Abstract
Several oxidative reactions can be effected with MnO2 in the presence of sub-stoichiometric quantities of DDQ. These transformations include oxidative cyclization, deprotection, and dehydrogenation reactions. The use of MnO2 as a terminal oxidant for DDQ-mediated reactions is attractive based on economical and environmental factors.
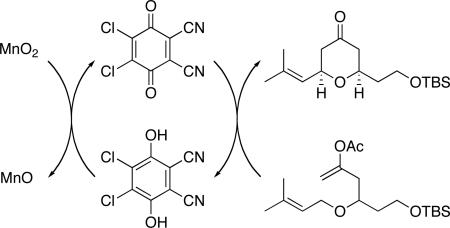
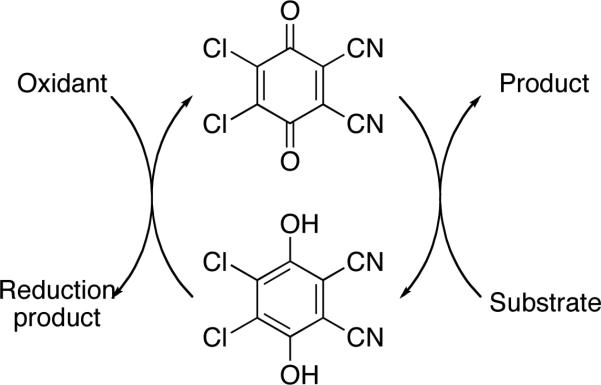
DDQ (2,3-dichloro-5,6-dicyano-1,4-benzoquinone) is a highly effective oxidant for a number of chemical transformations including protecting group removal, aromatization, acetal formation, and biaryl construction.1 DDQ is moderately expensive, however, with a cost of $526/mol according to the 2009-2010 Aldrich catalog.2 In addition to concerns about cost, DDQ also poses modest toxicity concerns, with an LD50 of 82 mg/kg,3 and the potential for HCN liberation upon exposure to H2O. These issues, coupled with the potential purification difficulties associated with utilizing stoichiometric amounts of an organic oxidant, have led to the search for economical and environmentally benign oxidants that regenerate DDQ from its reduced hydroquinone form (Scheme 1). HNO3 is a suitable oxidant for this purpose,4 though its strong acidity is often not compatible with functionalized organic compounds and necessitates that the reoxidation be conducted as an independent step. Chandrasekhar and co-workers reported5 that DDQ could be used as a catalytic oxidant with FeCl3 serving as the stoichiometric oxidant. This system is economically and ecologically attractive, with FeCl3 costing only $41/mol, and having an LD50 of 450 mg/kg, though the Lewis acidity of FeCl3 again makes it incompatible with many functional groups. Sharma and co-workers showed6 that Mn(OAc)3 is also an effective agent for DDQ regeneration that does not exhibit strong Lewis acidity. However Mn(OAc)3•2H2O is actually more expensive than DDQ at a price of $647/mol. The cost factor is compounded by the fact that Mn(OAc)3 is a single electron oxidant, making two equivalents necessary to regenerate DDQ.7
Scheme 1.
DDQ as a catalytic oxidant.
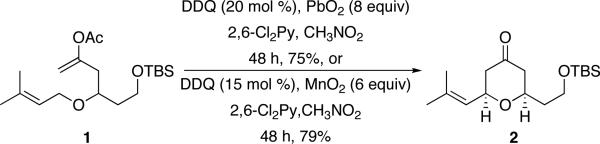
In accord with our efforts8 and those of other groups9 to use DDQ as a reagent for oxidative carbon–carbon bond forming reactions and our interest in developing catalytic oxidative transformations,10 we have initiated an effort to identify an inexpensive, non-acidic, and environmentally benign reagent that can be used as a terminal oxidant in the presence of sub-stoichiometric DDQ loadings. Following the observation that the use of FeCl3 as a terminal oxidant for DDQ-catalyzed carbon–hydrogen bond functionalization reactions induced substrate decomposition, we directed our initial screen for terminal oxidants toward metal oxides that are known to effect phenol oxidations. This search led us to explore PbO211 ($117/mol, LD50 = 220 mg/kg). Exposing prenyl ether 1 to DDQ (20 mol %) and PbO2 in CH3NO2 provided tetrahydropyrone 2 in 75% yield after 48 h (Scheme 2). Incorporating 2,6-dichloropyridine made this reaction and subsequent transformations proceed more efficiently because it can act as a non-oxidizable base and because it quenches the acylium ion that forms when the enol acetate group reacts with the oxocarbenium ion. Cognizant of the long term toxicity of lead, we explored other metal oxides as terminal oxidants. Exposing 1 to 15 mol % DDQ and MnO2 in CH3NO2 provided 2 in 79% yield. While the reaction time was long (48 h) this result was quite gratifying in consideration of the low cost of MnO2 ($28/mol) and its negligible toxicity (LD50 = 3,478 mg/kg). The products of these reactions were quite easy to isolate, with the excess metal-containing reagents and by-products being removed with a simple filtration over silica gel. In general oxidative cyclizations are slower in CH3NO2 than in less polar solvents, such as 1,2-dichlorothane. However the regeneration of DDQ was clearly faster in CH3NO2, making it the solvent of choice for this process. No conversion of 1 to 2 was observed when PbO2 or MnO212 were used in the absence of DDQ, confirming that the reactions were mediated by DDQ and that the metal oxides function to oxidize the hydroquinone. Additional evidence in support of DDQ regeneration was provided by observing the formation of DDQ upon subjecting 2,3-dichloro-5,6-dicyanohydroquinone13 to MnO2 in CH3NO2.14
Scheme 2.
PbO2 and MnO2 as terminal oxidants for a DDQ-mediated oxidation.
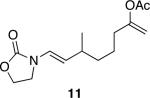
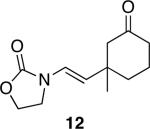
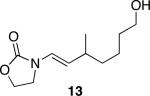
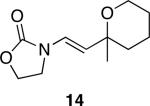
The successful demonstration of MnO2-mediated DDQ turnover for our oxidative cyclization protocol led us to examine the generality of the protocol. A representative group of these reactions is shown in Table 1. Allylic ethers, benzylic ethers, and vinyl oxazolidinones serve as substrates for the protocol. While we did not study a broad range of nucleophiles, we observed that alcohols are tolerated by the reaction conditions in addition to electron rich alkenes. Quaternary centers can be formed in the process, and sterically hindered ethers can be used as substrates. In general the yields of the reactions were within approximately 10% of the yields for the corresponding reactions that employ 2.0 eqivalents of DDQ. The rates of these reactions roughly correlate to the corresponding stoichiometric reactions, though the long reaction times that are observed for even the most reactive substrates show that hydroquinone oxidation is the rate limiting step. The only significant problem was encountered when slowly reacting substrate 19 (entry 9) was examined. This problem can be attributed to the change in solvent, because these cyclizations proceed more slowly in CH3NO2 than DCE, and to the low reaction rates that result from employing a substoichiometric amount of oxidant.
Table 1.
DDQ-catalyzed oxidative cyclization reactions.a
| entry | substrate | product | time (h) | yield (%)b |
|---|---|---|---|---|
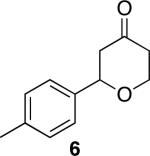
| 1 |

|
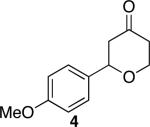
|
41 | 76 |
| 2 |

|
|
44 | 83 |
| 3 |
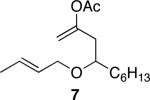
|
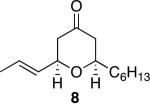
|
40 | 92 |
| 4c |
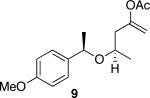
|

|
48 | 75 |
| 5 |
|
|
36 | 68 |
| 6 |
|
|
24 | 87 |
| 7 |
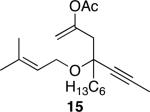
|

|
40 | 70 |
| 8 |
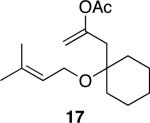
|

|
30 | 81 |
| 9 |

|
– | – | – |
Representative procedure: DDQ was added in three equal portions over the course of the reaction (0.15 equiv total) to a suspension of the substrate, 2,6-dichloropyridine (2 equiv), and activated MnO2 (6 equiv) in CH3NO2 (0.1 M). The reaction stirred at room temperature for the indicated period of time.
Yields refer to isolated, purified products.
Reaction required 0.2 equiv DDQ.
We examined the applicability of using MnO2 as a terminal oxidant to other commonly employed DDQ-mediated transformations to explore the generality of the process. A number of these reactions are shown in Scheme 3. PMB ether cleavage can be effected, as seen in the conversion of 20 to 21. Water deactivates MnO2, so MeOH was used as the nucleophile in this transformation. Oxidizing hydroxyl-containing PMB ether 22 in the absence of MeOH provided PMP acetal 23. Dehydrogenation reactions are quite effective, with dihydronaphthalene (24) being converted to naphthalene (25) in 96% yield. This process can also be applied to the conversion of oxazolines to oxazoles, as seen in the conversion of 26 to 27.15 This method of oxazole preparation is an improvement over a previously reported variant16 in which specially prepared high surface area MnO2 (25 equiv) is used for oxazoline oxidation in the absence of DDQ,17 and should be quite beneficial for economical syntheses of these important structures.18 The protocol can be utilized for Li's cross dehydrogenative coupling19 between acetophenone (28) and isochroman 29 to form alkylation product 30. While the yield for the reaction was modest, the transformation illustrated the capability of forming carbon–carbon bonds from readily-available precursors by using an inexpensive oxidant. Attempts to conduct a biaryl formation of 31 through Rathore's variant20 of the Scholl reaction were unsuccessful, most likely indicating that the MeSO3H that is necessary for the coupling deactivates MnO2.The ability to conduct large scale transformations with minimal DDQ loading would be a major benefit of this protocol. We confirmed the capacity to apply the procedure to large scale reactions by converting 24 to 25 on 4.1 g (31.5 mmol) scale. The reaction proceeded in comparable yield (94%) to the smaller scale reaction (96%) and reached completion more rapidly (16 h compared to 24 h). The rate enhancement can be attributed to the reaction being conducted at approximately 3 M as opposed to the 0.1 M concentration that was used in the smaller scale transformations.
Scheme 3.
MnO2-mediated DDQ-catalyzed reactions.
We have shown that a number of oxidations can be effected by using catalytic DDQ with MnO2 serving as an inexpensive and environmentally benign terminal oxidant. These transformations include oxidative cyclizations, ether cleavages, dehydrogenations, and cross dehydrogenative couplings. The processes, while significantly slower than the corresponding stoichiometric reactions, are comparable with respect to yield and the resulting products are easier to purify. The protocol is incompatible with very slow oxidations and with the presence of water or strong acid. The range of successful transformations reported herein and the capacity to conduct reactions on large scale, however, suggest that this process should be useful for most DDQ-mediated oxidations, and the cost advantages and minimal toxicity benefits could prove to be very valuable for multigram syntheses.
Supplementary Material
Acknowledgment
We thank the National Institutes of Health, Institute of General Medicine (GM062924), for generous support of this work. We thank Professor Yoshito Kishi (Harvard University) for suggesting the use of polar solvents to improve oxidant turnover.
Footnotes
Supporting Information Available. Experimental details and characterization of all new compounds. The material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Buckle DR. In: Encyclopedia of Reagents for Organic Synthesis. Paquette LA, editor. Vol. 3. John Wiley & Sons; Chichester, UK: 1995. p. 1699. [Google Scholar]
- 2.All price quotes are based on 100 g bottles of reagent grade material.
- 3.Rat LD50 values can be found on MSDS sheets.
- 4.Newman MS, Khanna VK. Org. Prep. Proced. Int. 1985;17:422. [Google Scholar]
- 5.Chandrasekhar S, Sumithra G, Yadav JS. Tetrahedron Lett. 1996;37:1645. [Google Scholar]
- 6.Sharma GVM, Lavanya B, Mahalingam AK, Krisha PR. Tetrahedron Lett. 2000;41:10323. [Google Scholar]
- 7.For a review of Mn(OAc)3 chemistry, see: Snider BB. Chem. Rev. 1996;96:339. doi: 10.1021/cr950026m.
- 8.a Tu W, Liu L, Floreancig PE. Angew. Chem., Int. Ed. 2008;47:4184. doi: 10.1002/anie.200706002. [DOI] [PubMed] [Google Scholar]; b Tu W, Floreancig PE. Angew. Chem., Int. Ed. 2009;48:4567. doi: 10.1002/anie.200901489. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Liu L, Floreancig PE. Org. Lett. 2009;11:3152. doi: 10.1021/ol901188q. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Jung HH, Floreancig PE. Tetrahedron. 2009;65:10830. doi: 10.1016/j.tet.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Liu L, Floreancig PE. Angew. Chem., Int. Ed. 2010;49:3069. doi: 10.1002/anie.201000033. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Cui Y, Tu W, Floreancig PE. Tetrahedron. 2010;66:4867. doi: 10.1016/j.tet.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Liu L, Floreancig PE. Angew. Chem., Int. Ed. 2010;49:5894. doi: 10.1002/anie.201002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a Ying B-P, Trogden BG, Kohlman DT, Liang SX, Xu Y-C. Org. Lett. 2004;6:1523. doi: 10.1021/ol036314j. [DOI] [PubMed] [Google Scholar]; b Zhang Y, Li C-J. Angew. Chem., Int. Ed. 2006;45:1949. doi: 10.1002/anie.200503255. [DOI] [PubMed] [Google Scholar]; c Li Z, Li H, Guo X, Cao L, Yu R, Li H, Pan S. Org. Lett. 2008;10:803. doi: 10.1021/ol702934k. [DOI] [PubMed] [Google Scholar]; d Cheng D, Bao W. J. Org. Chem. 2008;73:6881. doi: 10.1021/jo8010039. [DOI] [PubMed] [Google Scholar]; e Tsang AS-K, Todd MH. Tetrahedron Lett. 2009;50:1199. [Google Scholar]; f Yu B, Jiang T, Li J, Su Y, Pan X, She X. Org. Lett. 2009;11:3442. doi: 10.1021/ol901291w. [DOI] [PubMed] [Google Scholar]; g Benfatti F, Capdevila MG, Zoli L, Benedetto E, Cozzi PG. Chem. Commun. 2009:5919. doi: 10.1039/b910185c. [DOI] [PubMed] [Google Scholar]; h He Z, Lin X, Zhu Y, Wang Y. Heterocycles. 2010;81:965. [Google Scholar]; i Guo C, Song J, Luo S-W, Gong L-Z. Angew. Chem., Int. Ed. 2010;49:5558. doi: 10.1002/anie.201002108. [DOI] [PubMed] [Google Scholar]
- 10.Kumar VS, Aubele DL, Floreancig PE. Org. Lett. 2001;3:4123. doi: 10.1021/ol016996f. [DOI] [PubMed] [Google Scholar]
- 11.Cook CD, English ES, Wilson BJ. J. Org. Chem. 1958;23:755. [Google Scholar]
- 12.For an example of a manganese-catalyzed ether oxidation, see: Kamijo S, Amaoka Y, Inoue M. Chem. Asian J. 2010;5:486. doi: 10.1002/asia.200900420.
- 13.van Haeringen CJ, West JS, Davis FJ, Gilbert A, Hadley P, Pearson S, Wheldon AE, Henbest RGC. Photochem. Photobiol. 1998;67:407. [Google Scholar]
- 14.We thank an anonymous reviewer for suggesting this experiment.
- 15.For recently reported oxidative approaches to oxazole synthesis, see: Graham TH. Org. Lett. 2010;12:3614. doi: 10.1021/ol101346w. Phillips AJ, Uto Y, Wipf P, Reno MJ, Williams DR. Org. Lett. 2000;2:1165. doi: 10.1021/ol005777b.
- 16.Aoyama T, Sonoda N, Yamauchi M, Toriyama K, Anzai M, Ando A, Shioiri T. Synlett. 1998:35. [Google Scholar]
- 17.We did not observe oxazoline oxidation using activated reagent grade MnO2 in the absence of DDQ.
- 18.Jin Z. Nat. Prod. Rep. 2006;23:464. doi: 10.1039/b502166a. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Li C-J. J. Am. Chem. Soc. 2006;128:4242. doi: 10.1021/ja060050p. For a review of cross dehydrogenative coupling reactions, see: Li C-J. Acc. Chem. Res. 2009;42:335. doi: 10.1021/ar800164n.
- 20.Zhai L, Shukla R, Rathore R. Org. Lett. 2009;11:3474. doi: 10.1021/ol901331p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.