Abstract
Mice have increasingly been used as a model for studies of myopia. The key to successful use of mice for myopia research is the ability to obtain accurate measurements of refractive status of their eyes. In order to obtain accurate measurements of refractive errors in mice, the refraction needs to be performed along the optical axis of the eye. This represents a particular challenge, because mice are very difficult to immobilize. Recently, ketamine-xylazine anesthesia has been used to immobilize mice before measuring refractive errors, in combination with tropicamide ophthalmic solution to induce mydriasis. Although these drugs have increasingly been used while refracting mice, their effects on the refractive state of the mouse eye have not yet been investigated. Therefore, we have analyzed the effects of tropicamide eye drops and ketamine-xylazine anesthesia on refraction in P40 C57BL/6J mice. We have also explored two alternative methods to immobilize mice, i.e. the use of a restraining platform and pentobarbital anesthesia. We found that tropicamide caused a very small, but statistically significant, hyperopic shift in refraction. Pentobarbital did not have any substantial effect on refractive status, whereas ketamine-xylazine caused a large and highly significant hyperopic shift in refraction. We also found that the use of a restraining platform represents good alternative for immobilization of mice prior to refraction. Thus, our data suggest that ketamine-xylazine anesthesia should be avoided in studies of refractive development in mice and underscore the importance of providing appropriate experimental conditions when measuring refractive errors in mice.
1. Introduction
Several vertebrate species, including non-human primates (Wiesel and Raviola, 1977), tree shrews (Sherman et al., 1977) and chickens (Wallman et al., 1978), have been historically used for myopia research. Myopia can be experimentally induced in these species by eyelid fusion, diffusers or negative spectacle lenses. Several recent reports have also suggested that visual form deprivation and imposed hyperopic defocus can lead to development of myopia in mice (Barathi et al., 2008; Schaeffel et al., 2004; Tejedor and de la Villa, 2003; Tkatchenko et al., 2010b). Therefore mice increasingly become a popular model for myopia research. However, small body size, small size of mouse eyes and lack of well-established procedures for restraining the animals cause difficulty in obtaining accurate measurements of the refractive state of the eye, which complicates the use of mice for myopia research.
Several different approaches have been used to measure refractive errors in mice, i.e. streak retinoscopy of animals anesthetized with ketamine-xylazine (Barathi et al., 2008), photorefraction of animals anesthetized with ketamine-xylazine (Pardue et al., 2008), and photorefraction of freely moving (Schaeffel et al., 2004; Schmucker and Schaeffel, 2004; Zhou et al., 2008) or physically restrained (Tkatchenko et al., 2010a, b) alert mice using a high-resolution automated eccentric infrared photorefractor. In some of these studies, animals have been immobilized by ketamine-xylazine anesthesia before refraction (Barathi et al., 2008; Pardue et al., 2008), and/or tropicamide was used to induce mydriasis (Pardue et al., 2008; Schaeffel et al., 2004).
Ketamine hydrochloride is a N-methyl-D-aspartate (NMDA) receptor antagonist, which is used for induction of analgesia and anesthesia. Xylazine hydrochloride is an α2-adrenergic receptor agonist, which causes sedat ion, anesthesia, muscle relaxation and analgesia. Tropicamide, a muscarinic acetylcholine receptor antagonist, is widely used in clinical practice to induce mydriasis and cycloplegia.
Although ketamine-xylazine anesthesia is increasingly used to immobilize mice and rats before refraction, xylazine alone and in combination with ketamine was shown to cause transient opacification of the crystalline lens of the eye in rodents (Calderone et al., 1986; Kufoy et al., 1989) that may interfere with the accurate measurement of refractive errors. The effects of tropicamide on refractive state of the mouse eye have not been investigated.
In the present study, we have analyzed the effects of tropicamide eye drops, ketamine-xylazine and pentobarbital anesthesia on refractive state of the eye in C57BL/6J mice. We found that pentobarbital did not have any substantial effect on refractive status, whereas ketamine-xylazine caused a large hyperopic shift in refraction. Tropicamide caused statistically significant, but very small hyperopic shift in refraction.
2. Materials and methods
2.1. Animals
C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and were maintained as an in-house breeding colony at the Wayne State University School of Medicine. The experimental group was composed of 40-day-old (P40) animals from the same litter to minimize the impact of individual variations within the mouse population. C57BL/6J mice are known to have a relatively high incidence of microphthalmia, which affects from 4.4% to 10% of animals (Chase, 1942; Kalter, 1968). Therefore, animals were screened for the presence of microphthalmia and other ophthalmic abnormalities such as corneal opacities and anterior polar cataracts often associated with this condition (Koch and Gowen, 1939). Animals found to have microphthalmia, corneal opacities or cataract were removed from the study (~10% in our colony). All procedures adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Wayne State University Institutional Animal Care and Use Committee.
2.2. Drug treatment and dosages
Mydriasis was induced by 1% tropicamide ophthalmic solution (Alcon Laboratories, Fort Worth, TX), which was used as eye drops. One drop of the solution was instilled in each eye; excess of the solution was carefully removed with Kimwipes (Kimberly-Clark, Roswell, GA). Mydriasis was complete in 2-3 min and remained stable for at least 2 hours (the longest we have carried out the observation). Application of the eye drops caused transient eye irritation, resulting in partial eyelid closure that lasted for 10-15 min.; therefore, mice were refracted 20 min. after application of tropicamide. Following the measurement, animals were anesthetized via intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg), and refracted again within 10 min. after the injection. The same animals were anesthetized via intraperitoneal injection of pentobarbital (40 mg/kg) next day and refracted again within 10 min. after injection. The pentobarbital injection was preceded by the application of tropicamide eye drops as described above.
2.3. Photorefraction
The refractive state of both left and right eyes was determined using a high-resolution automated eccentric infrared photorefractor as previously described (Schaeffel et al., 2004; Tkatchenko et al., 2010a). The animal to be refracted was immobilized using either restraining platform (alert mice) or drug-induced anesthesia (ketamine-xylazine- or pentobarbital-anesthetized mice), and each eye was refracted in dim room light (< 1 lux). To ensure refraction along the optical axis, the first Purkinje image was aligned with the center of the pupil. In case of off-axis refraction, the first Purkinje image was positioned at the periphery of the pupil (~23° off the optical axis) by rotating the animal in the horizontal plane. Five independent measurements (5-10-sec-long each) were taken for each eye. Each successful measurement was marked by a green LED flash, which was registered by the photorefractor software. Sixt y points (automatically acquired by the system every 16 msec.) from each measurement immediately preceding the green LED flash were combined, and a total of 300 points were used to calculate the mean and standard deviation.
2.4. Data analysis
Data modeling was performed with SigmaPlot version 10.0 (Systat Software, San Jose, CA). P-values were calculated using paired t test for two independent samples as implemented in STATISTICA version 7.1 (StatSoft, Tulsa, OK). All data are presented as mean ± SD.
3. Results
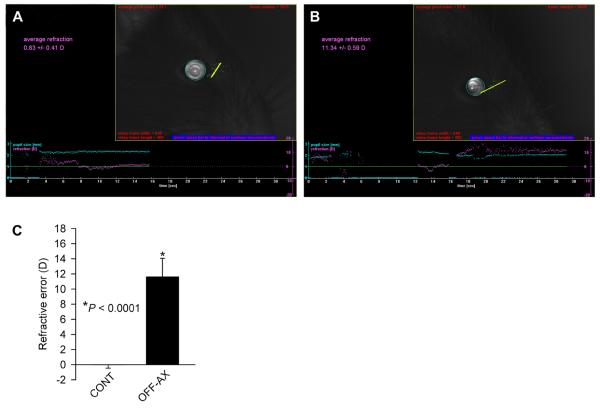
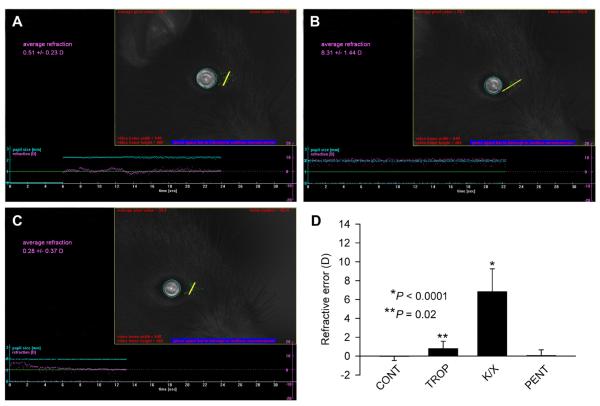
We found that animals in our population of 40-day-old C57BL/6J mice were emmetropic (0.0 ± 0.4 D, n = 9) when refracted along the optical axis of the eye, and highly hyperopic (+11.6 ± 2.4 D, P < 0.0001, n = 9) when refracted off the optical axis (Fig. 1, Table 1). Topical application of a 1% tropicamide ophthalmic solution resulted in only a slight, but statistically significant, hyperopic shift in refraction. The average on-axis refractive errors after the tropicamide treatment were +0.8 ± 0.8 D (P = 0.02, n = 9) (Table 1). Tropicamide did not have any adverse effects on the reflective properties of the cornea or the crystalline lens. However, ketamine-xylazine anesthesia delivered via intraperitoneal injection resulted in the development of a transient cataract as previously reported (Calderone et al., 1986; Kufoy et al., 1989). The cataract developed within 10-20 min. after the ketamine-xylazine injection and persisted for several hours. In spite of the cataract development, animals could still be refracted between the onset of anesthesia (3-5 min. after injection) and the appearance of cataract (10-20 min. after injection). We found that ketamine-xylazine anesthesia caused a substantial and highly significant hyperopic shift in on-axis refraction. Refractive errors in ketamine-xylazine-anesthetized animals were +6.9 ± 2.4 D (P < 0.0001, n = 9) (Fig. 2, Table 1). However, pentobarbital anesthesia did not have any significant effect on refraction. Refractive errors in animals anesthetized via intraperitoneal injection of pentobarbital (40 mg/kg) were +0.1 ± 0.6 D (P = 0.6, n = 9) (Fig. 2, Table 1).
Fig. 1.
Mice are emmetropic if measured along the optical axis, but peripheral refractive errors have hyperopic values. (A) Image showing a photorefractor measurement of the refractive error along the optical axis in a P40 C57BL/6J mouse. (B) Image showing a photorefractor measurement of the refractive error off the optical axis in the same eye shown in (A). (C) Graph summarizing refraction data (Table 1) in the same eye refracted along the optical axis (CONT) and off the optical axis (OFF-AX). P, significance value. Vertical error bars, SD. n = 9.
Table 1.
Refractive errors in P40 C57BL/6J mice before and after application of drugs
| Eye No | Control** | Off-axis*** | Tropicamide | Ketamine/Xylazine* | Pentobarbital* |
|---|---|---|---|---|---|
| 1 | −0.4 ± 1.5 | +8.7 ± 1.2 | +1.2 ± 0.5 | +7.1 ± 0.8 | +0.3 ± 1.3 |
| 2 | −0.2 ± 1.0 | +15.8 ± 2.0 | −0.4 ± 1.3 | +5.4 ± 1.1 | +0.5 ± 0.5 |
| 3 | +0.1 ± 0.8 | +9.8 ± 1.1 | +1.0 ± 1.2 | +6.2 ± 1.1 | +0.4 ± 0.9 |
| 4 | 0.0 ± 1.3 | +13.6 ± 3.9 | +1.1 ± 0.8 | +6.9 ± 0.8 | −0.7 ± 0.7 |
| 5 | +0.2 ± 1.3 | +13.6 ± 2.9 | +1.2 ± 1.0 | +5.7 ± 1.6 | +1.2 ± 0.6 |
| 6 | −0.6 ± 1.0 | +9.1 ± 4.1 | −0.4 ± 1.0 | +4.1 ± 0.6 | −0.1 ± 0.5 |
| 7 | +0.1 ± 1.0 | +10.1 ± 2.3 | +0.9 ± 1.3 | +9.0 ± 2.0 | +0.1 ± 0.5 |
| 8 | −0.4 ± 1.1 | +12.8 ± 2.7 | +2.1 ± 0.6 | +5.2 ± 0.9 | −0.5 ± 0.8 |
| 9 | +0.8 ± 1.1 | +11.2 ± 1.4 | +0.7 ± 1.0 | +12.1 ± 0.7 | −0.4 ± 0.8 |
| Mean | 0.0 ± 0.4 | +11.6 ± 2.4 | +0.8 ± 0.8 | +6.9 ± 2.4 | +0.1 ± 0.6 |
Refractive errors are shown in diopters as mean ± SD
Anesthetic injection followed tropicamide application (see Materials and methods)
Control refractions were measured along the optical axis of the eye
Maximum off-axis values (~23° off the optical axis) registered by the photorefractor are reported
Fig. 2.
Ketamine-xylazine anesthesia caused hyperopic shift in refraction. (A) Image showing a photorefractor measurement of the refractive error in a tropicamide-treated P40 C57BL/6J mouse. (B) Image showing a photorefractor measurement of the refractive error in the same eye shown in (A) after ketamine-xylazine injection. (C) Image showing a photorefractor measurement of the refractive error in the same eye shown in (A) after pentobarbital injection. (D) Graph summarizing refraction data (Table 1) in the untreated control (CONT), tropicamide-treated (TROP), tropicamide-ketamine-xylazine-treated (K/X) and tropicamide-pentobarbital-treated (PENT) animals. In the photorefractor images, refractive error values are shown in purple (horizontal line, right axis); pupil size is shown in cyan (horizontal line, left axis). A video frame on the right shows the surface brightness profile of a measured pupil with the brightness profile across the vertical meridian (yellow line). P, significance value. Vertical error bars, SD. n = 9.
4. Discussion
In the current study, we have analyzed the effects of tropicamide eye drops as well as ketamine-xylazine and pentobarbital anesthesia on refractive state of the mouse eye as measured by a high-resolution automated eccentric infrared photorefractor. Both tropicamide eye drops and ketamine-xylazine anesthesia have been used to facilitate refraction in mice (Barathi et al., 2008; Pardue et al., 2008; Schaeffel et al., 2004). We found that tropicamide caused a very small, but statistically significant, hyperopic shift in refraction, similar to what occurs in humans (Jorge et al., 2005). Pentobarbital did not have any substantial effect on either physical properties of the ocular media or refractive state of the eye. Conversely, ketamine-xylazine anesthesia caused a large hyperopic shift in refraction followed by the development of cataract.
Adult mice have previously been reported to have highly hyperopic refractive errors (Barathi et al., 2008; Pardue et al., 2008; Schaeffel et al., 2004; Schmucker and Schaeffel, 2004; Zhou et al., 2008). According to our data the hyperopic refractive errors reported in two of these studies (Barathi et al., 2008; Pardue et al., 2008) may be explained by the effect of ketamine-xylazine anesthesia. In other studies, animals were not immobilized and refractive errors were determined on freely moving alert mice using a high-resolution automated eccentric infrared photorefractor (Schaeffel et al., 2004; Schmucker and Schaeffel, 2004; Zhou et al., 2008). Our data suggest that hyperopic refractive errors reported in these studies may have resulted from off-axis refractions. When we compared refraction data with and without the use of a restraining platform, which physically immobilizes an animal and facilitates alignment of the first Purkinje image with the center of the pupil that ensures refraction along the optical axis of the eye, we noticed a persistent hyperopic shift if animals were refracted without restraint. Considering that the photorefractor collects data as long as the pupil is ≥ 1.7 mm, independently of the Purkinje image position (Fig. 1), and that mice are highly hyperopic just a few degrees off the optical axis as our data demonstrate (Fig. 1, Table 1), relatively hyperopic values previously reported in freely moving mice may be explained by the contribution of off-axis measurements.
The highly hyperopic refractive errors found in mice have been historically attributed to the “small eye artifact” that was suggested to result from reflection of the retinoscope light from the boundary between the vitreous and the retina (Glickstein and Millodot, 1970). However, further detailed studies of the optical properties of ocular tissues did not support the “small eye artifact” hypothesis and concluded that light is primarily reflected by the retinal pigment epithelium (RPE) (Delori and Pflibsen, 1989; Gorrand, 1986; Hammer et al., 1995; Knighton et al., 1992; Knighton et al., 1989; Preece and Claridge, 2002). The reflective properties of the fundus at the level of the RPE are even more pronounced in infrared light (Hammer et al., 1995; Preece and Claridge, 2002); therefore, the hyperopic refractive errors previously reported in mice cannot be explained by the “small eye artifact” and are likely to represent an artifact of measurement caused by ketamine-xylazine anesthesia or difficulty to refract along the optical axis of the eye.
We observed that tropicamide caused a small hyperopic shift in refraction, similar to what occurs in humans with drug-induced cycloplegia (Jorge et al., 2005). Although mice lack classical accommodative apparatus due to the rigidity of the lens and the absence of the ciliary muscle (Chalupa and Williams, 2008; Woolf, 1956), they possess a rudimentary capability for accommodation, which is afforded by the retractor bulbi muscle (Grimsdale, 1921; Isomura, 1981). This muscle, which is attached to the posterior segment of the eye, pulls on the eye when an animal looks down at near objects. This would cause slight elongation of the eyeball and result in a myopic shift in refraction. Considering that muscarinic agonists and antagonists can directly or indirectly influence activity of the neuromuscular junctions (Akk and Auerbach, 1999; Arenson, 1989; Ganguly and Das, 1979; Nathanson, 2000; Parnas et al., 2000; Santafe et al., 2003), the hyperopic shift observed in the tropicamide-treated mice might be explained by partial relaxation of the retractor bulbi muscle resulting in a forward displacement of the retina.
The hyperopic shift in refraction observed in ketamine-xylazine-anesthetized mice theoretically may be caused by either reduction in the axial length of the eye or changes in the refractive indices of the ocular media. Although xylazine was shown to reduce intraocular pressure in mice (Avila et al., 2001), it was recently demonstrated that the axial length and the vitreous chamber depth of the eye are not affected by ketamine-xylazine anesthesia (Tkatchenko et al., 2010a). Conversely, ketamine-xylazine anesthesia is known to cause transient anterior polar cataract in rodents (Calderone et al., 1986; Kufoy et al., 1989), which is likely to be accompanied by changes in the optical power of the crystalline lens. The opacifying effect of ketamine-xylazine anesthesia on the crystalline lens in rodents has been attributed to xylazine (Calderone et al., 1986). This ocular effect of xylazine was shown to be enhanced by ketamine (Calderone et al., 1986) and is similar to that of a number of other drugs known to produce acute reversible lens opacities in rodents (Weinstock and Scott, 1967). The opacifying effect of ketamine-xylazine on the lens has been explained by increased loss of water through the cornea, which was suggested to lead to increased osmolarity of the aqueous humor in the anterior chamber of the eye causing opacification of the anterior cortex of the lens (Fraunfelder and Burns, 1970; Weinstock and Scott, 1967). These data suggest that the appearance of the opacities is preceded by an increase in osmolarity of the lens fiber cytoplasm in the anterior cortex of the lens resulting in an increase of the anterior cortex density and its refractive index. Such increase in the refractive index of the anterior cortex is expected to lead to the reduction or disappearance of the refractive index gradient and reduction in the lens optical power similar to what takes place in the aging crystalline lens (Augusteyn et al., 2008; Glasser and Campbell, 1999; Hemenger et al., 1995; Kasthurirangan et al., 2008). This, in turn, would cause a hyperopic shift in refraction. Depression of blinking caused by the central effect of xylazine on α2-adrenergic receptors has been postulated to be responsible for increased loss of water through the cornea and opacification of the lens in ketamine-xylazine-anesthetized mice (Hsu, 1981; Hsu et al., 1981a; Hsu et al., 1981b); however, we observed that blinking was suppressed in both ketamine-xylazine- and pentobarbital-anesthetized mice with completely different outcomes. While ketamine-xylazine-anesthetized animals exhibited visibly dry eyes, production of tears in mice anesthetized with pentobarbital was visibly increased. Therefore, the difference in the effects of ketamine-xylazine (opacification of the lens and hyperopic shift in refraction) and pentobarbital (absence of lens opacification and no effect on refraction) may be linked to the increased production of tears in pentobarbital-anesthetized animals. Although our data seem to support dehydration hypothesis of transient lenticular opacification in ketamine-xylazine-anesthetized mice, the exact mechanism of this phenomenon remains unclear. Considering that sigma receptor 1 is present in the crystalline lens (Ola et al., 2001) and its activation is influenced by ketamine (Narita et al., 2001), ketamine-xylazine anesthesia may exert a direct effect on the lens.
In conclusion, we have shown that ketamine-xylazine anesthesia causes a large hyperopic shift in refraction in mice, whereas pentobarbital anesthesia does not cause any changes in the refractive status. We have also found that tropicamide eye drops cause statistically significant, but very small hyperopic shift in refraction, which might be associated with the presence of rudimentary accommodation in the mouse. Taken together these results underscore the importance of providing appropriate experimental conditions when measuring refraction in mice. Ketamine-xylazine anesthesia should be avoided in studies of refractive eye development in mice, while the use of a restraining platform and pentobarbital anesthesia represent useful alternatives.
Acknowledgments
The authors thank Frank Schaeffel for help with the mouse photorefractor and Harry Goshgarian for help with the manuscript. This work was supported by National Eye Institute grant R21EY018902 and Core Grant for Vision Research P30EY004068, Midwest Eye-Banks, and Research to Prevent Blindness.
References
- Akk G, Auerbach A. Activation of muscle nicotinic acetylcholine receptor channels by nicotinic and muscarinic agonists. Br J Pharmacol. 1999;128:1467–76. doi: 10.1038/sj.bjp.0702941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenson MS. Muscarinic inhibition of quantal transmitter release from the magnesium-paralysed frog sartorius muscle. Neuroscience. 1989;30:827–36. doi: 10.1016/0306-4522(89)90174-7. [DOI] [PubMed] [Google Scholar]
- Augusteyn RC, Jones CE, Pope JM. Age-related development of a refractive index plateau in the human lens: evidence for a distinct nucleus. Clin Exp Optom. 2008;91:296–301. doi: 10.1111/j.1444-0938.2007.00244.x. [DOI] [PubMed] [Google Scholar]
- Avila MY, Carre DA, Stone RA, Civan MM. Reliable measurement of mouse intraocular pressure by a servo-null micropipette system. Invest Ophthalmol Vis Sci. 2001;42:1841–6. [PubMed] [Google Scholar]
- Barathi VA, Boopathi VG, Yap EP, Beuerman RW. Two models of experimental myopia in the mouse. Vision Res. 2008;48:904–16. doi: 10.1016/j.visres.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Calderone L, Grimes P, Shalev M. Acute reversible cataract induced by xylazine and by ketamine-xylazine anesthesia in rats and mice. Exp Eye Res. 1986;42:331–7. doi: 10.1016/0014-4835(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Chalupa LM, Williams RW. Eye, retina, and visual system of the mouse. MIT Press; Cambridge, Mass.: 2008. [Google Scholar]
- Chase HB. Studies on an Anophthalmic Strain of Mice. III. Results of Crosses with Other Strains. Genetics. 1942;27:339–48. doi: 10.1093/genetics/27.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delori FC, Pflibsen KP. Spectral Reflectance of the Human Ocular Fundus. Applied Optics. 1989;28:1061–77. doi: 10.1364/AO.28.001061. [DOI] [PubMed] [Google Scholar]
- Fraunfelder FT, Burns RP. Acute reversible lens opacity: caused by drugs, cold, anoxia, asphyxia, stress, death and dehydration. Exp Eye Res. 1970;10:19–30. doi: 10.1016/s0014-4835(70)80005-7. [DOI] [PubMed] [Google Scholar]
- Ganguly DK, Das M. Effects of oxotremorine demonstrate presynaptic muscarinic and dopaminergic receptors on motor nerve terminals. Nature. 1979;278:645–6. doi: 10.1038/278645a0. [DOI] [PubMed] [Google Scholar]
- Glasser A, Campbell MC. Biometric, optical and physical changes in the isolated human crystalline lens with age in relation to presbyopia. Vision Res. 1999;39:1991–2015. doi: 10.1016/s0042-6989(98)00283-1. [DOI] [PubMed] [Google Scholar]
- Glickstein M, Millodot M. Retinoscopy and eye size. Science. 1970;168:605–6. doi: 10.1126/science.168.3931.605. [DOI] [PubMed] [Google Scholar]
- Gorrand JM. Separation of the reflection by the inner limiting membrane. Ophthalmic Physiol Opt. 1986;6:187–96. [PubMed] [Google Scholar]
- Grimsdale H. A note on the centre of rotation of the eye. Tr Ophth Soc U Kingdom. 1921;41:357. [Google Scholar]
- Hammer M, Roggan A, Schweitzer D, Muller G. Optical properties of ocular fundus tissues--an in vitro study using the double-integrating-sphere technique and inverse Monte Carlo simulation. Phys Med Biol. 1995;40:963–78. doi: 10.1088/0031-9155/40/6/001. [DOI] [PubMed] [Google Scholar]
- Hemenger RP, Garner LF, Ooi CS. Change with age of the refractive index gradient of the human ocular lens. Invest Ophthalmol Vis Sci. 1995;36:703–7. [PubMed] [Google Scholar]
- Hsu WH. Xylazine-induced depression and its antagonism by alpha adrenergic blocking agents. J Pharmacol Exp Ther. 1981;218:188–92. [PubMed] [Google Scholar]
- Hsu WH, Betts DM, Lee P. Xylazine-induced mydriasis: possible involvement of a central postsynaptic regulation of parasympathetic tone. J Vet Pharmacol Ther. 1981a;4:209–14. doi: 10.1111/j.1365-2885.1981.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Hsu WH, Lee P, Betts DM. Xylazine-induced mydriasis in rats and its antagonism by alpha-adrenergic blocking agents. J Vet Pharmacol Ther. 1981b;4:97–101. doi: 10.1111/j.1365-2885.1981.tb00717.x. [DOI] [PubMed] [Google Scholar]
- Isomura G. Comparative anatomy of the extrinsic ocular muscles in vertebrates. Anat Anz. 1981;150:498–515. [PubMed] [Google Scholar]
- Jorge J, Queiros A, Gonzalez-Meijome J, Fernandes P, Almeida JB, Parafita MA. The influence of cycloplegia in objective refraction. Ophthalmic Physiol Opt. 2005;25:340–5. doi: 10.1111/j.1475-1313.2005.00277.x. [DOI] [PubMed] [Google Scholar]
- Kalter H. Sporadic congenital malformations of newborn inbred mice. Teratology. 1968;1:193–9. doi: 10.1002/tera.1420010208. [DOI] [PubMed] [Google Scholar]
- Kasthurirangan S, Markwell EL, Atchison DA, Pope JM. In vivo study of changes in refractive index distribution in the human crystalline lens with age and accommodation. Invest Ophthalmol Vis Sci. 2008;49:2531–40. doi: 10.1167/iovs.07-1443. [DOI] [PubMed] [Google Scholar]
- Knighton RW, Baverez C, Bhattacharya A. The directional reflectance of the retinal nerve fiber layer of the toad. Invest Ophthalmol Vis Sci. 1992;33:2603–11. [PubMed] [Google Scholar]
- Knighton RW, Jacobson SG, Kemp CM. The spectral reflectance of the nerve fiber layer of the macaque retina. Invest Ophthalmol Vis Sci. 1989;30:2392–402. [PubMed] [Google Scholar]
- Koch FLP, Gowen JW. Spontaneous ophthalmic mutation in a laboratory mouse. Arch Pathol Lab Med. 1939;28:171–6. [Google Scholar]
- Kufoy EA, Pakalnis VA, Parks CD, Wells A, Yang CH, Fox A. Keratoconjunctivitis sicca with associated secondary uveitis elicited in rats after systemic xylazine/ketamine anesthesia. Exp Eye Res. 1989;49:861–71. doi: 10.1016/s0014-4835(89)80045-4. [DOI] [PubMed] [Google Scholar]
- Narita M, Yoshizawa K, Aoki K, Takagi M, Miyatake M, Suzuki T. A putative sigma1 receptor antagonist NE-100 attenuates the discriminative stimulus effects of ketamine in rats. Addict Biol. 2001;6:373–6. doi: 10.1080/13556210020077091. [DOI] [PubMed] [Google Scholar]
- Nathanson NM. A multiplicity of muscarinic mechanisms: enough signaling pathways to take your breath away. Proc Natl Acad Sci U S A. 2000;97:6245–7. doi: 10.1073/pnas.97.12.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ola MS, Moore P, El-Sherbeny A, Roon P, Agarwal N, Sarthy VP, Casellas P, Ganapathy V, Smith SB. Expression pattern of sigma receptor 1 mRNA and protein in mammalian retina. Brain Res Mol Brain Res. 2001;95:86–95. doi: 10.1016/s0169-328x(01)00249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue MT, Faulkner AE, Fernandes A, Yin H, Schaeffel F, Williams RW, Pozdeyev N, Iuvone PM. High susceptibility to experimental myopia in a mouse model with a retinal on pathway defect. Invest Ophthalmol Vis Sci. 2008;49:706–12. doi: 10.1167/iovs.07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas H, Segel L, Dudel J, Parnas I. Autoreceptors, membrane potential and the regulation of transmitter release. Trends Neurosci. 2000;23:60–8. doi: 10.1016/s0166-2236(99)01498-8. [DOI] [PubMed] [Google Scholar]
- Preece SJ, Claridge E. Monte Carlo modelling of the spectral reflectance of the human eye. Phys Med Biol. 2002;47:2863–77. doi: 10.1088/0031-9155/47/16/303. [DOI] [PubMed] [Google Scholar]
- Santafe MM, Salon I, Garcia N, Lanuza MA, Uchitel OD, Tomas J. Modulation of ACh release by presynaptic muscarinic autoreceptors in the neuromuscular junction of the newborn and adult rat. Eur J Neurosci. 2003;17:119–27. doi: 10.1046/j.1460-9568.2003.02428.x. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Burkhardt E, Howland HC, Williams RW. Measurement of refractive state and deprivation myopia in two strains of mice. Optom Vis Sci. 2004;81:99–110. doi: 10.1097/00006324-200402000-00008. [DOI] [PubMed] [Google Scholar]
- Schmucker C, Schaeffel F. A paraxial schematic eye model for the growing C57BL/6 mouse. Vision Res. 2004;44:1857–67. doi: 10.1016/j.visres.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Norton TT, Casagrande VA. Myopia in the lid-sutured tree shrew (Tupaia glis) Brain Res. 1977;124:154–7. doi: 10.1016/0006-8993(77)90872-1. [DOI] [PubMed] [Google Scholar]
- Tejedor J, de la Villa P. Refractive changes induced by form deprivation the mouse eye. Investigative Ophthalmology & Visual Science. 2003;44:32–6. doi: 10.1167/iovs.01-1171. [DOI] [PubMed] [Google Scholar]
- Tkatchenko TV, Shen Y, Tkatchenko AV. Analysis of postnatal eye development in the mouse with high-resolution small animal magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2010a;51:21–7. doi: 10.1167/iovs.08-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkatchenko TV, Shen Y, Tkatchenko AV. Mouse experimental myopia has features of primate myopia. Invest Ophthalmol Vis Sci. 2010b;51:1297–303. doi: 10.1167/iovs.09-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest change in early visual experience. Science. 1978;201:1249–51. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Scott JD. Effect of various agents on drug-induced opacities of the lens. Exp Eye Res. 1967;6:368–75. doi: 10.1016/s0014-4835(67)80011-3. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266:66–8. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- Woolf D. A comparative cytological study of the ciliary muscle. Anat Rec. 1956;124:145–63. doi: 10.1002/ar.1091240203. [DOI] [PubMed] [Google Scholar]
- Zhou X, Shen M, Xie J, Wang J, Jiang L, Pan M, Qu J, Lu F. The development of the refractive status and ocular growth in C57BL/6 mice. Invest Ophthalmol Vis Sci. 2008;49:5208–14. doi: 10.1167/iovs.07-1545. [DOI] [PubMed] [Google Scholar]