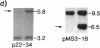Abstract
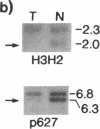
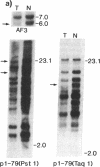
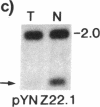
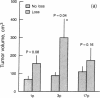
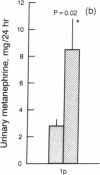
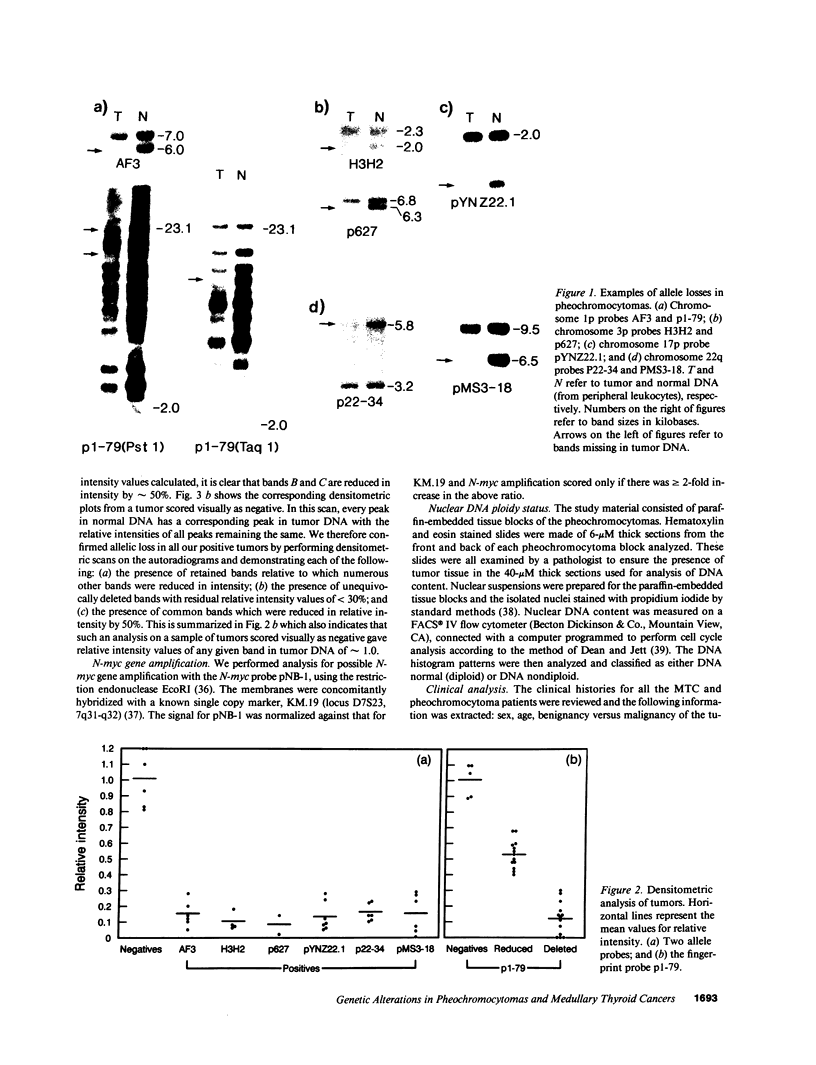
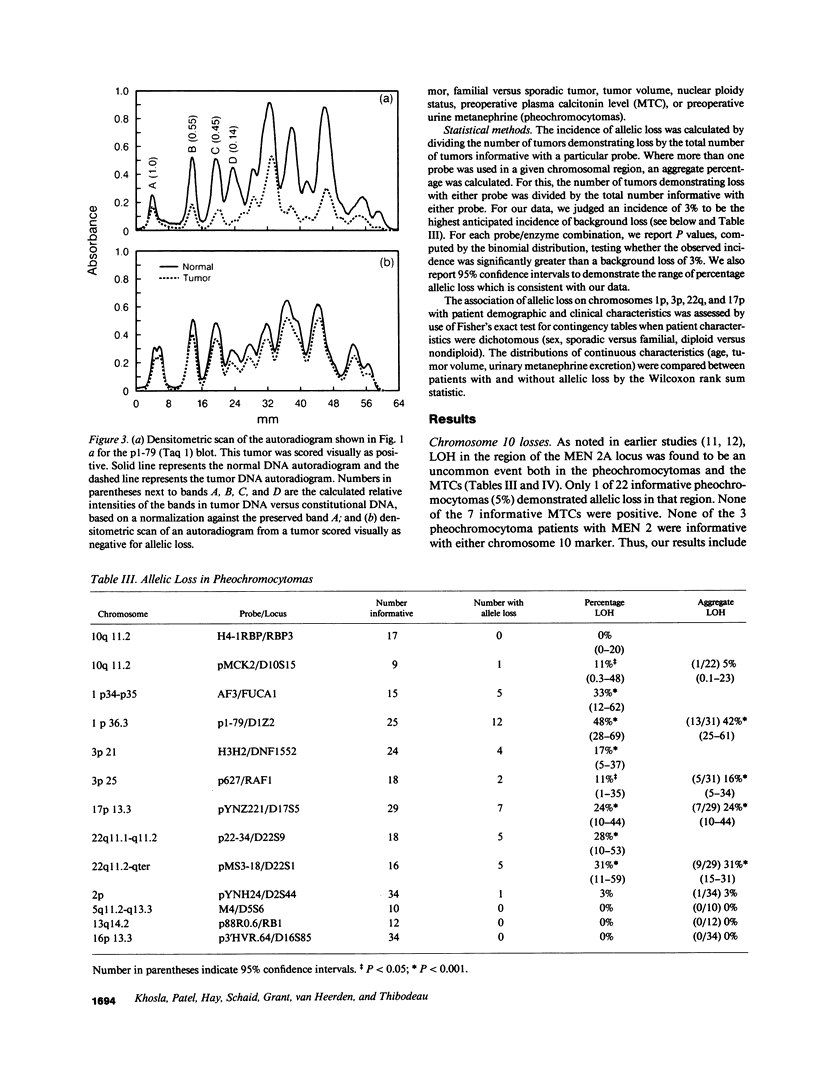
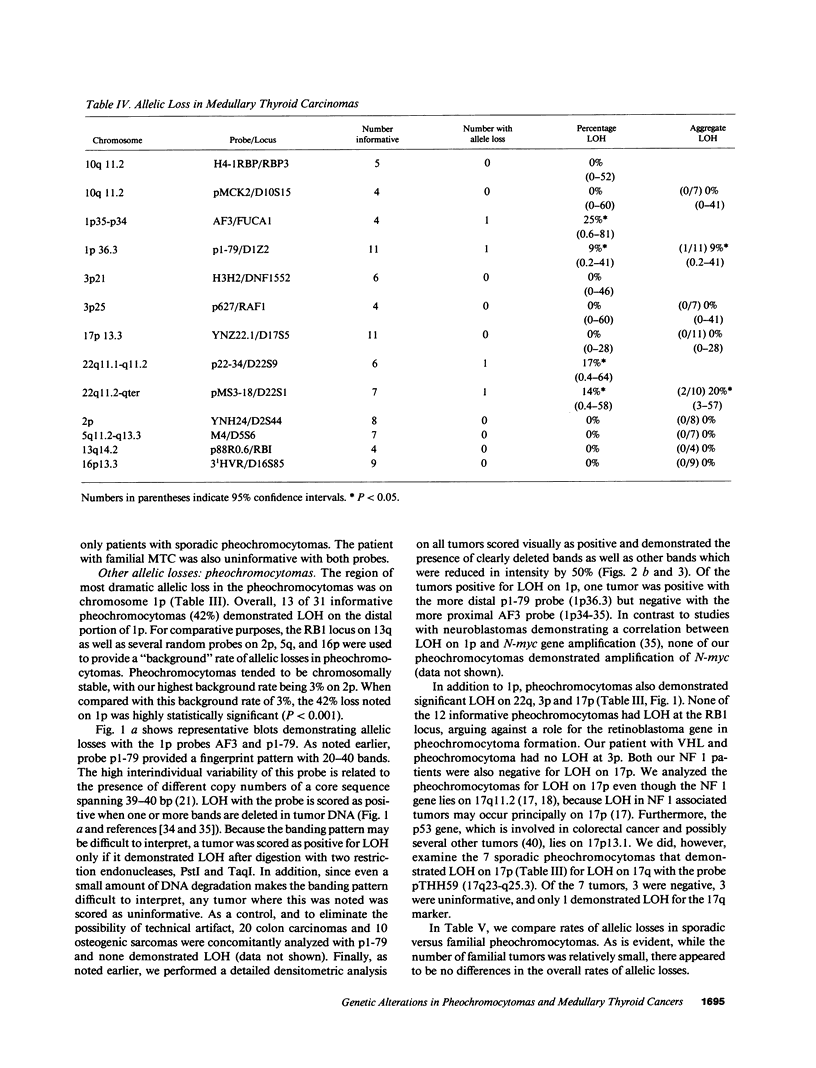
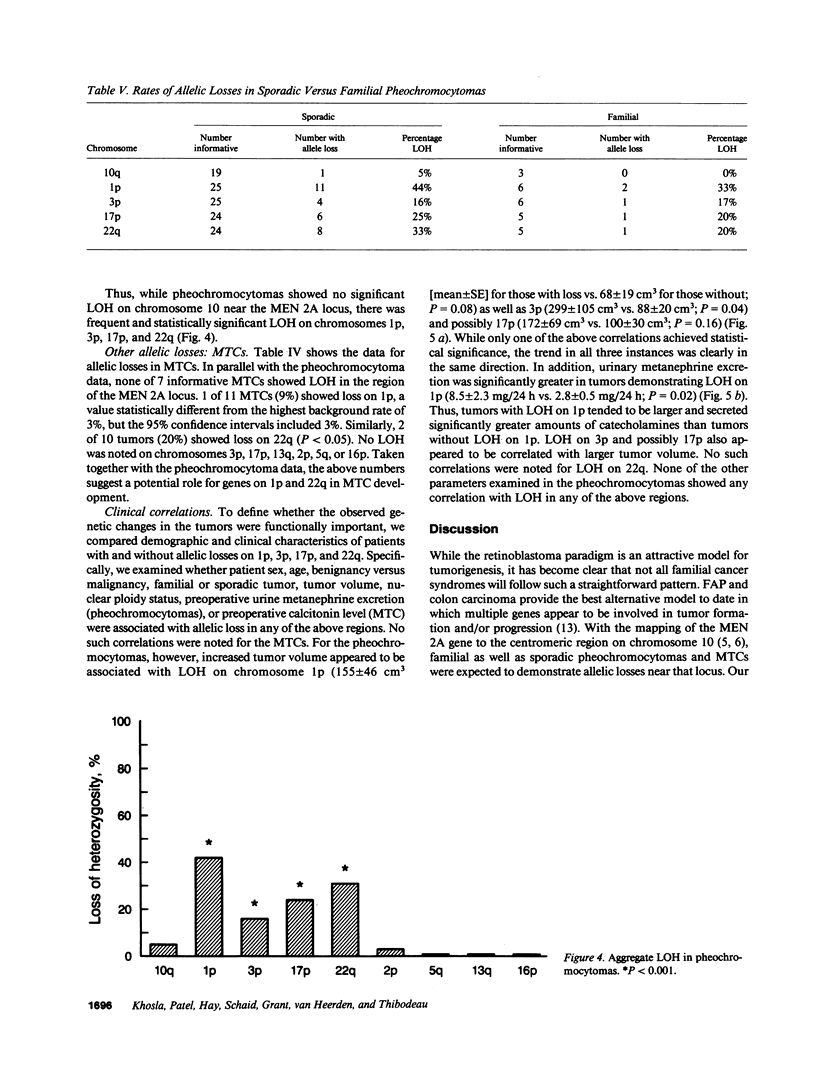
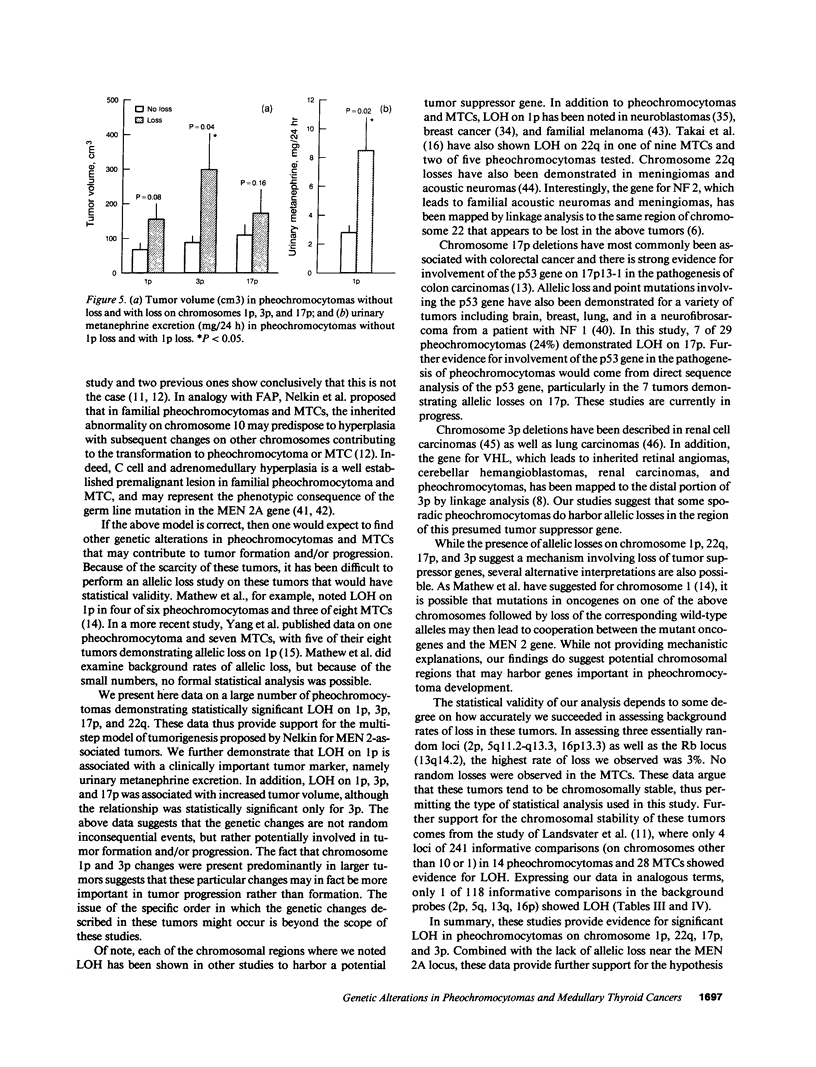
Loss of heterozygosity (LOH) at specific loci may help localize tumor suppressor genes involved in the formation of various familial and sporadic tumors. In addition, the genetic loci for a number of familial tumor syndromes have been mapped by linkage analysis. To explore the possible role of tumor suppressor genes in endocrine tumors, we tested 41 pheochromocytomas (34 sporadic and 7 familial) and 11 medullary thyroid cancers (MTC) (10 sporadic and 1 familial) for LOH near a variety of potentially important genetic loci: (a) the multiple endocrine neoplasia type 2A (MEN 2A) locus on chromosome 10; (b) the von Hippel-Lindau locus on 3p; and (c) the p53 and neurofibromatosis 1 loci on 17. We also examined chromosomes 1p and 22q because previous studies in a small number of pheochromocytomas and MTCs suggested LOH in these regions. Background rates for LOH were assessed using several "random" probes. Finally, we examined a number of clinical and histologic characteristics of these tumors for possible correlations with specific genetic alterations. LOH in the region of the MEN 2A locus was uncommon (0% for MTCs, 5% for pheochromocytomas). However, we found significant allelic losses in pheochromocytomas on chromosomes 1p (42%), 3p (16%), 17p (24%), and 22q (31%). We also noted a correlation between LOH on 1p and urinary excretion of metanephrine by these patients (P = 0.02). LOH on 1p, 3p, and 17p also appeared to be associated with increased tumor volume. Analysis of the smaller number of MTCs demonstrated allelic losses on chromosomes 1p and 22q. Our results suggest that tumor formation and/or progression in pheochromocytomas and MTCs involves multiple genes, analogous with the model proposed for colon carcinoma.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bale S. J., Dracopoli N. C., Tucker M. A., Clark W. H., Jr, Fraser M. C., Stanger B. Z., Green P., Donis-Keller H., Housman D. E., Greene M. H. Mapping the gene for hereditary cutaneous malignant melanoma-dysplastic nevus to chromosome 1p. N Engl J Med. 1989 May 25;320(21):1367–1372. doi: 10.1056/NEJM198905253202102. [DOI] [PubMed] [Google Scholar]
- Barker D., Schafer M., White R. Restriction sites containing CpG show a higher frequency of polymorphism in human DNA. Cell. 1984 Jan;36(1):131–138. doi: 10.1016/0092-8674(84)90081-3. [DOI] [PubMed] [Google Scholar]
- Bodmer W. F., Bailey C. J., Bodmer J., Bussey H. J., Ellis A., Gorman P., Lucibello F. C., Murday V. A., Rider S. H., Scambler P. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature. 1987 Aug 13;328(6131):614–616. doi: 10.1038/328614a0. [DOI] [PubMed] [Google Scholar]
- Bonner T., O'Brien S. J., Nash W. G., Rapp U. R., Morton C. C., Leder P. The human homologs of the raf (mil) oncogene are located on human chromosomes 3 and 4. Science. 1984 Jan 6;223(4631):71–74. doi: 10.1126/science.6691137. [DOI] [PubMed] [Google Scholar]
- Buroker N., Bestwick R., Haight G., Magenis R. E., Litt M. A hypervariable repeated sequence on human chromosome 1p36. Hum Genet. 1987 Oct;77(2):175–181. doi: 10.1007/BF00272388. [DOI] [PubMed] [Google Scholar]
- Carritt B., Welch H. M., Parry-Jones N. J. Sequences homologous to the human D1S1 locus present on human chromosome 3. Am J Hum Genet. 1986 Apr;38(4):428–436. [PMC free article] [PubMed] [Google Scholar]
- Cawthon R. M., Weiss R., Xu G. F., Viskochil D., Culver M., Stevens J., Robertson M., Dunn D., Gesteland R., O'Connell P. A major segment of the neurofibromatosis type 1 gene: cDNA sequence, genomic structure, and point mutations. Cell. 1990 Jul 13;62(1):193–201. doi: 10.1016/0092-8674(90)90253-b. [DOI] [PubMed] [Google Scholar]
- Chin K. S., Mathew C. G., Fong S. L., Bridges C. D., Ponder B. A. Styl RFLP recognised by a human IRBP cDNA localised to chromosome 10. Nucleic Acids Res. 1988 Feb 25;16(4):1645–1645. doi: 10.1093/nar/16.4.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLellis R. A., Wolfe H. J., Gagel R. F., Feldman Z. T., Miller H. H., Gang D. L., Reichlin S. Adrenal medullary hyperplasia. A morphometric analysis in patients with familial medullary thyroid carcinoma. Am J Pathol. 1976 Apr;83(1):177–196. [PMC free article] [PubMed] [Google Scholar]
- Dean P. N., Jett J. H. Mathematical analysis of DNA distributions derived from flow microfluorometry. J Cell Biol. 1974 Feb;60(2):523–527. doi: 10.1083/jcb.60.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzsch E., Retief A. E., Lotze M. J., Warnich L., Nicholson D. L., Fox M. F., Fricke J., du Plessis L., Oosthuizen C. J. An anonymous human single copy genomic clone, D5S6 (M4) on chromosome 5 identifies a three allele RFLP. Nucleic Acids Res. 1986 Feb 25;14(4):1923–1923. doi: 10.1093/nar/14.4.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estivill X., Scambler P. J., Wainwright B. J., Hawley K., Frederick P., Schwartz M., Baiget M., Kere J., Williamson R., Farrall M. Patterns of polymorphism and linkage disequilibrium for cystic fibrosis. Genomics. 1987 Nov;1(3):257–263. doi: 10.1016/0888-7543(87)90052-8. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Fong C. T., Dracopoli N. C., White P. S., Merrill P. T., Griffith R. C., Housman D. E., Brodeur G. M. Loss of heterozygosity for the short arm of chromosome 1 in human neuroblastomas: correlation with N-myc amplification. Proc Natl Acad Sci U S A. 1989 May;86(10):3753–3757. doi: 10.1073/pnas.86.10.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler M. L., Nakai H., Byers M. G., Fukushima H., Eddy R. L., Henry W. M., Haley L. L., O'Brien J. S., Shows T. B. Chromosome 1 localization of the human alpha-L-fucosidase structural gene with a homologous site on chromosome 2. Cytogenet Cell Genet. 1986;43(1-2):103–108. doi: 10.1159/000132304. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Dryja T. P., Weinberg R. A. Oncogenes and tumor-suppressing genes. N Engl J Med. 1988 Mar 10;318(10):618–622. doi: 10.1056/NEJM198803103181007. [DOI] [PubMed] [Google Scholar]
- Genuardi M., Tsihira H., Anderson D. E., Saunders G. F. Distal deletion of chromosome Ip in ductal carcinoma of the breast. Am J Hum Genet. 1989 Jul;45(1):73–82. [PMC free article] [PubMed] [Google Scholar]
- Higgs D. R., Wainscoat J. S., Flint J., Hill A. V., Thein S. L., Nicholls R. D., Teal H., Ayyub H., Peto T. E., Falusi A. G. Analysis of the human alpha-globin gene cluster reveals a highly informative genetic locus. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5165–5169. doi: 10.1073/pnas.83.14.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs G., Erlandsson R., Boldog F., Ingvarsson S., Müller-Brechlin R., Klein G., Sümegi J. Consistent chromosome 3p deletion and loss of heterozygosity in renal cell carcinoma. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1571–1575. doi: 10.1073/pnas.85.5.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsvater R. M., Mathew C. G., Smith B. A., Marcus E. M., te Meerman G. J., Lips C. J., Geerdink R. A., Nakamura Y., Ponder B. A., Buys C. H. Development of multiple endocrine neoplasia type 2A does not involve substantial deletions of chromosome 10. Genomics. 1989 Apr;4(3):246–250. doi: 10.1016/0888-7543(89)90327-3. [DOI] [PubMed] [Google Scholar]
- Larsson C., Skogseid B., Oberg K., Nakamura Y., Nordenskjöld M. Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature. 1988 Mar 3;332(6159):85–87. doi: 10.1038/332085a0. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Shew J. Y., Hong F. D., Sery T. W., Donoso L. A., Young L. J., Bookstein R., Lee E. Y. The retinoblastoma susceptibility gene encodes a nuclear phosphoprotein associated with DNA binding activity. Nature. 1987 Oct 15;329(6140):642–645. doi: 10.1038/329642a0. [DOI] [PubMed] [Google Scholar]
- Leppert M., Dobbs M., Scambler P., O'Connell P., Nakamura Y., Stauffer D., Woodward S., Burt R., Hughes J., Gardner E. The gene for familial polyposis coli maps to the long arm of chromosome 5. Science. 1987 Dec 4;238(4832):1411–1413. doi: 10.1126/science.3479843. [DOI] [PubMed] [Google Scholar]
- Lothe R. A., Nakamura Y., Woodward S., Gedde-Dahl T., Jr, White R. VNTR (variable number of tandem repeats) markers show loss of chromosome 17p sequences in human colorectal carcinomas. Cytogenet Cell Genet. 1988;48(3):167–169. doi: 10.1159/000132617. [DOI] [PubMed] [Google Scholar]
- Mathew C. G., Chin K. S., Easton D. F., Thorpe K., Carter C., Liou G. I., Fong S. L., Bridges C. D., Haak H., Kruseman A. C. A linked genetic marker for multiple endocrine neoplasia type 2A on chromosome 10. Nature. 1987 Aug 6;328(6130):527–528. doi: 10.1038/328527a0. [DOI] [PubMed] [Google Scholar]
- Mathew C. G., Smith B. A., Thorpe K., Wong Z., Royle N. J., Jeffreys A. J., Ponder B. A. Deletion of genes on chromosome 1 in endocrine neoplasia. Nature. 1987 Aug 6;328(6130):524–526. doi: 10.1038/328524a0. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Carlson M., Krapcho K., Gill J., O'Connell P., Leppert M., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence pMCK2 on chromosome 10 [D10S15]. Nucleic Acids Res. 1988 Jan 11;16(1):374–374. doi: 10.1093/nar/16.1.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Gillilan S., O'Connell P., Leppert M., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence pYNH24 on chromosome 2 (D2S44). Nucleic Acids Res. 1987 Dec 10;15(23):10073–10073. doi: 10.1093/nar/15.23.10073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Holm T., Gillilan S., Leppert M., O'Connell P., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence (pTHH59) on chromosome 17q [D17S4]. Nucleic Acids Res. 1988 Apr 25;16(8):3598–3598. doi: 10.1093/nar/16.8.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelkin B. D., Nakamura Y., White R. W., de Bustros A. C., Herman J., Wells S. A., Jr, Baylin S. B. Low incidence of loss of chromosome 10 in sporadic and hereditary human medullary thyroid carcinoma. Cancer Res. 1989 Aug 1;49(15):4114–4119. [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Okazaki M., Nishisho I., Tateishi H., Motomura K., Yamamoto M., Miki T., Hayakawa T., Takai S., Honjo T., Mori T. Loss of genes on the long arm of chromosome 22 in human meningiomas. Mol Biol Med. 1988 Feb;5(1):15–22. [PubMed] [Google Scholar]
- Ponder B. Cancer. Gene losses in human tumours. Nature. 1988 Sep 29;335(6189):400–402. doi: 10.1038/335400a0. [DOI] [PubMed] [Google Scholar]
- Ryan J. J., Hay I. D., Grant C. S., Rainwater L. M., Farrow G. M., Goellner J. R. Flow cytometric DNA measurements in benign and malignant Hürthle cell tumors of the thyroid. World J Surg. 1988 Aug;12(4):482–487. doi: 10.1007/BF01655427. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., Rouleau G. A., Ozelius L. J., Lane A. H., Farmer G. E., Lamiell J. M., Haines J., Yuen J. W., Collins D., Majoor-Krakauer D. Von Hippel-Lindau disease maps to the region of chromosome 3 associated with renal cell carcinoma. Nature. 1988 Mar 17;332(6161):268–269. doi: 10.1038/332268a0. [DOI] [PubMed] [Google Scholar]
- Simpson N. E., Kidd K. K., Goodfellow P. J., McDermid H., Myers S., Kidd J. R., Jackson C. E., Duncan A. M., Farrer L. A., Brasch K. Assignment of multiple endocrine neoplasia type 2A to chromosome 10 by linkage. Nature. 1987 Aug 6;328(6130):528–530. doi: 10.1038/328528a0. [DOI] [PubMed] [Google Scholar]
- Sithanandam G., Dean M., Brennscheidt U., Beck T., Gazdar A., Minna J. D., Brauch H., Zbar B., Rapp U. R. Loss of heterozygosity at the c-raf locus in small cell lung carcinoma. Oncogene. 1989 Apr;4(4):451–455. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanton L. W., Schwab M., Bishop J. M. Nucleotide sequence of the human N-myc gene. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1772–1776. doi: 10.1073/pnas.83.6.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai S., Tateishi H., Nishisho I., Miki T., Motomura K., Miyauchi A., Kato M., Ikeuchi T., Yamamoto K., Okazaki M. Loss of genes on chromosome 22 in medullary thyroid carcinoma and pheochromocytoma. Jpn J Cancer Res. 1987 Sep;78(9):894–898. [PubMed] [Google Scholar]
- Wallace M. R., Marchuk D. A., Andersen L. B., Letcher R., Odeh H. M., Saulino A. M., Fountain J. W., Brereton A., Nicholson J., Mitchell A. L. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990 Jul 13;249(4965):181–186. doi: 10.1126/science.2134734. [DOI] [PubMed] [Google Scholar]
- Wertelecki W., Rouleau G. A., Superneau D. W., Forehand L. W., Williams J. P., Haines J. L., Gusella J. F. Neurofibromatosis 2: clinical and DNA linkage studies of a large kindred. N Engl J Med. 1988 Aug 4;319(5):278–283. doi: 10.1056/NEJM198808043190505. [DOI] [PubMed] [Google Scholar]
- Wiggs J., Nordenskjöld M., Yandell D., Rapaport J., Grondin V., Janson M., Werelius B., Petersen R., Craft A., Riedel K. Prediction of the risk of hereditary retinoblastoma, using DNA polymorphisms within the retinoblastoma gene. N Engl J Med. 1988 Jan 21;318(3):151–157. doi: 10.1056/NEJM198801213180305. [DOI] [PubMed] [Google Scholar]
- Wolfe H. J., Melvin K. E., Cervi-Skinner S. J., Saadi A. A., Juliar J. F., Jackson C. E., Tashjian A. H., Jr C-cell hyperplasia preceding medullary thyroid carcinoma. N Engl J Med. 1973 Aug 30;289(9):437–441. doi: 10.1056/NEJM197308302890901. [DOI] [PubMed] [Google Scholar]
- Yang K. P., Nguyen C. V., Castillo S. G., Samaan N. A. Deletion mapping on the distal third region of chromosome 1p in multiple endocrine neoplasia type IIA. Anticancer Res. 1990 Mar-Apr;10(2B):527–533. [PubMed] [Google Scholar]