Abstract
Exercise intolerance, muscle fatigue and weakness are often-reported, little-investigated concerns of patients with osteogenesis imperfecta (OI). OI is a heritable connective tissue disorder hallmarked by bone fragility resulting primarily from dominant mutations in the proα1(I) or proα2(I) collagen genes and the recently discovered recessive mutations in post-translational modifying proteins of type I collagen. In this study we examined the soleus (S), plantaris (P), gastrocnemius (G), tibialis anterior (TA) and quadriceps (Q) muscles of mice expressing mild (+/oim) and moderately severe (oim/oim) OI for evidence of inherent muscle pathology. In particular, muscle weight, fiber cross-sectional area (CSA), fiber type, fiber histomorphology, fibrillar collagen content, absolute, relative and specific peak tetanic force (Po, Po/mg and Po/CSA respectively) of individual muscles were evaluated. Oim/oim mouse muscles were generally smaller, contained less fibrillar collagen, had decreased Po and an inability to sustain Po for the 300 ms testing duration for specific muscles; +/oim mice had a similar but milder skeletal muscle phenotype. +/oim mice had mild weakness of specific muscles but were less affected than their oim/oim counterparts which demonstrated readily apparent skeletal muscle pathology. Therefore muscle weakness in oim mice reflects inherent skeletal muscle pathology.
Keywords: muscle weight, peak tetanic force, skeletal muscle, type I collagen
1. Introduction
Osteogenesis imperfecta (OI) is a heritable connective tissue disorder characterized by small stature, reduced bone mineral density, and frequent fractures (Byers, 1993). Greater than 85% of patients with OI fall into four (Types I-IV OI) of nine potential subtypes, due to predominantly dominant mutations in either of the type I collagen genes, COL1A1 and COL1A2 (Basel et al., 2009; Marini et al., 2007b). Though the gene defects responsible for types V and VI remain unknown, recent discoveries have attributed the rare recessive types (VII–IX) of OI mutations in collagen posttranslational modifying enzymes and proteins including prolyl-3-hydroxylase-1 (LEPRE1), cartilage-associated protein (CRTAP) and peptidyl-prolyl isomerase B (PPIB) genes, respectively (Baldridge et al., 2008; Barnes et al., 2006; Barnes et al., 2010; Cabral et al., 2007; Marini et al., 2007a; Morello et al., 2006; Van Dijk et al., 2009; Willaert et al., 2009). Of the four classical types, type I OI is the mildest clinically and is characterized by blue sclerae, premature deafness, and mild to moderate bone fragility. Type II is perinatal lethal and type III OI (the most severe viable form) is characterized by short stature, deformity of the long bones and spine due to fractures, as well as premature hearing loss (Gajko-Galicka, 2002). Type IV OI has a moderate variable phenotype between types I and III.
Fatigue and muscle weakness are also associated with OI (Engelbert et al., 1997; Takken et al., 2004), and can be the presenting symptom in patients with the disease (Boot et al., 2006). A case study of a patient with OI performed by Boot et al. documented increased acid phosphatase and swollen skeletal muscle mitochondria. However, muscle morphology, nerve conduction studies and electromyography were normal (Boot et al., 2006). This led Boot to conclude that “the etiology of decreased muscle force in patients with OI is unclear but may be due to an intrinsic muscle defect” (Boot et al., 2006). Takken at al. studied cardiopulmonary fitness and muscle strength in children with OI type I. They found that while no pulmonary or cardiac abnormalities at rest could be found, type I patients had decreased exercise tolerance and muscle strength compared to reference values for healthy pediatric peers (Takken et al., 2004). These findings were attributed to a combination of proximal muscle weakness and joint hypermobility, but it remained unclear whether the reduced peak oxygen consumption (VO2peak) and muscle force were a consequence of inactive lifestyle or a specific consequence of the impaired type I collagen synthesis.
The purpose of this study is to determine if an inherent skeletal muscle defect exists in the osteogenesis imperfecta murine (oim) mouse model. The oim mouse is the most widely used model of osteogenesis imperfecta and was first described in 1993. Oim/oim are homozygous for a spontaneous nucleotide deletion which causes a frameshift in the COL1A2 gene resulting in the absence of functional α2(I) chains of type I collagen. Oim/oim mice produce exclusively homotrimeric type I collagen [α1(I)3] (Chipman et al., 1993) instead of heterotrimeric type I collagen, α1(I)2α2(I). The oim/oim mouse phenotype most closely correlates with human OI type III. Heterozygous (+/oim) mice have a milder phenotype similar to human OI type I (Camacho et al., 1999; McBride et al., 1998; Phillips et al., 2000; Saban et al., 1996),with bone biomechanical integrity intermediate to wildtype (wt) and oim/oim mice (Saban et al., 1996).
Though the impact of type I collagen mutations on bone strength and integrity is clearly evident (Byers, 2001; Marini et al., 2007b), whether type I collagen mutations impact skeletal muscle structure and function is unknown. In this study we investigated skeletal muscle characteristics of four month old age-matched male and female wildtype (wt), +/oim and oim/oim mice. We examined skeletal muscles with different fiber types and functional demands (soleus (S), plantaris (P), gastrocnemius (G), tibialis anterior (TA) and quadriceps (Q) muscles) and determined muscle weights, fibrillar collagen content, fiber cross-sectional area (CSA), fiber type distribution, fiber histomorphology, and peak tetanic force (Po) of individual muscles.
2. Materials and methods
2.1 Experimental Model
Mature male and female wt, +/oim and oim/oim mice maintained on a C57BL/6J background (Carleton et al., 2008) were evaluated. However, as there was no apparent sex predilection for the muscle phenotype described and similar findings in males and females, only data from male mice are presented. Mice were 4 months of age at time of study to avoid the characterization of animals in the rapid growth phase of development. In addition, 4 months of age is when peak bone mass in mice is achieved and extensive studies have been performed characterizing the bone in oim mice at this age (Carleton et al., 2008; Phillips et al., 2000). The protocols used for this study comply with the guidelines of the American Physiological Society. All experimental manipulations were performed under an approved University of Missouri Animal Care and Use Protocol.
2.2 Contractile properties
The soleus (S), plantaris (P), gastrocnemius (G), tibialis anterior (TA), and quadriceps (Q) muscles were chosen based on their differing fiber type compositions, architecture and contribution to movement. The S, P, G, TA and Q muscles are uni- or multi-pennate; span one or more joints and function as anti-gravity, postural or locomotor muscles. The S, P and G muscles serve as plantar flexors and the TA is a dorsal flexor in mice (Hesselink et al., 2002). The Q extends the knee as well as flexes the hip (Lieber, 2002).
To determine contractile properties of the S, P, G, and TA muscles in wt, +/oim and oim/oim mice, the mice were anesthetized with pentobarbital sodium (0.15ml pentobarbital with 0.85ml saline) with 0.15ml as first injection, and anesthesia was maintained with 0.05ml injection given as needed. Each mouse was placed side lying on a water-jacketed heating pad which maintained body temperature at 37°C. The left S, P, G, and TA muscle were surgically exposed only at their distal insertions. The distal tendon of each muscle was attached in turn to the Grass force transducer with 4.0 silk. The sciatic nerve was isolated and placed on a bipolar stimulating electrode. The exposed tendon of each muscle was continually bathed in saline solution and the nerve was bathed continuously with 37°C mineral oil.
For contractile testing the left hind-limb and mouse torso were rigidly immobilized, and muscles were attached in the order of S→P→G→TA to a force transducer by the distal tendon and adjusted in length so that passive tension was zero grams. A twitch was obtained at that position with the parameters: 0.5ms, 0.3Hz, at 6V, and subsequently the micromanipulator was used to progressively lengthen each muscle to the point where peak twitch was attained (Lo). At optimal length, a peak tetanic contraction (Po) was elicited by pulses delivered at 150Hz, 300-ms duration, and an intensity of 6V for each type muscle (Brown et al., 2009). Preliminary studies revealed 6V to be supramaximal; the 300-ms duration was greater than what was required to achieve Po. Force curves generated at 15, 50, 75, 100 and 125 Hz revealed that all muscles were maximally recruited by the time 100 Hz was reached. All data were collected using Power Lab®. The duration of contractile function testing was approximately 15 minutes.
In pilot studies, nonsystematic testing of muscles was done as well as testing in the order TA→G→P→S and no differences in tension were observed, regardless of stimulation order. Repeat testing of S and P during preliminary studies and subsequently during actual stimulation indicated the protocol did not result in reduced force production.
2.3 Tissue Harvest
After contractile properties were obtained, left (stimulated) S, P, G, TA, and Q muscles were removed, cleaned of extraneous tissue, blotted, and weighed. These muscles were then placed in 4% paraformaldehyde solution for 24 hours followed by transfer to 70% ethanol for future staining with hematoxylin and eosin (H&E) for morphologic evaluation. Right sided muscles, those that were not electrically stimulated, were placed at their in situ length, embedded in OCT tissue-freezing medium, frozen slowly in chilled 2-methylbuterol, and then placed in liquid nitrogen and stored at −80°C until analysis for myofibrillar ATPase activity.
2.4 Histochemistry, cross-sectional myofiber area measurements, and fibrillar collagen content determination
Left-sided paraformaldehyde and ethanol prepared muscles from wt, +/oim, and oim/oim mice were transversely sectioned at the middle of the muscle belly and then sectioned at 5 μm and separate sections were stained with H&E to reveal evidence of potential muscle damage or inflammation or with picrosirius red stain to visualize and quantify fibrillar collagen content. From H&E stained sections fiber areas were obtained. Digital images were taken from the cross-section at 10x magnification to obtain an average of 300 fibers to evaluate muscle fiber morphology and for fiber area measures. Myofiber cross sectional areas were used to measure evidence of atrophy or hypertrophy of the individual muscle fibers, as well as in the calculation of specific Po [peak tetanic force (Po) / CSA (μm2)]. Six sections of 50 contiguous myofibers were circled for each muscle evaluated to obtain an average of 300 fibers for fiber area measures. Area determinations were done using a calibrated pen by circling each fiber. Image J software (NIH) was used to derive area data which were subsequently transferred into an Excel spreadsheet.
Digital images of muscle fibers (10X magnification) stained with picrosirius red stain were captured using an Olympus D11 digital camera (Olympus America Inc., NY) attached to a Zeiss (Carl Zeiss Inc., NY) microscope. Images were opened in Adobe Photoshop and the tiff files were used for morphological studies of fibrillar collagen content using Fovea Pro v.3 from Reindeer Graphics. ( P. O. Box 2281 Asheville, NC 28802 ). Fibrillar collagen content was determined using BiLevel Thresholding with the threshold value of 50 to ensure optimal tissue:fibrillar collagen contrast. Total area of the muscle tissue was determined by subtracting out surrounding empty space in the visible field. Artifacts (marking ink, tissue folds, birefringent muscle fibers, large, brightly staining vessels, and epimysium when visible) were blackened out and fibrillar collagen content was calculated (mm2) corresponding to picrosirius red staining. Fovea Pro.software was used to derive area data which were subsequently transferred into an Excel spreadsheet. Fibrillar collagen area was divided by total muscle tissue area to determine the percent collagen:muscle tissue area ratio for each muscle.
2.5 Myofibrillar ATPase activity
Right-sided S, P, G, TA and Q muscles, which were harvested immediately after contractile studies and stored at −80°C, were thawed to −20°C in a microtome, oriented vertically, and sectioned at 10 μm. Sections were stained using traditional acid-stable myofibrillar ATPase stain (4.2 pH acid preincubation) to reveal muscle fiber type (Carson, 1997).
2.7 Statistical analysis
All statistical analyses were performed using SAS (SAS Institute Inc., Cary, NC). Data from the three groups (males and females) were analyzed as a 3×1 factorial (3 genotypes, 1 sex). When heterogeneous variations made it necessary, a log transformation was used to stabilize heterogeneous variation. If this log transformation failed to stabilize the variation, a ranking procedure was used (Conover et al., 1982). Mean differences were determined using Fisher's Least Significant Difference (LSD). Means and standard errors presented are untransformed values, although P values are for the transformed or ranked data. All results are presented as mean ± standard error (SE). Differences were considered to be statistically significant at a p value ≤0.05. Data obtained for male and female mice were nearly identical, and so only findings from males are included in the results.
3. Results
3.1 Muscle Weight
Consistent with previous studies (Carleton et al., 2008; Chipman et al., 1993; McBride et al., 1997), oim/oim mouse body weights were significantly less (p < 0.05) than the body weights of wt and +/oim littermates (Table 1). Whole muscle wet weights for the S, P, G, TA and Q muscles in oim/oim mice were 77%, 70%, 70%, 78% and 82% (p < 0.05) of their wt counterpart muscle weights, respectively. Whole muscle weights of +/oim S, P, G, TA and Q muscles were 97%, 94%, 98%, 95% and 99% of wt whole S, P, G, TA and Q weights (not significant). Whole muscle weights for the S, P, G, TA and Q muscles in oim/oim mice were 80%, 76%, 72%, 72%, 82% and 83% (p < 0.05) of +/oim mice, respectively. In addition, relative muscle wet weights (expressed as a percent body weight) of oim/oim P, G and TA muscles were 82%, 82% and 92% of wt, respectively. Relative muscle weights of +/oim S, P, G, TA and Q muscles were 99%, 97%, 100%, 97% and 101% of relative weights of wt S, P, G, TA and Q (not significant). Relative muscle weights of oim/oim S, P, G, and TA muscles were 90%, 85%, 82% and 94% (p < 0.05) compared to +/oim relative muscle weights (Table 1).
Table 1.
Body and muscle weight of four month old wt, +/oim and oim/oim mice.
| MOUSE GENOTYPE | ||||||
|---|---|---|---|---|---|---|
| PARAMETERS | wt (n=13) |
+/oim (n=17) |
+/oim % wt values |
oim/oim (n=13) |
oim/oim % wt values |
oim/oim % +/oim values |
| Body weight (g) | 29.53 ± 0.63 | 28.88 ± 0.56 | 98 | 25.30 ± 0.63*† | 86 | 88 |
| Muscle wet weight (mg) | ||||||
| S | 8.51 ± 0.31 | 8.26 ± 0.26 | 97 | 6.58 ± 0.31*† | 77 | 80 |
| P | 20.16 ± 0.51 | 19.01 ± 0.44 | 94 | 14.17 ± 0.51*† | 70 | 76 |
| G | 144.04 ± 3.87 | 140.91 ± 3.38 | 98 | 101.17 ± 3.87*† | 70 | 72 |
| TA | 54.32 ± 1.17 | 51.77 ± 1.03 | 95 | 42.55 ± 1.17*† | 78 | 82 |
| Q | 227.44 ± 6.68 | 225.03 ± 5.84 | 99 | 186.88 ± 6.68*† | 82 | 83 |
|
Relative Wet Muscle Weight [Wet wt (mg)/ Body wt (g)] | ||||||
| S | 0.029 ± 0.001 | 0.029 ± 0.001 | 99 | 0.026 ± 0.001† | 89 | 90 |
| P | 0.068 ± 0.001 | 0.066 ± 0.001 | 97 | 0.056 ± 0.001*† | 82 | 85 |
| G | 0.489 ± 0.012 | 0.490 ± 0.010 | 100 | 0.400 ± 0.012*† | 82 | 82 |
| TA | 0.184 ± 0.004 | 0.180 ± 0.003 | 97 | 0.169 ± 0.004*† | 92 | 94 |
| Q | 0.771 ± 0.021 | 0.781 ± 0.019 | 101 | 0.741 ± 0.021 | 96 | 95 |
Values expressed as mean ± SE. G, gastrocnemius; oim, osteogenesis imperfecta murine; P, plantaris; S, soleus; TA, tibialis anterior; Q, quadriceps;
p < 0.05 compared to wt,
p < 0.05 compared to +/oim
3.2 Histomorphometry
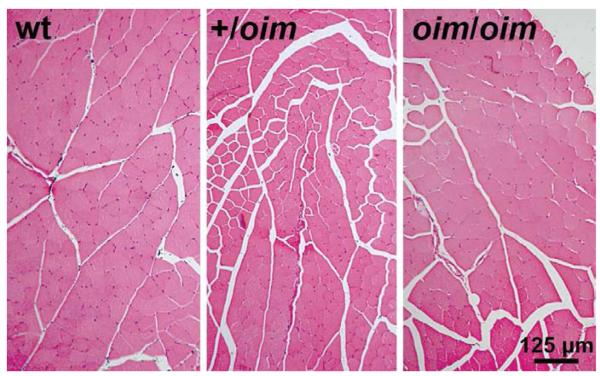
Histology cross-sections of the S, P, G, TA and Q muscles stained with hematoxylin and eosin (H&E) showed no evidence of necrosis, regeneration, degeneration, fibrosis or infiltration by inflammatory cells (Fig. 1).
Figure 1.
Hematoxylin and eosin (H&E) stained cross sections of the tibialis anterior (TA) muscle from 4 month old wt (left), +/oim(middle) and oim/oim(right) mice showed no evidence of necrosis, regeneration, degeneration, or infiltration by inflammatory cells, 10X magnification.
3.3 Muscle fibrillar collagen content
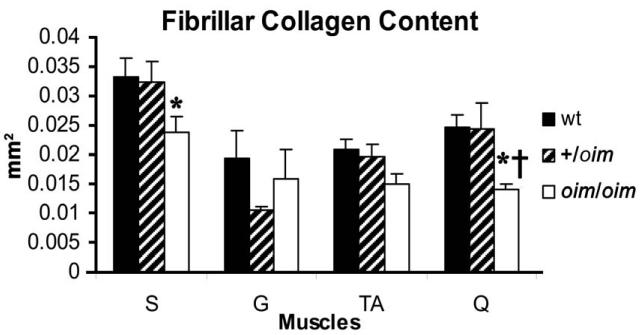
Fibrillar collagen content of the oim/oim S muscle was less (p < 0.05) compared to wt. Fibrillar collagen content of the Q muscle in oim/oim and +/oim mice was less (p < 0.05) compared to wt. Fibrillar collagen content was not different in the G or TA muscles between the genotypes (Fig. 2). Fibrillar collagen as a whole represented less than 3.5% of the tissue area (mm2).
Figure 2.
Fibrillar collagen content as determined by picrosirius red staining of the S, G, TA and Q muscles of wt (n=6; solid), +/oim (n=6; dashed) and oim/oim (n=6; open) mice. Oim/oim S had less (p < 0.05) collagen compared to wt S. Q had significantly less (p < 0.05) collagen compared to wt and +/oim Q.
3.4 Myofibrillar ATPase
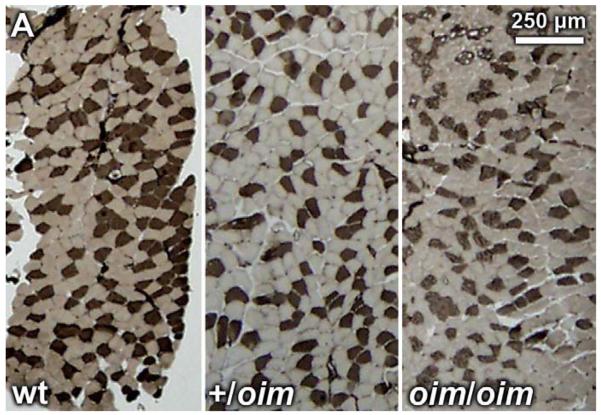
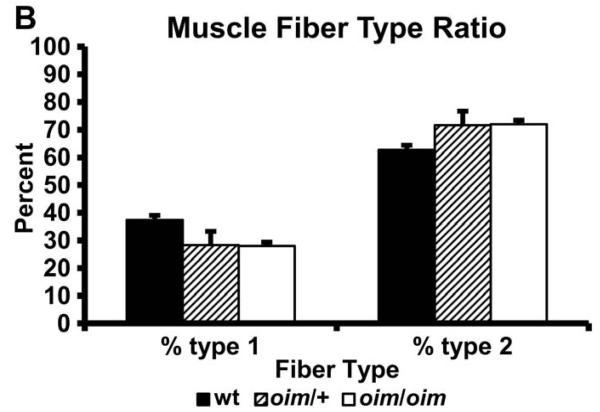
Fiber-type analysis of the S, P, G, TA and Q muscles of wt, +/oim and oim/oim mice by histochemical staining for ATPase activity (acid preincubation) revealed no significant differences in the absolute number of slow fibers in +/oim or oim/oim mice as compared to wt muscles (Fig. 3A,B).
Figure 3.
(A) Myofibrillar ATPase stained (acid preincubation) +/oim (n=3; center) and oim/oim (n=4; right) soleus (S) muscle from 4 month old mice did not show differences in distribution of type I or type II fibers compared to wt (n=3; left) S muscle, 2.5X magnification. The same was true for plantaris (P), gastrocnemius (G), tibialis anterior (TA) and quadriceps (Q) muscles (results not shown). (B) Distribution of type I and type 2 fibers of the S muscle expressed as a percent of total muscle fiber number (mean ± SE).
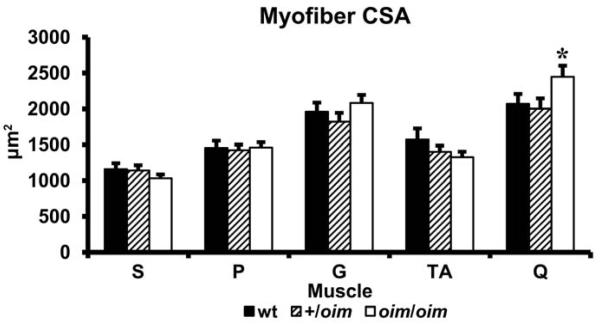
3.5 Myofiber Cross sectional Area
Evaluation of cross-sectional area (CSA) of the muscle fibers of the S, P, G, TA and Q muscles indicate that oim mice do not have smaller muscle fiber CSAs than wt mice. Interestingly, oim/oim mice did exhibit larger (p < 0.05) CSA of their Q myofibers compared to their +/oim counterparts (Fig. 4).
Figure4.
Cross-sectional area (CSA (μm2)) of soleus (S), plantaris (P), gastrocnemius (G), tibialis anterior (TA) and quadriceps (Q) muscles of wt (n=12; solid), +/oim (n=14; diagonal), and oim/oim (n=13; open) mice (mean ± SE). Q CSA is significantly larger in oim/oim mice compared to their +/oim counterparts. * p < 0.05 compared to +/oim.
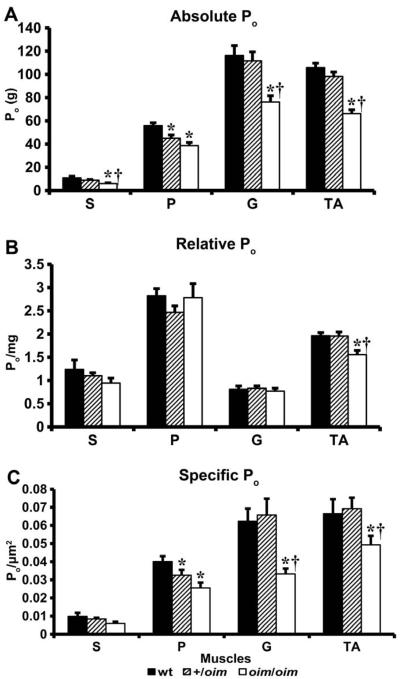
3.6 Contractile Force Generating Capacity
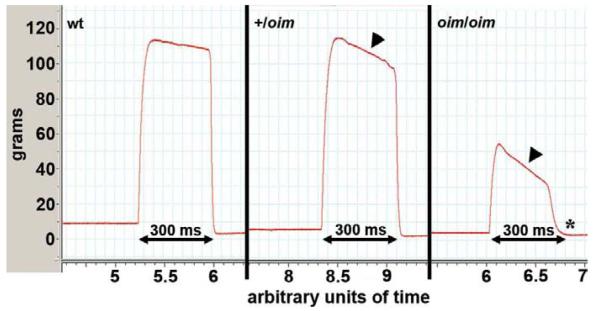
Absolute whole muscle contractile generating capacity [peak tetanic force (Po)] was less in oim/oim S (p ≤ 0.055), G and TA (p < 0.05) muscles compared to wt and +/oim muscles. Po for P was significantly impaired in +/oim and oim/oim compared to wt (Fig. 5A). Relative muscle contractile generating capacity [peak tetanic force (Po) / muscle weight in milligrams (mg)] was impaired (p < 0.05) in the TA muscle of oim/oim mice relative to their wt and +/oim counterparts (Fig. 5B). Specific Po [peak tetanic force (Po) / CSA (μm2)] was decreased (p < 0.05) in oim/oim G and TA muscles compared to wt and +/oim (Fig. 5C). P Po/CSA was reduced (p < 0.05) in +/oim and oim/oim relative to wt mice (Fig. 5C). In addition to determining the contractile force generating capacity, we observed a severe decay of tetanic force in the S, G and TA muscles of oim/oim mice and a mild decay of tetanic force in the G and TA muscles of +/oim relative to wt mice (Fig. 6). The oim muscles could not sustain Po for the 300 ms duration. A representative example of this decay and inability to sustain Po during 300 ms of stimulation can be seen with the force curves of the TA of +/oim and oim/oim mice (Fig. 6). The equivalent shape and duration of the toe region in contractile force recordings of the S, P, G and TA between wt, +/oim and oim/oim mice, and passive tensions, suggested that there was no difference between the genotypes in the ability of the tendon to maintain tension (data not shown).
Figure 5.
(A) Absolute tetanic force (Po) for soleus (S) (p < 0.055), gastrocnemius (G) and tibialis anterior (TA) muscles is reduced in 4 month old oim/oim (n=11; open) compared to wt (n=12; solid) and +/oim (n=12; diagonal) mice. Po for the plantaris (P) is reduced in +/oim and oim/oim mice compared to wt (mean ± SE). (B) Relative tetanic force (Po / mg) for the TA muscle is significantly reduced in oim/oim mice compared to their +/oim and wt counterparts (mean ± SE). (C) Specific tetanic force (Po / CSA) is significantly less in the P of +/oim and oim/oim mice compared to wt. Po/CSA of G and TA muscles is significantly less in oim/oim compared to wt and +/oim mice (mean ± SE). * p < 0.05 compared to wt, † p < 0.05 compared to +/oim
Figure 6.
Tetanic stimulation of the tibialis anterior (TA) muscle of 4 month old mice shows a precipitous decay in tetanic force in oim/oim (right, arrowhead) and mild decay in +/oim (center, arrowhead) compared to wt (left) mice (300 ms duration, 150 Hz). In addition, oim/oim mice were unable to sustain Po contraction during the entire 300 ms stimulation period (right, asterisk).
4. Discussion
We found that oim/oim mice had and weaker muscles compared to their wt and +/oim counterparts. Since oim/oim mice are significantly smaller than their wt littermates (Carleton et al., 2008; Chipman et al., 1993; Phillips et al., 2000), we anticipated that muscle weight would also be decreased. Though whole muscle wet weights of oim/oim mice were 75% and 80% of wt and +/oim mice, respectively, the muscles remained smaller even when normalized to body weight; oim/oim relative muscle weights were approximately 88% and 90% of wt and +/oim muscles, respectively. This percent loss of whole and relative muscle weight is of potential clinical significance and suggests that reported muscle weakness by OI patients with moderate to severe forms of the disease may be due, in part, to decreased muscle size. Currently the biological significance of these findings is unknown, and the percent loss of muscle that correlates to a subsequent loss of function is not well characterized in mice or humans. Female mice exhibited the same trends as males and had similar decreases in wet muscle weights and contractile capacity. There appears no predilection in clinical presentation of the disease for either males or females.
There were no differences in the distribution of muscle fiber types in the S, P, G, TA and Q muscles between wt, +/oim or oim/oim genotypes, suggesting that neither inherent differences in fiber type nor fiber type switching can account for the observed increased CSA of the Q or decreased contractile function of the S, P, G, or TA muscles in +/oim or oim/oim mice. Mice have primarily a type II fiber makeup (Burkholder et al., 1994) even for muscles that are considered “slow twitch” such as the soleus muscle (Glaser et al., 2009). A limitation of the ATPase methodology for muscle fiber typing is that only type I and type II fibers are clearly distinguishable, and thus, subtle differences in type II fiber subtype composition can not be ruled out (Burkholder et al., 1994; Lieber, 2002).
Though relative muscle weight was decreased in most of the oim/oim muscles compared to wt and +/oim mice, CSA did not appear negatively affected in oim/oim mice. In particular, while whole muscle weights of the Q muscle in oim/oim male mice were decreased relative to wt and +/oim littermates, the relative Q muscle weight was not reduced and there was even an increase in average Q myofiber CSA.
Fiber area affects fiber force and fiber type distribution affects muscle speed and endurance (Lieber, 2002). Skeletal muscle contractile properties depend on many factors including muscle fiber size, fiber properties (i.e. cross-bridge formation, Ca+2 influx into the sarcoplasmic reticulum), and the arrangement and number of fibers within the muscle (i.e., muscle architecture) (Burkholder et al., 1994). Carleton et al. have shown that oim/oim femur lengths are 3-4% shorter than wt femurs (Carleton et al., 2008), whereas tibial lengths between wt, +/oim and oim/oim were not different (data not shown). Perhaps the oim/oim Q fiber CSA reflects altered bone geometry and / or altered muscle attachment sites and architecture which are not present in the tibia associated muscles, the S, P, G and TA.
Decreased muscle strength may also result from a decrease in skeletal muscle capillary density. Takken et al. found that OI children had significantly lower peak oxygen consumption (VO2peak) compared with healthy subjects, though there was no evidence of circulatory abnormalities (Takken et al., 2004). It has been noted that subjects which are more sedentary compared to those with active lifestyles have fewer capillaries in their muscles per CSA (Takken et al., 2004). Sedentary subjects also have a reduction of oxidative enzyme which can result in reduced skeletal muscle capacity for oxygen extraction from the blood, leading to reduced VO2peak (Takken et al., 2004). Activity levels and capillary density in oim mice remain to be evaluated.
The P muscle of +/oim mice was significantly weaker compared to wt. In addition, +/oim mice displayed a mild decay of tetanic tension during the stimulation period. This indicates that +/oim mice (representing individuals with clinically mild OI type I) may have mild skeletal muscle weakness, consistent with findings by Takken et al. (Takken et al., 2004). Although no obvious disruptions in the muscle cell morphology were noted, mitochondria and enzymatic parameters were not examined. In addition to the reduced Po in S, G and TA muscles of oim/oim compared to wt and +/oim mice, as well as the decreased Po/CSA in oim/oim P, G and TA muscles compared to wt mice, we observed a decay of tension during tetanic stimulation in the TA, S and G muscles in oim/oim mice, which was less apparent in the P muscle of oim/oim. We also observed a mild decay of tension in both the TA and G muscles of +/oim which lead us to believe that the skeletal muscle deficits observed have physiologic causes (Fig. 6). These phenomena may be due to alterations in the regulation of intracellular Ca+2 handling and thus muscle contractility. There may also be reduced or altered functional status of store-operated Ca+2 (SOCE) resulting in defective Ca+2 homeostasis in oim/oim muscle cells. It would be valuable to perform single muscle studies in order to address this phenomenon.
We also observed reduced levels of fibrillar collagen in oim/oim skeletal muscle compared to wt and +/oim, which is consistent with the markedly lower myocardial collagen content and reduced collagen fiber diameter found in the oim/oim heart, contributing to decreased ventricular function (Weis et al., 2000).
Tendons of oim/oim mice are also known to have reduced type I collagen levels and to be biomechanically compromised (Misof et al., 1997; Sims et al., 2003). Tail tendon of oim/oim mice contains approximately 60% less collagen and possesses 40% lower tensile strength than wt controls, and oim/oim tendon fibers are thinner than age-matched and weight-matched controls (McBride et al., 1997).
Force transduction measurements in our experiments were performed by attaching silk thread to the tendons in the oim mice, which may have resulted in possible discrepancies in the amount of force measured due to alterations in type I collagen strength and content. However, examination of the contractile force recordings and passive tensions of wt, +/oim and oim/oim S, P, G and TA muscles suggest that there is no difference between the genotypes in the ability of the tendon to maintain tension.
Although the oim mouse is the most widely used mouse model of osteogenesis imperfecta, the oim gene defect, which causes the absence of functional α2(I) chains and it's recessive mode of inheritance are rare causes of OI in the human population (Nicholls et al., 1984; Pihlajaniemi et al., 1984).
We demonstrate that oim/oim mice, which are phenotypically similar to the clinical presentation of type III OI in humans, have impairment of certain hindlimb muscles. Although all muscles evaluated appear morphologically normal, whole muscle wet weights and fibrillar collagen content of the S and Q muscles are significantly reduced compared to their wt and +/oim counterparts. P, G and TA muscles of oim/oim mice are reduced in weight compared to wt and +/oim mice to a greater extent than what would be expected due to their overall decreased body size. Po of all muscles evaluated is compromised in oim/oim mice. Muscle fiber type distribution is similar for all muscles evaluated between genotypes. It appears also that +/oim mice (whose phenotype is similar to the mild form of OI type I in humans) have mild weakness of specific muscles and are less affected than their oim/oim counterparts, which have readily apparent skeletal muscle pathology. Our findings suggest that the skeletal muscle weakness in oim mice may reflect the decreased muscle strength observed in patients with OI (Boot et al., 2006; Takken et al., 2004). Suskauer et al. looked at physical performance in children with OI and found that they were significantly less active than their nondisabled peers (Suskauer et al., 2003). In children the cycle of fractures, pain and casting likely leads to minimized activity. Although it is also possible that reduced physical activity compounds the inherent skeletal muscle pathology in oim mice, the fiber CSAs were not different for oim/oim mice compared to controls, which undermines the potential contribution of reduced physical activity as an explanation for our findings. Future studies are needed to investigate muscle weakness at the single fiber and ultrastructural levels. Additionally, further investigations are needed of skeletal muscle in other mouse models of OI such as Brtl (Forlino et al., 1999) and G610C (Daley et al., 2009) as well as the CRTAP deficient (Morello et al., 2006) and Cyclophilin-B deficient mice (Choi et al., 2009) to clarify the role of OI causing mutations on skeletal muscle weakness and function.
5. Acknowledgements
We would like to acknowledge and thank Dr. Stephanie Carleton for her contributions to this project as well as Jill Gruenkemeyer, Jan Adair and Bonita Cowan of the RADIL histology lab for their technical assistance in slide preparation. We thank Dave Timm for his help generating data for the CSA measurements.
6. Role of the funding source NIH grants: T32 RR007004 (CF), AR055907 (CLP), HD058834 (MB); Phi Zeta (BG); Leda J Sears (CLP); University of Missouri Research Board (CLP, MB)
The funding sources had no involvement in the study design, collection, analysis or interpretation of data, writing of the report or in the decision to submit the paper for publication.
Abbreviations
- ATP
adensosine triphoshate
- CSA
cross-sectional area
- CRTAP
cartilage-associated protein
- G
gastrocnemius
- LEPRE1
prolyl-3-hydroxylase-1
- LSD
least significant difference
- OI
osteogenesis imperfecta
- oim
osteogenesis imperfecta murine
- P
plantaris
- Po
absolute whole muscle peak tetanic force
- Po/mg
relative muscle tetanic force
- Po/CSA
specific muscle tetanic force
- PPIB
peptidyl-prolyl isomerase B
- S
soleus
- SE
standard error
- SOCE
store-operated Ca+2
- TA
tibialis anterior
- Q
quadriceps
- VO2peak
peak oxygen consumption
- wt
wildtype
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this work were presented at the: Experimental Biology Meeting (San Diego, CA April 8, 2008), the American Society for Matrix Biology Meeting (San Diego, CA December 9, 2008) and the Experimental Biology Meeting (New Orleans, LA April 22, 2009)
7. References
- 1.Baldridge D, Schwarze U, Morello R, et al. CRTAP and LEPRE1 mutations in recessive osteogenesis imperfecta. Hum Mutat. 2008;29:1435–1442. doi: 10.1002/humu.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes AM, Chang W, Morello R, et al. Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. N Engl J Med. 2006;355:2757–2764. doi: 10.1056/NEJMoa063804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes AM, Carter EM, Cabral WA, et al. Lack of cyclophilin B in osteogenesis imperfecta with normal collagen folding. N Engl J Med. 2010;362:521–528. doi: 10.1056/NEJMoa0907705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basel D, Steiner RD. Osteogenesis imperfecta: recent findings shed new light on this once well-understood condition. Genet Med. 2009;11:375–385. doi: 10.1097/GIM.0b013e3181a1ff7b. [DOI] [PubMed] [Google Scholar]
- 5.Boot AM, de Coo RF, Pals G, et al. Muscle weakness as presenting symptom of osteogenesis imperfecta. Eur J Pediatr. 2006;165:392–394. doi: 10.1007/s00431-006-0083-6. [DOI] [PubMed] [Google Scholar]
- 6.Brown M, Ning J, Ferreira JA, et al. Estrogen receptor-alpha and -beta and aromatase knockout effects on lower limb muscle mass and contractile function in female mice. Am J Physiol Endocrinol Metab. 2009;296:E854–861. doi: 10.1152/ajpendo.90696.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burkholder TJ, Fingado B, Baron S, et al. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol. 1994;221:177–190. doi: 10.1002/jmor.1052210207. [DOI] [PubMed] [Google Scholar]
- 8.Byers PH. Osteogenesis Imperfecta. 2 ed. Wiley-Liss; New York: 1993. [Google Scholar]
- 9.Byers PH. Disorders of Collagen Biosynthesis and Structure. In: Scriver C, Beadudet A, Sly W, Valle D, Childs B, Vogelstein B, editors. Metabolic and Molecular Bases of Inherited Disease. 8th Ed McGraw-Hill; 2001. pp. 5241–5285. [Google Scholar]
- 10.Cabral WA, Chang W, Barnes AM, et al. Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat Genet. 2007;39:359–365. doi: 10.1038/ng1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camacho NP, Hou L, Toledano TR, et al. The material basis for reduced mechanical properties in oim mice bones. J Bone Miner Res. 1999;14:264–272. doi: 10.1359/jbmr.1999.14.2.264. [DOI] [PubMed] [Google Scholar]
- 12.Carleton SM, McBride DJ, Carson WL, et al. Role of genetic background in determining phenotypic severity throughout postnatal development and at peak bone mass in Col1a2 deficient mice (oim) Bone. 2008;42:681–694. doi: 10.1016/j.bone.2007.12.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carson FL. Histotechnology A Self-Instructional Text. 2nd ed. ASCP Press; Chicago: 1997. [Google Scholar]
- 14.Chipman SD, Sweet HO, McBride DJ, Jr., et al. Defective pro alpha 2(I) collagen synthesis in a recessive mutation in mice: a model of human osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1993;90:1701–1705. doi: 10.1073/pnas.90.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi JW, Sutor SL, Lindquist L, et al. Severe osteogenesis imperfecta in cyclophilin B-deficient mice. PLoS Genet. 2009;5:e1000750. doi: 10.1371/journal.pgen.1000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conover WJ, Iman RL. Analysis of covariance using the rank transformation. Biometrics. 1982;38:715–724. [PubMed] [Google Scholar]
- 17.Daley E, Streeten EA, Sorkin JD, et al. Variable Bone Fragility Associated with an Amish COL1A2 Variant and a Knock-in Mouse Model. J Bone Miner Res. 2009 doi: 10.1359/jbmr.090720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engelbert RH, van der Graaf Y, van Empelen R, et al. Osteogenesis imperfecta in childhood: impairment and disability. Pediatrics. 1997;99:E3. doi: 10.1542/peds.99.2.e3. [DOI] [PubMed] [Google Scholar]
- 19.Forlino A, Porter FD, Lee EJ, et al. Use of the Cre/lox recombination system to develop a non-lethal knock-in murine model for osteogenesis imperfecta with an alpha1(I) G349C substitution. Variability in phenotype in BrtlIV mice. J Biol Chem. 1999;274:37923–37931. doi: 10.1074/jbc.274.53.37923. [DOI] [PubMed] [Google Scholar]
- 20.Gajko-Galicka A. Mutations in type I collagen genes resulting in osteogenesis imperfecta in humans. Acta Biochim Pol. 2002;49:433–441. [PubMed] [Google Scholar]
- 21.Glaser BW, You G, Zhang M, et al. Relative proportions of hybrid fibers are unaffected by 6 weeks of running exercise in mouse skeletal muscles. Exp Physiol. 2009 doi: 10.1113/expphysiol.2009.049023. [DOI] [PubMed] [Google Scholar]
- 22.Hesselink RP, Gorselink M, Schaart G, et al. Impaired performance of skeletal muscle in alpha-glucosidase knockout mice. Muscle Nerve. 2002;25:873–883. doi: 10.1002/mus.10125. [DOI] [PubMed] [Google Scholar]
- 23.Lieber RL. Skeletal muscle structure, function & plasticity : the physiological basis of rehabilitation. 2nd ed. Lippincott Williams & Wilkins; Philadelphia: 2002. [Google Scholar]
- 24.Marini JC, Cabral WA, Barnes AM, et al. Components of the collagen prolyl 3-hydroxylation complex are crucial for normal bone development. Cell Cycle. 2007a;6:1675–1681. doi: 10.4161/cc.6.14.4474. [DOI] [PubMed] [Google Scholar]
- 25.Marini JC, Forlino A, Cabral WA, et al. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum Mutat. 2007b;28:209–221. doi: 10.1002/humu.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McBride DJ, Jr., Choe V, Shapiro JR, et al. Altered collagen structure in mouse tail tendon lacking the alpha 2(I) chain. J Mol Biol. 1997;270:275–284. doi: 10.1006/jmbi.1997.1106. [DOI] [PubMed] [Google Scholar]
- 27.McBride DJ, Jr., Shapiro JR, Dunn MG. Bone geometry and strength measurements in aging mice with the oim mutation. Calcif Tissue Int. 1998;62:172–176. doi: 10.1007/s002239900412. [DOI] [PubMed] [Google Scholar]
- 28.Misof K, Landis WJ, Klaushofer K, et al. Collagen from the osteogenesis imperfecta mouse model (oim) shows reduced resistance against tensile stress. J Clin Invest. 1997;100:40–45. doi: 10.1172/JCI119519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morello R, Bertin TK, Chen Y, et al. CRTAP is required for prolyl 3-hydroxylation and mutations cause recessive osteogenesis imperfecta. Cell. 2006;127:291–304. doi: 10.1016/j.cell.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 30.Nicholls AC, Osse G, Schloon HG, et al. The clinical features of homozygous alpha 2(I) collagen deficient osteogenesis imperfecta. J Med Genet. 1984;21:257–262. doi: 10.1136/jmg.21.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips CL, Bradley DA, Schlotzhauer CL, et al. Oim mice exhibit altered femur and incisor mineral composition and decreased bone mineral density. Bone. 2000;27:219–226. doi: 10.1016/s8756-3282(00)00311-2. [DOI] [PubMed] [Google Scholar]
- 32.Pihlajaniemi T, Dickson LA, Pope FM, et al. Osteogenesis imperfecta: cloning of a pro-alpha 2(I) collagen gene with a frameshift mutation. J Biol Chem. 1984;259:12941–12944. [PubMed] [Google Scholar]
- 33.Saban J, Zussman MA, Havey R, et al. Heterozygous oim mice exhibit a mild form of osteogenesis imperfecta. Bone. 1996;19:575–579. doi: 10.1016/s8756-3282(96)00305-5. [DOI] [PubMed] [Google Scholar]
- 34.Sims TJ, Miles CA, Bailey AJ, et al. Properties of collagen in OIM mouse tissues. Connect Tissue Res. 2003;44(Suppl 1):202–205. [PubMed] [Google Scholar]
- 35.Suskauer SJ, Cintas HL, Marini JC, et al. Temperament and physical performance in children with osteogenesis imperfecta. Pediatrics. 2003;111:E153–161. doi: 10.1542/peds.111.2.e153. [DOI] [PubMed] [Google Scholar]
- 36.Takken T, Terlingen HC, Helders PJ, et al. Cardiopulmonary fitness and muscle strength in patients with osteogenesis imperfecta type I. J Pediatr. 2004;145:813–818. doi: 10.1016/j.jpeds.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Van Dijk FS, Pals G, Van Rijn RR, et al. Classification of Osteogenesis Imperfecta revisited. Eur J Med Genet. 2009;53:1–5. doi: 10.1016/j.ejmg.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Weis SM, Emery JL, Becker KD, et al. Myocardial mechanics and collagen structure in the osteogenesis imperfecta murine (oim) Circ Res. 2000;87:663–669. doi: 10.1161/01.res.87.8.663. [DOI] [PubMed] [Google Scholar]
- 39.Willaert A, Malfait F, Symoens S, et al. Recessive osteogenesis imperfecta caused by LEPRE1 mutations: clinical documentation and identification of the splice form responsible for prolyl 3-hydroxylation. J Med Genet. 2009;46:233–241. doi: 10.1136/jmg.2008.062729. [DOI] [PubMed] [Google Scholar]