Abstract
Mice are excellent subjects for use of genetic-manipulation techniques to study the basis of pathological and normal physiology and behavior; however behavioral analyses of associated phenotypes is often limited. To improve the accuracy and specificity of repeated measurements of vestibular function, we developed a miniaturized, contact-lens scleral search coil to measure mouse eye movements. We describe the physical attributes and document its functionality by measuring vestibulo-ocular responses in normal mice. This coil should greatly improve the sensitivity and documentation of vestibular dysfunction in mouse models of pathology and dysfunction while allowing screening of significant numbers of subjects.
Keywords: Eye coil, vestibular assessment, oculomotor function, screening, testing
INTRODUCTION
In recent years, there has been an explosion in the application of genetics and molecular genetic techniques to studies ranging from the etiology of disease to motor learning. One area where there is tremendous potential for advancement is in the correlation of genetic deficits and manipulations (e.g., knockouts) with a variety of functions and dysfunctions (i.e., phenotypes), especially those related to the sophisticated behaviors of higher animals and man. Unfortunately, animals that lend themselves to genetic manipulation often do not display the same important behaviors seen in higher animals. One solution is to attempt to bridge that gap by performing the genetic manipulations in animals that lend themselves to such techniques (e.g., mice) and correlate the resulting mouse phenotypes with those in higher animals (e.g., primates and man) where more sophisticated behavioral tests can be applied, much more is known about the underlying physiological mechanisms, and clinically relevant dysfunctions have been extensively characterized. The closer the match between the mouse and human phenotypes, the greater the confidence that the mouse model is appropriate.
Vestibular function is an area where genetic techniques are beginning to be applied to both basic research and clinical pathology using mouse models. Unfortunately, the most commonly used tests for vestibular dysfunction in mouse clinical models are not specific. For example, the rotorod test is currently used to assess vestibular and motor function (e.g., Drago et al., 1996; Rampello and Drago, 1999; Deiss et al., 2000; Fasipe et al., 2004; Jones et al., 2004). It uses an apparatus that consists of a thin horizontally oriented spinning rod with disks that define test compartments. The test subject must run perpendicular to the axis of rotation to keep from falling off. Clearly, such a crude test is not designed to differentiate between vestibular function and ataxia or other motor deficits, visual and other non-vestibular sensory deficits, disorientation, or any other of a variety of potential contributing factors. Other common tests are the murine swimming test, which is essentially a sink or swim test (e.g., Kaiser et al., 2001; Kaartinen et al., 2002), and the drop test (Bergstrom et al., 1998; Murakami et al., 2002) that asks whether the mice land on their feet when knocked from a perch. All of these measures have been represented as tests of vestibular and even otolith function but involve a lot more. Such tests are, at best, relatively crude and do not lend themselves to quantification of deficits. In addition, similar tests are not performed on human subjects, for obvious reasons. In contrast, there are a number of well-defined tests of vestibular function that are used currently in humans and in non-human primates. These tests have also been applied in mice, but they require a high degree of technical sophistication due to the imaging and surgical techniques used (Stahl et al., 2000; Harrod and Baker, 2003). It is clear that tests commonly employed in testing of human and non-human primate vestibular physiology would improve greatly the description of the vestibular deficits in genetically manipulated mice and allow comparison between the mouse phenotypes and the plethora of knowledge about primate normal vestibular function and vestibular dysfunction in humans. It is also clear that a simplification of the techniques used would make this approach more accessible to the wider community.
Eye movements offer a unique opportunity to assess vestibular function quantitatively and precisely. In addition, because of the abundance of information regarding the underlying physiology and anatomy of vestibulo-ocular function, quantitative measurements can point immediately to potential underlying etiology. Unfortunately, methods used by basic scientists to study mouse eye movements require time-consuming and delicate surgery (Stahl et al. 2000) or result in highly variable eye movements that require extensive statistical manipulation and estimation in order reflect the actual eye movement (van Alphen and De Zeeuw 2002).
While eye movement measurement in mice has come a long way since simple observation (Mitchner et al. 1976) initial improvements were inherently noisy and inconsistent (electro-oculography; e.g., Grusser-Cornehls and Böhm 1988) or painstaking (video tape; e.g., Mangini et al. 1985). Even modern techniques like the scleral search coil (Fuchs and Robinson 1966) or high-speed video recording (e.g., Satakani and Isa 2004) have not been easily adapted to recording mouse eye movements. In fact, results using video techniques give different answers than those using coils (Stahl et al. 2000) and, worse, it depends on who is constructing the coils. Coil measurements of the gain of the vestibulo-ocular reflex (VOR), measured for sinusoidal stimulation at 0.5Hz, can range from values of ~0.15 ± 0.1 (Boyden and Raymond 2003) to 0.51 ± 0.09 (Harrod and Baker 2003) to ~0.6 ± 0.08 (Stahl et al. 2000). Thus, at least a portion of the inconsistencies between studies reflects the inability to quantitatively measure and specify the actual behavioral deficit. In addition, few screening studies would be willing to take the time or put in the effort necessary to develop workable mouse coils (Boyden and Raymond 2003; Harrod and Baker 2003; Stahl et al. 2000).
Our aim was to provide an incrementally improved technique for use with clinically relevant mouse models that would provide more comparable (to primate), quantitative data and be easier to use. Thus, we have developed a new scleral coil for eye movement measurement in mice that is simple to use and could be used for screening of genetically manipulated mice in large enough numbers to provide statistically meaningful comparisons. In addition such a device may allow a more detailed comparison of the phenotypes of mice, monkey, and man to assure their congruence.
There are several limitations of current methods that use coils. The first is the mass of the coil, which is significant when compared to the mass of the mouse globe (~18 mg on average; Zhou and Williams 1999). The second is the volume of the coils currently being produced which creates additional drag that impedes eye movement. A third is the problem of affixing the coil to the eye. A fourth is that the current types either occlude vision (e.g., Harrod and Baker, 2003) or require surgical implantation in a side pouch next to the muscles (e.g., Boyden and Raymond 2003) that may interfere with the movement of the eye. Finally, the construction of current coils is also a limitation because of the difficulty in manufacturing the coils consistently (J Baker, personal communication). While vestibulo-ocular scientists have been successful in dealing with these limitations, they form a major impediment to use by researchers who are simply trying to establish a quantitative phenotype. Here, we describe a new coil that should allow not only more quantitative and repeatable measurements but also overcome many of the previous limitations, especially manufacture and implantation, so that clinical researchers will find them straightforward to use.
The rationale behind our approach was to take advantage of modern electronic manufacturing techniques to produce an eye coil that was light enough not to load the mouse eye, annular so as not to obscure vision completely, and simple to apply. In addition, to facilitate and encourage adoption by researchers who work with genetic manipulation of mice but are not necessarily familiar with measuring mouse eye movements, we wanted something that could be used with existing eye movement measurement systems currently available in dozens of labs and clinics around the world.
To validate our methods, the second part of this description details the testing of vestibular and optokinetic reflexes in which we measured murine VOR for comparison with previous studies.
MATERIALS AND METHODS
Eye Coil
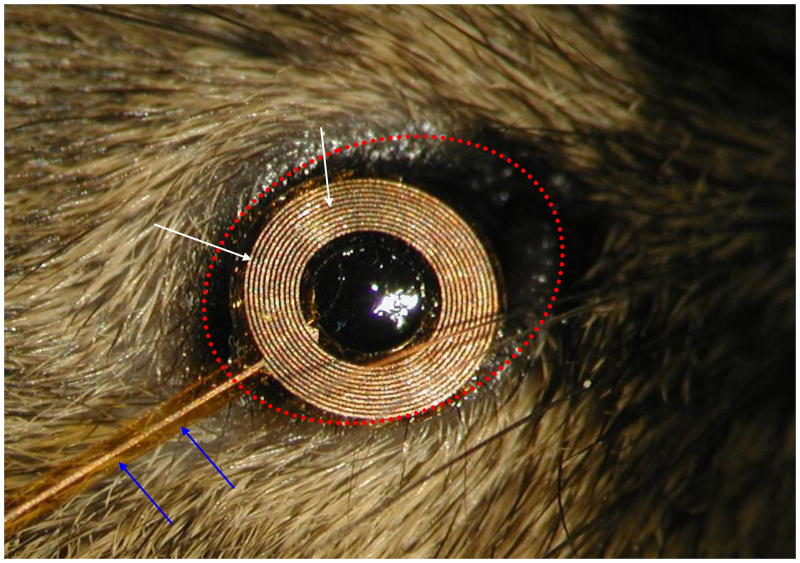
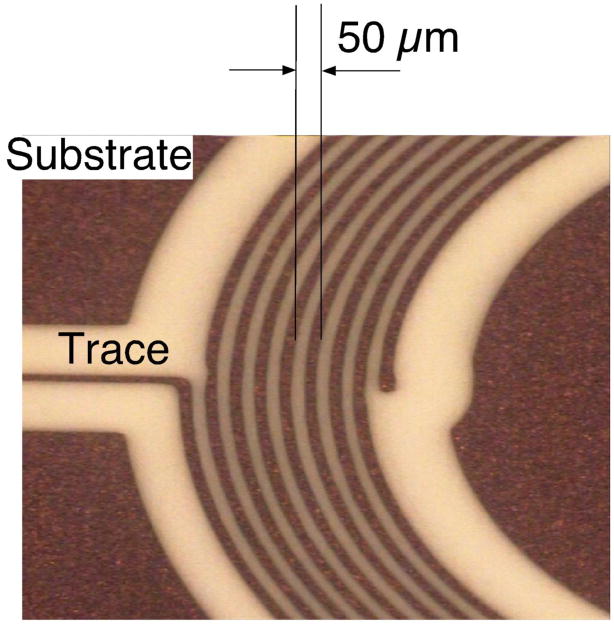
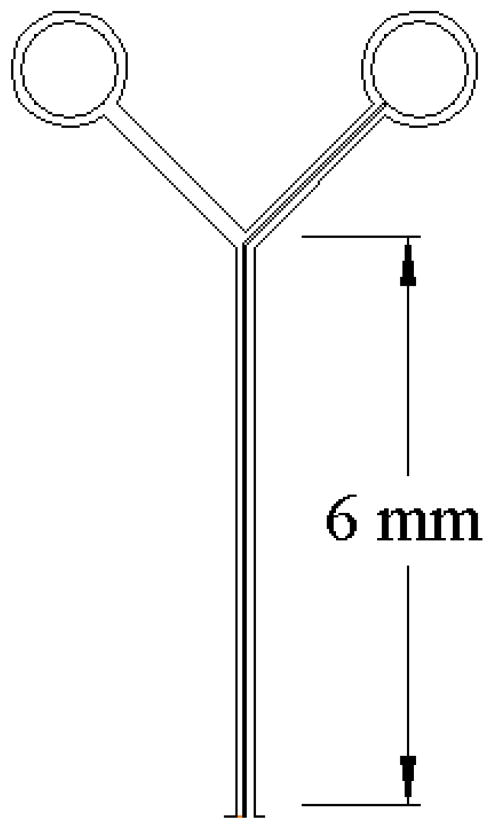
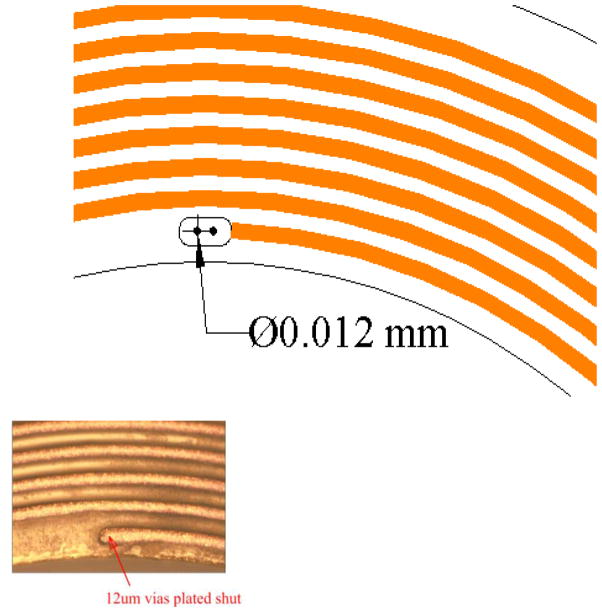
Our initial requirements for the mouse coil specified an annulus of outside diameter of 2.8 mm with 10 turns on each side and leads that we could solder to (Fig. 1). This had to be modified in the course of testing in 3 significant ways. First, because most mice extrude their globe when restrained, thus protruding the cornea, and because the diameter of the globe is 3.2 mm (Katoh et al. 1998), the initially flat annulus had to be curved significantly to fit the curvature of the eye. The “target” curvature was 3/32″ (~.094″) but after thermoform it was closer to 0.080″. Second, the diameter was reduced slightly, to avoid the eyelids, by reducing the number of turns to 8 on each side, 16 total. Third, the leads were altered because they were too stiff. The substrate was trimmed, the traces were reduced in width and the overall lead (Fig. 1, blue arrows) was shortened. We used silver paint (Silver Print 842, MG Chemicals, Toronto, Canada; www.abra-electronics.com/catalog/chemicals/mg842_20.html) to attach very flexible lightweight wire (50 Ga) to act as a flexible coupling for the electrical connection with the preamplifier headstage. A superior contact resulted when the paint was thinned slightly with methyl methacrylate. We used enamel coated magnet wire (Fig. 2, blue arrows) from an old spool (insulated, awg 50) we had, but nearly any fine wire would serve the same purpose for strain relief. This allowed us to keep load on the eye to a minimum and improved unrestricted movement. After many modifications and iterations of testing, the current MicroConnex (http://www.microconnex.com/) mouse eye coil is constructed as follows. It starts out as a flat double-sided coil with a total of 16 turns. Traces on the coil are 6 μm thick, 25 μm wide, with a 50 μm pitch (Fig. 3). The traces are wound so that they are continuous from the outside edge of one side of the annulus to the inside in an inward spiral, through a via in the substrate to the other side (making electrical connection between the sides), and then from the inside of the other side of the annulus to the outside edge. The turns are wound in opposite directions so that the signals add. MicroConnex formed the traces with a variation of the print-and-etch process. The leads from the coil to solder pads (Fig. 2) come off the outer edge of each side and are 35 μm wide, with the lead substrate being 0.15 mm in width and 8 mm in length, including a branched section leading to two solder pads of 1.0 mm diameter (Fig. 4). The two sides are connected by 12 μm vias between two opposing 40 μm × 85 μm pads (oval, Fig. 5) to electrically connect the coil layers on each side (Fig. 5). The Cu coil is electrodeposited on a 25 μm liquid crystal polymer (LCP) substrate, with an approximately 15-μm thick polyimide covercoat, applied by MicroConnex in liquid form to insulate and electrically isolate the traces from the globe. The coil is then thermoformed to fit the sclera. The final weight average ~1.1mg (n=10), or less than 10% the mass of the globe (Zhou and Williams 1999).
Fig. 1.
Photomicrograph of prototype eye coil laid on mouse eye. Note, even the prototype fits without major obstruction of the lids (dotted red oval). The substrate under the lead wires (blue arrows) can be made out and was trimmed to improve flexibility. The turns of the coil (white arrows) are clearly visible at this magnification.
Fig. 2.
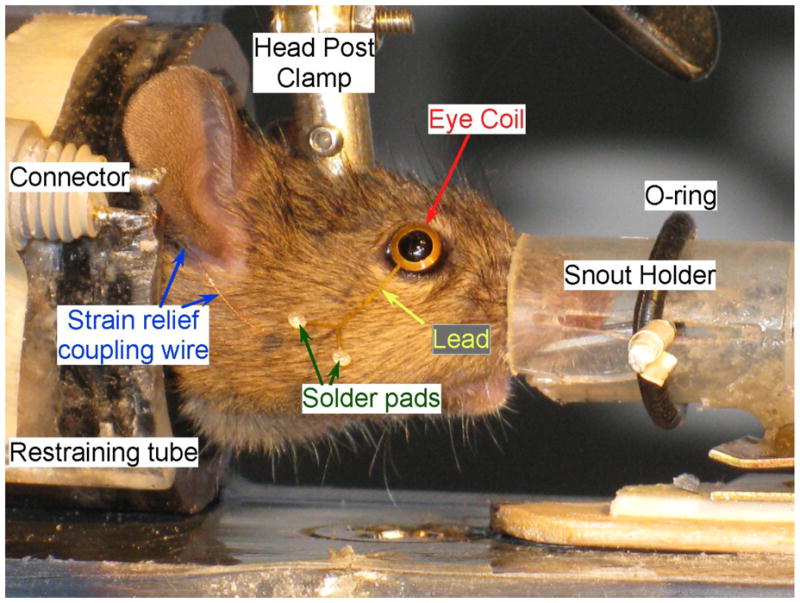
Testing setup. Lower power photo of mouse in restraining tube with homemade snout holder to deliver gas anesthesia (not shown) while coil is placed on the eye. The O-ring holds the nose in the modified syringe by securing the paper clip (barely visible) that slides in the slots. The strain relief-connecting wire leads from the solder pads to the connector and from there to the headstage. The curvature of the eye coil can be seen in this photo compared to the original in Fig. 1.
Figure 3.
Photomicrograph of the eye coil traces. Sector showing the dimensions of the mouse eye coil. The outer turn is initially imaged larger, since it is exposed to more etchant and tends to etch faster.
Figure 4.
Detail of the coil lead traces and their connection to the soldering pads that come off the ends of each trace, one on each side.
Figure 5.
Detail of the via for connecting traces on both sides to form the continuous coil. Inset, photomicrgraph of via.
Preparation
To secure the mouse for testing, we restricted its movement in two ways. We minimized head movement by securing a head bolt (Fig. 2) made of a nylon flat-head screw (2-56, 3/8″). The screw was filed down to a minimum head thickness and attached to the skull, head-down, with 000 stainless steel screws (TX000-1-1/2; http://www.smallparts.com) and dental acrylic during a clean surgery under isoflurane anesthesia. We restricted body movement using a modified “box and collar restrain system”, consisting of an acrylic tube (Fig. 2) fit with a rubber collar (instead of an Elizabethan collar) on the head end and a plunger with a tail notch on the other (not shown). When the tube was closed, the grommet fit snugly around the mouse’s neck so that it could neither withdraw into the tube nor escape out of it. The plunger adjusted to fit the mouse snuggly in the grommet end of the tube and was notched so that the tail could protrude backward out of the tube. The tube was split in half and hinged on one side to allow for easy insertion of the mouse and it was closed with velcro tape (http://www.velcro.com) for quick, secure encasement. We used initially, a 60 cc disposable syringe that worked nearly as well but was not as easy to clean.
To secure the mouse and apply the coil to the mouse eye, the animal is initially anesthetized with isoflurane via a standard nose cone (e.g., A5-01-0525; http://www.summitmedicalequipment.com/page22.html) and then placed into the acrylic tube. The mouse was placed into the field coil on a testing platform and the tube was held in place with a rubber band. Anesthesia was maintained by administering isofluorane through the snout holder/bite bar (e.g., MyNeurolab #462952; http://www.myneurolab.com/myneurolab/default.asp) while the head was stabilized using the head post and the eye coil was applied. We actually preferred a homemade snout grabber made of a disposable 3 cc plastic syringe and a bent paper clip (Fig. 2). Before placing the coil the eye was numbed using 1 drop of Proparacaine Hydrochloride solution (0.5%, Falcon Pharmaceuticals; http://www.falconpharma.com) directly on the eye for 30 s.
The eye coil was placed on the eye using no- or low-power magnification by laying it on the sclera. With some adjustment, the fit of the coil was sufficiently contoured to produce reasonable and consistent signals (see RESULTS), perhaps because the protruding cornea helped to maintain the position of the coil. Adjustments during placement usually included avoiding the lashes by lifting them away from the coil and centering the coil so that the coil avoided the lids. Nevertheless, if the mouse closed it’s eye fully, either voluntarily or during a blink, the coil was often dislodged and had to be replaced on the eye. The coil lead was positioned to avoid the whiskers (Fig. 2). While this placement process could be tedious initially, a practiced investigator always succeeded in obtaining a satisfactory placement (judged by lack of fast or slow, atypical displacements of the eye movement trace) in a few tries.
Since the coils are pre-formed, they can be pre-calibrated. We accomplished this using a jig that centered the coil on a gimbaled fixture equipped with a compass for measuring horizontal and vertical angular displacement (e.g., CNC calibration fixture, Seattle, WA). Once calibrated, there was virtually no variability in the gain settings over time.
Eye movements were measured using a standard CNC system (CNC Engineering, Seattle, WA). The coil signal was connected to a preamplifier with slightly increased gain to compensate for the lower signal levels from the small mouse coil. While we ordered a higher gain preamplifier from CNC to facilitate our extensive testing, our original feasibility tests were done with one of our standard monkey systems and required no modification, other than adjusting the gain on the phase detectors. The testing platform and coil form were bolted to the top of a 10 ft-lb, servo-controlled, DC torque motor (Neuro Kinetics; http://www.neuro-kinetics.com). The VOR was tested using en bloc sinusoidal, whole body rotation in the dark. Mice were rotated at frequencies from 0.1 to 1.5 Hz, and amplitudes of ±5° or ±10°. The servomotor was driven by a sinusoidal waveform generated from a homemade program running on Spike2 software (Cambridge Electronic Design; http://www.ced.co.uk/indexu.shtml). The program also collected and digitized (at 1 kHz) the resulting eye movement and the chair movement signals. Data were analyzed as described previously (Kaneko & Fukushima 1998) using a program that calculated the velocities from the eye position signals using a low pass (125 Hz), finite impulse filter and allowed the experimenter to remove saccades and fit the resultant slow eye movements with a sinusoid using a least squares method. During each session, we tested 0.1, 0.25, 0.5, 1.0, and 1.5 Hz at either of the designated amplitudes. Animals were kept restrained for no more than 1 hour at a time and were tested no more then 3 times per week and over a 4 to 6 week period of testing until a minimum of three good sessions were collected to for each animal. These data yielded the values reported in the results. The choice of 3 sessions was arbitrary and used only to assure we had consistent measurements from each animal.
Animals
Mice used during development and testing of the scleral search coil were inbred CBA/CaJ, obtained from The Jackson Laboratory (Bar Harbor, ME) and bred in the specific pathogen free (SPF) facility at the University of Washington.
All experiments were performed in accordance with the recommendations of the National Research Council (Guide for the Care and Use of Laboratory Animals, 1997; Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research, 2003), the Society for Neuroscience, and exceeded the minimal requirements recommended by the Institute of Laboratory Animal Resources and the Association for Assessment and Accreditation of Laboratory Animal Care International. All procedures were evaluated and approved by the local Animal Care and Use Committee of the University of Washington (ACC 4146-02 and 2199-09).
RESULTS
The removable mouse scleral eye coil worked very well. The consistency of the measurements improved as the mice adapted to the apparatus and the handling. Before adaptation, the mice would occasionally squirm in the apparatus and such movements would distort the coil signal or even dislodge the coil, which would require re-placing the coil. After mastering the fine control necessary to place the coil on the eye, we were able to measure routinely eye movements and the results were quite consistent and equivalent to those reported previously for implanted eye coils in normal control CBA/CaJ mice (van Alphen et al. 2001; their figure 7). Figure 6 shows an example of raw data (inset) and pooled results of repeated measurements of the VOR in the dark, on different days and across different CBA/CaJ animals. Our gains (peak-to-peak eye velocity/peak-to-peak chair velocity) are slightly higher that those of van Alphen et al. (2001) with values around 0.4 at 0.5 Hz for ± 10°(black squares) sinusoidal oscillation but are about the same for ± 5° (blue circles; ~0.2 and 0.5 Hz). The phase leads (Fig. 6, bottom) of the eye relative to chair velocity are also about the same as those of van Alphen et al. (2001). We averaged values of around 40° phase lead at 0.5 Hz for ± 10° and 30° lead for ± 5° while they reported a few degrees larger leads for ± 10° and smaller lags for ± 5°. In addition, our measurements were slightly more variable as judged by the size of the standard deviations (vertical brackets surrounding each symbol). Most of this variance is due to differences between animals as intra-animal variance was usually between 1 and 10%.
Figure 6.
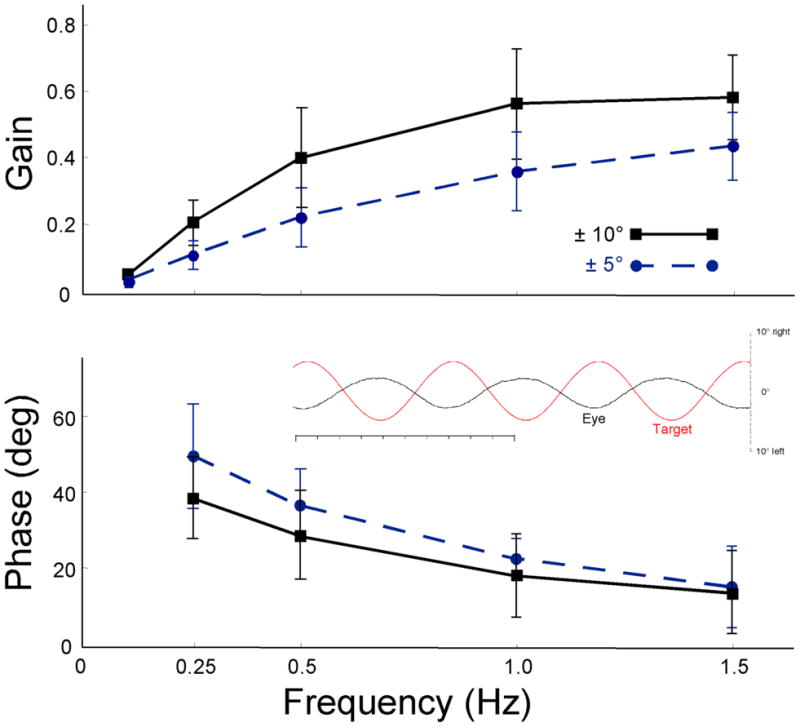
Overall average VOR Bode plots. Gain (upper) and phase lead re chair (lower) as a function of frequency for ± 10° (solid black) and ± 5° (dashed blue). Error bars are standard deviations (SD) for repeated measures (3–6 repetitions) on 4–5 mice. Inset: exemplary raw records of vor at 1.5 Hz, ± 5°. Target (red) and eye (black), scale on right. Upward deflection is rightward. Time scale is 1.0 s total in 100 ms tic marks.
DISCUSSION
Genetic manipulation in man and monkeys is impractical so the usefulness of mouse models depends on, and is successful to the extent that both the genetic and the correlated phenotypic anomalies are identical in mice and primates. To increase the accessibility of high resolution testing of phenotypic deficits in mouse models, especially vestibular dysfunctions, we have developed an eye coil suitable for use in mice. We have shown that our coil produces consistent measurement of the VOR comparable to those measured by implanted miniature coils without the necessity for difficult micro surgery. This device improves the potential for using such measurements to screen mouse mutants, knockouts or knockins.
There are still limitations to the comparisons between primates and mouse models afforded by more quantitative eye movement measurements even if the vestibular dysfunctions are essentially identical. If the phenotypes reflect broad pleiotropic responses to the underlying genetic manipulation, the models are much less useful. For example, while the p/q-type calcium channel mouse mutant Rocker is the specific product of a single gene mutation that encodes the ion pore of the voltage activated calcium channel, that channel is expressed broadly in neural and muscle tissue. The correlated behaviors (e.g., Stahl 2004) are thus much less useful as phenotypic markers or clinical or research tools because the behavioral dysfunction may be due to pathology in the cerebellum, the brainstem, at the motoneurons or in the muscle as well as a variety of less obvious structures. Thus, for each different behavioral deficit, in this case ataxia, each potentially affected element that contributes to the various behaviors (e.g., decreased velocity of fast phases of nystagmus, low VOR gain, downbeat nystagmus, vertical position offset, etc.; Stahl 2004) would have to be checked physiologically.
On the other end of the spectrum, sophisticated behaviors have often advanced our understanding considerably only to be met by a roadblock for want of appropriate molecular techniques. For example, in the case of the adaptive plasticity of the VOR, years of study of VOR adaptive plasticity in a number of animal models from many labs has implicated long-term depression (LTD; see recent review by Ito 2002) at the cerebellar parallel fiber-purkinje cell synapse as being important in the learning process. However, selective manipulation of LTD using molecular techniques has proven difficult until recently because of the broad pleiotropic affects of those mutations (e.g., Katoh et al.1998). Thus, inability to induce VOR plasticity in type I metabotropic glutamate receptor mutant mice that have deficient LTD is not a very telling finding because metabotropic glutamate receptors are quite ubiquitous.
An area where mouse mutant models are beginning to be useful is in clinical research. A number of mouse mutants have been studied in parallel with human families that seem to show similar genetic deficits. An example are the deaf waddler mutants which were originally identified in the mouse Atp2b2 gene, encoding the plasma membrane ATPase type 2 protein (PMCA2) (Street et al., 1998; Kozel et al., 1998) and later identified in human families with hearing loss and mutations in, or deletion of the human ATP2B2 gene (Schultz et al., 2005; McCullough et al., 2007; Ficarella et al., 2007).
This lack of sophisticated testing techniques is a major impediment in relating mouse genetic studies and human genetic dysfunction. Point mutations produce effects that are distributed across populations of cells with distinct functional roles, each contributing to a range of processes. Current, widely used testing protocols have limited ability to draw distinctions between deficits in each of these processes, and therefore fail to elucidate the mechanism underlying current phenotypes.
It is our hope that the ability to use more simplified version of sophisticated vestibular testing techniques using our new coil will add another armament to screening, phenotyping, and testing of genetically manipulated mouse models of pathology as well as normal behavior. With regard to the latter, the current form of our mouse coil is not optimized for chronic recording of murine eye movements. For such long-term recording, modifications in the shape and the leads would seem necessary. For mice, a ring shape that can be implanted rather than an annulus would seem more appropriate and still offer advantages of reduced size and weight compared with wound coils. For larger animals, it seems likely that scaling up the size (i.e., larger diameter) should be much more straightforward. Indeed, we have already developed a prototype suitable in size for use in humans and MicroConnex has expressed a willingness to cooperate in the development of similar coils for use in other applications.
Research Highlights.
To aid in the assessment of vestibular dysfunction in genetically-manipulated mouse models, we developed a new scleral coil for mice.
The pre-formed coil should improve assessment of vestibular dysfunction and allow screening without adding unnecessary technical complication to the measurement.
We tested the coils in normal mice and obtained measurements comparable to those obtained with implanted eye coils.
Acknowledgments
The development of this coil was supported by NIDCD grant DC008085. The commercialization of this product, if any, will be through MicroConnex. Ethan Fontaine remains employed by MicroConnex and John Yarno is a retired employee. The other authors have no current commercial affiliation with MicroConnex. Please contact MicoConnex for pricing and availability (For information go to: http://www.microconnex.com/)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergstrom RA, You Y, Erway LC, Lyon MF, Schimenti JC. Deletion mapping of the head tilt (het) gene in mice: a vestibular mutation causing specific absence of otoliths. Genetics. 1998;150:815–22. doi: 10.1093/genetics/150.2.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Raymond JL. Active reversal of motor memories reveals rules governing memory encoding. Neuron. 2003;11:1031–42. doi: 10.1016/s0896-6273(03)00562-2. [DOI] [PubMed] [Google Scholar]
- Deiss V, Strazielle C, Lalonde R. Regional brain variations of cytochrome oxidase activity and motor co-ordination in staggerer mutant mice. Neuroscience. 2000;95:903–11. doi: 10.1016/s0306-4522(99)00464-9. [DOI] [PubMed] [Google Scholar]
- Drago F, Nardo L, Rampello L, Raffaele R. Vestibular compensation in aged rats with unilateral labyrinthectomy treated with dopaminergic drugs. Pharmacol Res. 1996;33:135–40. doi: 10.1006/phrs.1996.0020. [DOI] [PubMed] [Google Scholar]
- Fasipe FR, Ubawike AE, Eva R, Fabry ME. Arginine supplementation improves rotorod performance in sickle transgenic mice. Hematology. 2004;9:301–5. doi: 10.1080/10245330410001714185. [DOI] [PubMed] [Google Scholar]
- Ficarella R, Di Leva F, Bortolozzi M, Ortolano S, Donaudy F, Petrillo M, Melchionda S, Lelli A, Domi T, Fedrizzi L, et al. A functional study of plasma-membrane calcium-pump isoform 2 mutants causing digenic deafness. Proc Natl Acad Sci. 2007;104:1516–1521. doi: 10.1073/pnas.0609775104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EK, Priestley MD, Waters J, Maliszewska C, Latif F, Maher ER. Detailed mapping of a congenital heart disease gene in chromosome 3p25. J Med Genet. 2000;37:581–7. doi: 10.1136/jmg.37.8.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guide for the Care and Use of Laboratory Animals. Compiled by The National Research Council. Washington, D.C: Natl. Acad. Press; 1997. [Google Scholar]
- Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. Compiled by The National Research Council. Washington, D.C: Natl. Acad. Press; 2003. [PubMed] [Google Scholar]
- Harrod CG, Baker JF. The vestibulo ocular reflex (VOR) in otoconia deficient head tilt (het) mutant mice versus wild type C57BL/6 mice. Brain Res. 2003;972:75–83. doi: 10.1016/s0006-8993(03)02505-8. [DOI] [PubMed] [Google Scholar]
- Ito M. The molecular organization of cerebellar long-term depression. Nat Rev Neurosci. 2002;3:896–902. doi: 10.1038/nrn962. [DOI] [PubMed] [Google Scholar]
- Jones SM, Erway LC, Johnson KR, Yu H, Jones TA. Gravity receptor function in mice with graded otoconial deficiencies. Hear Res. 2004;191:34–40. doi: 10.1016/j.heares.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Nagy A, Gonzalez-Gomez I, Groffen J, Heisterkamp N. Vestibular dysgenesis in mice lacking Abr and Bcr Cdc42/RacGAPs. Dev Dyn. 2002;223:517–25. doi: 10.1002/dvdy.10071. [DOI] [PubMed] [Google Scholar]
- Kaiser A, Fedrowitz M, Ebert U, Zimmermann E, Hedrich HJ, Wedekind D, Loscher W. Auditory and vestibular defects in the circling (ci2) rat mutant. Eur J Neurosci. 2001;14:1129–42. doi: 10.1046/j.0953-816x.2001.01726.x. [DOI] [PubMed] [Google Scholar]
- Kaneko CRS, Fukushima K. Discharge Characteristics of Vestibular Saccade Neurons in Alert Monkeys. J Neurophysiol. 1998;79:835–847. doi: 10.1152/jn.1998.79.2.835. [DOI] [PubMed] [Google Scholar]
- Katoh A, Kitazawa H, Itohara S, Nagao S. Dynamic characteristics and adaptability of mouse vestibulo-ocular and optokinetic response eye movements and the role of the flocculo-olivary system revealed by chemical lesions. Proc Natl Acad Sci. 1998;95:7705–7710. doi: 10.1073/pnas.95.13.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel PJ, Friedman R, Erway LC, Yamoah EN, Liu LH, Riddle T, Duffy JJ, Doetschman T, Miller ML, Cardell EL, Shull GE. Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2. J Biol Chem. 1998;273:18693–18696. doi: 10.1074/jbc.273.30.18693. [DOI] [PubMed] [Google Scholar]
- Libby RT, Kitamoto J, Holme RH, Williams DS, Steel KP. Cdh23 mutations in the mouse are associated with retinal dysfunction but not retinal degeneration. Exp Eye Res. 2003;77:731–9. doi: 10.1016/j.exer.2003.07.007. [DOI] [PubMed] [Google Scholar]
- McCullough BJ, Adams JC, Shilling DJ, Feeney MP, Sie KCY, Tempel BL. 3p-syndrome defines a hearing loss locus in 3p25.3. Hearing Res. 2007;8(224):51–60. doi: 10.1016/j.heares.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami DM, Erkman L, Hermanson O, Rosenfeld MG, Fuller CA. Evidence for vestibular regulation of autonomic functions in a mouse genetic model. Proc Natl Acad Sci. 2002;99:17078–82. doi: 10.1073/pnas.252652299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterstedde CR, Spandau U, Blankenagel A, Kimberling WJ, Reisser C. A new clinical classification for Usher’s syndrome based on a new subtype of Usher’s syndrome type I. Laryngoscope. 2001;111:84–6. doi: 10.1097/00005537-200101000-00014. [DOI] [PubMed] [Google Scholar]
- Rampello L, Drago F. Nicergoline facilitates vestibular compensation in aged male rats with unilateral labyrinthectomy. Neurosci Lett. 1999;267:93–6. doi: 10.1016/s0304-3940(99)00328-6. [DOI] [PubMed] [Google Scholar]
- Schultz JM, Yang Y, Caride AJ, Filoteo AG, Penheiter AR, Lagziel A, Morell RJ, Mohiddin SA, Fananapazir L, Madeo AC, et al. Modification of human hearing loss by plasma-membrane calcium pump PMCA2. N Engl J Med. 2005;352:1557–1564. doi: 10.1056/NEJMoa043899. [DOI] [PubMed] [Google Scholar]
- Stahl JS. Eye movements of the murine p/q calcium channel mutant Rocker, and the impact of aging. J Neurophysiol. 2004;91:2066–2078. doi: 10.1152/jn.01068.2003. [DOI] [PubMed] [Google Scholar]
- Stahl JS, van Alper AM, De Zeeuw CI. A comparison of video and magnetic search coil recordings of mouse eye movements. J Neurosci Meth. 2000;99:101–110. doi: 10.1016/s0165-0270(00)00218-1. [DOI] [PubMed] [Google Scholar]
- Street VA, McKee-Johnson JW, Fonseca RC, Tempel BL, Noben-Trauth K. Mutations in a plasma membrane Ca2+-ATPase gene cause deafness in deafwaddler mice. Nat Genet. 1998;19:390–394. doi: 10.1038/1284. [DOI] [PubMed] [Google Scholar]
- van Alphen AM, Stahl JS, De Zeeuw CI. The dynamic characteristics of the mouse horizontal vestibulo-ocular and optokinetic response. Brain Res. 2001;890:296–305. doi: 10.1016/s0006-8993(00)03180-2. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Householder DB, Coppola V, Tessarollo L, Fritzsch B, Lee EC, Goss D, Carlson GA, Copeland NG, Jenkins NA. Mutations in Cdh23 cause nonsyndromic hearing loss in waltzer mice. Genomics. 2001;74:228–33. doi: 10.1006/geno.2001.6554. [DOI] [PubMed] [Google Scholar]
- Zhou G, Williams RW. Mouse models for the analysis of myopia: an analysis of variation in eye size of adult mice. Optometry and Vision Science. 1999;76:408–418. doi: 10.1097/00006324-199906000-00021. [DOI] [PubMed] [Google Scholar]