Abstract
Attention-deficit hyperactivity disorder (ADHD) is characterized by persistent and impairing developmentally inappropriate levels of inattention, hyperactivity, and impulsivity. Such behavioral dysregulation may be a consequence of deficits in self-monitoring or adaptive control, both of which are required for adaptive behavior. Processing of contextual demands, ongoing monitoring of one’s behavior to evaluate whether it is appropriate for a particular situation, and adjusting behavior when it is suboptimal are components of self-regulation. This review examines and integrates the emerging literature on error-processing and adaptive control as components of self-regulation into the prominent etiological theories of ADHD. Available data on error-processing, as reflected in event-related potentials (ERN and Pe) and behavioral performance, suggest that both early error detection and later error-evaluation may be diminished in ADHD, thereby interfering with adaptive control processes. However, variability in results limit broad conclusions, particularly for early error detection. A range of methodological issues, including ERP parameters and sample and task characteristics, likely contribute to this variability, and recommendations for future work are presented. The emerging literature on error-processing and adaptive control informs etiological theories of ADHD in general and may provide a method for testing self-regulation models in particular.
Keywords: ADHD, self-regulation, error-processing, error-related brain activity, ERN, Pe
ADHD is one of the most commonly diagnosed childhood disorders, occurring in approximately 5% of the world population (Polanczyk, de Lima, Horta, Biederman, & Rohde, 2007). Children receiving a diagnosis of ADHD display persistent levels of inattentive and/or hyperactive and impulsive behavior that is developmentally inappropriate and causes significant impairment across situations (DSM-IV; American Psychiatric Association, 2000). Stimulant medication and contingency management improve behavior (Faraone & Buitelaar, 2009; Pelham & Fabiano, 2008) and the cognitive processes that are implicated in ADHD (Luman, Oosterlaan, & Sergeant, 2005; Pietrzak, Mollica, Maruff, & Snyder, 2006). The purpose of this review is to elaborate prominent etiological theories of ADHD through integration with the relevant cognitive neuroscience literature regarding self-regulation. Empirical evidence for impairments in neurophysiological and behavioral correlates of self-regulatory processes including self-monitoring (e.g., error-processing) and adaptive control in ADHD will be reviewed to facilitate and improve research on this important topic.
Etiological Theories of ADHD: Cognition, Motivation, and Self-Regulation
Heterogeneity of ADHD and variability of symptom presentation in particular settings (Douglas, 1999) pose a significant challenge for etiological theories of the disorder. Until recently, such theories have emphasized deficits in either cognition or motivation, though there are recent attempts at integration of these domains. Cognitive dysfunction theories of ADHD initially postulated single cognitive deficits in sustained attention, response inhibition (i.e., the ability to withhold a prepotent response) (Barkley, 1997), working memory (i.e., temporary storage and manipulation of the information necessary for complex cognitive tasks) (Baddeley, 1992) and executive functions (i.e., neurocognitive processes that maintain an appropriate action towards a goal) (see review by Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005). However, there is substantial evidence that sensitivity and specificity of any cognitive deficit is not high enough to support a single deficit as the cause of most cases of ADHD, since fewer than half of the children with ADHD exhibit significant impairment on any specific cognitive task (see review by Nigg, 2005). In contrast to the cognitive dysfunction theories of ADHD, several researchers have emphasized the role of reinforcement processes in ADHD, hypothesizing an elevated reward threshold (Haenlein & Caul, 1987), reduced response to punishment and non-reward (Quay, 1997), sensitivity to removal of reward (Douglas & Parry, 1994), delay aversion (Sonuga-Barke, 2003), and deficient extinction processes (Sagvolden, Johansen, Aase, & Russell, 2005).
Regulatory deficit models offer an alternative conceptual framework, integrating cognitive and motivational processes across levels of information processing to understand behavioral regulation. Maintaining adaptive behavior is complex, requiring an awareness of the contextual demands, monitoring of one’s behavior to evaluate whether it is appropriate for the context (i.e., self-monitoring) and adjusting behavior once a discrepancy is detected between the expected and actual outcomes (i.e., adaptive control). Adaptive control refers to processes which enable the human information processing system to flexibly and continuously configure itself to be consistent with an internally represented goal through appropriate adjustments in perceptual selection, response biasing, and the on-line maintenance of contextual information (Botvinick, Braver, Barch, Carter, & Cohen, 2001)1. Since self-monitoring and adaptive control processes work together to produce goal-directed behavior, deficits in either or both of these regulatory processes may result in maladaptive or suboptimal behavior. Given heterogeneity of ADHD and the lack of a common core neuropsychological deficit among children with this disorder, regulatory models provide a plausible alternative to core-cognitive/motivational-deficit models of ADHD.
There have been two models of ADHD (Douglas, 1999; Sergeant, 2000) which move beyond identification of a cognitive or motivational deficit and describe the interplay between cognition and motivation to select actions that are appropriate for a given context as part of the broad domain of self-regulation (Pennington, 2005). These models attempt to account for the variability associated with ADHD across several domains, including symptom presentation (Castellanos & Tannock, 2002), response times across numerous cognitive tasks (Leth-Steensen, Elbaz, & Douglas, 2000), and physiological activity (Borger et al., 1999). Beyond ADHD, understanding self-regulation has been identified as the “single most crucial goal for advancing an understanding of development and psychopathology” (Posner & Rothbart, 2000, pg. 427).
According to Douglas, (1985, 1999, 2008) deficient self-regulation is responsible for impaired performance of children with ADHD on cognitive, information-processing and neuropsychological tasks. Douglas (1999) emphasized that an adequate conceptualization of cognitive impairments in ADHD must account for attentional and inhibitory deficits, diminished regulation of arousal-activation levels to meet situational demands, an abnormal reward response, and increased behavioral variability. The self-regulation model postulated by Douglas was among the first to shift focus from a specific cognitive deficit such as sustained attention, response inhibition, or working memory, to processes that may influence all of these constructs. A recent formulation of Douglas’ model (2008) suggests that complex effortful (or adaptive) control processes contribute to efficient attention and inhibition. Effortful control is conceptually related to self-regulation and executive functions (Martel & Nigg, 2006; Rothbart, Ellis, Rueda, & Posner, 2003) and is thought to modulate other cognitive processes implicated in ADHD, including working memory, self-monitoring, and planning. Although this model provides an interesting and plausible alternative to the cognitive deficit models of ADHD, empirical examination of regulatory deficits has proven difficult given their complexity and poor operationalization.
The cognitive-energetic model, originally proposed by Sanders (1983) and later applied to individuals with ADHD by Sergeant and colleagues (Sergeant, 2000, 2005; Sergeant, Geurts, Huijbregts, Scheres, & Oosterlaan, 2003; Sergeant, Oosterlaan, & van der Meere, 1999), adopts a similar explanation for the cognitive and behavioral impairments associated with ADHD as Douglas’ deficient regulation hypothesis. Both models incorporate adaptive control and regulatory concepts rather than attempting to identify a core cognitive deficit. According to the cognitive-energetic model, the overall efficiency of information processing is determined by process (i.e., computational mechanisms of attention) and state (i.e., effort, arousal and activation) factors, that are monitored by an evaluation mechanism (Sergeant et al., 2003, see Figure 1).
Figure 1.
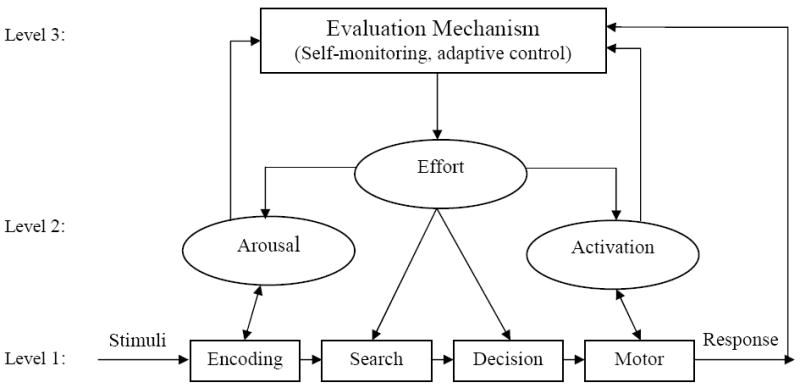
The Cognitive-Energetic Model (adapted from Sergeant, 2000; van der Meere, 2005)
The concept of state regulation is central to this model and refers to “energy mobilization which is necessary to change the current state of the organism in the direction of that which is optimal for a task or situation” (van der Meere & Stemerdink, 1999, pg. 214). Van der Meere (2005) hypothesizes that state regulation is impaired in children with ADHD and the deficit becomes manifest particularly in boring conditions, whereas group differences would be minimal when children with ADHD are in an optimal state. Deficits in state regulation could explain impaired performance on a wide range of neuropsychological measures. From one perspective, children with ADHD may be chronically hypoaroused, with distractibility and hyperactivity serving as attempts to modulate underarousal by seeking increased levels of stimulation/novelty (Zentall & Zentall, 1983). Alternatively, van der Meere (2005) hypothesizes that children with ADHD are unable to regulate their state toward the task demands because the range of their optimal state is too narrow to fulfill boring and exciting task requirements.
The energetic pools that are thought to be involved in state regulation include the effort, arousal and activation pools, all of which are monitored and adjusted by an evaluation mechanism (Sergeant, 2000). The evaluation mechanism receives feedback from the activation and arousal pools, as well as the behavioral response, and is thought to be responsible for planning, monitoring, detection of errors and their correction (Sergeant, 2005). It is proposed that a suboptimal state is identified by the evaluation mechanism and may be compensated for by effort once this has been detected. Sergeant and colleagues (2000, 2005) hypothesize that deficits seen in ADHD are due to failure to correct and adjust responding following an error, although the precise mechanisms for error detection and correction are not clearly defined in this model (Luman et al., 2005).
The cognitive-energetic model is an appealing heuristic for understanding the deficits and behavioral characteristics of ADHD because it integrates cognitive and motivational factors into a single, interactive model. In the past several years, studies that have directly attempted to test this model have provided some support for state regulation deficits in ADHD (e.g., Borger & van der Meere, 2000; van der Meere & Stemerdink, 1999; Wiersema, van der Meere, Antrop, & Roeyers, 2006). Moreover, the inconsistency that has been found in the ADHD neuropsychology literature may partially be due to a failure to consider energetic factors when designing or interpreting the results of empirical studies (see discussion by Kuntsi & Stevenson, 2000). Despite the conceptual appeal of this model it has received far less attention than alternative etiological theories of ADHD. This may be partially due to the lack of methods to directly assess various components of the model and to specify the optimal state, which may depend on the context and the particular child, making it difficult to evaluate the model empirically (Johnson, Wiersema, & Kuntsi, 2009; Luman et al., 2005). The management or evaluation mechanism, in particular, seems to serve as a homunculus in this model, lacking a clear description and operationalization of the self-monitoring and adaptive control processes involved.
Cognitive Neuroscience Models of Self-Regulation
Cognitive neuroscience researchers use neurophysiological indices of error processing to examine components of self-regulation and related factors2. This approach may fill an important gap in the regulatory deficit models of ADHD by providing methods to parse aspects of self-monitoring and adaptive control. At a neurophysiological level, self-regulation must involve the integrated functioning of structures that serve high-level cognition, such as the prefrontal cortices, and structures that serve motivation, such as the limbic system (Pennington, 2005). Adaptive control is considered to be an important part of self-regulation, involving the frontal systems of the brain including the prefrontal cortex, the anterior cingulate cortex, and the basal ganglia (Holroyd & Coles, 2002). Executive function systems are thought to regulate the most global aspects of human behavior, such as planning and decision-making, particularly when a task is novel or difficult. Importantly, deficits in regulation of cognition, emotion, or behavior, may depend on an individual’s ability to determine when adaptive control is required. Thus, regulatory deficits may reflect problems in self-monitoring of behavior, which is necessary but not sufficient for effective regulation of behavior.
Self-Regulation: Self-Monitoring Processes
The role of self-monitoring has been neglected in most etiological theories of ADHD. The cognitive-energetic model (Sergeant et al., 2003) may come closest in this regard. It includes an overriding management system that is responsible for the detection of suboptimal performance, including errors, via self-monitoring and the correction of behavior to meet the task demands via influence on the effort pool. Similarly, Douglas’ (1999) regulatory deficiency model has been extended to include self-monitoring as an important part of self-regulation (Douglas, 2008). Douglas also mentions the role of error detection under the rubric of specific control processes, but the precise mechanism is not operationalized. In addition to the regulative dimensions of control, there must also exist an evaluative component that monitors information processing, making an assessment of current demands and actual performance. Thus, ADHD may involve deficits in self-monitoring and adaptive control, both of which are necessary for effective self-regulation but have proven difficult to study.
Research in cognitive neuroscience has identified a reliable electrophysiological index of performance monitoring, the error-related negativity (ERN or Ne; Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; Gehring, Goss, Coles, Meyer, & Donchin, 1993). The ERN is an event-related brain potential (ERP), a negative deflection in the electroencephalogram (EEG), seen when people commit errors in a wide variety of psychological tasks3. The ERN is a response-locked ERP; onset coincides with response initiation and peaks 50-100 ms thereafter. There is general agreement that the ERN reflects the activity of a generic response monitoring system, since it has been observed across different stimulus and response modalities (Holroyd & Coles, 2002) and it occurs for various types of errors, including commission errors (response on trials requiring response inhibition), errors of choice (incorrect button press in choice reaction-time tasks), (Falkenstein, Hoormann, Christ, & Hohnsbein, 2000), and errors of inaction (taking too long to respond) (e.g., Luu, Flaisch, & Tucker, 2000). Recent developmental studies (Davies, Segalowitz, & Gavin, 2004a, 2004b) have also shown that the amplitude of the ERN increases with age.
The ERN has a frontocentral distribution over the scalp and there is considerable evidence that the ERN is generated in the anterior cingulate cortex (ACC) (Dehaene, Posner, & Tucker, 1994; Dikman & Allen, 2000; Luu, Tucker, Derryberry, Reed, & Poulsen, 2003; Miltner, Braun, & Coles, 1997; van Veen & Carter, 2002), a brain structure involved in self-monitoring and behavioral regulation (Bush, Luu, & Posner, 2000) and in the pathophysiology of ADHD (e.g., Fallgatter et al., 2004). The precise functional significance of the ERN and the cognitive processes associated with the ACC continues to be debated. The conflict monitoring hypothesis (Botvinick et al., 2001; Carter et al., 1998) proposes that the ERN reflects activation of the ACC, which is at the core of a system that monitors for the presence of conflict between simultaneously active but incompatible information processing streams. The major competing perspective is the reinforcement learning theory (Holroyd & Coles, 2002), which suggests that the ERN is generated as part of a dopamine-dependent reinforcement learning process. In contrast, the affect evaluation hypothesis of the ERN (Luu & Tucker, 2004) proposes that the ACC is part of a system involved in the representation of adaptive goals, incorporating the concept of motivational control, which appears to influence self-regulation and may be highly relevant for understanding regulatory deficits in ADHD. Despite the controversy among these competing theories, there is general agreement that the ERN reflects activation of the brain’s mechanism for on-line action monitoring, suggesting that it is conceptually related to the evaluation mechanism from the cognitive-energetic model.
Self-Regulation: Adaptive Control Processes
In addition to self-monitoring, self-regulation requires adaptive control processes to adjust behavior when an error is detected. Post-error slowing, or an increase in response time on trials following an error, is a common behavioral indicator of adaptive control. This post-error slowing is construed as a compensatory mechanism intended to improve performance on subsequent trials (Rabbitt, 1966). Thus, a failure to slow responding on post-error trials has been interpreted as reflecting deficient adaptive control. However, deficits in self-monitoring may result in a failure to detect the error and preclude adaptive control, complicating the interpretation of post-error slowing.
Neurophysiological indices of self-regulation may provide information that cannot be obtained with purely behavioral measures about the role of error processing in deficient post-error slowing among children with ADHD. The ERN is frequently followed by a positive deflection (with a slightly more posterior but diffuse scalp distribution and a peak at 200-400 ms post-response) referred to as the error positivity (Pe). Several hypotheses as to the functional significance of the Pe have been proposed, including conscious error recognition, adjustment of response strategies after an error, and subjective/emotional evaluation of the error event (Falkenstein et al., 1991; Falkenstein et al., 2000; Nieuwenhuis, Ridderinkhof, Blom, Band, & Kok, 2001). It has recently been suggested that the Pe may actually be a delayed P3b, which is an ERP component in response to the stimulus when errors occur thought to reflect stimulus updating or categorization (Shalgi, Barkan, & Deouell, 2009). It has also been proposed that the Pe/P3b reflect a phasic response of the locus-coeruleus-noradrenaline system to the outcome of internal decision-making (Nieuwenhuis, Aston-Jones, & Cohen, 2005). These hypotheses have not yet been directly tested against one another, but they share the presumption that the Pe is related to adaptive control of subsequent behavior.
The ERN and Pe both reflect error processing, but they are thought to index different cognitive processes for a number of reasons (see review by Overbeek, Nieuwenhuis, & Ridderinkhof, 2005). Developmental studies of error monitoring have found that the ERN increases with age (thought to reflect development of the ACC), but the Pe does not (Davies et al., 2004b). In fact, a robust Pe was seen in children despite the absence of an observable ERN, suggesting that these components, while both involved in error processing, may be functionally different. In addition, several studies (Nieuwenhuis et al., 2001; O’Connell et al., 2009; Shalgi et al., 2009) found the ERN to be present following both recognized and unrecognized errors whereas the Pe was present exclusively on trials on which subjects were aware of their error. Pe amplitude has also been positively associated with post-error slowing, whereas this association has not been consistently found with the ERN (Hajcak, McDonald, & Simons, 2003b). Based on these dissociations, it appears that the ERN may index error monitoring whereas the Pe reflects evaluation of the error response and its motivational significance, along with the initiation of adaptive control processes. Thus, examination of both ERP components may provide information regarding regulatory deficits in ADHD.
Error Processing in ADHD: ERN and Pe
Over the past 5 years, the literature on the neurophysiology of error-processing in ADHD has gone from nonexistent to 13 studies, 9 of which have been conducted with children. These studies were identified via PubMed using the search terms “ADHD AND (“ERN” or “Pe”). This search produced 27 results with an available Epub prior to March 2010. Studies that did not include a group of children diagnosed with ADHD were excluded from this review. In addition, given developmental trends in the ERN and complexities in relating child and adult ADHD, studies conducted with only adolescents and adults (Groom et al., 2009; McLoughlin et al., 2009; O’Connell et al., 2009; Wild-Wall, Oades, Schmidt-Wessels, Christiansen, & Falkenstein, 2009) were also excluded. Examination of the remaining studies suggests the rapidly emerging body of research examining neurophysiological indices of error processing among individuals with ADHD has produced equivocal results. After briefly describing each study, the review will focus on factors that may contribute to the variability in findings and make recommendations for the next wave of research on self-monitoring in ADHD.
Individual studies
Table 1 provides sample and task characteristics and primary results for each of the studies reviewed.
Table 1.
Characteristics of studies examining neurophysiological measures of error-processing (ERN and Pe) among children with ADHD and typically-developing children.
| Authors | Participants | Comorbidity1 | Task | Results 2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Behavioral | ERN | Pe | |||||||||
| a. Liotti et al (2005) | ADHD | 10 | ANX | Excl | Stop Signal Task | Accuracy | + | FCz | + | n/a | |
| Subtype (C:I: H) | 10:0:0 | DEP | Excl | 30 min | MRT | 0 | 30-80 ms | ||||
| CTRL | 10 | DBD | Excl (CD) | Adjusted task difficulty | SDRT | + | Peak amplitude | ||||
| Sex (%M) | 100% | LD | NR | Post-error RT | NR | Errors: discrimination | |||||
| Age | 9-11 | DD | NR | Post-error ACC | NR | Correct: discrimination | |||||
| b. Wiersema et al (2005) | ADHD | 21 | ANX | NR | Task 1: Go/No-Go | Accuracy | + | Fz, Cz, Pz | 0 | Fz, Cz, Pz | + |
| Subtype (C:I: H) | 22:0:0 | DEP | NR | 11 min | MRT | 0 | 60-120 ms | 200-500 ms | |||
| CTRL | 15 | DBD | Incl | Task 2: S1-S23 | SDRT | NR | Peak amplitude | Mean amplitude | |||
| Sex (%M) | 65% | LD | NR | 10 min | Post-error RT | + | Errors: No-Go (Task 1); Discrimination (Task 2) | ||||
| Age | 7-13 | DD | NR | 19 ADHD; 12 Control | Post-error ACC | NR | Correct: not included in analysis | ||||
| c. Burgio-Murphy-etal (2007) | ADHD | 259 | ANX | NR | Rare & Equiprobable | Accuracy | 0 | Fz | - | Fz | 0 |
| Subtype (C:I: H) | 155:27:77 | DEP | NR | Discrimination Task | MRT | 0 | 0-60 ms | 205-400 ms | |||
| CTRL | 60 | DBD | Incl | 25 min per task | SDRT | 0 | Peak amplitude | Mean amplitude | |||
| Sex (%M) | 72% | LD | Incl | Post-error RT | NR | Errors: discrimination | |||||
| Age | 7-13.5 | DD | Excl | Post-error ACC | NR | Correct: not included in analysis | |||||
| d. van Meel et al (2007) | ADHD | 16 | ANX | NR | Flanker Task | Accuracy | + | Midlines | + | n/a | |
| Subtype (C:I: H) | 12:4:0 | DEP | NR | 90 min (with breaks) | MRT | 0 | 50-90 ms | ||||
| CTRL | 16 | DBD | Incl | RT deadline | SDRT | 0 | Mean amplitude | ||||
| Sex (%M) | 100% | LD | Excl | Post-error RT | 0 | Errors: discrimination | |||||
| Age | 8-12 | DD | Excl | Post-error ACC | NR | Correct: discrimination | |||||
| e. Jonkman et al (2007) | ADHD | 10 | ANX | NR | Flanker Task | Accuracy | + | Fz, Cz, Pz | 0 | Fz, Cz, Pz | + |
| Subtype (C:I: H) | NR | DEP | NR | 21 min (without breaks) | MRT | + | 25-180 ms | 200-450 ms | |||
| CTRL | 10 | DBD | NR | SDRT | NR | Peak amplitude | Mean amplitude | ||||
| Sex (%M) | NR | LD | NR | Post-error RT | 0 | Errors: discrimination | |||||
| Age | 8-12 | DD | NR | Post-error ACC | NR | Correct: discrimination | |||||
| f. Albrecht et al (2008) | ADHD | 68 | ANX | Incl | Flanker Task | Accuracy | + | FCz | + | Cz, Pz | 0 |
| Subtype (C:I: H) | 68:0:0 | DEP | Incl | 13 min (after long task) | MRT | + | 0-150 ms | 200-500 ms | |||
| CTRL | 22 | DBD | Incl | Feedback (block) | SDRT | + | Peak amplitude (PNe) | Mean amplitude | |||
| Sex (%M) | 100% | LD | NR | Post-error RT | NR | Errors: discrimination | |||||
| Age | 8-15 | DD | Incl | Post-error ACC | NR | Correct: not included in analysis | |||||
| g. Groen et al (2008) | ADHD | 18 | ANX | Incl | Probabilistic Learning | Accuracy | + | Fz, Cz, Pz | + | Fz, Cz, Pz | + |
| Subtype (C:I: H) | 18:0:0 | DEP | Incl | 60-75 min | MRT | 0 | -300-100 ms | 100-800 ms | |||
| CTRL | 18 | DBD | Incl | RT deadline | SDRT | + | Mean amplitude | Mean amplitude | |||
| Sex (%M) | 86% | LD | NR | Post-error RT | NR | Errors: discrimination | |||||
| Age | 10-12 | DD | NR | Post-error ACC | NR | Correct: discrimination | |||||
| h. Zhang et al (2009) | ADHD | 12 | ANX | NR | Go/No-Go Task | Accuracy | 0 | Fz, Cz, Pz | 0 | Fz, Cz, Pz | + |
| Subtype (C:I: H) | 0:0:12 | DEP | NR | 8 min | MRT | 0 | 0-100 ms | 0-400 ms | |||
| CTRL | 14 | DBD | NR | Immediate accuracy feedback | SDRT | NR | Peak amplitude | Peak amplitude | |||
| Sex (%M) | NR | LD | NR | Post-error RT | NR | Errors: failure to inhibit | |||||
| Age | 7-11 | DD | NR | Post-error ACC | NR | Correct: go response | |||||
| i. Van De Voore et al (2010) | ADHD | 29 | ANX | Excl | Go/No-Go Task | Accuracy | + | FCz | NR | CPz | + |
| Subtype (C:I: H) | 22:9:0 | DEP | Excl | 20 min | MRT | 0 | -25 to 75 ms | 175 to 345 ms | |||
| CTRL | 31 | DBD | Excl (CD) | SDRT | + | Mean amplitude | Mean amplitude | ||||
| Sex (%M) | 58% | LD | Incl (RD)4 | Post-error RT | 0 | Errors: failure to inhibit | |||||
| Age | 8-12 | DD | Excl | Post-error ACC | + | Correct: go response | |||||
Notes: Sample size reflects sample for ERP analyses.
Comorbidity: Excl=Excluded, Incl=Included, NR=not reported.
Results: + = consistent with predictions, - = opposite of predictions, 0 = no group differences, NR = not reported
An ERN was not observed in either group of participants for this task.
This study included children with ADHD-only (n=18), ADHD+RD (n=16), RD-only (n=15), and typically developing controls (n=16).
The first study to examine the ERP components of error-processing in children with ADHD used the Stop Signal task to examine the ERN among 10 9- to 11-year-old children with ADHD-combined type and 10 comparison children. The stop signal task (Logan, Schachar, & Tannock, 1997) is a widely used measure of response inhibition during which participants are first trained to rapidly make a simple two-choice discrimination. Once the “go” process is well trained, participants are instructed to withhold this prepotent response on infrequent trials in which a “stop” signal (such as an auditory beep) is presented within several hundred ms of the onset of the “go” stimulus. As predicted, ERN amplitude was reduced on “Go” trials in the ADHD group compared to non-ADHD children (Liotti, Pliszka, Perez, Kothmann, & Woldorff, 2005)a. Pe was not assessed, nor was post-error adaptive behavior.
Subsequent studies demonstrated a range of findings regarding deficits in error processing in ADHD. Wiersema et al. (2005) investigated error processing in ADHD using a brief Go/No-Go task and an S1-S2 (i.e., stimulus 1-stimulus 2) task. The Go/No-Go task used in this study consisted of trials on which participants were instructed to press a button for a frequently (e.g., 75% of trials) presented “Go” stimulus and to withhold responding for an infrequently presented “No-Go” stimulus. The S1-S2 task involved the presentation of two neutral warning stimuli prior to the presentation of the imperative stimulus, which required a button press depending on whether an “L” or “R” was presented. Although children with ADHD demonstrated impaired performance on both tasks, ADHD and control children did not reliably differ in ERN amplitude on the Go/No-Go task, suggesting intact error detection in ADHD. Neither group displayed an ERN on the simple S1-S2 task. However, children with ADHD demonstrated diminished awareness or conscious evaluation of the error as indicated by reduced Pe amplitude on error trials in both tasks.
A study by Burgio-Murphy et al., (2007)c found that children with ADHD exhibited a larger ERN in comparison to non-ADHD children (both groups included a relatively high percentage of children with learning disabilities, a second focus of the paper, which complicates the interpretation of results) during two simple response tasks which varied target probability (i.e., press one button when ‘O’ appears and another button when ‘X’ appears) and failed to replicate the finding of diminished Pe amplitude in ADHD. The authors acknowledged that the task was very simple and suggested that perhaps children with ADHD had to exert more effort than controls to achieve comparable performance on this task, resulting in a larger ERN as found in this study. From a cognitive-energetic perspective, deficient self-monitoring may become apparent as task difficulty increases. This may occur because children with ADHD fail to increase the amount of effort necessary to meet the increasing task demands, or motivation may decline as the demands increase (Brehm & Self, 1989). Thus, it may be that self-monitoring deficits are most apparent in children with ADHD during more difficult tasks that require higher levels of effortful control.
The first published studies of ERN in ADHD using the Flanker task also appeared in 2007. The Flanker task is (Eriksen & Eriksen, 1974) a common paradigm for assessing the ERN in which a central target stimulus is ‘flanked’ by either congruent (e.g., SSSSS) or incongruent (e.g., HHSHH) stimuli and participants are instructed to respond depending on a characteristic of the character in the center of the stimulus array and ignore the surrounding stimuli. In this task, incongruent trials are thought to generate conflict requiring greater effortful control to efficiently process the target stimuli. Van Meel and colleagues (van Meel, Heslenfeld, Oosterlaan, & Sergeant, 2007)d conducted a long (90 minute) Flanker task with a response deadline to increase the number of errors on the task. They found that children with ADHD tended to have a diminished ERN (the effect was marginally statistically significant) compared to non-ADHD children and concluded that a specific deficit in monitoring ongoing behavior gives rise to performance limitations in ADHD. Pe was not reported. The task included feedback on response speed (i.e., a warning tone and visual feedback saying “faster” when RT exceeded the deadline), which may have helped individuals with ADHD meet the task demands. Indeed, in tasks with a response deadline and feedback, post-feedback speeding may be a better index of adaptive control than is post-error slowing.
Despite a similar paradigm, albeit shorter and without a response deadline, results of a second flanker study conflicted with those of van Meel et al. Jonkman and colleagues (2007)e found that children with ADHD did not differ from control children in ERN amplitude on error trials during a Flanker task. Both groups exhibited a pronounced negativity after making an error as well as a large positivity (Pe) following the ERN on error trials compared to correct trials. However, the Pe amplitude was reliably smaller for ADHD children suggesting reduced error awareness or evaluation. These findings might also suggest normal early error detection in children with ADHD but impaired error evaluation, although it is important to note that this study was quite small (n=10 per group), limiting interpretation of the null effects. In contrast, a substantially larger study examining neurophysiological measures of error processing in ADHD (n=68 for the ADHD group) using a Flanker task (Albrecht et al., 2008)f found reduced ERN amplitude in children with ADHD compared to controls, although Pe amplitude did not differ between groups.
In the past year, three additional studies on error processing in children with ADHD have been published (Groen et al., 2008; Van De Voorde, Roeyers, & Wiersema, 2010; Zhang, Wang, Cai, & Yan, 2009). Groen and colleagues (2008)g examined error processing in children with and without ADHD using a Probabilistic Learning task, which is considerably different from the tasks described above. The Probabilistic Learning task (Holroyd & Coles, 2002) examines the transition over trials from external (i.e., feedback) to internal (i.e., response) monitoring as a child learns the appropriate response for each of several stimuli. In this study, children with ADHD demonstrated reduced ERN and Pe amplitude in comparison to typically developing participants. Zhang and colleagues (2009)h reported reduced Pe amplitude among a small sample of children with ADHD Hyperactive/Impulsive subtype compared to typically developing controls in a very brief visual Go/No-Go task; group differences in ERN were not observed.
The most recent study examined error processing in children with ADHD compared to typically developing controls, children with a reading disorder (RD), and a combined ADHD+RD group (Van De Voorde et al., 2010)i using a Go/No-Go task. The results of this study suggest impaired performance in children with ADHD on a range of variables, including diminished accuracy on trials immediately following an error (post-error slowing was not observed in this study). The authors conclude that children with ADHD displayed reduced Pe amplitude and equivalent ERN amplitude compared to children without ADHD, whereas children with RD showed reduced ERN amplitude and equivalent Pe amplitude compared to children without RD. Despite many strengths of this study, including the explicit consideration of comorbidity, the results for ADHD are difficult to interpret. The ADHD test contrasted ADHD with and without RD to a comparison group that also included about 50% children with RD. Given that RD was associated with diminished ERN (see also Burgio-Murphy et al., 2007), this seems an unusual control group. Moreover, no inferential statistic or effect size information was presented for the ADHD effect or the interaction with RD for the ERN, and visual inspection of the grand average waveforms for each group suggests diminished ERN in all three patient groups relative to the typically developing controls. Thus, clear conclusions about ERN and ADHD are difficult to draw from this study.
Integration across studies
No literature is completely consistent, but a simple box-score for ERN deficits in ADHD is not encouraging. There are four positive results (Albrecht et al., 2008; Groen et al., 2008; Liotti et al., 2005; van Meel et al., 2007), four studies with null findings (Jonkman et al., 2007; Van De Voorde et al., 2010; Wiersema et al., 2005; Zhang et al., 2009), and one reversal of the expected group effect (Burgio-Murphy et al., 2007). The results appear more consistent for Pe, with five studies reporting diminished Pe among children with ADHD (Groen et al., 2008; Jonkman et al., 2007; Van De Voorde et al., 2010; Wiersema et al., 2005; Zhang et al., 2009) two failures to replicate (c.f., Albrecht et al., 2008; Burgio-Murphy et al., 2007), and no reversals. This may suggest deficient error evaluation or conscious error processing in ADHD, which is the most common interpretation (Overbeek et al., 2005). There are many possible reasons for the inconsistent results among studies examining error-processing in ADHD, including sample characteristics, task characteristics, and data reduction methods used to quantify ERP components.
In terms of sample characteristics, consideration of temperament, comorbidity, sample size, and age may be very important. Self-monitoring and adaptive control have been associated with personality and temperament (Amodio, Master, Yee, & Taylor, 2008; Boksem, Tops, Wester, Meijman, & Lorist, 2006; Dikman & Allen, 2000; Pailing & Segalowitz, 2004; Potts, George, Martin, & Barratt, 2006; Santesso, Segalowitz, & Schmidt, 2005), development (Davies et al., 2004b; Eppinger, Mock, & Kray, 2009; Segalowitz & Davies, 2004), externalizing psychopathology (e.g., Franken, van Strien, Franzek, & van de Wetering, 2007; Hall, Bernat, & Patrick, 2007; Stieben et al., 2007; additional studies listed in Table 1), and internalizing psychopathology (Gehring, Himle, & Nisenson, 2000; Hajcak, McDonald, & Simons, 2003a, 2004; Hajcak & Simons, 2002; Ladouceur, Dahl, Birmaher, Axelson, & Ryan, 2006; Olvet & Hajcak, 2008). Though a complete review of this growing literature is beyond the scope of this paper, a few key points warrant mention given their relevance for ADHD. The personality research has focused on the relationship between error processing and individual differences in conscientiousness, socialization, impulsivity, and the behavioral approach and inhibition systems proposed by Gray (1982). Collectively, these studies, along with studies of psychopathology, have generally found that individuals characterized by high levels of behavioral inhibition or negative affect tend to show an enhanced ERN, whereas individuals high on impulsivity or low on socialization (resembling antisocial traits) tend to show diminished error processing. These findings suggest it may be important to consider temperament when examining self-monitoring in ADHD.
Although ERN is enhanced among individuals with internalizing psychopathology and personality traits (Olvet & Hajcak, 2008), only two studies examining error processing in children with ADHD excluded children with comorbid internalizing disorders (e.g., Liotti et al., 2005; Van De Voorde et al., 2010). Further emphasizing the role of comorbidity, Stieben et al. (2007) recently reported that the reduction in ERN observed in children with externalizing problems was weakened by the presence of co-occuring internalizing problems. Interestingly, the one study that did exclude comorbid internalizing disorders and also failed to find reliably reduced ERN in ADHD (Van De Voode et al., 2010) included another comorbidity (RD) in both the ADHD and comparison group. The examination of RD is clearly warranted, and the factorial approach taken in the manuscript is laudable, but the atypical group contrast and modest sample sizes (especially for detecting an ADHD x RD interaction) suggest the null effect should be interpreted cautiously.
Indeed, small sample sizes used in most of the studies conducted to date make failures to replicate particularly difficult to interpret. Seven out of 9 studies had fewer than 21 participants per group for ERP analyses with most studies having 10-15 individuals in each group. With sample sizes in this range, the magnitude of the effect would have to be quite large (upwards of Cohen’s d=.90) for it to be consistently detected. This is a very high standard. Indeed, the neuropsychological processes that are most consistently and strongly associated with ADHD (e.g., response inhibition, spatial working memory, sustained attention) do not measure up to this standard (Nigg, 2005; Willcutt et al., 2005). For example, the weighted mean effect size for a Stop Task response inhibition deficit in children with ADHD, perhaps the most robust effect in the cognitive literature on ADHD, is d=0.61 (Willcutt et al., 2005). Thus, future studies of ERN and Pe in ADHD must have larger samples if null effects are to be meaningful.
In addition, several studies included individuals with a fairly large age range for such a small sample size (see Table 1). As noted above, the ERN appears to mature across childhood (e.g., Davies et al., 2004b). Most of the studies in this review did not report reliable group differences in age (c.f., Van De Voorde et al., 2010). However, inspection of the available descriptive statistics for the only study which found an enhanced ERN in children with ADHD (Burgio-Murphy et al., 2007) suggests that children with ADHD may have been older (ADHD M = 10.37, comparison groups M = 9.34). Even in the absence of group differences in age, consideration of age variability, which can contribute to within-group variability, remains important. Indeed, the two studies that included participants with a narrow age range of only two years both observed reduced ERN amplitude in children with ADHD (Groen et al., 2008; Liotti et al., 2005); the remaining studies had age ranges of 4 to 7 years. This suggests that many of these studies may have been underpowered to detect group differences in error processing and this literature would benefit from studies with larger and/or more homogeneous participant groups.
Additional possibilities for the inconsistent findings in the ERN and ADHD literature may be due to the nature and difficulty of the task, which varied markedly across studies and influenced error rate. Importantly, the amplitude of the ERN and perhaps the Pe may be influenced by an individual’s error rate either as a consequence of the data reduction process (i.e., fewer trials often result in a less clear peak and broader ERP component of the waveform) or an increase in error expectancy, both of which may reduce the amplitude of the ERN (and possibly Pe) (Holroyd & Coles, 2002). It is difficult to evaluate whether the number of error trials included in the average waveforms for error and correct ERPs contributed to the inconsistent results, as this information is often not reported (c.f., Liotti et al., 2005) or explicitly evaluated (c.f., Jonkman et al., 2007). Related to measurement of the error-related ERPs, it is very useful to also present data on comparable correct trials, thus providing a within-subjects comparison for whether any differences are specific to error trials – ERN versus correct-related negativity (CRN) and Pe versus the correct-positivity (Pc). Interestingly, neither the study reporting the group reversal nor two of the failures to demonstrate ERN differences included such data (Burgio-Murphy et al., 2007; Wiersema et al., 2005; Zhang et al., 2009), whereas all but one of the positive ERN findings (Albrecht et al., 2008) included ERPs for error and correct trials.
The other issue with task difficulty, error expectancy, is conceptually very interesting for self-regulatory models of ADHD, but it is difficult to evaluate methodologically. Perhaps individuals with ADHD exhibit a reduced ERN or Pe because the experience of making an error is more consistent with their predictions compared to children without ADHD rather than due to a self-monitoring deficit. This could be dependent both on the local context of the specific task and the global context of their experience outside the lab. Interestingly, in the two studies (Burgio-Murphy et al., 2007; Zhang et al., 2009) that did not observe reduced accuracy among children with ADHD, ERN was not reduced. We tentatively hypothesize that alterations in error expectancy, at least at the local level, may be an important moderator of group differences in error-processing. Explicit manipulations of task difficulty or repeated testing across sessions may be ways to address this issue, but no study to date has done so.
From a theoretical perspective, task characteristics, such as difficulty, inter-trial or inter-stimulus interval, provision of feedback, and task duration, are important to consider in terms of self-regulatory models (Douglas, 1999; Sergeant, 2000). Each of these task parameters may influence various aspects of self-regulatory and motivational processes, so they should be considered when designing a study and can either be controlled for or manipulated directly to examine whether they influence error-processing in ADHD. Several studies have examined the impact of motivational and state factors on neurophysiological correlates of self-regulation (Boksem, Meijman, & Lorist, 2006; Dikman & Allen, 2000; Gehring et al., 1993; Gehring & Willoughby, 2002; Hajcak, Moser, Yeung, & Simons, 2005; Pailing & Segalowitz, 2004). These studies have shown that ERN amplitude decreases with fatigue and increases as the motivational level is enhanced. For example, consistent with the postulate that the error detection system is sensitive to the significance of errors, the ERN was significantly larger on high-value errors and during evaluation (Hajcak et al., 2005).
In the ADHD literature, there are several studies in which state factors likely contributed to performance. Zhang et al. (2009), in which trial-by-trial feedback regarding response accuracy was provided, did not find differences in the ERN or performance between children with and without ADHD. Though manipulations of feedback are of considerable theoretical and practical interest, the study did not include a no-feedback condition for comparison. The absence of a group difference in ERN in the presence of trialwise feedback may indicate that such reinforcement ameliorated problems in error-processing among children with ADHD (e.g., Luman et al., 2005)4.
Task duration, which varied across studies from 8 to 90 minutes, may be another critical variable. Self-regulation models of ADHD suggest that performance deficits appear as time on task increases because it is difficult to maintain persistent adaptive control on long tasks. Notably, of the four studies that found reduced ERN amplitude in children with ADHD, 3 of them had tasks ranging from 30-90 minutes (Groen et al., 2008; Liotti et al., 2005; van Meel et al., 2007). The remaining study (Albrecht et al., 2008) with the predicted ERN results had a shorter task (13 minutes) but this followed a “highly-demanding” task and had one of the largest ADHD sample sizes (n=68). The studies reporting null ERN effects had task durations of 8 to 21 minutes (Jonkman et al., 2007; Van De Voorde et al., 2010; Wiersema et al., 2005; Zhang et al., 2009). This pattern is consistent with conceptual models emphasizing the contribution of time on task to self-regulatory processes and suggests task duration should be considered when designing and evaluating future studies.
Behavioral Indices of Adaptive Control: Relation to Error Processing
A goal in many of the studies of error processing is to consider how self-monitoring is related to behavioral outcomes. Studies that have examined behavioral post-error processes among children with ADHD have also produced inconsistent results. Sergeant and van der Meere (1988) found that ADHD participants failed to show adaptive post-error slowing as the task demands increased relative to controls. Diminished post-error slowing in children with ADHD was also found in studies involving a Stop Signal task (Schachar et al., 2004) and a Go/No-Go task (Wiersema et al., 2005) in which they slowed less following failed inhibitions or commission errors, respectively, compared to controls.
In contrast, two recent studies (Jonkman et al., 2007; van Meel et al., 2007) reported intact post-error slowing in ADHD children compared to typically developing children using a Flanker task. Importantly, one of these studies incorporated time pressure and auditory feedback on response speed, (van Meel et al., 2007) which would greatly reduce reaction time variability and likely weaken the post-error slowing effect. It may also be that post-error slowing is not adaptive in a task with a response deadline because if the child slows too much, they may still miss the response deadline. Instead, examining speeding of response after receiving feedback that a response was too slow may be a more accurate measure of adaptive behavior. In addition, error awareness may influence post-error responding, which is more apparent in certain tasks (i.e., Stop Signal task). This would be consistent with recent evidence indicating that individuals with ADHD tend to be less aware of their errors as measured by the error awareness task (e.g., O’Connell et al., 2009), which requires participants to indicate whether they think they made an error. Thus, it may be that error awareness is more difficult in the Flanker task compared to the Stop Signal task, which is necessary in order for adaptive control (i.e., post-error slowing, posterror accuracy, or post too-slow speeding) to occur.
Many studies examining neurophysiological indices of error-processing have not reported post-error slowing (c.f., Jonkman et al., 2007; van Meel et al., 2007; Wiersema et al., 2005), preventing a clear understanding of behavioral and physiological correlates of self-regulation in ADHD. Future research should examine behavioral measures of adaptive control, such as post-error slowing and post-error accuracy (e.g., Van De Voorde et al., 2010), to provide a more comprehensive picture of these components of self-regulation. In addition, examining whether ERN or Pe amplitude is predictive of behavioral measures of error-processing and adaptive control may improve our understanding of self-regulatory process models of ADHD.
Summary and Recommendations
Self-regulation has been implicated in prominent theories of ADHD (Douglas, 1999; Sergeant, 2000) and is gaining research attention as single core deficit models are increasingly viewed as insufficient to understand this heterogeneous disorder (Nigg, 2006; Pennington, 2006; Willcutt et al., 2005). However, empirical studies of self-regulation have proven difficult due to the complexity of this construct and the relative lack of reliable and valid measures of the relevant processes. Fortunately, over the past several years there has been extensive research on the neurophysiological correlates of self-regulation. The identification of the ERN and Pe as neurophysiological correlates of error processing enables detailed analysis of the components of self-regulation that complement behavioral measures, although there are certainly some important issues which need to be resolved in future research.
As reviewed above, several studies have found impaired error processing among individuals with personality traits that are associated with the externalizing spectrum (i.e., low socialization, low conscientiousness) (Dikman & Allen, 2000; Pailing & Segalowitz, 2004), presaging the emergence of the studies of ADHD reviewed here. However, studies examining error processing among individuals with ADHD have produced inconsistent results in terms of deficits in early error detection (ERN). Although Pe has been examined less frequently, most studies conducted to date suggest that children with ADHD are characterized by a diminished conscious or affective evaluation of a variety of types of errors. It may be reasonable to conclude based on the available literature that the behavioral dysregulation which characterizes individuals with ADHD is more reflective of later, more conscious aspects of error-processing. That said, to the extent that null results in this area are less likely to be published (Rosenthal’s 1979 ‘file drawer problem’), even this conclusion may be too optimistic. However, we believe it is premature to make strong conclusions on either front given the inconsistencies in the literature and the conceptual and methodological issues discussed in the above review.
In order to advance this line of research, we offer a number of recommendations for future studies. The first set of recommendations pertains to characteristics of the sample, specifically age range, comorbidity, and criteria for the control group. One strength of the studies reviewed in this paper is that groups tended to be matched for age, which is particularly important given the developmental studies on the ERN (Davies et al., 2004b). The next step may be to select participants with a smaller age range or to include enough participants to systematically examine the influence of age. A similar point can be made for comorbidity of internalizing psychopathology, which has received limited attention in these studies. There now exists substantial theoretical and empirical evidence suggesting that error processing must be considered across internalizing and externalizing dimensions (see review by Olvet & Hajcak, 2008).
There may be other moderators which should be considered, including subtype, sex, intelligence, socioeconomic status, and other comorbid disorders such as reading disorders (e.g., Burgio-Murphy et al., 2007; Van De Voorde et al., 2010) and Autistic Spectrum Disorders (Groen et al., 2008). At a minimum, future work should better characterize the sample in terms of comorbid conditions and provide effect size data for various subgroups. Ideally, studies would increasingly either exclude relevant comorbidities or employ sample sizes large enough to evaluate comorbidity with adequate power. As the literature grows, it may be important to test the generalizability of these results to girls, particularly since the neuropsychological profile of girls with ADHD has been shown to differ from boys (see review by Mahone & Wodka, 2008). The repetition of our call for larger samples and effect sizes is particularly important, given the very small samples in most studies to date (c.f., Albrecht et al., 2008; Burgio-Murphy et al., 2007).
In addition to sample characteristics, there are several task parameters which are important to consider when conducting this type of research including task difficulty, time on task, feedback, type of task, and task instructions. Task difficulty may be particularly important to consider, as it influences the number of errors participants will make, which carries psychological and methodological implications, and it influences group differences in behavioral performance. From a theoretical perspective, individualizing task difficulty may reduce the contribution of error expectancy to group differences and problems associated with including different amounts of error trials in the ERP average, but it could also reduce group differences in performance which may be of interest. At the very least, it seems reasonable for future studies to report the number of trials included in the ERP averages and to examine whether error rates affect the ERP and behavioral data of interest. Relatedly, the review above suggests task duration may be important, with group differences in ERN more apparent on longer tasks, which is broadly consistent with evidence for a vigilance deficit in ADHD (Huang-Pollock, Nigg, & Halperin, 2006). It will be relatively straightforward but important for studies to include longer tasks and evaluate the role of task duration.
The analytical framework is another important issue to consider in this literature. Studies varied in their use of mean versus peak amplitude for quantification of the ERN, whereas Pe was typically measured using mean amplitude. Mean amplitude is generally regarded as a superior measure to peak amplitude (Luck, 2005) in part because of the variability in response latency of the ERP components, which may reduce the amplitude of ERP components. This is even more important in ADHD, where variability is the rule rather than the exception (Leth-Steensen et al., 2000). In addition, it is recommended that ERN and Pe amplitude are examined in comparison to amplitude on response-locked waveforms for correct responses to reduce the influence of between-subject variability in physiological responses and to ensure that the error-processing indices are indeed specific to error processing.
Future research should also examine interrelationships among ERN, Pe, and behavior to improve our understanding of the associations among and meaning of these processes. Because of method variance, we do not expect particularly large correlations between electrophysiological and behavioral measures, but consideration of these interrelationships is essential for coherent models of error-processing and self-regulation. Similarly, processing of the error response should be understood in relation to processing of the stimulus to evaluate potential relationships and even confounds in stimulus- and response-locked ERPs. Certainly, how one processes the stimulus should have some impact on accuracy and error-related ERPs. Similarly, an error on one trial might lead to enhanced processing of the subsequent stimulus.
Regarding confounds, it has recently been suggested that Pe may actually be a P3b response to the target (Shalgi et al., 2009). Only one study reviewed above reported the association between the P3b and Pe (Albrecht et al., 2008) finding a strong effect of stimulus-locked P3b for incongruent error trials on Pe amplitude. Thus, it will be important to determine whether the Pe is truly a consequence of making an erroneous response.
More broadly, we believe the field will benefit by increasingly contextualizing error-processing within a developmental and theoretical framework to both understand parameters and to test theory-based predictors. For example, examination of error-processing may provide a method for testing one component of the cognitive-energetic model, the evaluation mechanism that has proven difficult to operationalize. Furthermore, particular tasks may be more appropriate for testing certain theories such as using the Probabilistic Learning task to assess reinforcement learning processes and the error awareness task to examine explicit error detection.
Finally, future directions include examinations of the impact of the effective treatments for ADHD, which includes behavior therapy, stimulant medication, and their combination (American Academy of Pediatrics, 2001), on error-processing and post-error adaptive behavior. Both stimulant medication and performance-based incentives should improve error-processing as reflected in ERN and Pe amplitude and adaptive control. Emerging data suggest that at least Pe is sensitive to medication effects in (Groen et al., 2008; Jonkman et al., 2007). The effect of reward or response contingencies on these neurophysiological measures has not yet been evaluated in ADHD (c.f., Pakulak, 2007), although incentives have been shown to enhance ERN amplitude in other populations (e.g., Hajcak et al., 2005; Pailing & Segalowitz, 2004).
Overall, the rapidly growing interest in error processing in ADHD and the theoretical relevance of examining components of self-regulation suggest that there will be considerably more work in this area. Whether error-processing reflects one or more “core deficits” in ADHD remains to be seen. Regardless, the neurophysiological and behavioral indices discussed here provide a window into understanding the regulatory processes that may be broadly important in the disorder yet have previously been challenging to operationalize. Of course, there is much work to be done in characterizing these processes in ADHD. It is hoped that the current qualitative review is useful for the next wave of increasingly theory-based, methodologically-sophisticated work on self-regulation in ADHD.
Acknowledgments
Preparation of this manuscript was supported in part by R01MH069434 to LWH and the University at Buffalo Department of Psychology Jacobs Scholarship to KS
Footnotes
The terms effortful control and executive control are often used interchangeably with the term adaptive control, although adaptive control is more commonly used in the error processing literature and throughout this paper.
It is important to note that this review was selective in terms of the psychophysiological measures associated with cognitive control or self-regulatory processes. Several studies have examined additional ERP components such as the stimulus-locked N2 (e.g., Albrecht et al., 2008; Jonkman, van Melis, Kemner, & Markus, 2007), the Contingent Negative Variation (CNV) (see review by Barry, Johnstone, & Clarke, 2003) , the pre-feedback Stimulus Preceding Negativity (SPN) (e.g., Groen et al., 2008), and heart-rate variability (e.g., Groen, Mulder, Wijers, Minderaa, & Althaus, 2009; Luman, Oosterlaan, Hyde, van Meel, & Sergeant, 2007) as correlates of self-regulatory processes.
ERP methods involve recording EEG signals at the surface of the scalp and time-locking them to the presentation of stimuli or to motor responses. Of the available neural assessment tools, ERP methodologies offer millisecond temporal resolution, although this is at the cost of poor localization of activity, and are relatively inexpensive and less invasive than alternative procedures.
The provision of external feedback – from a parent, teacher, or task – provides important information about performance and may initiate adaptive control processes even in the context of disrupted error-processing. The cognitive-energetic model (Sergeant et al., 1999) includes a role for knowledge of results and cognitive neuroscience provides a potential tool for evaluating this process. Specifically, performance feedback elicits the feedback error-related negativity, more simply the feedback-related negativity (FRN), a negative deflection in the ERP that is distributed over frontal areas of the scalp and reaches maximum amplitude about 250 ms following the onset of negative feedback stimuli (Holroyd, Hajcak, & Larsen, 2006). This component is considered to be functionally similar to the ERN in that non-satisfying outcomes result in a greater negative deflection than do optimal outcomes. Only two recent studies have examined the FRN among children with and without ADHD (Holroyd, Baker, Kerns, & Muller, 2008; van Meel, Oosterlaan, Heslenfeld, & Sergeant, 2005); both reported an enhanced FRN in the context of a guessing task. Examining feedback processing in a task with true performance feedback on response accuracy may provide information regarding the extent to which children with ADHD rely on external information to regulate their behavior (as indicated by FRN amplitude) rather than an internal monitoring system (as indicated by ERN amplitude), improving our understanding of hypothesized self-regulatory deficits in ADHD.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albrecht B, Brandeis D, Uebel H, Heinrich H, Mueller UC, Hasselhorn M, et al. Action Monitoring in Boys With Attention-Deficit/Hyperactivity Disorder, Their Nonaffected Siblings, and Normal Control Subjects: Evidence for an Endophenotype. Biological Psychiatry. 2008 doi: 10.1016/j.biopsych.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics. Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108(4):1033–1044. doi: 10.1542/peds.108.4.1033. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Amodio DM, Master SL, Yee CM, Taylor SE. Neurocognitive components of the behavioral inhibition and activation systems: Implications for theories of self-regulation. Psychophysiology. 2008;45(1):11–19. doi: 10.1111/j.1469-8986.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255(5044):556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Johnstone SJ, Clarke AR. A review of electrophysiology in attention-deficit/hyperactivity disorder: II. Event-related potentials. Clinical Neurophysiology. 2003;114(2):184–198. doi: 10.1016/S1388-2457(02)00363-2. [DOI] [PubMed] [Google Scholar]
- Boksem MA, Meijman TF, Lorist MM. Mental fatigue, motivation and action monitoring. Biological Psychology. 2006;72(2):123–132. doi: 10.1016/j.biopsycho.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Boksem MA, Tops M, Wester AE, Meijman TF, Lorist MM. Error-related ERP components and individual differences in punishment and reward sensitivity. Brain Research. 2006;1101(1):92–101. doi: 10.1016/j.brainres.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Borger N, van der Meere J. Motor control and state regulation in children with ADHD: a cardiac response study. Biological Psychology. 2000;51(2-3):247–267. doi: 10.1016/s0301-0511(99)00040-x. [DOI] [PubMed] [Google Scholar]
- Borger N, van der Meere J, Ronner A, Alberts E, Geuze R, Bogte H. Heart rate variability and sustained attention in ADHD children. Journal of Abnormal Child Psychology. 1999;27(1):25–33. doi: 10.1023/a:1022610306984. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–652. doi: 10.1037//0033-295X.I08.3.624. [DOI] [PubMed] [Google Scholar]
- Brehm JW, Self EA. The intensity of motivation. Annual Review of Psychology. 1989;40:109–131. doi: 10.1146/annurev.ps.40.020189.000545. [DOI] [PubMed] [Google Scholar]
- Burgio-Murphy A, Klorman R, Shaywitz SE, Fletcher JM, Marchione KE, Holahan J, et al. Error-related event-related potentials in children with attention-deficit hyperactivity disorder, oppositional defiant disorder, reading disorder, and math disorder. Biological Psychology. 2007;75(1):75–86. doi: 10.1016/j.biopsycho.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nature Reviews Neuroscience. 2002;3(8):617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of error-monitoring event-related potentials in adolescents. Annals of the New York Academy of Sciences. 2004a;1021:324–328. doi: 10.1196/annals.1308.039. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7- to 25-year-olds. Developmental Neuropsychology. 2004b;25(3):355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–305. [Google Scholar]
- Dikman ZV, Allen JJ. Error monitoring during reward and avoidance learning in high- and low-socialized individuals. Psychophysiology. 2000;37(1):43–54. [PubMed] [Google Scholar]
- Douglas VI. The response of ADD children to reinforcement: theoretical and clinical implications. In: Bloomingdale LL, editor. Attention Deficit Disorder Identification, Course, and Rationale. New York: Spectrum; 1985. pp. 49–66. [Google Scholar]
- Douglas VI. Cognitive control processes in attention-deficit/hyperactivity disorder. In: Quay HC, Hogan AE, editors. Handbook of disruptive behavior disorders. New York: Kluwer Academic/Plenum Publishing; 1999. pp. 105–138. [Google Scholar]
- Douglas VI. “Core Deficits” and Contingency Management in Attention Deficit Hyperactivity Disorder. Buffalo, NY: University at Buffalo Center for Children and Families Speaker Series; 2008. [Google Scholar]
- Douglas VI, Parry PA. Effects of reward and nonreward on frustration and attention in attention deficit disorder. Journal of Abnormal Child Psychology. 1994;22(3):281–302. doi: 10.1007/BF02168075. [DOI] [PubMed] [Google Scholar]
- Eppinger B, Mock B, Kray J. Developmental differences in learning and error processing: Evidence from ERPs. Psychophysiology. 2009 doi: 10.1111/j.1469-8986.2009.00838.x. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon identification of a target letter in a nonsearch task. Perception and Psychophysics. 1974;16:143–149. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78(6):447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biological Psychology. 2000;51(2-3):87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Ehlis AC, Seifert J, Strik WK, Scheuerpflug P, Zillessen KE, et al. Altered response control and anterior cingulate function in attention-deficit/hyperactivity disorder boys. Clinical Neurophysiology. 2004;115(4):973–981. doi: 10.1016/j.clinph.2003.11.036. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. European Child & Adolescent Psychiatry. 2009 doi: 10.1007/s00787-009-0054-3. [DOI] [PubMed] [Google Scholar]
- Franken IH, van Strien JW, Franzek EJ, van de Wetering BJ. Error-processing deficits in patients with cocaine dependence. Biological Psychology. 2007;75(1):45–51. doi: 10.1016/j.biopsycho.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4(6):385–390. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychological Science. 2000;11(1):1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295(5563):2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gray JA. The neuropsychology of anxiety: An enquiry into the functions of the septohippocampal system. New York: Oxford University Press; 1982. [Google Scholar]
- Groen Y, Mulder LJ, Wijers AA, Minderaa RB, Althaus M. Methylphenidate improves diminished error and feedback sensitivity in ADHD: An Evoked Heart Rate analysis. Biological Psychology. 2009 doi: 10.1016/j.biopsycho.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Groen Y, Wijers AA, Mulder LJ, Waggeveld B, Minderaa RB, Althaus M. Error and feedback processing in children with ADHD and children with Autistic Spectrum Disorder: An EEG event-related potential study. Clinical Neurophysiology. 2008;119(11):2476–2493. doi: 10.1016/j.clinph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Groom MJ, Cahill JD, Bates AT, Jackson GM, Calton TG, Liddle PF, et al. Electrophysiological indices of abnormal error-processing in adolescents with attention deficit hyperactivity disorder (ADHD) Journal of Child Psychology and Psychiatry. 2009 doi: 10.1111/j.1469-7610.2009.02128.x. [DOI] [PubMed] [Google Scholar]
- Haenlein M, Caul WF. Attention deficit disorder with hyperactivity: a specific hypothesis of reward dysfunction. Journal of the American Academy of Child and Adolescent Psychiatry. 1987;26(3):356–362. doi: 10.1097/00004583-198705000-00014. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Anxiety and error-related brain activity. Biological Psychology. 2003a;64(1-2):77–90. doi: 10.1016/S0301-0511(03)00103-0. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. To err is autonomic: error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology. 2003b;40(6):895–903. doi: 10.1111/1469-8986.00107. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Error-related psychophysiology and negative affect. Brain and Cognition. 2004;56(2):189–197. doi: 10.1016/j.bandc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42(2):151–160. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Simons RF. Error-related brain activity in obsessive-compulsive undergraduates. Psychiatry Research. 2002;110(1):63–72. doi: 10.1016/s0165-1781(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Hall JR, Bernat EM, Patrick CJ. Externalizing psychopathology and the error-related negativity. Psychological Science. 2007;18(4):326–333. doi: 10.1111/j.1467-9280.2007.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Baker TE, Kerns KA, Muller U. Electrophysiological evidence of atypical motivation and reward processing in children with attention-deficit hyperactivity disorder. Neuropsychologia. 2008 doi: 10.1016/j.neuropsychologia.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109(4):679–709. doi: 10.1037//0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Hajcak G, Larsen JT. The good, the bad and the neutral: electrophysiological responses to feedback stimuli. Brain Research Bulletin. 2006;1105(1):93–101. doi: 10.1016/ j.brainres.2005.12.01 5. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock CL, Nigg JT, Halperin JM. Single dissociation findings of ADHD deficits in vigilance but not anterior or posterior attention systems. Neuropsychology. 2006;20(4):420–429. doi: 10.1037/0894-4105.20.4.420. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Wiersema JR, Kuntsi J. What would Karl Popper say? Are current psychological theories of ADHD falsifiable? Behavioral and Brain Functions. 2009;5 doi: 10.1186/1744-9081-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman LM, van Melis JJM, Kemner C, Markus CR. Methylphenidate improves deficient error evaluation in children with ADHD: An event-related brain potential study. Biological Psychology. 2007;76(3):217–229. doi: 10.1016/j.biopsycho.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Stevenson J. Hyperactivity in children: a focus on genetic research and psychological theories. Clinical Child and Family Psychology Review. 2000;3(1):1–23. doi: 10.1023/a:1009580718281. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry. 2006;47(10):1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychologica. 2000;104(2):167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Liotti M, Pliszka SR, Perez R, Kothmann D, Woldorff MG. Abnormal brain activity related to performance monitoring and error detection in children with ADHD. Cortex. 2005;41(3):377–388. doi: 10.1016/s0010-9452(08)70274-0. [DOI] [PubMed] [Google Scholar]
- Logan GD, Schachar R, Tannock R. Impulsivity and inhibitory control. Psychological Science. 1997;8(1):60–64. [Google Scholar]
- Luck SJ. An Introduction to the Event-Related Potential Technique. Cambridge, MA: The MIT Press; 2005. [Google Scholar]
- Luman M, Oosterlaan J, Hyde C, van Meel C, Sergeant JA. Heart rate and reinforcement sensitivity in ADHD. Journal of Child Psychology and Psychiatry. 2007;48(9):890–898. doi: 10.1111/j.1469-7610.2007.01769.x. [DOI] [PubMed] [Google Scholar]
- Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clinical Psychology Review. 2005;25(2):183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Luu P, Flaisch T, Tucker DM. Medial frontal cortex in action monitoring. Journal of Neuroscience. 2000;20(1):464–469. doi: 10.1523/JNEUROSCI.20-01-00464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu P, Tucker DM. Self-regulation by the medial frontal cortex: limbic representation of motive set-points. In: Beauregard M, editor. Consciousness, emotional self-regulation and the brain. Amsterdam: John Benjamin; 2004. pp. 123–161. [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychological Science. 2003;14(1):47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Mahone EM, Wodka EL. The neurobiological profile of girls with ADHD. Developmental Disabilities Research Reviews. 2008;14(4):276–284. doi: 10.1002/ddrr.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Nigg JT. Child ADHD and personality/temperament traits of reactive and effortful control, resiliency, and emotionality. J Child Psychol Psychiatry. 2006;47(11):1175–1183. doi: 10.1111/j.1469-7610.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- McLoughlin G, Albrecht B, Banaschewski T, Rothenberger A, Brandeis D, Asherson P, et al. Performance monitoring is altered in adult ADHD: A familial event-related potential investigation. Neuropsychologia. 2009 doi: 10.1016/j.neuropsychologia.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltner WHR, Braun CH, Coles MGH. Event-related brain potentials following incorrect feedback in a time-estimation task: evidence for a “generic” neural system for error detection. Journal of Cognitive Neuroscience. 1997;9(6):788–798. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychological Bulletin. 2005;131(4):510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology. 2001;38(5):752–760. [PubMed] [Google Scholar]
- Nigg JT. Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: the state of the field and salient challenges for the coming decade. Biological Psychiatry. 2005;57(11):1424–1435. doi: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Nigg JT. What Causes ADHD? Understanding what goes wrong and why. New York: Guilford Press; 2006. [Google Scholar]
- O’Connell RG, Bellgrove MA, Dockree PM, Lau A, Hester R, Garavan H, et al. The neural correlates of deficient error awareness in attention-deficit hyperactivity disorder (ADHD) Neuropsychologia. 2009;47(4):1149–1159. doi: 10.1016/j.neuropsychologia.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: Toward an endophenotype. Clinical Psychology Review. 2008 doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek TJM, Nieuwenhuis S, Ridderinkhof KR. Dissociable components of error processing: on the functional significance of the Pe vis-a-vis the ERN/Ne. Federation of European Psychophysiology Societies. 2005;19(4):319–329. doi: 10.1027/0269-8803.19.4.319. [DOI] [Google Scholar]
- Pailing PE, Segalowitz SJ. The error-related negativity as a state and trait measure: motivation, personality, and ERPs in response to errors. Psychophysiology. 2004;41(1):84–95. doi: 10.1111/1469-8986.00124. [DOI] [PubMed] [Google Scholar]
- Pakulak A. Examining the mechanisms of error monitoring: implications for attention deficit hyperactivity disorder. PhD Dissertation Ontario Institute for Education of the University of Toronto; Toronto: 2007. [Google Scholar]
- Pelham WE, Fabiano GA. Evidence-based psychosocial treatments for attention-deficit/hyperactivity disorder. Journal of Clinical Child & Adolescent Psychology. 2008;37(1):184–214. doi: 10.1080/15374410701818681. [DOI] [PubMed] [Google Scholar]
- Pennington BF. Toward a new neuropsychological model of attention-deficit/hyperactivity disorder: subtypes and multiple deficits. Biological Psychiatry. 2005;57(11):1221–1223. doi: 10.1016/j.biopysch.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Pennington BF. From single to multiple deficit models of developmental disorders. Cognition. 2006;101(2):385–413. doi: 10.1016/j.cognition.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Mollica CM, Maruff P, Snyder PJ. Cognitive effects of immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. Neuroscience & Biobehavioral Reviews. 2006;30(8):1225–1245. doi: 10.1016/j.neubiorev.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. American Journal of Psychiatry. 2007;164(6):942–948. doi: 10.1176/appi.ajp.164.6.942. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Developing mechanisms of self-regulation. Development and Psychopathology. 2000;12(3):427–441. doi: 10.1017/s0954579400003096. [DOI] [PubMed] [Google Scholar]
- Potts GF, George MR, Martin LE, Barratt ES. Reduced punishment sensitivity in neural systems of behavior monitoring in impulsive individuals. Neuroscience Letters. 2006;397(1-2):130–134. doi: 10.1016/j.neulet.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Quay HC. Inhibition and attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology. 1997;25(1):7–13. doi: 10.1023/a:1025799122529. [DOI] [PubMed] [Google Scholar]
- Rabbitt PMA. Errors and error correction in choice reaction tasks. Journal of Experimental Psychology. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. The “file drawer problem” and tolerance for null results. Psychological Bulletin. 1979;86(3):638–641. [Google Scholar]
- Rothbart MK, Ellis LK, Rueda MR, Posner MI. Developing mechanisms of temperamental effortful control. Journal of Personality. 2003;71(6):1113–1143. doi: 10.1111/1467-6494.7106009. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behavioral and Brain Sciences. 2005;28(3):397–419. doi: 10.1017/S0140525X05000075. discussion 419-368. [DOI] [PubMed] [Google Scholar]
- Sanders AF. Towards a model of stress and performance. Acta Psychologica. 1983;53:61–97. doi: 10.1016/0001-6918(83)90016-1. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ, Schmidt LA. ERP correlates of error monitoring in 10-year olds are related to socialization. Biological Psychology. 2005;70(2):79–87. doi: 10.1016/j.biopsycho.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Schachar RJ, Chen S, Logan GD, Ornstein TJ, Crosbie J, Ickowicz A, et al. Evidence for an error monitoring deficit in attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology. 2004;32(3):285–293. doi: 10.1023/b:jacp.0000026142.11217.f2. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Davies PL. Charting the maturation of the frontal lobe: an electrophysiological strategy. Brain and Cognition. 2004;55(1):116–133. doi: 10.1016/S0278-2626(03)00283-5. [DOI] [PubMed] [Google Scholar]
- Sergeant JA. The cognitive-energetic model: an empirical approach to attention-deficit hyperactivity disorder. Neuroscience and Biobehavioral Reviews. 2000;24(1):7–12. doi: 10.1016/s0149-7634(99)00060-3. [DOI] [PubMed] [Google Scholar]
- Sergeant JA. Modeling attention-deficit/hyperactivity disorder: a critical appraisal of the cognitive-energetic model. Biological Psychiatry. 2005;57(11):1248–1255. doi: 10.1016/j.bps.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Sergeant JA, Geurts H, Huijbregts S, Scheres A, Oosterlaan J. The top and the bottom of ADHD: a neuropsychological perspective. Neuroscience and Biobehavioral Reviews. 2003;27:583–592. doi: 10.1016/j.neubiorev.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Sergeant JA, Oosterlaan J, van der Meere J. Information processing and energetic factors in attention deficit/hyperactivity disorder. In: Quay H, Hogan A, editors. Handbook of Disruptive Behavior Disorders. New York: Kluwer Academic; 1999. pp. 75–104. [Google Scholar]
- Sergeant JA, Van der Meere J. What happens after a hyperactive child commits an error? Psychiatry Research. 1988;24:157–164. doi: 10.1016/0165-1781(88)90058-3. [DOI] [PubMed] [Google Scholar]
- Shalgi S, Barkan I, Deouell LY. On the positive side of error processing: error-awareness positivity revisited. European Journal of Neuroscience. 2009;29(7):1522–1532. doi: 10.1111/j.1460-9568.2009.06690.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neuroscience and Biobehavioral Reviews. 2003;27(7):593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Stieben J, Lewis MD, Granic I, Zelazo PD, Segalowitz S, Pepler D. Neurophysiological mechanisms of emotion regulation for subtypes of externalizing children. Development and Psychopathology. 2007;19(2):455–480. doi: 10.1017/0S0954579407070228. [DOI] [PubMed] [Google Scholar]
- Van De Voorde S, Roeyers H, Wiersema JR. Error Monitoring in Children with Adhd or Reading Disorder: An Event-Related Potential Study. Biological Psychology. 2010 doi: 10.1016/j.biopsycho.2010.01.011. [DOI] [PubMed] [Google Scholar]
- van der Meere J. State regulation and attention-deficit hyperactivity disorder. In: D M, Gozal DL, editors. Attention deficit hyperactivity disorder: From genes to patients. Totowa, NJ: Human Press Inc; 2005. pp. 413–433. [Google Scholar]
- van der Meere J, Stemerdink N. The development of state regulation in normal children: An indirect comparison with children with ADHD. Developmental Neuropsychology. 1999;16(2):213–225. [Google Scholar]
- van Meel C, Heslenfeld D, Oosterlaan J, Sergeant J. Adaptive control deficits in attention-deficit/hyperactivity disorder (ADHD): The role of error processing. Psychiatry Research. 2007;151(3):211–220. doi: 10.1016/j.psychres.2006.05.011. [DOI] [PubMed] [Google Scholar]
- van Meel C, Oosterlaan J, Heslenfeld D, Sergeant J. Motivational effects on motor timing in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(5):451–460. doi: 10.1097/01.chi.0000155326.22394.e6. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology & Behavior. 2002;77(4-5):477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Wiersema R, van der Meere J, Antrop I, Roeyers H. State regulation in adult ADHD: an event-related potential study. Journal of Clinical and Experimental Neuropsychology. 2006;28(7):1113–1126. doi: 10.1080/13803390500212896. [DOI] [PubMed] [Google Scholar]
- Wiersema R, van der Meere JJ, Roeyers H. ERP correlates of impaired error monitoring in children with ADHD. Journal of Neural Transmission. 2005;112(10):1417–1430. doi: 10.1007/s00702-005-0276-6. [DOI] [PubMed] [Google Scholar]
- Wild-Wall N, Oades RD, Schmidt-Wessels M, Christiansen H, Falkenstein M. Neural activity associated with executive functions in adolescents with attention-deficit/hyperactivity disorder (ADHD) International Journal of Psychophysiology. 2009;74(1):19–27. doi: 10.1016/j.ijpsycho.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Zentall SS, Zentall TR. Optimal stimulation: a model of disordered activity and performance in normal and deviant children. Psychological Bulletin. 1983;94(3):446–471. [PubMed] [Google Scholar]
- Zhang JS, Wang Y, Cai RG, Yan CH. The brain regulation mechanism of error monitoring in impulsive children with ADHD--an analysis of error related potentials. Neuroscience Letters. 2009;460(1):11–15. doi: 10.1016/j.neulet.2009.05.027. [DOI] [PubMed] [Google Scholar]


