or in those whose cholesterol levels are relatively unresponsive to statins. It is tempting to speculate that the measurement of new biomarkers such as FOS may provide additional insights into the biology of cardiovascular diseases to improve treatment outcome.
Supplementary Material
Acknowledgement
The authors wish to thank Drs. Ping-yuan Wang, Myron A. Waclawiw, Michael Sack and Toren Finkel for helpful discussions. We also thank Amy Linn, Danielle Singer, Paul Gobourne and Laurel Mendelsohn and Dr. George Csako for sample and data collection. This research was supported by the Intramural Research Program of the NHLBI and NHGRI, NIH, and the National Center for Research Resources (NCRR) Grant M01-RR00039 for the Emory General Clinical Research Center (GCRC).
Glossary
Abbreviations
- CAD
coronary artery disease
- CVD
cardiovascular diseases
- FOS
Finkel-Biskis-Jinkins osteosarcoma gene
- HMG CoA reductase
3-hydroxy-3-methylglutaryl coenzyme A reductase
- hsCRP
high sensitivity C-reactive protein
- statin
HMG CoA reductase inhibitor
Footnotes
Disclosure
Dr. Quyyumi has received research funding from Pfizer, which manufactures atorvastatin, a product related to the research described in this paper and has received honoraria as a consultant. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4:977–87. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 3.Robinson JG, Smith B, Maheshwari N, Schrott H. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta-regression analysis. J Am Coll Cardiol. 2005;46:1855–62. doi: 10.1016/j.jacc.2005.05.085. [DOI] [PubMed] [Google Scholar]
- 4.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 5.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–38. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seo D, Ginsburg GS, Goldschmidt-Clermont PJ. Gene expression analysis of cardiovascular diseases: novel insights into biology and clinical applications. J Am Coll Cardiol. 2006;48:227–35. doi: 10.1016/j.jacc.2006.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chittenden TW, Sherman JA, Xiong F, et al. Transcriptional profiling in coronary artery disease: indications for novel markers of coronary collateralization. Circulation. 2006;114:1811–20. doi: 10.1161/CIRCULATIONAHA.106.628396. [DOI] [PubMed] [Google Scholar]
- 8.Sinnaeve PR, Donahue MP, Grass P, et al. Gene expression patterns in peripheral blood correlate with the extent of coronary artery disease. PLoS One. 2009;4:e7037. doi: 10.1371/journal.pone.0007037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liebermann DA, Gregory B, Hoffman B. AP-1 (Fos/Jun) transcription factors in hematopoietic differentiation and apoptosis. Int J Oncol. 1998;12:685–700. doi: 10.3892/ijo.12.3.685. [DOI] [PubMed] [Google Scholar]
- 10.Patino WD, Mian OY, Kang JG, et al. Circulating transcriptome reveals markers of atherosclerosis. Proc Natl Acad Sci U S A. 2005;102:3423–8. doi: 10.1073/pnas.0408032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang ZQ, Ovitt C, Grigoriadis AE, Mohle-Steinlein U, Ruther U, Wagner EF. Bone and haematopoietic defects in mice lacking c-fos. Nature. 1992;360:741–5. doi: 10.1038/360741a0. [DOI] [PubMed] [Google Scholar]
- 12.Lavezzi AM, Milei J, Grana DR, Flenda F, Basellini A, Matturri L. Expression of c-fos, p53 and PCNA in the unstable atherosclerotic carotid plaque. Int J Cardiol. 2003;92:59–63. doi: 10.1016/s0167-5273(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 13.Marinissen MJ, Chiariello M, Tanos T, Bernard O, Narumiya S, Gutkind JS. The small GTP-binding protein RhoA regulates c-jun by a ROCK-JNK signaling axis. Mol Cell. 2004;14:29–41. doi: 10.1016/s1097-2765(04)00153-4. [DOI] [PubMed] [Google Scholar]
- 14.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 15.Biesecker LG, Mullikin JC, Facio FM, et al. The ClinSeq project: piloting large-scale sequencing for research in genomic medicine. Genome. 2009 doi: 10.1101/gr.092841.109. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.