Abstract
Despite the eradication of smallpox, there is heightened concern that it could be reintroduced as a result of intentional release of Variola major virus through an act of bioterrorism. The live vaccine that was pivotal in the eradication of smallpox though considered a gold standard for its efficacy still retains sufficient residual virulence that can cause life-threatening sequelae especially in immune deficient individuals. Therefore, a safer smallpox vaccine that can match the efficacy of first generation vaccines is urgently needed. We previously reported that the integration of human IL-15 cytokine into the genome of Wyeth strain of vaccinia (Wyeth/IL-15), the same strain as the licensed vaccine, generates a vaccine with superior immunogenicity and efficacy in a mouse model. We now demonstrate that Wyeth/IL-15 is non-lethal to athymic nude mice when administered intravenously at a dose of 107 plaque forming units and it undergoes enhanced in vivo clearance in these immune deficient mice. Furthermore, a majority of cynomolgus monkeys vaccinated with vaccinia viruses with integrated IL-15, when challenged 3 years later with a lethal dose of monkeypox virus displayed milder clinical manifestations with complete recovery supporting the utility of Wyeth/IL-15 for contemporary populations as a safer and efficacious smallpox vaccine.
Keywords: Smallpox vaccine, IL-15, Monkeypox
1. Introduction
Global eradication of smallpox in 1977 as a result of a sustained word-wide campaign under the auspices of the World Health Organization represents one of the most significant medical triumphs of the 20th century [1]. Pivotal in this eradication endeavor was the availability of a live vaccine derived from a related Orthopoxvirus vaccinia (Wyeth strain in the US, Lister/Elstree strain in Britain, EM63 strain in Russia, Ikeda strain in Japan and Tian Tan strain in China) with stellar efficacy against smallpox [1], [2]. Despite the elimination of smallpox as a natural disease, the etiological agent of smallpox, Variola major virus still remains in more than one designated high security repositories in the world. In addition, the possibility of undocumented existence of V. major virus has long been suspected, fuelling concerns that smallpox could reemerge due to malicious release of this virus in an act of bioterrorism with devastating consequences. In recognition of this potential threat, the US Department of Defense has vaccinated more than 1.5 million individuals in the military vaccination program since December of 2002, and the Department of Health and Human Services has vaccinated close to 40,000 in the civilian first-responder program, along with the establishment of a strategic national stockpile of smallpox vaccines by the US government and many other nations (reviewed in [3]).
The Dryvax vaccine that was used in the smallpox eradication campaigns, despite being efficacious against smallpox still retained residual virulence that contributed to serious adverse complications in a small proportion of vaccinees or their close contacts especially with underlying immunological deficiencies or atopic skin disease [4]. Although, the production of Dryvax vaccine has been discontinued and its license withdrawn, the currently licensed, cell culture grown ACAM 2000 vaccine (Wyeth strain) is a derivative of the original Dryvax vaccine and has the same contraindications as Dryvax and is not recommended for individuals with immune deficiencies, atopic skin diseases, cardiac disorders or pregnancy [5]. Based on these recommendations, should mass vaccination against smallpox be required as a consequence of bioterror attack involving smallpox, nearly 25% of the contemporary population would be ineligible for vaccination with the ACAM 2000 smallpox vaccine. Therefore, the development of a smallpox vaccine that can match the efficacy and immunogenicity of Dryvax and yet be devoid of its residual virulence and thus better suited for contemporary populations with greater numbers of immunodeficient individuals, either due to HIV infections or iatrogenic consequences such as therapy for autoimmune disorders or organ transplantations, remains a priority.
We recently reported that the integration of IL-15, a cytokine with pleiotropic immune modulatory activities into the Wyeth strain of vaccina virus derived from the Dryvax vaccine resulted in the development of a smallpox vaccine candidate with superior efficacy and immunogenicity that out-performed the parental vaccine in protecting mice when lethally challenged with the neurotropic WR strain of vaccinia virus intranasally [6]. Similarly, the integration of IL-15 into the modified vaccinina Ankara (MVA), a weakened vaccinia strain that is under consideration for licensure also displayed enhanced immunogenicity and efficacy [6]. In continuation with further preclinical development of these IL-15 integrated vaccines, we now demonstrate with in vivo imaging techniques that the replication competent Wyeth/IL-15 vaccine we generated, undergoes enhanced in vivo clearance in T-cell deficient nude mice without causing any mortality when administered intravenously at a dose of 107 pfu (plaque forming units), unlike the wild-type Wyeth strain that causes a progressively fatal infection in these immune deficient nude mice. Furthermore, when cynomolgus macaque (Macaca fasicularis) monkeys were vaccinated with a single dose of Wyeth/IL-15 vaccine, the vaccine not only prevented the death of vaccinated monkeys when challenged intravenously with a high lethal dose of monkeypox virus (Zaire 79 strain) after a period of three years following vaccination, but two challenged animals out of three developed fewer than 15 skin lesions, whereas animals vaccinated with wild-type Wyeth, Wyeth/IL-2 (Wyeth strain of vaccinia with integrated human IL-2), MVA, MVA/IL-15 or MVA/IL-2 displayed much greater numbers of pock lesions on their skin. These results further strengthen the notion that our Wyeth/IL-15 vaccine is superior in both efficacy and safety than the currently licensed smallpox vaccine and is a better suited alternative for contemporary populations.
2. Materials and methods
2.1. Recombinant vaccinia viruses
The Wyeth New York Board of Health strain of vaccinia was obtained from Wyeth Ayerst Laboratories (Marietta, PA). Modified vaccinia virus Ankara was kindly provided by Dr. Bernard Moss at the National Institute of Allergy and Infectious Diseases. The creation of recombinant viruses that express human IL-15 (Wyeth/IL-15 and MVA/IL-15) has been described previously [6]. To create recombinant vaccinia viruses that express human IL-2, a cDNA clone of human IL-2 was obtained from ATCC (cat# 36673) and the coding region of the IL-2 gene was excised by digesting with Pst 1 enzyme. An 800 bp Pst 1 fragment carrying the coding segment of IL-2 was then cloned into a transfer vector that carries vaccinia hemagglutinin gene segments and E. coli gpt gene derived from the pTFHA plasmid [7] for the creation of IL-2 expressing recombinant vaccinia in either Wyeth or MVA backbones (Wyeth/IL-2 and MVA/IL-2 respectively). To create, Wyeth/Luc, the coding region of luciferase gene was excised from the pGL3 basic vector (Promega Corp.) and cloned into the same transfer vector described above for IL-2 recombinants whereas for the creation of Wyeth/IL-15/Luc, both human IL-15 and luciferase genes (coding segments) with synthetic vaccinia promoter sequences were placed in a head to tail configuration in the same transfer vector. In all vaccinia recombinants used in the present study, heterologous genes were recombined into the hemagglutinin locus of the respective vaccinia strain genome. The Wyeth strain of vaccinia and its recombinant derivatives were grown and titered in a CV-1 monkey kidney cell line from ATCC while the MVA strain and its recombinant derivatives were grown in a BHK-21 cell line from ATCC.
2.2. Animals and immunizations
Eight to twelve weeks old, female BALB/c and athymic congenic nude mice (CAnN.Cg-Foxn1 nu/Crl) were obtained from the Veterinary Resources Program, National Institutes of Health (Bethesda, MD) and Charles River respectively. Twenty adult male cynomolgus macaques (Macaca fascicularis) approximately 10 years of age were obtained from a NCI sponsored facility colony and were housed at the NIH primate center during the immunization period, prior to being transferred to Southern Research Institute, Frederick, MD for monkeypox challenge experiments. Cell-culture grown vaccine viruses were purified by sedimentation through a sucrose cushion. A single dose of vaccine in a volume of 50 μl containing 1 × 108 pfu of virus was administered intradermally in a shaved area between the two scapulae of the cynomolgus monkeys. Housing and caring of monkeys were carried out in accordance with the American Association for Accreditation of Laboratory Animal Care standards in accredited facilities. The experimental design of this study was approved by the Institutional Animal Care and Use Committees.
2.3. Intracellular cytokine staining
Freshly isolated, 3 × 106 peripheral blood mononuclear cells (PBMC) were mixed with an equal number of irradiated (3000 rads) autologous PBMC that had been infected with Wyeth vaccinia at a multiplicity of infection of 5 for 4 h prior to being irradiated, and co-cultured in RPMI medium containing 20% fetal calf serum with anti CD49d (cat# 556634) and anti CD28 (cat# 556620) both at a final concentration of 0.25 μg/ml. After 4 h of co-culture with irradiated vaccinia infected cells at 37 °C, Brefeldin A was added (1 μg/ml) to the cultures and incubated for an additional 8 h before staining for intracellular interferon gamma and surface CD4 and CD8 markers. All antibodies were purchased from Pharmingen and the background staining was controlled for by using isotype matched control antibodies. Cells stained with appropriate antibodies were analyzed on a FACSCalibur instrument (Becton Dickinson).
2.4. Plaque-reduction neutralization test (PRNT)
Pre-immunization and post immunization serum samples were heat inactivated for 30 min at 56 °C. Anti-vaccinia neutralizing antibody titers (80% PRNT) were determined as described previously [6]. To determine anti-monkeypox virus neutralizing antibody titers (50% PRNT), serum samples that were serially diluted 2-fold were mixed (225 μl) with an equal volume of medium containing 2000 pfu of monkeypox virus (Zaire strain) and incubated overnight at 37 °C, followed by the removal of 100 μl of this mixture and the addition onto E6 Vero cell monolayers in 24-well culture plates. After a period of 1 h incubation, cells were overlaid with DMEM containing 2% FBS, 1% carboxy-methylcellulose (Sigma: cat# M-0512). The plates were then incubated at 37 °C for 72 h and then stained with a solution containing 10% formaldehyde, 5% acetic acid, 60% ethanol and 1% crystal violet. Each well was then evaluated for the number of plaques and the reciprocal serum dilution yielding 50% reduction in the plaque count was determined and expressed as the neutralization titer. Each sample was assayed in triplicate.
2.5. In vivo bioluminescence measurements in mice
Vaccinia viruses (1 × 107 pfu) with an integrated luciferase gene were administered via the tail vein in a volume of 50 μl. For imaging of vaccinia infected animals, mice were first lightly anesthetized using isoflurane/O2 (1.5–5%, v/v) and injected with 100 μl (30 mg/ml solution in phosphate buffered saline) of luciferin intraperitoneally (Caliper Life Sciences, Alameda, CA). Imaging was performed 10 min after the administration of luciferin with an IVIS 100 imager from Xenogen. Overlay images and luminescence measurements were made using Living Image software (version 2.50.1; Xenogen).
2.6. Virus challenge
Monkeypox virus (Zaire 79 MA-104 11/26/03) was the challenge agent used in this study and was obtained from the NIAID Biodefense and Emerging Infections Research Resources Repository. All macaques in Groups 1–7 were administered an intravenous dose of 5 × 107 pfu of monkeypox virus in a final volume of 1 ml.
2.7. Clinical observations
All study animals were observed for clinical illness or changes in behavior twice daily. On study Days 0, 3, 6, 9, 12, 15, 18, 21, 24, and 27, all study animals were monitored for number and quality of pock lesions (lesions were photographed), weight, and body temperature.
2.8. Quantitation of plasma monkeypox viral loads
On study days −1, 0 (day of challenge), 3, 6, 9, 12, 15, 18, 21, 24, and 27, blood samples were collected from all study animals for determination of viral load by Real-Time PCR. DNA was extracted from 200 μl of whole blood using Qiagen DNA mini kits according to the manufacturer's instructions. Monkeypox virus genomic DNA was measured with the LightCycler Quantitative Pan-orthopox HA PCR Assay. Primers and probes used for targeting the HA gene are listed below: forward primers: OPHA F89 5′-ATGTACTATCTCAACGTAGTAG-3′, reverse primer: OPHA R219 5′-CTGCAGAACATAAAACTATTAATATG-3′, probe: OPHA-P143S-MGB 6FAM AGTGCTTGGTATAAGGAG MGBNFQ. Viral load data are reported as the number of monkeypox virus DNA genome copies per milliliter of blood. The limit of detection for the assay was 5000 viral DNA genome copies per milliliter.
2.9. Statistical analysis
Analysis of variance was used to determine the effect of different immunization protocols on the magnitude of cellular and antibody responses. Student's t test was used to compare immunization protocols and significance levels were set at a P value of 0.05.
3. Results and discussion
Because the second generation cell-culture grown smallpox vaccine ACAM 2000 displays similar reactogenicity and residual virulence profiles as the calf lymph derived Dryvax vaccine [5], many approaches are being explored to develop a smallpox vaccine that can match the immunogenicity of Dryvax, but without its residual virulence. The use of strains with deleted putative virulence genes from the genome of the vaccine strain as in NYVAC or the use of spontaneous deletant mutants with attendant attenuation such as the modified virus Ankara (MVA) has been proposed and considered as potential replacements that are more suited for contemporary populations. However, several immunogenicity studies conducted either in non-human primates and/or human volunteers indicate that both NYVAC and MVA are less immunogenic than Dryvax [8], [9]. Although, the efficacy of NYVAC or MVA against now eradicated smallpox cannot be determined in humans, challenge studies with monkeypox in vaccinated non-human primates, which is considered as a reasonable model of smallpox in humans have revealed that both NYVAC and MVA are inferior to the protection conferred by Dryvax in this model, thus raising concerns whether these attenuated deletant viruses could confer sufficient protection against smallpox in a situation, where deliberate release of V. major virus in a concentrated fashion occurs as a result of bioterrorism.
3.1. Impact of IL-15 on the replication and clearance of vaccinia virus in immune competent mice
We are of the view that approaches to enhance the engagement of the host immune system to attenuate the residual virulence of the Wyeth strain of vaccinia without reducing the genetic content of the viral genome is more likely to yield a reliable vaccine candidate that can match the efficacy/immunogenicity of the Dryvax vaccine. Recently, we reported the generation of a smallpox vaccine candidate (Wyeth/IL-15) with superior immunogenicity and efficacy as determined by protection against a lethal intranasal challenge with WR vaccinia in vaccinated mice by integrating the human IL-15 cytokine into the hemagglutinin locus of the Wyeth strain of vaccinia derived from the Dryvax vaccine [6]. The only vaccinia gene disrupted in creating this Wyeth/IL-15 vaccine is the hemagglutinin gene, which does not appreciably affect the replication or pathogenicity of the virus unlike the thymidine kinase gene locus that has been widely used for constructing an array of recombinant vaccinia viruses [10]. However, when we assessed the lethality of Wyeth/IL-15 in athymic nude mice, the lethal dose 50 (LD50) of Wyeth/IL-15 was approximately 500–1000-fold higher than the LD50 of parental Wyeth vaccinia virus (data not shown) suggesting that the integration of IL-15 considerably attenuates the in vivo virulence of Wyeth vaccinia. In order to delineate whether the greater LD50 seen with our Wyeth/IL-15 is reflective of diminished in vivo viral replication or alternatively, an indication of enhanced viral clearance, we utilized in vivo imaging to monitor the progression of intravenously inoculated virus by integrating the firefly luciferase gene into the viral genome. In Wyeth/Luc virus, the luciferase gene is integrated into the hemagglutinin locus of wild-type Wyeth virus derived from the Dryvax vaccine, whereas in the Wyeth/IL-15/Luc, the luciferase and human IL-15 genes are integrated into the hemagglutinin locus of Wyeth vaccinia virus in a head to tail configuration. To evaluate in vivo replication and subsequent clearance of these two viruses in immune competent BALB/c mice, 107 pfu were administered intravenously to a group of five mice and in vivo imaging performed longitudinally starting 30 min post administration of the virus at which time point no appreciable signal emanated from the infected mice (see Fig. 1 ). Of note, it is the luminescence elicited by virus infected cells expressing luciferase, but not free virus in the plasma that gets detected by in vivo imaging. In contrast, 12 h post infection images revealed rampant viral activity in multiple organs. At this period of peak viral activity, the robustness of Wyeth/IL-15/Luc replication was similar if not slightly higher than that of Wyeth/Luc in these immune competent mice. The duration of infection in immune competent BALB/c mice with both Wyeth/Luc and Wyeth/IL-15/Luc vaccinia virus was rather abbreviated with the viruses being cleared almost completely within 48 h post administration, without any overt differences in the kinetics of virus clearance for the two viruses as can be seen in Fig. 1. This is in contrast to what has been reported for the more virulent WR strain of vaccinia in similar in vivo imaging studies indicating longer periods, up to 5 days of persistence in immune competent mice consistent with the heightened virulence of neurovirulent WR strain of vaccinia in comparison to Wyeth strain [11].
Fig. 1.

In vivo biodistribution and clearance of Wyeth strain of vaccinia in immune competent BALB/c mice. Wyeth vaccinia derived from the Dryvax vaccine with the firefly luciferase gene integrated (Wyeth/Luc) or the firefly luciferase gene plus human IL-15 gene integrated (Wyeth/IL-15/Luc) into the hemagglutinin locus was injected intravenously (1 × 107 pfu) in a volume of 50 μl via the tail vein to a group of 5 female BALB/c mice. Luciferase expression in mice was then sequentially visualized by luminescence imaging (IVIS 100 system from Xenogen, Caliper Life Sciences) starting 30 min post virus administration and continued every 12 h. Representative longitudinal images are shown. An integration time of 2 min was used for luminescence image acquisition.
3.2. Impact of IL-15 on the replication and clearance of vaccinia virus in T-cell deficient nude mice
However, when we administered Wyeth/Luc or Wyeth/IL-15/Luc to T-cell deficient immunocompromised nude mice the course of infection was dramatically different as shown in Fig. 2 . Widespread intense viral activity was demonstrable within 2 h post inoculation in these nude mice and this high viral replicative activity, without any discernible differences between the two viruses continued for the first 26 h. But, imaging analysis performed at 38 h post inoculation revealed a clear cut reduction in viral activity in mice inoculated with Wyeth/IL-15/Luc over animals inoculated with Wyeth/Luc. It should be mentioned that a dose of 107 pfu of Wyeth/Luc by intravenous route was lethal to athymic nude mice although the occurrence of death varied from 5 to 21 days with loss of body weight and emaciation. Another important finding to emerge from the imaging studies was that in immune competent mice, no appreciable viral activity was detectable in the brain after intravenous administration of the Wyeth/Luc or Wyeth/IL-15/Luc vaccinia throughout the course of infection. In striking contrast, images taken from T cell deficient nude mice 114 h following the intravenous administration of Wyeth/Luc, but not before, abundant viral activity was apparent in the brains of all animals and the viral replication in the brain continued to intensify although the clearance of the virus from the other organs continued such that at the moribund stage viral activity was almost exclusively confined to the brain in animals given Wyeth/Luc. But, in the Wyeth/IL-15/Luc group, no loss of body weight or deaths occurred in any of the inoculated animals and only a solitary animal displayed some viral activity in the brain by Day 13 post inoculation (Fig. 2). Yet, even this animal subsequently cleared the viral infection and survived. This observation underscores the fact that despite the integration of IL-15, Wyeth/IL-15 is not totally avirulent in immune compromised host and the possible existence of host associated variability in the reduction of virulence.
Fig. 2.
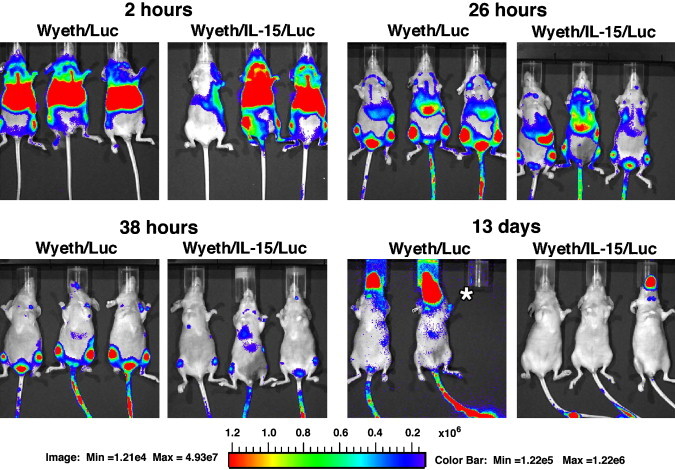
In vivo biodistribution and clearance of Wyeth strain of vaccinia in T cell-deficient nude mice. Wyeth vaccinia derived from the Dryvax vaccine with the firefly luciferase gene integrated (Wyeth/Luc) or the firefly luciferase gene plus human IL-15 gene integrated (Wyeth/IL-15/Luc) into the hemagglutinin locus was injected intravenously and luminescence was monitored longitudinally over a period of 3 weeks as described in the legend to Fig. 1. Asterisk in 13 days panel indicates the unavailability of the mouse due to death at that time point.
The imaging data shown in Fig. 1, Fig. 2 collectively demonstrate that the integration of IL-15 and its expression had no impact on the initial vaccinia virus replication in vivo. Because of the relatively short and self-limiting infection of Wyeth virus in immune competent mice, the impact of virally expressed IL-15 on the clearance of vaccinia virus was not apparent. However, the progressive course of infection in T-cell deficient nude mice, clearly revealed the impact of virally expressed IL-15 on the course of vaccinia infection allowing the host to limit the progressive spread of the virus and facilitate its clearance along with the resolution of an otherwise fatal vaccinia virus infection in these immune deficient mice.
IL-15 is a cytokine with profound pleiotropic effects on both innate and adaptive immune systems. It is involved in the activation, proliferation and differentiation of CD8+ T-cells and the maintenance of CD8+ memory T cells, in addition to supporting the survival of mature dendritic cells (reviewed in [12], [13]). IL-15 is also pivotal in NK cell function and differentiation, an innate cell component that is implicated in the control and clearance of vaccinia virus [14], [15].
3.3. Immunogenicity and efficacy of IL-15 integrated vaccinia virus in cynomolgus monkeys against a lethal monkeypox virus challenge
Despite the eradication of smallpox with a highly efficacious vaccine, the correlates of vaccine mediated protection yet remain to be fully defined. The efficacy assessment and subsequent licensure of a safer smallpox vaccine more suited for our present day populations remain extraordinarily complex for a fatal infection that does not exist as a natural disease any more. The U.S. Food and Drug Administration (FDA) animal efficacy rule that provides a pathway for licensure of vaccines for such diseases mandates testing of a candidate vaccine in more than one sufficiently well characterized animal model that recapitulates the pathophysiology and protective endpoints in humans [16], [17]. Having confirmed the superior immunogenicity, efficacy (see ref [6]) and safety of our Wyeth/IL-15 vaccine in mice, we proceeded to evaluate the efficacy of this vaccine in a cohort of cynomolgus macaque monkeys (Macaca fascicularis) against a high dose lethal intravenous monkeypox virus (Zaire 79 strain) challenge that is considered a valid non-human primate model of smallpox.
A cohort of 20 cynomolgus macaque monkeys were assigned to 7 groups. As shown in Table 1 , for vaccination with 6 different vaccine candidates, namely, the wild type Wyeth, Wyeth/IL-2, Wyeth/IL-15, MVA, MVA/IL-2, MVA/IL-15. Three animals that were not vaccinated with any virus but included in the challenge study served as controls. Because MVA is under consideration for licensure and the integration of IL-15 significantly improved the immunogenicity and efficacy of parental wild-type MVA as a vaccine in a mouse model of smallpox as we reported previously [6], we included the wild-type MVA and its cytokine integrated derivatives in the present non-human primate study for a direct comparison with the replication competent Wyeth virus and its derivatives. The rationale to include IL-2 integrated vaccinia viruses in the present study was based on previous reports indicating that the integration of IL-2 gene into the WR strain of vaccinia results in dramatic attenuation of the virulence of this neurotropic virus [18], [19], [20], [21] and the fact that both IL-2 and IL-15 share many biological functions including the augmentation of NK cell activation with enhanced interferon gamma secretion that is pivotal for the control of vaccinia infection [12], [13], [14], [15]. IL-2 integrated, attenuated vaccinia viruses have not been evaluated for their potential use as safer smallpox vaccines and therefore we generated two new vaccine candidates in the Wyeth and MVA backbone, Wyeth/IL-2 and MVA/IL-2 by integrating the human IL-2 gene into the hemagglutinin locus of the respective genomes so that we could make direct comparisons between the IL-15 integrated versions versus IL-2 integrated versions for their efficacy and suitability. The key elements in the design of the study that enable us to make direct comparisons among the 6 vaccine candidates included: (i) a single dose of vaccine containing 108 pfu of virus in a volume of 50 μl given intradermally for all six vaccine candidates tested, (ii) an intravenous challenge with a dose of 5 × 107 pfu of monkeypox virus (Zaire 79 strain) administered 3 years after the initial vaccination so that vaccine induced immune responses are in the waning phase and thus likely to permit us to detect any subtle differences in the protective responses induced by the respective vaccines.
Table 1.
Study groups with post vaccination vims neutralizing antibody titers.
| Study group | Monkey ID | Anti-vaccinia titer (PRNT 80%) |
Anti-monkeypox titer (PRNT 50%) |
||
|---|---|---|---|---|---|
| 6 Weeks post vaccination | 3 Years post vaccination | Pre-challenge | Post-challenge | ||
| Wyeth | CY1990 | 1:25 | <l:10 | <l:10 | 1:4593 |
| CY1992 | 1:25 | <l:10 | <l:10 | >1:10,240 | |
| CY128175 | 1:25 | <l:10 | <l:10 | 1:1600 | |
| Wyeth/IL-2 | CY3856 | 1:50 | <l:10 | <l:10 | >1:10,240 |
| CY10619 | 1:50 | <l:10 | <l:10 | >1:10,240 | |
| CY66400 | 1:50 | <l:10 | <l:10 | >1:10,240 | |
| Wyeth/IL-15 | CY19248 | 1:50 | <l:10 | <l:10 | 1:7680 |
| CY19249 | 1:50 | <l:10 | <l:10 | >1:10,240 | |
| CY19251 | 1:50 | 1:50 | 1:100 | >1:10,240 | |
| MVA | CY66393 | 1:200 | <l:10 | <l:10 | 1:7314 |
| CY128497 | 1:200 | <l:10 | <l:10 | >1:10,240 | |
| MVA/IL-2 | CY10621 | 1:200 | <l:10 | <l:10 | N/A |
| CY19279 | 1:200 | <l:10 | <l:10 | 1:7447 | |
| CY48447 | 1:200 | 1:25 | 1:40 | >1:10,240 | |
| MVA/IL-15 | CY3887 | 1:200 | <l:10 | <l:10 | 1:1173 |
| CY10627 | 1:200 | 1:25 | 1:40 | 1:10,240 | |
| CY66406 | 1:200 | <l:10 | <l:10 | >1:10,240 | |
In animals inoculated with Wyeth and Wyeth/IL-15, the site of inoculation became erythematous and slightly raised around 48 h post vaccination and progressed into classic vesicular lesions with swelling and erythema by Day 4. The lesions became erosive by Day 10 with a diameter of approximately 10 mm with some scab formation as shown in Fig. 3 . However, in animals vaccinated with Wyeth/IL-2, the lesions were much milder in intensity with less erythema and swelling consistent with an earlier report indicating a significant reduction in the area of induration in Patas monkeys vaccinated with a WR recombinant virus expressing IL-2 [16]. Complete healing and resolution of the lesions were slightly faster for cytokine integrated versions of Wyeth virus and shown in Fig. 3, are the healed lesions of three macaques vaccinated with Wyeth/IL-15 thirty days post vaccination. Surprisingly, we also noted some induration and erythema at the site of intradermal vaccination with MVA or its cytokine integrated derivatives which appeared within 24 h post vaccination, but lacked the typical characteristics of vaccinia skin lesions with bullae or vesicle formation. These lesions resolved within a few days and are likely due to some host inflammatory response to BHK-21 cell components present in the vaccine preparation rather than due to the non-replicating MVA virus itself (data not shown).
Fig. 3.

Post vaccination skin lesions induced by the Wyeth strain of vaccinia or its derivatives with integrated cytokines. Each candidate smallpox vaccine, Wyeth, Wyeth/IL-2 and Wyeth/IL-15 was administered intradermally in a volume of 50 μl containing 1 × 108 pfu to a shaved area between the scapulae. Lesions formed were photographed at the indicated time points after vaccination.
Previous reports indicate that monkeys vaccinated with either the Wyeth strain or MVA are protected against a lethal monkeypox virus infection [22], [23], [24], [25], [26], [27], [28], although it should be noted that in some of those studies, the lethal monkeypox challenge dose employed was relatively low (1 × 105 pfu) compared to the dose of 5 × 107 pfu used in the present study. Therefore, to determine an optimal time to challenge the vaccinated monkeys that would permit us to discern any differences, if any, among the six vaccine candidates in their protective efficacy against a lethal monkeypox virus challenge, we considered the data from our own work in mice with these vaccine candidates [6], as well as published reports [22], [28] and reached the view that in the late phase of post vaccination when vaccine induced immune responses are waning is most likely to reveal any impact of cytokine mediated enhancement of protective efficacy especially considering the effects of IL-15 on CD8+ memory cells [12], [29]. In addition, during the smallpox eradication campaigns in the 1960s, the WHO recommendation was to revaccinate individuals every three years because of the waning immunity at this time [1]. Therefore, we decided to challenge the vaccinated monkeys three years after they have been vaccinated with a single inoculation of the respective vaccine. In the interim, vaccinated monkeys were periodically monitored for their humoral and cell mediated immune responses against vaccinia virus to assess the long term persistence of their immune status.
As shown in Table 1, at 6 weeks post vaccination, vaccinia plaque reduction neutralizing antibody titers (PRNT 80%) were approximately 4-fold higher in the monkeys vaccinated with MVA or MVA derivatives with integrated cytokines compared to their counterparts vaccinated with Wyeth or Wyeth derivatives with integrated cytokines. Interestingly, among the MVA vaccines, the integration of IL-2 or IL-15 had no measurable impact on the antibody levels induced in vaccinated monkeys. In contrast, with the Wyeth vaccines, the integration of IL-2 or IL-15 resulted in a slight increase (2-fold) in the antibody levels that was consistent and reproducible in the early phase of post vaccination. When the serum vaccinia neutralizing antibody levels were re-assessed three years later, except for three animals that displayed low yet detectable levels (a titer of 50 in animal #19251 vaccinated with Wyeth/IL-15, a titer of 25 in animal #48447 vaccinated with MVA/IL-2 and a titer of 25 in animal #10627 vaccinated with MVA/IL-15) in all other 14 animals no vaccinia neutralizing antibodies were detectable at the lowest serum dilution tested (1:10). Similarly, as shown in Table 1, except for the three animals with detectable anti-vaccinia neutralizing antibodies, all other 14 animals also lacked any detectable neutralizing antibodies against monkeypox virus, a closely related orthopoxvirus at 3 years post vaccination, just prior to being challenged with monkeypox virus.
We also evaluated vaccinia-specific CD8+ T cell responses in vaccinated monkeys longitudinally following vaccination. As shown in Fig. 4 , all animals vaccinated with the Wyeth viruses displayed measurable vaccinia-specific CD8+ T cell responses and the 3 animals vaccinated with Wyeth/IL-15 as a group had the most robust response 10 days post vaccination. This superior vaccinia-specific CD8+ T cell response induced by Wyeth/IL-15 was statistically significant at 1-month, 2-month and 3-month post vaccination over the parental Wyeth vaccine-induced CD8+ T cell response (p < 0.05). However, when tested at 6 months post vaccination we were unable to detect vaccinia specific CD8+ cells by this assay in any of the vaccinated animals. Of interest, we were also unable to detect any vaccinia-specific CD8+ T cell responses in any of the monkeys vaccinated with the MVA vaccinia viruses, starting at Day 10 post vaccination or anytime thereafter. Our observation of lack of any demonstrable anti-vaccinia CD8+ response following a single dose of MVA administration is consistent with a similar observation in humans who had received a single dose of MVA or a much attenuated response in monkeys [26], [30]. The absence of any detectable anti-vaccinia CD8+ responses after a single MVA vaccination contrasts with the robust vaccinia specific CD8+ T cell responses observed after multiple doses of MVA both in human subjects and non-human primates [22], [28], [30].
Fig. 4.

Induction of vaccinia specific, interferon gamma producing CD8+ T cells. Fresh peripheral blood mononuclear cells (PBMC) isolated from vaccinated animals at indicated time points (3 × 106cells) mixed with an equal number of irradiated (3000 rads) autologous PBMC that were infected with Wyeth vaccinia at a multiplicity of infection of 5 for 4 h prior to being irradiated. After 4 h of co-culture at 37 °C, Brefeldin A was added and incubation continued for further 8 h. Cells were then stained for surface CD3, CD8 and intracellular IFN gamma as described previously [6]. Cells stained with appropriate antibodies were analyzed on a FACSCalibur instrument (Becton Dickinson) using FloJo software.
Recent reports have indicated long term persistence of both cellular and humoral immune responses in Dryvax vaccinees for decades even after a single vaccination which is in contrast to what we observed here in cynomolgus monkeys perhaps reflecting species-specific differences in the longevity of vaccinia induced immune responses (reviewed in [31], [32]). Despite having very low (three animals with low yet detectable anti-monkeypox antibody titers) or no measurable immune activity against vaccinia or monkeypox, at three years post vaccination, when we challenged these monkeys intravenously with a high lethal dose of monkeypox virus (5 × 107 pfu of Zaire 79 strain) in a blinded study, a rapid rise (1000–10,000-fold) in serum anti-monkeypox antibody titers was observed in all vaccinated animals (sera collected 21 days post-challenge) except macaque #CY10621 (vaccinated with MVA/IL-2) that succumbed to monkeypox infection before the collection of serum reflecting a robust recall response in the cohort of vaccinated animals in our study (see Table 1). A commercial vaccinia immunoglobulin (VIg) preparation which displayed a PRNT 50% titer between 3413 and 6784 against monkeypox was included in the assay for comparison.
3.4. Post-challenge clinical observations
Post-challenge body temperatures for all study animals were monitored and recorded for the duration of the study as indicated in Table 2 . In general, the majority of the animals did not have temperatures greater than 103 °F with the exception of macaques #A13345 and #A13465 (both unvaccinated controls). These two animals had temperatures above 103 F at Day 3 post-challenge and returned to acceptable levels by Day 6 post-challenge. Macaque #A13465 (unvaccinated control) did have a slightly elevated temperature at the time of euthanasia (Day 11 post-challenge). But all macaques that progressed to fatal monkeypox disease [macaque #CY10621 (vaccinated with MVA/IL-2), and macaques #A13345, #A13465, and #A13778 (unvaccinated controls)] demonstrated peak elevated body temperatures at Day 3 post-challenge. Analgesics were not administered during these time points.
Table 2.
Post-challenge body temperature observations (F) for all study animals.
| Monkey ID | Study day |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day −1 | Day 0 | Day 3 | Day 6 | Day 9 | Day 11 | Day 12 | Day 15 | Day 18 | Day 21 | Day 24 | Day 27a | ||
| Wyeth | CY1990 | 101.5 | 103.0 | 98.8 | 98.6 | 101.9 | – | 100.2 | 100.3 | 99.9 | 99.4 | 98.9 | 101.1 |
| CY1992 | 100.5 | 100.7 | 101.4 | 102.3 | 101.1 | – | 100.7 | 99.9 | 99.7 | 99.7 | 98.9 | 100.9 | |
| CY128175 | 100.5 | 100.7 | 98.6 | 100.5 | 102.3 | – | 101.8 | 100.7 | 101.3 | 101.0 | 100.0 | 101.4 | |
| Wyeth/IL-2 | CY3856 | 99.2 | 101.4 | 99.4 | 100.7 | 100.8 | – | 99.6 | 99.9 | 99.2 | 98.8 | 96.8 | 99.4 |
| CY10619 | 98.6 | 101.9 | 101.8 | 100.2 | 100.5 | – | 100.1 | 100.9 | 98.5 | 98.5 | 97.7 | 100.0 | |
| CY66400 | 100.3 | 100.7 | 101.1 | 100.8 | 95.3 | – | 100.7 | 99.9 | 101.1 | 100.0 | 100.0 | 101.2 | |
| Wyeth/IL-15 | CY19248 | 101.0 | 102.6 | 101.1 | 100.9 | 102.0 | – | 99.5 | 100.3 | 101.1 | 99.0 | 100.2 | 101.3 |
| CY19249 | 101.0 | 101.2 | 101.1 | 100.5 | 101.5 | – | 100.7 | 101.4 | 100.3 | 99.4 | 99.5 | 100.7 | |
| CY19251 | 101.1 | 102.1 | 100.5 | 102.3 | 101.9 | – | 100.9 | 101.4 | 99.7 | 100.0 | 99.0 | 101.6 | |
| MVA | CY66393 | 100.5 | 101.2 | 101.5 | 100.4 | 101.6 | – | 98.8 | 99.3 | 98.4 | 98.7 | 97.8 | 98.9 |
| CY128497 | 101.3 | 100.8 | 100.4 | 100.0 | 101.8 | – | 100.0 | 101.3 | 100.6 | 99.3 | 98.7 | 98.3 | |
| MVA/IL-2 | CY10621 | 98.7 | 98.7 | 100.9 | 100.5 | 99.8a | – | – | – | – | – | – | – |
| CY19279 | 102.1 | 102.7 | 101.6 | 101.3 | 102.4 | – | 101.3 | 101.3 | 99.4 | 99.7 | 98.2 | 101.1 | |
| CY48447 | 100.1 | 100.9 | 100.3 | 98.0 | 101.4 | – | 100.0 | 99.7 | 99.0 | 98.6 | 97.8 | 98.9 | |
| MVA-IL-15 | CY3887 | 98.6 | 101.1 | 100.1 | 101.4 | 101.5 | – | 99.2 | 99.3 | 96.4 | 97.5 | 98.1 | 99.7 |
| CY10627 | 100.4 | 101.7 | 101.2 | 100.4 | 101.2 | – | 100.8 | 100.9 | 99.9 | 99.5 | 98.7 | 100.5 | |
| CY66406 | 100.3 | 101.1 | 101.4 | 100.6 | 100.7 | – | 100.9 | 101.1 | 99.2 | 98.2 | 98.8 | 100.6 | |
| Control | A13345 | 100.8 | 99.5 | 103.7 | 102.1 | 102.9a | – | – | – | – | – | – | – |
| A13465 | 102.2 | 101.2 | 103.7 | 100.8 | 102.9 | 103.3a | – | – | – | – | – | – | |
| A13778 | 100.8 | 101.1 | 102.8 | 100.1 | 100.3 | – | 100.3 | 99.4a | – | – | – | – | |
–: Not done.
Euthannized.
Post-challenge body weight data for all study animals are provided in Table 3 . All weights were monitored in kilograms (kg). All study animals were monitored for weight loss of greater than 4% (determined from Day 0 when monkeys were challenged). Three of the four animals that were euthanized prior to the completion of the study due to the progression of fatal disease [macaques #A13465, #A13778 (unvaccinated controls), and #CY10621 (MVA/IL-2)] demonstrated weight loss greater than 4% by Day 6. Curiously, animal #A13345 (unvaccinated control) had gained weight at the time of euthanasia. Interestingly, six other animals had demonstrable weight loss at Day 12 that persisted until the end of the study. Even with this pattern of weight loss, there was no apparent negative impact on the outcome of the disease course. It should be noted that several animals were placed on fluid support during the study to overcome dehydration.
Table 3.
Post-challenge weight observations (kg) for all study animals.
| Monkey ID | Study day |
Weight range (kg) ±4% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day −1 | Day 0 | Day 3 | Day 6 | Day 9 | Day 11 | Day 12 | Day 15 | Day 18 | Day 21 | Day 24 | Day 27a | |||
| Wyeth | CY1990 | 6.42 | 6.37 | 6.33 | 6.18 | 6.25 | – | 6.10 | 6.29 | 6.16 | 6.31 | 6.28 | 6.25 | 6.16–6.66 |
| CY1992 | 8.73 | 8.52 | 8.47 | 8.40 | 8.36 | – | 8.15 | 8.03 | 8.01 | 8.06 | 8.05 | 7.97 | 8.35–9.08 | |
| CY128175 | 7.36 | 7.30 | 7.21 | 7.08 | 7.24 | – | 7.17 | 7.16 | 7.22 | 7.16 | 7.24 | 7.18 | 7.06–7.66 | |
| Wyeth/IL-2 | CY3856 | 5.90 | 5.92 | 5.99 | 5.75 | 5.90 | – | 5.77 | 5.81 | 5.88 | 6.00 | 5.88 | 6.03 | 5.66–6.14 |
| CY10619 | 10.71 | 10.56 | 10.39 | 10.23 | 10.28 | – | 10.04 | 10.01 | 9.78 | 9.89 | 9.54 | 9.52 | 10.28–11.14 | |
| CY66400 | 8.95 | 8.84 | 8.68 | 8.44 | 8.66 | – | 8.46 | 8.59 | 8.28 | 8.33 | 8.15 | 8.07 | 8.59–9.31 | |
| Wyeth/IL-15 | CY19248 | 7.10 | 7.10 | 7.15 | 6.76 | 6.86 | – | 6.63 | 6.77 | 6.57 | 6.59 | 6.38 | 6.56 | 6.82–7.38 |
| CY19249 | 10.86 | 10.72 | 10.47 | 10.49 | 10.38 | – | 10.26 | 10.31 | 10.11 | 10.06 | 9.87 | 9.86 | 10.46–11.23 | |
| CY19251 | 7.94 | 7.73 | 7.78 | 7.78 | 7.93 | – | 7.89 | 7.80 | 7.84 | 7.92 | 7.90 | 7.95 | 7.62–8.26 | |
| MVA | CY66393 | 10.91 | 10.72 | 10.53 | 10.51 | 10.37 | – | 10.20 | 10.13 | 10.09 | 9.99 | 9.83 | 9.66 | 10.51–11.31 |
| CY128497 | 6.87 | 6.95 | 6.95 | 6.87 | 6.84 | – | 6.72 | 6.91 | 6.85 | 6.90 | 6.57 | 6.87 | 6.60–7.14 | |
| MVA/IL-2 | CY10621 | 7.98 | 8.02 | 7.66 | 7.62 | 7.37a | – | – | – | – | – | – | – | |
| CY19279 | 10.00 | 9.89 | 9.95 | 9.77 | 9.80 | – | 9.53 | 9.57 | 9.55 | 9.72 | 9.66 | 9.59 | 9.60–10.40 | |
| CY48447 | 6.58 | 6.57 | 6.53 | 6.47 | 6.55 | – | 6.25 | 6.43 | 6.53 | 6.56 | 6.58 | 6.54 | 6.32–6.84 | |
| MVA-IL-15 | CY3887 | 9.15 | 9.17 | 9.15 | 8.85 | 8.92 | – | 8.63 | 8.60 | 8.49 | 8.50 | 8.23 | 8.32 | 8.78–9.52 |
| CY10627 | 9.87 | 9.94 | 9.77 | 9.73 | 9.73 | – | 9.57 | 9.50 | 9.44 | 9.31 | 9.25 | 9.10 | 9.48–10.26 | |
| CY66406 | 8.54 | 8.32 | 8.33 | 8.22 | 8.48 | – | 8.12 | 8.24 | 8.30 | 8.47 | 8.02 | 8.32 | 8.20–8.88 | |
| Control | A13345 | 2.68 | 2.69 | 2.72 | 2.70 | 2.81a | – | – | – | – | – | – | – | 2.57–2.79 |
| A13465 | 2.64 | 2.56 | 2.53 | 2.43 | 2.45 | 2.41a | – | – | – | – | – | – | 2.53–2.75 | |
| A13778 | 2.76 | 2.83 | 2.81 | 2.64 | 2.56 | – | 2.42 | 2.41a | – | – | – | – | 2.65–2.87 | |
–: Not done. Bold = weight loss greater than 4% of body weight.
Euthannized.
3.5. Monkeypox virus lesion counts
Monkeypox virus lesion count data are provided in Table 4 . Animals with lesion counts greater than 200 were categorized as “Too Numerous to Count” (TNTC). Scabs, desquamations, and scars were counted as well; although, these were not considered as lesions. In general, lesions were not observed until Day 6 post-challenge. Six animals (30%) had greater than 200 counts or TNTC by Day 6. By Day 9, nine of the animals (45%) had TNTC lesions, whereas five (25%) had counts equal to or less than 100 lesions. It was observed that these four animals [#CY128175 (Wyeth vaccinated), #CY66406 (MVA/IL-15 vaccinated), #CY19251 (Wyeth/IL-15 vaccinated), #CY19249 (Wyeth/IL-15 vaccinated)] had fewer lesions which resolved sooner than the other animals. Four animals were euthanized on the following days: Macaques #A13345 (unvaccinated control with 100 lesions), and #CY10621 vaccinated with MVA/IL-2 at Day 9 (TNTC lesions); unvaccinated #A13465 at Day 11 (TNTC lesions); and unvaccinated #A13778 at Day 15 (TNTC lesions) due to complications from monkeypox virus infection. By Day 27, all vaccinated animals (94%) except one macaque vaccinated with MVA/IL-2 (#CY10621) had resolved their lesions and were protected from monkeypox virus infection, whereas all three unvaccinated control animals succumbed to monkeypox during the study period.
Table 4.
Monkeypox lesion count observations. Lesions counts >200 were categorized as “Too Numerous To Count” (TNTC).
| Monkey ID | Study day |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day −1 | Day 0 | Day 3 | Day 6 | Day 9 | Day 11 | Day 12 | Day 15 | Day 18 | Day 21 | Day 24 | Day 27a | ||
| Wyeth | CY1990 | 0 | 0 | 0 | 76 | TNTC | – | TNTC | TNTC (scabs) | 3 (scabs/desq) | Scabs/desq | Scabs/desq | Desq |
| CY1992 | 0 | 0 | 0 | TNTC | TNTC | – | TNTC | TNTC | Scabs/desq | Scabs/desq | Desq | 0 | |
| CY128175 | 0 | 0 | 0 | 34 | 34 | – | 33 | 33 | Scabs/desq | scabs/desq | Scabs/desq | Desq | |
| Wyeth/IL-2 | CY3856 | 0 | 0 | 0 | TNTC | 190 | – | 182 | 164 | Scabs/desq | Desq | 0 | 0 |
| CY10619 | 0 | 0 | 0 | TNTC | TNTC | – | TNTC | TNTC (scabs) | 85 (Scabs/desq) | Scabs/desq | Desq | Desq | |
| CY66400 | 0 | 0 | 0 | TNTC | 381 | – | TNTC | TNTC | TNTC (scabs/desq) | 18 (Scabs) | 18 (Scars) | 18 (Scars) | |
| Wyeth/IL-15 | CY19248 | 0 | 0 | 0 | TNTC | TNTC | – | TNTC | TNTC (scabs) | Scabs/desq | Scabs/desq | Desq | 0 |
| CY19249 | 0 | 0 | 0 | 2 | 14 | – | 14 | 14 | 14 | 14 | 0 | 0 | |
| CY19251 | 0 | 0 | 0 | 12 | 12 | – | 12 | Scabs | Desq | 0 | 0 | 0 | |
| MVA | CY66393 | 0 | 0 | 0 | 32 | TNTC | – | TNTC | TNTC (scabs) | 1 (Scabs/desq) | 1 (Scabs/desq) | Desq | Desq |
| CY128497 | 0 | 0 | 0 | 58 | 200 | – | TNTC | 172 (Scabs/desq) | Scabs/desq | Scabs/desq | Scabs/desq | 0 | |
| MVA/IL-2 | CY10621 | 0 | 0 | 0 | TNTC | TNTCa | – | – | – | – | – | – | – |
| CY19279 | 0 | 0 | 0 | 93 | TNTC | – | 146 | 111 (Scabs/desq) | Scabs/desq | Scabs/desq | Desq | 0 | |
| CY48447 | 0 | 0 | 0 | 196 | TNTC | – | 182 | 165 (Scabs) | 3 (Desq) | Scabs/desq | 0 | 0 | |
| MVA-IL-15 | CY3887 | 0 | 0 | 0 | 130 | TNTC | – | TNTC | 153 (Scabs) | 3 (Scabs/desq) | Scabs/desq | Scabs/desq | Desq |
| CY10627 | 0 | 0 | 0 | 173 | TNTC | – | TNTC | Scabs/desq | Scabs/desq | Desq | Desq | Desq | |
| CY66406 | 0 | 0 | 0 | 44 | 63 | – | 51 | 46 (Scabs) | Scabs/desq | Desq | 0 | 0 | |
| Control | A13345 | 0 | 0 | 0 | 30 | 100a | – | – | – | – | – | – | – |
| A13465 | 0 | 0 | 0 | 23 | 187 | TNTCa | – | – | – | – | – | – | |
| A13778 | 0 | 0 | 0 | 39 | 166 | – | TNTC | TNTCa | – | – | – | – | |
–: Not done.
Euthannized.
3.6. Monkeypox viral loads in plasma of challenged macaques
Post-challenge quantitative pan-orthopoxvirus DNA genome data are provided in Table 5 . The lower limit of detection of viral loads was 5000 DNA genome copies per milliliter of blood. By Day 3 post-challenge, five animals (25%) had detectable viral loads that ranged from 1.4 × 104 to 1.8 × 106 genome copies/milliliter. It was observed that by Day 6, only four animals [CY19249 (Wyeth/IL-15), CY19251 (Wyeth/IL-15), CY1990 (Wyeth), CY128497 (MVA)] had virus levels below the detection threshold. Interestingly, three of these four animals (CY19251, CY1990, and CY128497) did not have detectable viral loads during the entire course of the study even though they were vaccinated over three years earlier. Four animals were euthanized on the following days: A13345 and CY10621 at Day 9 (viral load: 7.2 × 107 and 9.2 × 106 respectively); A13465 at Day 11 (viral load: 8.0 × 108); and A13778 at Day 15 (peak viral load: 1.2 × 108 on Day 12) because of moribund monkeypox disease. By Day 15 all surviving animals had undetectable plasma viral loads with the exception of #A13778. This animal had DNA copies of 5.1 × 106 at Day 15 and was euthanized due to complications from monkeypox virus infection. At Day 27, all surviving animals had subsequently cleared the virus and were euthanized humanely at the termination of the study. An important unanticipated observation was that the lesion count did not exactly correlate with the magnitude of the circulating viremia as manifested by animals #CY1990 (Wyeth) and CY128497 (MVA) in whom repeated longitudinal assessment of plasma monkeypox viral DNA revealed no detectable viral DNA above the base-line threshold despite both animals developing more than 200 (TNTC) skin lesions during the post-challenge observation period. Although there are not many reported studies where careful assessment of plasma monkeypox viral loads have been performed and correlated with skin lesions, in a recent report Earl et al. [26], demonstrated a reasonable concordance between the plasma viral load and skin lesions. Also it is important to note that even in the present study, except for these two animals, all other animals did show a fairly good correlation between the number of skin lesions developed and the plasma viral loads detected. Therefore, our observation that certain animals manifesting skin lesions without detectable plasma viremia deserves further investigation.
Table 5.
Viral load measured by quantitative PCR and expressed in genome copies/milliliter of blood.
| Monkey ID | Study day |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day −1 | Day 0 | Day 3 | Day 6 | Day9 | Day 11 | Day 12 | Day 15 | Day 18 | Day 21 | Day 24 | Day 27a | ||
| Wyeth | CY1990 | <5000 | <5000 | <5000 | <5000 | <5000 | – | <5000 | <5000 | <5000 | <5000 | <5000 | <5000 |
| CY1992 | <5000 | <5000 | <5000 | 110,800 | 48,000 | – | <5000 | <5000 | <5000 | <5000 | <5000 | <5000 | |
| CY128175 | <5000 | <5000 | <5000 | 7120 | 12,960 | – | <5000 | <5000 | <5000 | <5000 | <5000 | <5000 | |
| Wyeth/IL-2 | CY3856 | <5000 | <5000 | <5000 | 99,600 | 19,360 | – | <5000 | <5000 | <5000 | <5000 | <5000 | <5000 |
| CY10619 | <5000 | <5000 | <5000 | 59,200 | 38,0000 | – | 11,720 | <5000 | <5000 | <5000 | <5000 | <5000 | |
| CY66400 | <5000 | <5000 | 15,580 | 26,6000 | <5000 | – | <5000 | <5000 | <5000 | <5000 | <5000 | <5000 | |
| Wyeth/IL-15 | CY19248 | <5000 | <5000 | <5000 | 21,0000 | 16,540 | – | 13,060 | <5000 | <5000 | <5000 | <5000 | <5000 |
| CY19249 | <5000 | <5000 | 39,0000 | <5000 | <5000 | – | <5000 | <5000 | <5000 | <5000 | <5000 | <5000 | |
| CY19251 | <5000 | <5000 | <5000 | <5000 | <5000 | – | <5000 | <5000 | <5000 | <5000 | <5000 | <5000 | |
| MVA | CY66393 | <5000 | <5000 | <5000 | 24,400 | 34,600 | – | 91,800 | <5000 | <5000 | <5000 | <5000 | <5000 |
| CY128497 | <5000 | <5000 | <5000 | <5000 | <5000 | – | <5000 | <5000 | <5000 | <5000 | <5000 | <5000 | |
| MVA/IL-2 | CY10621 | <5000 | <5000 | <5000 | 6300 | 9,240,000a | – | – | – | – | – | – | – |
| CY19279 | <5000 | <5000 | <5000 | 20,800 | 29,600 | – | <5000 | <5000 | <5000 | <5000 | <5000 | <5000 | |
| CY48447 | <5000 | <5000 | <5000 | 5620 | 12,680 | – | 16,920 | <5000 | <5000 | <5000 | <5000 | <5000 | |
| MVA-IL-15 | CY3887 | <5000 | <5000 | <5000 | 11,040 | 202,000 | – | 117,400 | <5000 | <5000 | <5000 | <5000 | <5000 |
| CY10627 | <5000 | <5000 | <5000 | 10,460 | 592,000 | – | 35,6000 | <5000 | <5000 | <5000 | <5000 | <5000 | |
| CY66406 | <5000 | <5000 | <5000 | 10,580 | 21,000 | – | <5000 | <5000 | <5000 | <5000 | <5000 | <5000 | |
| Control | A13345 | <5000 | <5000 | 17,0000 | 17,000,000 | 72,000,000a | – | – | – | – | – | – | – |
| A13465 | <5000 | <5000 | 14,020 | 2,400,000 | 17,740,000 | 802,000,000a | – | – | – | – | – | – | |
| A13778 | <5000 | <5000 | 1,792,000 | 3,320,000 | 38,800,000 | – | 116,800,000 | 5,120,000a | – | – | – | – | |
–: Not done.
Euthannized.
Although the primary objective of integrating IL-15 into the Wyeth strain of vaccinia was to reduce its residual virulence, the observation that out of four macaques that developed the least number of lesions (less than 100) throughout the post-challenge study period, three had received a vaccine with integrated IL-15, suggests that expressed IL-15 is still able to improve the efficacy of a vaccine considered a gold standard for effective infectious disease vaccines that were ever developed. However, to conclusively prove that the integration of IL-15 further enhances the efficacy of a vaccine that is already highly efficacious, an adequately powered study with a larger cohort is necessary. In contrast, the integration of IL-2 did not seem to enhance the efficacy of the Wyeth vaccine. Because the only vaccine break-through that resulted in the death of a vaccinated animal occurred with an IL-2 integrated vaccine (macaque #CY10621, MVA/IL-2), it is likely that the integration of IL-2 unlike IL-15 over-attenuates vaccinia viruses. Relevant in this regard is our previous observation in mice that the immune responses generated in the presence of IL-2 tend to be short-lived [29].
There has been considerable interest and enthusiasm for advancing third generation smallpox vaccines such as LC16M8 and MVA as optimal smallpox vaccines for contemporary populations [8], [33]. These vaccines have been administered safely to individuals even with immune deficiencies for whom the use of Drvvax or ACAM 2000 is contraindicated [8]. However, unlike Dryvax, proof that these attenuated vaccines can protect against smallpox under field conditions is lacking. Several recent macaque studies with monkeypox challenges, a relevant non-human primate model for smallpox, indicate that two doses of MVA can confer protection that equals Dryvax conferred protection [22], [23], [24], [25], [26], [27], [28]. However, in almost all of these studies the dose of MVA used was 100–1000-fold higher than the dose of Dryvax used [22], [26]. In addition, these studies utilized an intradermal route for Dryvax and an intramuscular route for MVA. In the present study, by administering both Dryvax and MVA identically with respect to both route (intradermal) and dose (1 × 108 pfu), we demonstrate that a single dose of MVA can confer long lasting protection for over 3 years that is no different than the protection elicited by a single equivalent dose of Wyeth vaccinia against monkeypox disease. It is possible that the intradermal route of vaccination for MVA that we have used in the present study which differs from most other MVA studies in non-human primates, elicits a qualitatively superior immune response than the intramuscular route because of the potential involvement of dermal Langerhans cells as has been recently shown for MVA and other vaccines [34], [35], [36].
Because of the uncertainty as to how correlative these protective studies with other orthopoxviral infections in animal models will be to smallpox caused by V. major virus in humans, our focus has been to attenuate the residual virulence of a vaccine that has proven to be efficacious against smallpox in both pre-exposure and post-exposure field settings. The integration of IL-15 in to Wyeth vaccinia not only attenuates its virulence as shown in Fig. 2 by in vivo imaging studies in immune deficient nude mice, but also improves its efficacy as demonstrated in this study in non-human primates against monkeypox disease. These results combined with our previous observation of its superior efficacy against a lethal intranasal vaccinia infection in mice merit its consideration as an effective and safe smallpox vaccine for contemporary populations.
Acknowledgments
This work was in part supported by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research, NIH and by a 3-year competitive research funding award to L.P.P. from the Trans-NIH/FDA Intramural Biodefense Program.
References
- 1.Fenner F., Henderson D.A., Arita I., Jecek Z., Ladnyi I.D. World Health Organization; Geneva, Switzerland: 1988. Smallpox and its eradication. History of International Public Health. [Google Scholar]
- 2.Fenner F. Risks and benefits of vaccinia vaccine use in the worldwide smallpox eradication campaign. Res Virol. 1989;140:465–466. doi: 10.1016/s0923-2516(89)80126-8. [DOI] [PubMed] [Google Scholar]
- 3.Poland G.A., Grabenstein J.D., Neff J.M. The US smallpox vaccination program: a review of a large modern era smallpox vaccination implementation program. Vaccine. 2005;23:2078–2081. doi: 10.1016/j.vaccine.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Lane J.M., Ruben F.L., Neff J.M., Millar J.D. Complications of smallpox vaccination, 1968. N Engl J Med. 1969;281(22):1201–1208. doi: 10.1056/NEJM196911272812201. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg R.N., Kennedy J.S. ACAM2000: a newly licensed cell culture-based live vaccinia smallpox vaccine. Expert Opin Investig Drugs. 2008;17(4):555–564. doi: 10.1517/13543784.17.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perera L.P., Waldmann T.A., Mosca J.D., Baldwin N., Berzofsky J.A., Oh S.K. Development of smallpox vaccine candidates with integrated interleukin-15 that demonstrate superior immunogenicity, efficacy, and safety in mice. J Virol. 2007;81(16):8774–8783. doi: 10.1128/JVI.00538-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander W.A., Moss B., Fuerst T.R. Regulated expression of foreign genes in vaccinia virus under the control of bacteriophage T7 RNA polymerase and the Escherichia coli lac repressor. J Virol. 1992;66(5):2934–2942. doi: 10.1128/jvi.66.5.2934-2942.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy J.S., Greenberg R.N. IMVAMUNE: modified vaccinia Ankara strain as an attenuated smallpox vaccine. Expert Rev Vaccines. 2009;8(1):13–24. doi: 10.1586/14760584.8.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Midgley C.M., Putz M.M., Weber J.N., Smith G.L. Vaccinia virus strain NYVAC induces substantially lower and qualitatively different human antibody responses compared with strains Lister and Dryvax. J Gen Virol. 2008;89(Pt 12):2992–2997. doi: 10.1099/vir.0.2008/004440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buller R.M.L., Palumbo GJ . In: Recombinant poxviruses. Binns M.M., Smith G.L., editors. CRC Press; Boca Raton, FL: 1992. Safety and attenuation of vaccinia viruses; pp. 235–267. [Google Scholar]
- 11.Hung C.F., Tsai Y.C., He L., Coukos G., Fodor I., Qin L., et al. Vaccinia virus preferentially infects and controls human and murine ovarian tumors in mice. Gene Ther. 2007;14(1):20–29. doi: 10.1038/sj.gt.3302840. [DOI] [PubMed] [Google Scholar]
- 12.Waldmann T.A. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 13.Rochman Y., Spolski R., Leonard W.J. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9(7):480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hügin A.W., Flexner C., Moss B. Clearance of recombinant vaccinia virus expressing IL-2: role of local host immune responses. Cell Immunol. 1993;152(2):499–509. doi: 10.1006/cimm.1993.1307. [DOI] [PubMed] [Google Scholar]
- 15.Perera L.P., Goldman C.K., Waldmann T.A. Comparative assessment of virulence of recombinant vaccinia viruses expressing IL-2 and IL-15 in immunodeficient mice. Proc Natl Acad Sci USA. 2001;98(9):5146–5151. doi: 10.1073/pnas.081080298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gronvall G.K., Trent D., Borio L., Brey R., Nagao L. Alliance for biosecurity. The FDA animal efficacy rule and biodefense. Nat Biotechnol. 2007;25(10):1084–1087. doi: 10.1038/nbt1007-1084. [DOI] [PubMed] [Google Scholar]
- 17.Food and Drug Administration HHS New drug and biological drug products; evidence needed demonstrate effectiveness of new drugs when human efficacy studies are not ethical or feasible. Final rule. Fed Regist. 2002;67(105):37988–37998. [PubMed] [Google Scholar]
- 18.Flexner C., Hügin A., Moss B. Prevention of vaccinia virus infection in immunodeficient mice by vector-directed IL-2 expression. Nature. 1987;330(6145):259–262. doi: 10.1038/330259a0. [DOI] [PubMed] [Google Scholar]
- 19.Ramshaw I.A., Andrew M.E., Phillips S.M., Boyle D.B., Coupar B.E. Recovery of immunodeficient mice from a vaccinia virus/IL-2 recombinant infection. Nature. 1987;329(6139):545–546. doi: 10.1038/329545a0. [DOI] [PubMed] [Google Scholar]
- 20.Flexner C., Moss B., London W.T., Murphy B.R. Attenuation and immunogenicity in primates of vaccinia virus recombinants expressing human interleukin-2. Vaccine. 1990;8(1):17–21. doi: 10.1016/0264-410x(90)90171-h. [DOI] [PubMed] [Google Scholar]
- 21.Ruby J., Brinkman C., Jones S., Ramshaw I. Response of monkeys to vaccination with recombinant vaccinia virus which coexpress HIV gp160 and human interleukin-2. Immunol Cell Biol. 1990;68(Pt 2):113–117. doi: 10.1038/icb.1990.16. [DOI] [PubMed] [Google Scholar]
- 22.Earl P.L., Americo J.L., Wyatt L.S., Eller L.A., Whitbeck J.C., Cohen G.H., et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428(6979):182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 23.Stittelaar K.J., van Amerongen G., Kondova I., Kuiken T., van Lavieren R.F., Pistoor F.H., et al. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J Virol. 2005;79(12):7845–7851. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edghill-Smith Y., Golding H., Manischewitz J., King L.R., Scott D., Bray M., et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11(7):740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 25.Marriott K.A., Parkinson C.V., Morefield S.I., Davenport R., Nichols R., Monath T.P. Clonal vaccinia virus grown in cell culture fully protects monkeys from lethal monkeypox challenge. Vaccine. 2008;26(4):581–588. doi: 10.1016/j.vaccine.2007.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Earl P.L., Americo J.L., Wyatt L.S., Espenshade O., Bassler J., Gong K., et al. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc Natl Acad Sci USA. 2008;105(31):10889–10894. doi: 10.1073/pnas.0804985105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao L., Huang D., Wei H., Wang R.C., Chen C.Y., Shen L., et al. Expansion, reexpansion, and recall-like expansion of Vgamma2Vdelta2 T cells in smallpox vaccination and monkeypox virus infection. J Virol. 2009;83(22):11959–11965. doi: 10.1128/JVI.00689-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nigam P., Earl P.L., Americo J.L., Sharma S., Wyatt L.S., Edghill-Spano Y., et al. DNA/MVA HIV-1/AIDS vaccine elicits long-lived vaccinia virus-specific immunity and confers protection against a lethal monkeypox challenge. Virology. 2007;366(1):73–83. doi: 10.1016/j.virol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh S., Berzofsky J.A., Burke D.S., Waldmann T.A., Perera L.P. Coadministration of HIV vaccine vectors with vaccinia viruses expressing IL-15 but not IL-2 induces long-lasting cellular immunity. Proc Natl Acad Sci USA. 2003;100(6):3392–3397. doi: 10.1073/pnas.0630592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Precopio M.L., Betts M.R., Parrino J., Price D.A., Gostick E., Ambrozak D.R., et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med. 2007;204(6):1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amanna I.J., Slifka M.K., Crotty S. Immunity and immunological memory following smallpox vaccination. Immunol Rev. 2006;211:320–337. doi: 10.1111/j.0105-2896.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 32.Taub D.D., Ershler W.B., Janowski M., Artz A., Key M.L., McKelvey J., et al. Immunity from smallpox vaccine persists for decades: a longitudinal study. Am J Med. 2008;121(12):1058–1064. doi: 10.1016/j.amjmed.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saito T., Fujii T., Kanatani Y., Saito M., Morikawa S., Yokote H., et al. Clinical and immunological response to attenuated tissue-cultured smallpox vaccine LC16m8. JAMA. 2009;301(10):1025–1033. doi: 10.1001/jama.2009.289. [DOI] [PubMed] [Google Scholar]
- 34.Prausnitz M.R., Mikszta J.A., Cormier M., Andrianov A.K. Microneedle-based vaccines. Curr Top Microbiol Immunol. 2009;333:369–393. doi: 10.1007/978-3-540-92165-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Künzi V., Klap J.M., Seiberling M.K., Herzog C., Hartmann K., Kürsteiner O., et al. Immunogenicity and safety of low dose virosomal adjuvanted influenza vaccine administered intradermally compared to intramuscular full dose administration. Vaccine. 2009;27(27):3561–3567. doi: 10.1016/j.vaccine.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 36.Liu L., Zhong Q., Tian T., Dubin K., Athale S.K., Kupper T.S., et al. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med. 2010;16(2):224–227. doi: 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]


