Abstract
The osteocyte is hypothesized to be the mechanosensory cell in bone. However, osteoblastic cell models have been most commonly used to investigate mechanisms of mechanosensation in bone. Therefore, we sought to determine if differences might exist between osteocytic and osteoblastic cell models relative to the activation of β-catenin signaling in MLO-Y4 osteocytic, 2T3 osteoblastic and primary neonatal calvarial cells (NCCs) in response to pulsatile fluid flow shear stress (PFFSS). β–catenin nuclear translocation was observed in MLO-Y4 cells at 2 and 16 dynes/cm2 PFFSS, but only at 16 dynes/cm2 in the 2T3 or NCC cultures. MLO-Y4 cells released high amounts of PGE2 into the media at all levels of PFFSS (2–24 dynes/cm2) and we observed a biphasic pattern of relative to the level of PFFSS. In contrast PGE2 release by 2T3 cells was only detected during 16 and 24 dynes/cm2 PFFSS starting at >1 hour and never reached the levels produced by MLO-Y4 cells. Exogenously added PGE2 was able to induce β–catenin nuclear translocation in all cells suggesting that the differences between the cell lines observed for β–catenin nuclear translocation was associated with the differences in PGE2 production. To investigate a possible mechanism for the differences in PGE2 release by MLO-Y4 and 2T3 cells we examined the regulation of Ptgs2 (Cox-2) gene expression by PFFSS. 2T3 cell Ptgs2 mRNA levels at both 0 and 24 hours after 2 hours of PFFSS showed biphasic increases with peaks at 4 and 24 dynes/cm2 and 24 hour levels were higher than 0 hour levels. MLO-Y4 cell Ptgs2 expression was similarly biphasic; however at 24 hours post flow Ptgs2 mRNA levels were lower. Our data suggest significant differences in the sensitivity and kinetics of the response mechanisms of 2T3 and neonatal calvarial osteoblastic versus MLO-Y4 osteocytic cells to PFFSS. Furthermore our data support a role for PGE2 in mediating the activation of β–catenin signaling in response to fluid flow shear stress.
Keywords: 2T3 osteoblast, MLO-Y4 osteocyte, PGE2, Ptgs2, shear stress
INTRODUCITON
The mammalian skeleton has evolved to be exquisitely suited to function in structural support for muscles, in protection of internal organs, and as a reservoir for mineral homeostasis. It is capable of detecting changes in mechanical loading and adapting to these changes to maintain the most structurally appropriate bone mass and architecture. The precise mechanism underlying bone responsiveness to changes in mechanical load is unknown. However, the mechanism clearly involves the orchestrated interplay of multiple bone cell types and multiple signaling pathways whose activity is tightly regulated.
The role of the osteocyte as the mechanosensory cell in bone has long been postulated given that it is the most abundant cell in bone and is ideally suited to perceive changes in mechanical load [1, 2]. Studies by Klein-Nulend and colleagues have shown that primary chicken osteoblasts are less sensitive to shear stress compared to primary chicken osteocytes [3] and that primary osteocytes subjected to fluid flow produce factors that inhibit osteoclast formation to a greater extent than osteoblasts [4]. Recently, in vivo evidence has accumulated that strongly supports a central role of the osteocyte in bone responsiveness to mechanical loading. Tatsumi et al. have elegantly demonstrated that the targeted ablation of the osteocyte induces rapid bone loss, osteoblast dysfunction, and the development of fragile bone [5]. Also deletion of the osteocyte protected against unloading-induced (hindlimb suspension) bone loss; providing strong evidence for its role as the mechanosensory cell in bone. At the molecular level it is interesting to note that much of the proposed models/mechanisms have relied heavily on in vitro studies using primary osteoblasts or osteoblastic cell lines as surrogates for the osteocyte. This is partially understandable from the perspective that osteocytes are in the same lineage as the osteoblast, primary osteocytes are much more difficult to isolate, and there are any number of osteoblastic cell lines that are readily available. However as has been previously discussed, the “osteocyte is not an osteoblast” [6] and there is ample evidence to support this important concept [3, 7–9].
Considerable evidence has accumulated in the literature in the past few years for a role of the Wnt/β-catenin signaling pathway in the response of bone / bone cells to various forms of mechanical loading. Norvell et al [10] have shown that fluid shear stress induces β–catenin nuclear translocation in primary rat neonatal calvarial osteoblasts and in MC3T3 osteoblastic cells and this regulates Cox-2 (Ptgs2) expression. Studies by Liedert et al. [11] demonstrated that in MC3T3-E1 cells subjected to mechanical stretch that estradiol and Wnt signaling interacted to regulate Ptgs2 gene expression. Lau et al [12] demonstrated the activation of Wnt, estrogen receptor, IGF-1 and BMP pathways in primary osteoblasts isolated from 8 week old calvaria or long bones of C57BL/6J mice but not C3H/HeJ mice. The role of the Wnt pathway in response to mechanical loading in vivo has been demonstrated in studies by Robinson et al [13] in which changes in the expression of a number of Wnt target genes was observed following tibia 4-point bending, while Sawakami et al [14] demonstrated that Lrp5, the Wnt co-receptor, is needed for new bone formation in response to loading. Armstrong et al [15] demonstrated β–catenin nuclear translocation in response to mechanical strain in ROS 17/2.8 cells and the critical role for ERα in mediating the signaling response. Rubin and colleagues have observed a similar result using uniform axial strain applied to the pre-osteoblastic CIMC-4 cells [16]. Rubin and colleagues also demonstrated that induction of β–catenin signaling controlled through GSK-3β in response to mechanical load in the form of uniform biaxial strain suppresses adipogenic differentiation of C3H10T1/2 and marrow-derived mesenchymal stem cells in favor of osteoblastic differentiation [17, 18]. Thus in a number of different types of loading systems and different osteoblastic bone cell lines a clear role for Wnt/β-catenin signaling has been established, however whether the same mechanisms are used by osteocytes remains to be fully investigated.
The creation of the MLO-Y4 osteocytic cell line [19] has provided a model that, although not perfect, possesses many of the properties of the early osteocyte [6] and provides an additional in vitro model to further investigate the pathways that are activated in response to mechanical loading. Previous work from our lab has shown an important role for fluid flow shear stress induced PGE2 release and β–catenin signaling in blocking glucocorticoid induced apoptosis in MLO-Y4 osteocytes [20]. Concomitant with those studies and presented in this paper, we have examined the response of the MLO-Y4 osteocytic cell line with respect to mechanical loading in the form of pulsatile fluid flow shear stress and compared that to the response of the 2T3 osteoblastic cell line [21]. Our findings demonstrate a number of differences in the responses of these two cell types/lines and raise a number of important considerations for our understanding of the pathways/mechanisms that bone may use to respond to mechanical loading.
MATERIALS AND METHODS
Cell Culture and Reagents
Cell culture reagents were purchased from Life Technologies and culture dishes and flasks were purchased from BD Biosciences. PGE2 was purchased from Cayman Chemicals. All cell cultures were conducted in 5%CO2/95% humidified air incubators maintained at 37°C. Two murine clonal cell lines, 2T3 osteoblastic (courtesy of Dr. Steve Harris, UTHSCSA) and MLO-Y4 osteocytic (courtesy of Dr. Lynda Bonewald, UMKC), were used in these experiments. Both cell lines were grown on rat-tail collagen type I (BD Biosciences) in media as described [19, 21].
For individual cultures, cells were plated 2–3 days before each experiment. 2T3 cells (passage 22) and MLO-Y4 cells (passage 27) were plated on 25 mm × 75 mm × 1 mm collagen-coated culture slides (Shandon SUPERFROST® positively charged, Thermo Scientific) at a density of 5 × 105 cells per slide. Slides were maintained in sets of three in 100 mm × 100 mm square culture dishes containing 15 ml of medium.
Primary mouse neonatal calvarial osteoblasts were isolated from 5–7 day old neonatal mouse calvaria as described by Mikuni-Takagaki et al. [22] with slight modifications. Neonatal mice were euthanized by decapitation and the calvaria surgically removed. After a quick wash in PBS the calvaria were digested twice with 0.2% collagenase-0.05% trypsin for 20 minutes. The first two digests are discarded as they contain largely fibroblastic cells. Four additional 20 minute digests were performed and the cell-containing supernatants were pooled, centrifuged, resuspended in 1 ml of α–MEM containing 10% fetal bovine serum and 1X pen-strep, and counted using a hemocytometer. This pool 3–6 is a predominantly primary osteoblast population of cells. All procedures using neonatal mice were approved by the UMKC IACUC.
Pulsatile Fluid Flow Shear Stress (PFFSS)
Fluid flow experiments were performed using the Flexcell® Streamer® Shear Stress Device (Flexcell International). For each experiment, 550 ml of cell culture medium was cycled over 6 slides in parallel through the closed loop for 2 hours. All fluid flow regimens began with a 20 second uniformly incremental ramp-up period until the target magnitude was reached. After this initial period, all regimens consisted of a base fluid shear stress (2.0 to 32.0 dynes/cm2) with cyclic pulses (± 0.6 dynes/cm2) at a frequency of 0.5 Hz. Three static control slides for each experiment were transferred to a new culture dish with fresh medium for each 2-hour experiment.
Immunostaining to Detect β-catenin Nuclear Translocation
Cells after PFFSS or various treatments were rinsed once in phosphate buffered saline (Ambion), fixed in 2% paraformaldehyde (Alfa Aesar) containing 0.2% Triton X-100 for 10 minutes and then washed 3 times in PBS at room temperature for 5 minutes each. Slides were then blocked with blocking solution [2.5% bovine serum albumin (BSA) – 1% non-immune donkey serum in PBS] for either 1 hour at room temperature or overnight at 4 °C. Primary goat antibody to total mouse β–catenin was obtained from Santa Cruz Biotechnology (used at 1:100 dilution in blocking solution). For immunostaining of cells, primary antibody was incubated for 4 hours at room temperature, washed, followed by secondary Cy3 conjugated donkey anti-goat antibody (Jackson ImmunoResearch Labs) (1:200 in blocking solution) for 1 hr at room temperature. Isotype matched nonimmune antibodies are used as a negative control for all immunostaining studies. After immunostaining the cells were photographed using a Nikon E800 microscope equipped with epifluorescence under 20X objective lens.
Phalloidin (Alexa Flour 488) was purchased from Invitrogen and used at a 1:200 working dilution in the buffer during the secondary antibody incubation. Images of β-catenin and phalloidin staining were overlaid in Photoshop. All photographs were taken at 20X magnification and the same exposure settings.
Immunoblotting
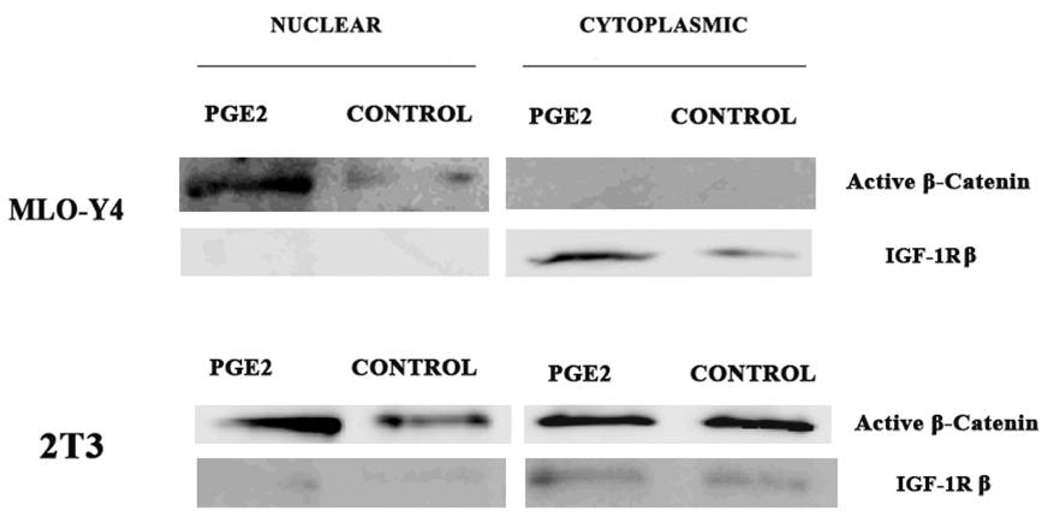
Antibodies against active (non-phospho Ser33/37/Thr41) β–catenin and the IGF-1 receptor β isoform (IGF-1Rβ) were obtained from Cell Signaling Technology. Nuclear and cytoplasmic protein extracts were obtained using a kit (Pierce Chemical, Inc.) exactly as described by the manufacturer. The protein concentration of extracts was determined using the BCA Protein Assay kit (Pierce Biotechnology, Inc.).
Cells were grown on collagen coated 6 wells dishes to ~70% confluency and then the media was changed to normal growth media with or without PGE2 at 1000 pg/ml for 2 hours before extraction. Total protein aliquots (5 µg) were denatured in 5X SDS sample buffer and then loaded onto 10% Tris-HCl pre-made gels (Bio-Rad Laboratories). The protein was then transferred onto nitrocellulose membrane (Bio-Rad Laboratories) by electroblotting at 60v for 2 hours. The membranes were blocked with 0.3% nonfat dried milk in PBS containing 0.1% tween-20 (PBS-T) using the S.N.A.P. ID system (Millipore Corp). The membrane was then incubated overnight at 4°C in primary antibody diluted 1:200 with 0.3% nonfat dried milk in PBS-T. Membranes were washed with PBS-T and incubated with HRP-conjugated anti-rabbit IgG for 1 hour at room temperature. Membranes were washed with PBS-T and stained with SuperSignal® West Dura Extended Duration Substrate (Pierce Biotechnology Inc). The blots were scanned using the Luminescent Image Analyzer LAS 4000 (FujiFilm Medical Systems USA, Inc.) and band intensity was determined using MultiGage software (FujiFilm Medical Systems USA).
PGE2 Assay
In a separate set of experiments, cells were subjected to 2, 4, 8, 16 or 24 +/− 0.6 dynes/cm2 of PFFSS for 2 hours as described above. 1–2 ml of circulating media was collected at 5, 15, 30, 60 and 120 minutes during the fluid flow through a T-valve that was inserted in the outflow line between the flow chamber and the media reservoir. The media was frozen immediately and stored at −80 °C until all samples were analyzed for PGE2 concentration using an EIA kit purchased from Cayman Chemical Company according to manufacturer’s directions. For 2T3 cells a 50 µl aliquot was assayed for each sample and for MLO-Y4 cells a 10 µl aliquot was assayed. Assays were performed in duplicate for each sample and the individual sample results were averaged. Triplicate experiments were performed for each fluid flow shear stress level.
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted using TRIzol reagent according to the manufacturer’s instructions. RNA pellets were re-hydrated in 30 µl of 1 mM sodium citrate pH 6.4 (Ambion) and quantitated by UV spectrophotometry. Isolated RNA (1.0 µg) was reverse transcribed in 20 µl replicate reactions at 42°C for 60 minutes using 2.5 µM oligo d(T)16 and 50 U Murine Leukemia Virus (MuLV) Reverse Transcriptase (Applied Biosystems). In order to reduce variability, replicate RT reactions from each slide were pooled.
Relative quantitation RT-PCR (TaqMan) was performed in 50 µl duplex reactions containing 1X TaqMan Universal Master Mix, 5 µl cDNA, 900 nM Ptgs2 forward primer, 900 nM Ptgs2 reverse primer, 350 nM Ptgs2 probe, 900 nM Gapdh forward primer, 900 nM Gapdh reverse primer, and 350 nM Gapdh probe. Amplifications for each slide were performed in triplicate and quantitated using an ABI Prism 7000 Sequence Detection System. Sequences of forward primers (FW), reverse primers (RV), and detection probes (P) were as follows: Ptgs2: TGTTGAGTCATTCACCAGACAGATTG (FW), TGTACAGCAATTGGCACATTTCTTC (RV), 6FAM-CCCAGCAACCCGGCCAG-MGBNFQ (P); Gapdh: TCAACGGGAAGCCCATCAC (FW), GCCTCACCCCATTTGATGTTAGT (RV), VIC-TCGCTCCTGGAAGATG-MGBNFQ (P).
Statistical Analysis
Changes in gene expression between experimental and control samples were detected using the Comparative Threshold Cycle (Ct) method of data analysis. Once a delta threshold cycle was calculated for each PCR reaction, the three replicates for each sample were averaged to give a mean delta threshold cycle (mean ΔCt). Along with this, a single average for all controls in each experiment was calculated, the mean control delta threshold cycle. This mean control delta threshold cycle allowed for the calculation of error in the control. The mean control delta threshold cycle in each experiment was then subtracted from mean experimental delta threshold cycle values, including control samples to give the delta delta threshold cycle (ΔΔCt). Finally, the delta delta threshold cycle values were converted to linear values for statistical analysis.
The fold difference values were then analyzed using one way analysis of variance (ANOVA) with Neuman- Keuls post hoc tests or two way ANOVA with Bonferroni post hoc tests for between group comparisons with the statistical software package in GraphPad Prism 4.03 to determine statistically significant changes in gene expression.
RESULTS
β-catenin nuclear translocation in response to PFFSS
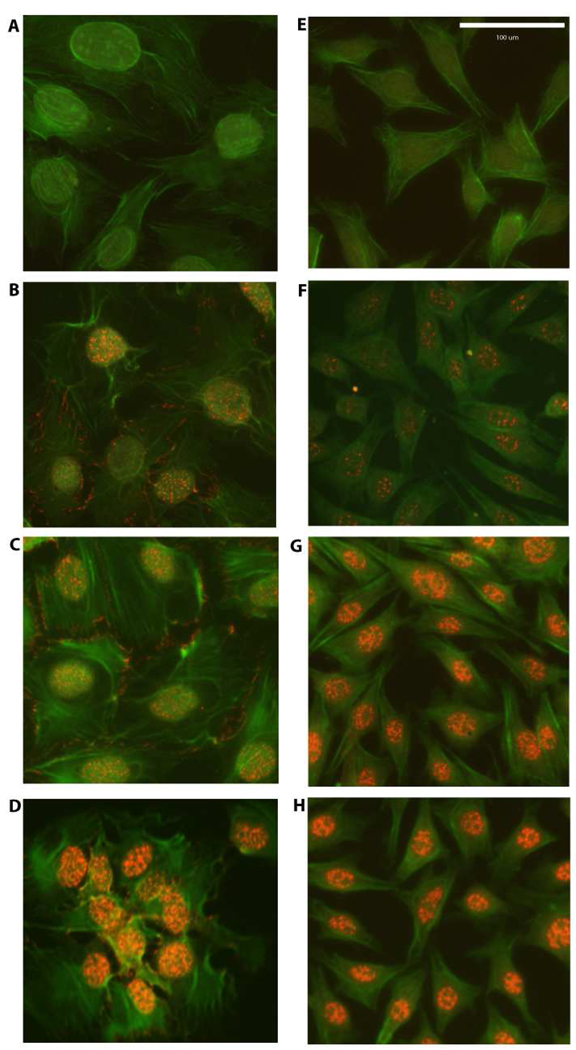
We first investigated whether the responsiveness of 2T3 and MLO-Y4 cells with regard to nuclear translocation of β–catenin was dependent on the magnitude of PFFSS (Figure 1). Cultures were subjected to PFFSS at 2 or 16 dynes/cm2 for 2 hours, immediately thereafter fixed and immunostained to localize β–catenin. As shown in Figure 1, static cultures show very faint nuclear staining for β–catenin (panels B, F). Interestingly, cell membrane staining for β–catenin was observed in 2T3 cells (panels B–D) but not detectable in MLO-Y4 cells (panels F–H). At 2 dynes/cm2 no evidence of β–catenin nuclear translocation was observed in the 2T3 osteoblastic cell line (Figure 1, panel C), whereas clear evidence for nuclear translocation of β–catenin was observed in the MLO-Y4 osteocytic cell line (Figure 1, panel G). At 16 dynes/cm2 evidence of β–catenin nuclear translocation was observed in both cell lines (Figure 1, panels D and H). Primary neonatal calvarial osteoblasts also demonstrated an identical β–catenin localization pattern in response to 2 and 16 dynes/cm2 PFFSS as that observed with 2T3 osteoblastic cells (Figure 2).
Figure 1.
β-catenin nuclear translocation detected by immunostaining in 2T3 osteoblastic (Panels A–D) and MLO-Y4 osteocytic cells (E–H). Panels A and B are non-immune IgG controls. Panels B and F are static controls. Panels C and G are 2 dynes/cm2 and Panels D and H are cells subjected to 16 dynes/cm2. All cells were photographed at 20X and the green (phalloidin) and red (β-catenin) channels were overlaid in Adobe Photoshop. Scale bar in Panel E is 100 µm and is the same for all panels.
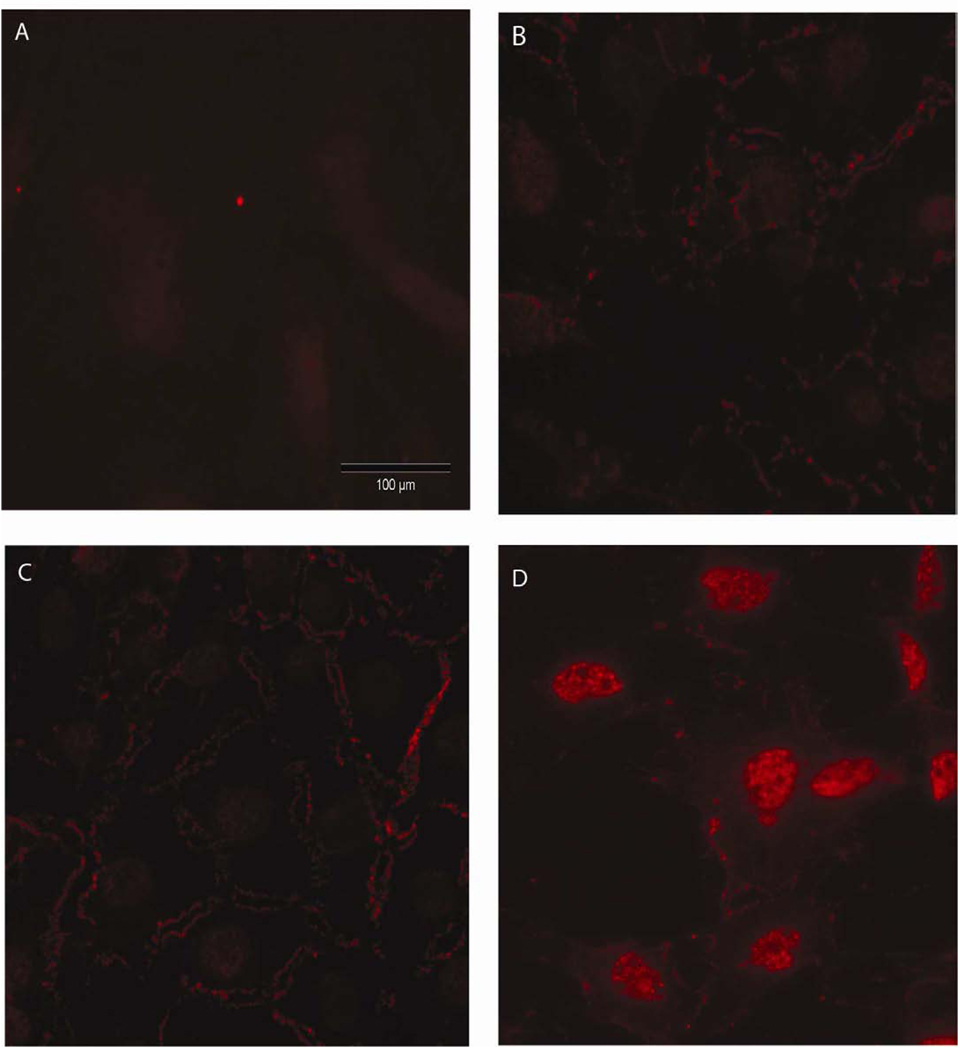
Figure 2.
β-catenin nuclear translocation in mouse neonatal calvarial osteoblastic cultures. Panel A shows non-immune control immunostaining. Panel B shows the immunostaining of static cultures. Panel C and D show immunostaining after 2 hours of PFFSS at 2 or 16 dynes/cm2. Note the significant amount of cytoplasmic membrane associated positive staining in Panels B–D, whereas nuclear staining for β–catenin was only visible in Panel D. All cells were stained with an antibody that recognizes total β–catenin (red staining) and photographed with a 20X objective lens. Scale bar is 100 µm and is the same for all panels.
PGE2 Production by 2T3 and MLO-Y4 Cells in Response to PFFSS
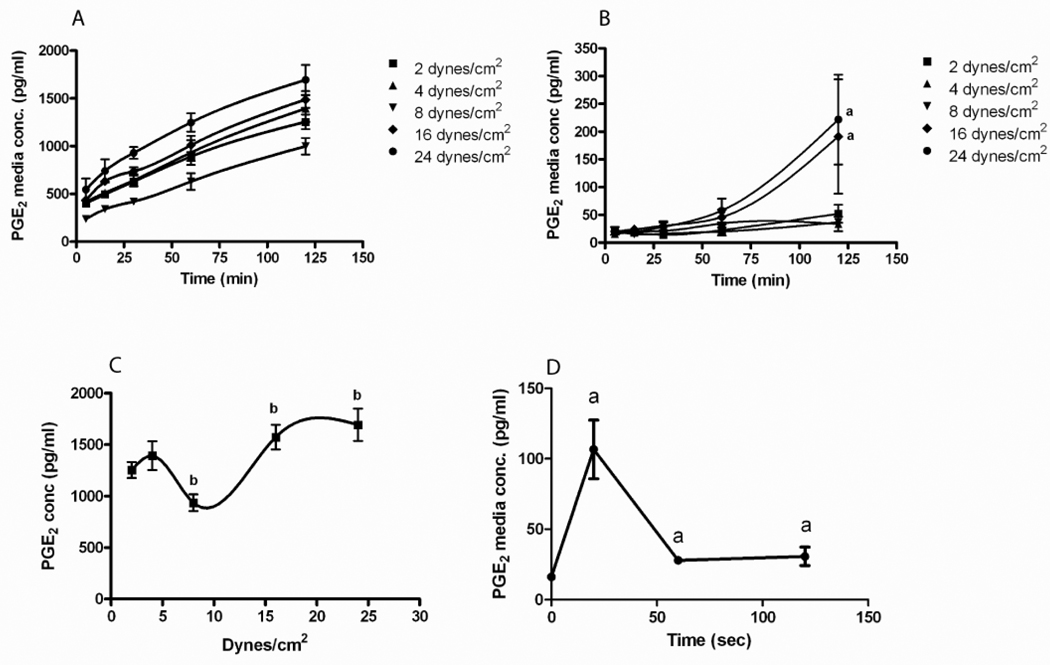
In order to understand the difference in the β–catenin nuclear translocation response of 2T3 versus MLO-Y4 cells we measured PGE2 production by these cells during the application of PFFSS. Prior to measuring PGE2 concentration in the media we performed an equilibration test to ascertain how fast at various levels of PFFSS a bolus of dye would dilute and reach a stable concentration in the recirculating media. At 2 dynes/cm2 this occurred within 3–4 minutes and at 16 dynes/cm2 this occurred between 1–2 minutes (data not shown). Thus we took our first sample at 5 minutes from the start of peak flow. The PGE2 concentration in the media circulating in the fluid flow system is shown in Figure 3. MLO-Y4 osteocytic cells produced higher levels of PGE2 compared to 2T3 osteoblastic cells at all levels of PFFSS. MLO-Y4 cells secreted a significant amount of PGE2 within the first 5 minutes of fluid flow and the concentration of PGE2 in the recirculating media increased over the two hours of applied PFFSS (Figure 3A). At 2, 4, and 8 dynes/cm2 of PFFSS, PGE2 remained undetectable in the recirculating media of 2T3 osteoblastic cells (Figure 3B) for the duration of the 2 hour experiment. At 16 and 24 dynes/cm2 2T3 cells produced detectable levels by 1 hour of PFFSS and significant levels by 2 hours of PFFSS. The level of PGE2 produced by MLO-Y4 cells at all magnitudes and times of PFFSS was greater than the highest amount produced by the 2T3 cells. Plotting the MLO-Y4 PGE2 data from the 2 hour time point demonstrated a biphasic curve for PGE2 accumulation in the media with respect to magnitude of PFFSS (Figure 3C).
Figure 3.
PGE2 accumulation in the fluid flow media during the application of various levels of pulsatile fluid flow shear stress. MLO-Y4 osteocytic cells (A) and 2T3 osteoblastic cells (B) were subjected to fluid shear stresses as indicated and media sampled at 5, 15, 30, 60 and 120 minutes. All time points in the MLO-Y4 are significantly different compared to static controls at p<.05. Panel C: PGE2 accumulation in the fluid flow media at 120 minutes was plotted versus the level of applied pulsatile fluid flow shear stress. Panel D: PGE2 release was measured at 20 sec, 1 and 2 minutes at 2 dynes/cm2. Data shown are mean ± std dev. n=3. ap<.05 compared to control. bp<.05 compared to 2 dynes/cm2.
To further explore the kinetics of PGE2 release in MLO-Y4 cells we performed a study (Figure 3D) in which media was sampled at the end of the slow ramp up to 2 dynes/cm2 (20 seconds) and at 1 and 2 minutes after the start of flow without recirculation of media. As seen in Figure 2D, there was a large release of PGE2 from the MLO-Y4 cells that occurred during the 20 second slow ramp.
Biphasic expression of Ptgs2 in response to pulsatile fluid flow
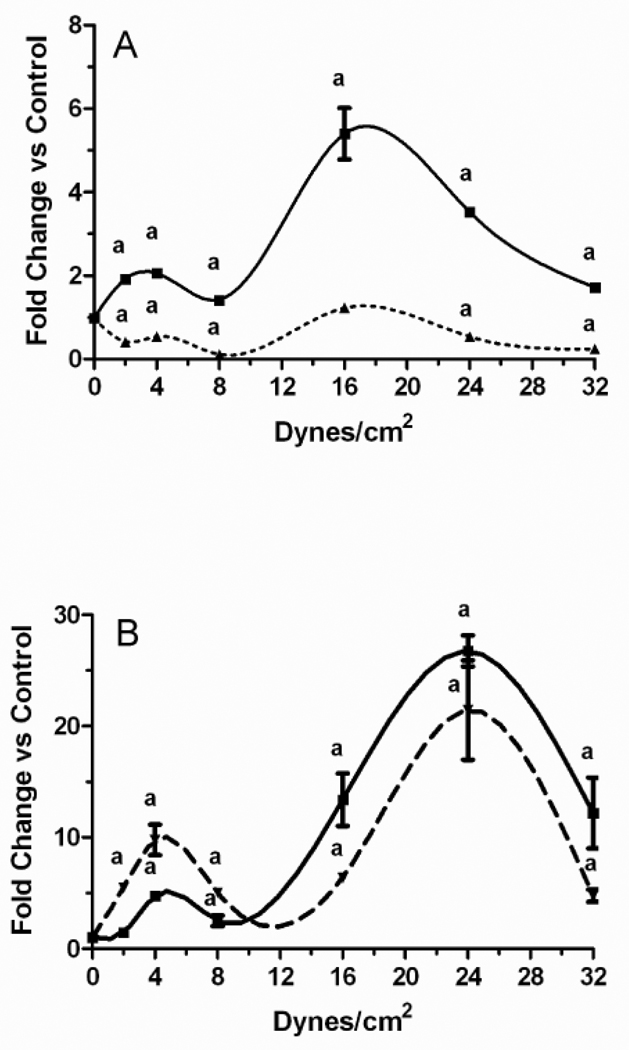
We next sought to understand the differences in PGE2 release by MLO-Y4 versus 2T3 cells. In all of these studies PFFSS was performed for 2 hours and RNA was extracted from the cells either immediately (zero hours post-flow) or after a subsequent incubation for 24 hours (24 hours post-flow). Ptgs2 (Cox-2) expression levels of each culture were normalized against matching static controls. Comparison of delta Ct values in MLO-Y4 versus 2T3 cells indicated a 4–8 fold higher baseline level of Ptgs2 mRNA in MLO-Y4 cells (not shown). Two separate peaks in Ptgs2 expression were evident in both 2T3 and MLO-Y4 cells in response to greater magnitudes of fluid shear stress. In MLO-Y4 cells, the first peak in expression was observed at 2–4 dynes/cm2 (Figure 4A). A much higher peak was observed at 16 dynes/cm2, while Ptgs2 mRNA levels in MLO-Y4 cells exposed to higher levels of fluid flow shear stress began to reduce towards static controls in a dose dependent fashion. In 2T3 cells, the two peaks, at 4 and 24 dynes/cm2 were evident at both zero hours post-flow as well as 24 hours post-flow (Figure 4B). Ptgs2 mRNA levels in 2T3 cells were greater at 24 hours post-flow at 2, 4, and 8 dynes/cm2, while they were greater at zero hours post-flow at 16, 24, and 32 dynes/cm2. Unlike 2T3 cells at 24 hours post-flow, expression of Ptgs2 in MLO-Y4 cells was less than controls at all time points, although what appeared to be a biphasic pattern with similar peaks seen at 0 hours post-flow was apparent.
Figure 4.
Fold change in Ptgs2 mRNA concentration in response to various levels of pulsatile fluid flow shear stress. MLO-Y4 osteocytic cells (A) and 2T3 osteoblastic cells (B) were subjected to fluid shear stresses of 2.0, 4.0, 8.0, 16.0, 24.0, and 32.0 dynes/cm2 with pulse heights of ±0.6 dynes/cm2 at a frequency of 0.5 Hz for 2 hours. Static controls were incubated for 2 hours in culture dishes. At the end of each fluid flow regimen, cells were either immediately lysed (0 hours post-flow, solid line) or returned to static culture in flow medium for 24 hours (24 hours post-flow, dashed line) followed by RNA extraction. Expression of Ptgs2 was quantitated by real-time PCR duplex reactions with Gapd as an endogenous control. ap < 0.01 compared to static controls. Data shown are mean ± sem. n=6.
PGE2 treatment-induced β–catenin nuclear translocation
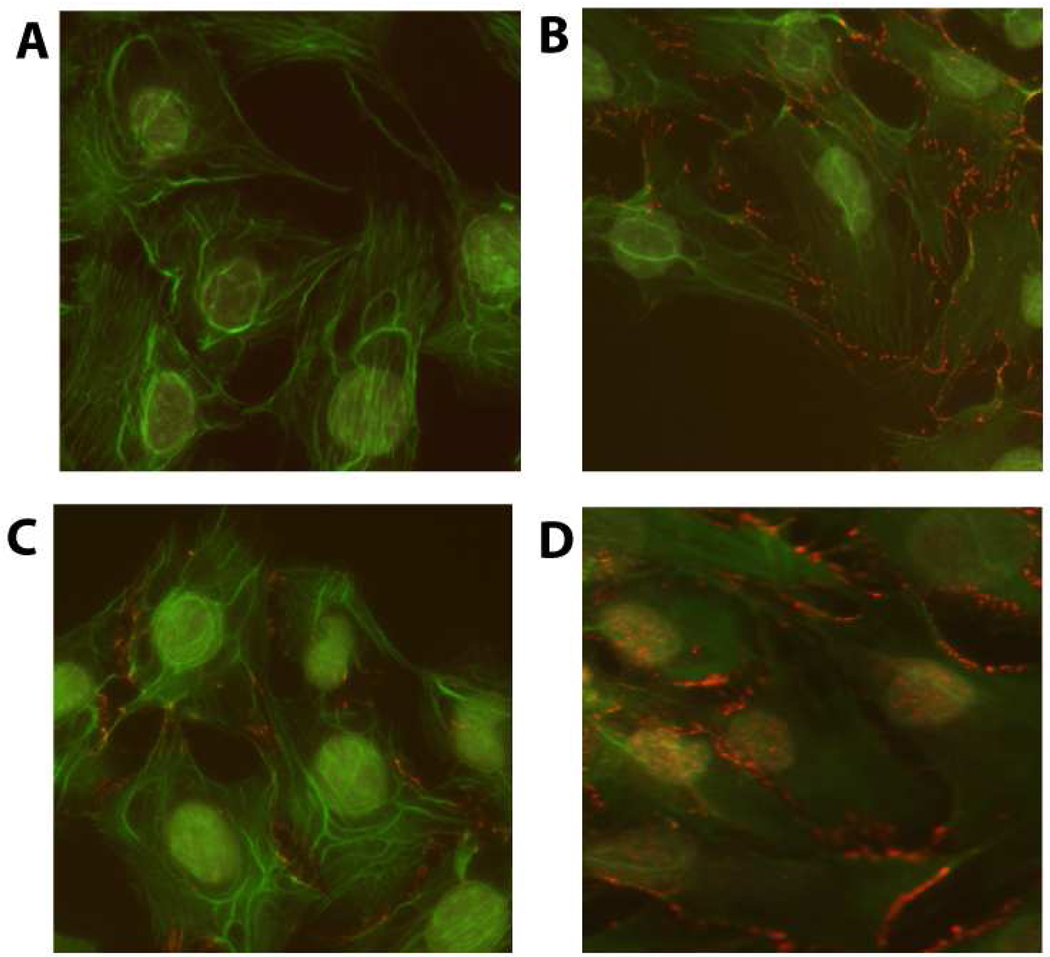
Given the large differences in the amount of PGE2 released by the MLO-Y4 versus 2T3 cells (Figure 3), we next asked if differences in PGE2 could explain the observed differences in β–catenin nuclear translocation between cell lines at various magnitudes of PFFSS. To test this we added PGE2 exogenously at 1000 pg/ml to static cultures to determine if this would induce the nuclear translocation of β-catenin. We fractionated non-treated and PGE2 treated cells into nuclear and cytoplasmic compartments and measured β–catenin levels using an antibody that detects the active form of β–catenin (Cell Signaling Technologies). As shown in Figure 5, the addition of 1000 pg/ml PGE2 (determined from Figure 3A) to static cultures resulted in increased active β–catenin in the nuclear fractions of both 2T3 and MLO-Y4 cell cultures after 2 hours of incubation. An antibody against IGF-1Rβ was used to assess the relative contamination of the nuclear fraction with cytoplasmic proteins. Shown in Figure 5, the IGF-1R immunoblot indicates that our nuclear fractions were not contaminated with significant amounts of cytoplasmic proteins. Interestingly, 2T3 cells showed a high level of active β–catenin in the cytoplasm of both non-treated and PGE2 treated cells. Immunostaining of 2T3 cells with this antibody (Figure 6) strong cell membrane associated immunofluorescence similar to that observed in Figure 1 (panels B–D). Application of 2 or 16 dynes/cm2 PFFSS for 2 hours resulted in little or no change in β–catenin associated with the cell membrane as was also observed in Figure 1, β–catenin nuclear staining was only observed at 16 dynes/cm2 (Figure 6C and 6D).
Figure 5.
Immunoblot detection of cytoplasmic and nuclear β–catenin. MLO-Y4 and 2T3 cells were grown on collagen coated dishes under static conditions and treated with PGE2 (1000 pg/ml) or vehicle (media) for 2 hours. Cells were harvested and cytoplasmic and nuclear extracts were prepared and immunoblotting performed with an antibody against the active form of β–catenin (Cell Signaling Technologies). To test the relative purity of the preparations the same blots were probed with an antibody against IGF-1R, which is a cytoplasmic protein.
Figure 6.
Active β-catenin nuclear translocation detected by immunostaining in 2T3 osteoblastic cells. Panel A is the non-immune control, Panel B are static cultures, Panels C and D are cultures treated with 2 or 16 dynes/cm2 PFFSS for 2 hours, respectively. All cells were photographed at 20X and the green (phalloidin) and red (β-catenin) channels were overlaid in Adobe Photoshop. Cytoplasmic membrane associated staining is visible in Panels B–D, while nuclear staining was only observed in Panel D.
DISCUSSION
The main goal of these studies was to determine if pulsatile fluid flow shear stress (PFFSS) would induce similar responses in the MLO-Y4 osteocytic cell line [19] in comparison to the 2T3 osteoblastic cell line [21] with respect to β–catenin signaling. We observed that PFFSS induced β–catenin nuclear translocation and increased the expression of Cox-2 (Ptgs2) gene expression in MLO-Y4 and 2T3 cells, but there were notable differences. PFFSS was able to induce β–catenin nuclear translocation in the MLO-Y4 osteocytic cell at much lower magnitude of PFFSS compared to the 2T3 osteoblastic cells. Our data also support a role for PGE2 as a key initiator of β–catenin signaling in these cells. The kinetics and amount of PGE2 release by MLO-Y4 osteocytic cells in response to PFFSS supports the model/mechanism by which β–catenin signaling is rapidly activated in osteocytes as we have proposed in a recent review [6].
We began by examining β–catenin nuclear translocation as a well established endpoint of bone cell response to mechanical loading [10, 12, 13, 15–18]. We observed significant β–catenin nuclear translocation in the MLO-Y4 osteocytic cells after 2 hours of PFFSS at both 2 and 16 dynes/cm2, but in the 2T3 osteoblastic cells this occurred only at 16 dynes/cm2 (Figure 1). Mouse neonatal calvarial osteoblasts also only showed β–catenin nuclear translocation at 16 dynes/cm2 (Figure 2). We have recently observed the same pattern of β–catenin nuclear translocation in fetal rat calvaria cells (not shown). One important consideration for in vitro studies is the form of mechanical loading to use; e.g. fluid flow shear stress versus mechanical stretch. As has been discussed in vivo the osteoblast is unlikely to experience fluid flow shear stress in the same form and magnitude as the osteocyte [6]. Thus it may be that the differential sensitivity we observed to the magnitude of PFFSS between MLO-Y4 osteocytic versus 2T3 osteoblastic cells could relate to the use of fluid flow versus mechanical stretch. Klein-Nulend and colleagues [23] have reported that in primary human bone cells (osteoblasts) that the response to pulsating fluid flow versus cyclic strain differed significantly and that primary chicken osteocytes are more sensitive to fluid shear stress that other bone cells [7, 24]. Zaman et al [25] have also shown that isolated chicken osteocytes release greater amounts of nitric oxide (NO) that primary osteoblasts and that calvarial osteoblasts are relatively unresponsive and fail to show increased NO production in response to loading. Conversely, Owan et al [26] reported that MC3T3-E1 osteoblastic cells were more responsive to fluid flow that mechanical strain. Our data suggest that the magnitude of applied fluid flow shear stress is critical and that the MLO-Y4 osteocytic cell line, similar to primary chicken osteocytes, is more sensitive to PFFSS than osteoblastic cells.
We next sought to determine a possible underlying mechanism that might account for the differences we observed in β–catenin nuclear translocation and magnitude of PFFSS between MLO-Y4 versus 2T3 cells. Castellone et al. [27] have shown that PGE2 signaling is able to crosstalk with β–catenin signaling through a mechanism involving Akt-mediated phosphorylation of GSK-3β. Both increased Akt phosphorylation and GSK-3β phosphorylation have been reported in osteoblasts in response to fluid flow using a high magnitude of PFFSS [10], although the connection to PGE2 was not made in that earlier work, recent studies from our group and others have implicated a role of Akt in mediating the crosstalk between PGE2 and β–catenin signaling [17, 20, 28]. Since PGE2 is capable of inducing β–catenin nuclear translocation it seems logical that one possible explanation for the lack of translocation in 2T3 and neonatal calvarial cells might be related to differences in PGE2 release by these cells versus MLO-Y4 cells in response to PFFSS. Mechanistically increased Cox-2 activity (Ptgs2 gene expression) and PGE2 secretion are known to be an early event in the response to mechanical loading [7, 29–36]. Our data demonstrated that MLO-Y4 osteocytic cells release significantly higher amounts of PGE2, at lower levels of applied shear stress and the kinetics of release is much more rapid than the 2T3 osteoblastic cells (Figure 3). Addition of exogenous PGE2 resulted in β–catenin nuclear translocation in both cell lines (Figure 5). Thus the differences in PGE2 release by MLO-Y4 versus 2T3 cells could explain the differences we observed in β–catenin nuclear translocation.
Several groups have previously reported measuring significant PGE2 levels in response to fluid flow shear stress in both primary osteoblasts and other osteoblastic cell lines. However, studies with osteoblastic cells have consistently demonstrated very slow kinetics for PGE2 production [35, 36] and required high magnitudes of shear stress. Additionally, previous studies with osteoblasts have routinely included a post-incubation period of 1–2 hours after flow before PGE2 could be measured. In our study we sampled the circulating media during the application of fluid flow shear stress and observed the release of PGE2 from MLO-Y4 osteocytic cells occurs within seconds (Figure 3D), implying that the mechanism and kinetics of response to loading in different bone cell types could vary substantially. Xia et al. [28] have shown an important role for connexin-43 hemichannels in mediating PGE2 release in the MLO-Y4 osteocytic cell. Our data agree with the work of Klein-Nulend et al. [3] who reported faster PGE2 release in chicken osteocytes relative to osteoblasts or periosteal fibroblasts. Future studies aimed at defining the exact temporal sequence of events involved in the perception of a physical signal by osteocytes in vivo will need to be performed.
Several studies have implicated an important role for PGE2 in the response of bone and bone cells to mechanical loading. Kunnel et al. have shown that Cox-2 and constitutive NOS are important in the anabolic responses of neonatal tibia to in vitro mechanical load [37], while Pilbeam and colleagues have shown that fluid flow induces Cox-2 gene expression in MC3T3-E1 osteoblastic cells via PKA and Erk signaling [38, 39]. Furthermore, Akhter et al [40] have shown that EP2 receptor knockout mice have weak biomechanical strength properties implicating this prostaglandin receptor subtype as being critical in bone. Our data are consistent with these and several other published studies and support a functional crosstalk signaling between the PGE2 signaling and β–catenin signaling pathways [6].
Another interesting finding of our studies was the relationship between the magnitude of PFFSS and the regulation of Ptgs2 (Cox-2) mRNA levels. In our experiments both the 2T3 osteoblastic and MLO-Y4 osteocytic cells displayed a similar biphasic induction of Ptgs2 mRNA in response to increasing magnitude of PFFSS. There was a peak of expression at 2–4 dynes/cm2 and a higher peak at 16 dynes/cm2 in both cell lines immediately after the end of a 2-hour fluid flow session. However, at 24 hours the Ptgs2 mRNA levels fell below those of the static control in MLO-Y4 cells (Figure 4A), whereas in the 2T3 cell lines the Ptgs2 mRNA levels continued to increase (Figure 4B). The greater fold induction by the 2T3 cells (Figure 4B) is probably reflective of the 4–8 fold lower basal level of Ptgs2 mRNA in these cells versus the MLO-Y4 cells (data not shown). While we did not absolutely quantitate Ptgs2 mRNA levels in 2T3 or MLO-Y4 cells, based on differences in the delta Ct values and the fold induction observed we estimate that the peak induced levels in 2T3 cells at 2 or 24 hours were equivalent to those in the MLO-Y4 cells at 2 hours after the start of PFFSS. PGE2 production by MLO-Y4 cells also showed a similar biphasic response and the high level of PGE2 released rapidly by MLO-Y4 cells (Figure 3) compared to 2T3 would seem to suggest that MLO-Y4 cells store considerable higher amounts of PGE2, which could be due to the higher basal level of Cox-2 or possibly to Cox-1. In this regard it is interesting that Alam et al. have shown that mechanotransduction in vivo did not require the Cox-2 gene, but as noted by the authors these paradoxical findings may have resulted from induction of Cox-1 activity [41].
What might account for this biphasic pattern of expression? It seems unlikely that this is a phenomenon uniquely related to these cell lines. Murray and Rushton [42] found a nearly two-fold greater level of PGE2 secretion at 7,000 µε, followed by a reduction at 14,000 µε and a second peak at 28,000 µε that was over two-fold higher than controls. Reich and colleagues found a nearly 13-fold greater concentration of intracellular cAMP at 4.3 dynes/cm2 followed by a reduction at 9 dynes/cm2, and a second peak at 36 dynes/cm2 that was nearly 16-fold higher than controls [43]. Searby et al. [44] also observed what appears to be a biphasic effect of hypergravity on PGE2 in cultures of primary fetal rat calvarial osteoblasts subjected to increasing magnitudes of g-force. Therefore, this biphasic response does not appear to be an artifact of the cell lines being used in our studies. One possible explanation is that higher magnitudes of strain may activate additional pathways that further up-regulate Ptgs2 gene expression. This is another important consideration for studies with osteoblastic cells that uniformly require high levels of fluid flow shear stress to activate a response. Further studies will be required to determine the mechanism behind this biphasic pattern.
Our data are consistent with a number of studies using osteoblastic cell lines that demonstrate the ability of these cells to respond to fluid flow shear stress or mechanical stretch. One key question that needs further examination is whether the mechanisms used by osteoblastic cells and osteocytic cells to respond to fluid flow or stretch can be fully explained by a PGE2 mediated mechanism? While our studies presented here do not specifically address this question they do demonstrate that the MLO-Y4 cell is much more sensitive to the magnitude of PFFSS than the 2T3 osteoblastic cell and more rapidly activates the biochemical cascade of events subsequent to the perception of strain compared to the osteoblast. Findings of greater sensitivity of primary chicken osteocytes to fluid flow have previously been reported [3, 7] and differential sensitivity of MLO-Y4 to shear stress versus MC3T3-E1 cells has been reported [9, 45]. Although interestingly, Wadhwa et al. [39] have reported a detectable increase in Cox-2 mRNA levels using fluid flow shear stress as low as 0.14 dynes/cm2 for 4 hours. Another difference in the response mechanism between the 2T3 and MLO-Y4 cells that we observed was revealed in the 24-hour post PFFSS Ptgs2 mRNA response. While 2T3 cells continued to increase the expression of Ptgs2 mRNA and presumably Cox-2 protein levels, the increase in MLO-Y4 cells was more transient, with Ptgs2 expression falling below that observed at the end of PFFSS (2 hour) (Figure 4A). The significance of this difference in terms of understanding the pathways used by bone cells in response to load remains unanswered and will need further investigation.
As we have noted our studies do have several limitations. It is important to recognize that the MLO-Y4 osteocytic cell, while possessing many characteristics of an osteocyte [6] does not express many of the proteins shown to be expressed in mature primary osteocytes [46, 47]. Therefore while closer to the osteocyte in terms of its differentiation than osteoblastic cell lines, the MLO-Y4 cell line it is not the same as an osteocyte embedded in a mineralized matrix. Thus, even better in vitro and in vivo models of osteocyte will need to be developed before we fully understand the mechanism of how bone cells respond to mechanical load. Another important consideration is the type and magnitude of load to apply in vitro. Weinbaum and colleagues [48] have predicted that fluid shear stress of 8–30 dynes/cm2 occur on the osteocyte process with physiologic loading. We observed an MLO-Y4 cell response at 2 dynes/cm2, which is much lower than the Weinbaum model prediction. The exact explanation for this discrepancy is not clear, but one contributing factor may be the two dimensional nature of cell culture compared the three dimensional mineralized environment in vivo.
In conclusion, our studies illustrate a major difference in the sensitivity of the MLO-Y4 versus the 2T3 (and primary neonatal calvarial osteoblast) in terms of their ability to respond to fluid flow shear stress. PFFSS induced β–catenin nuclear translocation at low magnitudes in MLO-Y4, but only at high magnitudes in the osteoblastic cells. The MLO-Y4 produces at least a magnitude greater amount of PGE2 at low levels of shear stress, which do not stimulate detectable secretion in osteoblasts. At high levels of shear stress, as is often used in studies of osteoblastic cells, PGE2 secretion does occur, but its level does not reach the same amount as we observed being produced by the MLO-Y4 cells and the kinetics of accumulation are much slower. Importantly our data make a functional connection between PGE2 and β–catenin nuclear translocation and connect these signaling pathways as part of the mechanism by which bone cells respond to fluid flow shear stress.
ACKNOWLEDGEMENTS
This work was supported by Grant Number RO1AR053949 from the National Institutes of Arthritis and Musculoskeletal and Skin Diseases.
Portions of this manuscript were submitted by Jason L. Picconi to the Graduate School at Creighton University in partial fulfillment for the Ph.D. degree.
The first two authors share equal credit for this work and are therefore co-first authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Burger EH, Klein-Nulend J, Van Der Plas A, Nijweide PJ. Function of osteocytes in Bone - Their Role in Mechanotransduction. J Nutrition. 1995;125:2020S–2023S. doi: 10.1093/jn/125.suppl_7.2020S. [DOI] [PubMed] [Google Scholar]
- 2.Lanyon LE. Osteocytes, strain detection, bone modeling and remodeling. Calcif Tissue Int. 1993;53:S102–S107. doi: 10.1007/BF01673415. discussion S106-7. [DOI] [PubMed] [Google Scholar]
- 3.Klein-Nulend J, van der Plas A, Semeins CM, Ajubi NE, Frangos JA, Nijweide PJ, Burger EH. Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J. 1995;9:441–445. doi: 10.1096/fasebj.9.5.7896017. [DOI] [PubMed] [Google Scholar]
- 4.Tan SD, de Vries TJ, Kuijpers-Jagtman AM, Semeins CM, Everts V, Klein-Nulend J. Osteocytes Subjected to Fluid Flow Inhibit Osteoclast Formation and Bone Resorption. Bone. 2007;41:745–751. doi: 10.1016/j.bone.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westbroek I, Ajubi NE, Alblas MJ, Semeins CM, Klein-Nulend J, Burger EH, Nijweide PJ. Differential Stimulation of Prostaglandin G/H Synthase-2 in Osteocytes and Other Osteogenic Cells by Pulsating Fluid Flow. Biochem. Biophys. Res. Commun. 2000;268:414–419. doi: 10.1006/bbrc.2000.2154. [DOI] [PubMed] [Google Scholar]
- 8.Kamioka H, Sugawara Y, Murshid SA, Ishihara Y, Honjo T, Takano-Yamamoto T. Fluid shear stress induces less calcium response in a single primary osteocyte than in a single osteoblast: implication of different focal adhesion formation. J Bone Miner Res. 2006;21:1012–1021. doi: 10.1359/jbmr.060408. [DOI] [PubMed] [Google Scholar]
- 9.Ponik SM, Triplett JW, Pavalko FM. Osteoblasts and Osteocytes Respond Differently to Oscillatory and Unidirectional Fluid Flow Profiles. J Cell Biochem. 2007;100:794–807. doi: 10.1002/jcb.21089. [DOI] [PubMed] [Google Scholar]
- 10.Norvell SM, Alvarez M, Bidwell JP, Pavalko FM. Fluid Shear Stress Induces β-Catenin Signaling in Osteoblasts. Calcif Tissue Int. 2004;75:396–404. doi: 10.1007/s00223-004-0213-y. [DOI] [PubMed] [Google Scholar]
- 11.Liedert A, Wagner L, Seefried L, Ebert R, Jakob F, Ignatius A. Estrogen receptor and Wnt signaling interact to regulate early gene expression in response to mechanical strain in osteoblastic cells. Biochemical and Biophysical Research Communications. 394:755–759. doi: 10.1016/j.bbrc.2010.03.065. [DOI] [PubMed] [Google Scholar]
- 12.Lau K-HW, Kapur S, Kesavan C, Baylink DJ. Up-Regulation of the Wnt, Estrogen Receptor, Insulin-like Growth Factor-I, and Bone Morphogenetic Protein Pathways in C57BL/6J Osteoblasts as Opposed to C3H/HeJ Osteoblasts in Part Contributes to the Differential Anabolic Response to Fluid Shear. J Biol Chem. 2006;281:9576–9588. doi: 10.1074/jbc.M509205200. [DOI] [PubMed] [Google Scholar]
- 13.Robinson JA, Chatterjee-Kishore M, Yaworsky P, Cullen DM, Zhao W, Li C, Kharode YP, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ. Wnt/β-Catenin Signaling is a Normal Physiological Response to Mechanical Loading in Bone. J Biol Chem. 2006;281:31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 14.Mount JG, Muzylak M, Allen S, Althnaian T, McGonnell IM, Price JS. Evidence that the Canonical Wnt Signalling Pathway Regulates Deer Antler Regeneration. Develop Dynamics. 2006;234:1390–1399. doi: 10.1002/dvdy.20742. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong VJ, Muzylak M, Sunters A, Zaman G, Saxon LK, Price JS, Lanyon LE. Wnt/β-Catenin signaling is a Component of Osteoblastic Bone Cell Early Responses to Load-bearing and Requires Estrogen Receptor α. J Biol Chem. 2007;282:20715–20727. doi: 10.1074/jbc.M703224200. [DOI] [PubMed] [Google Scholar]
- 16.Case N, Ma M, Sen B, Xie Z, Gross TS, Rubin J. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem. 2008;283:29196–29205. doi: 10.1074/jbc.M801907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sen B, Styner M, Xie Z, Case N, Rubin CT, Rubin J. Mechanical loading regulates NFATc1 and beta-catenin signaling through a GSK3beta control node. J Biol Chem. 2009;284:34607–34617. doi: 10.1074/jbc.M109.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sen B, Xie Z, Case N, Ma M, Rubin C, Rubin J. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology. 2008;149:6065–6075. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato Y, Windle J, Koop B, Qiao M, Bonewald LF. Establishment of an Osteocyte-like Cell Line, MLO-Y4. J Bone Miner Res. 1997;12:2014–2023. doi: 10.1359/jbmr.1997.12.12.2014. [DOI] [PubMed] [Google Scholar]
- 20.Kitase Y, Barragan L, Jiang JX, Johnson ML, Bonewald LF. Mechanical induction of PGE(2) in osteocytes blocks glucocorticoid induced apoptosis through both the beta-catenin and PKA pathways. J Bone Miner Res. doi: 10.1002/jbmr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh-Choudhury N, Windle JJ, Koop BA, Harris MA, Guerero DL, Wozney JM, Mundy GR, Harris SE. Immortalized Murine Osteoblasts Derived from BMP-2-T-Antigen Expressing Transgenic Mice. Endocrinology. 1996;137:331–339. doi: 10.1210/endo.137.1.8536632. [DOI] [PubMed] [Google Scholar]
- 22.Mikuni-Takagaki Y, Suzuki Y, Kawase T, Saito H. Distinct Responses of Different Populations of Bone Cells to Mechanical Stress. Endocrinology. 1996;137:2028–2035. doi: 10.1210/endo.137.5.8612544. [DOI] [PubMed] [Google Scholar]
- 23.Mullender M, El Haj AJ, Yang Y, van Duin MA, Burger EH, Klein-Nulend J. Mechanotransduction of bone cells in vitro: mechanobiology of bone tissue. Med Biol Eng Comput. 2004;42:14–21. doi: 10.1007/BF02351006. [DOI] [PubMed] [Google Scholar]
- 24.Ajubi NE, Klein-Nulend J, Nijweide PJ, Vrijheid-Lammers T, Alblas MJ, Burger EH. Pulsating fluid flow increases prostaglandin production by cultured chicken osteocytes--a cytoskeleton-dependent process. Biochem Biophys Res Commun. 1996;225:62–68. doi: 10.1006/bbrc.1996.1131. [DOI] [PubMed] [Google Scholar]
- 25.Zaman G, Pitsillides AA, Rawlinson SC, Suswillo RF, Mosley JR, Cheng MZ, Platts LA, Hukkanen M, Polak JM, Lanyon LE. Mechanical Strain Stimulates Nitric Oxide Production by Rapid Activation of Endothelial Nitric Oxide Synthase in Osteocytes. J Bone Miner Res. 1999;14:1123–1131. doi: 10.1359/jbmr.1999.14.7.1123. [DOI] [PubMed] [Google Scholar]
- 26.Owan I, Burr DB, Turner CH, Qiu J, Tu Y, Onyia JE, Duncan RL. Mechanotransduction in bone: osteoblasts are more responsive to fluid forces than mechanical strain. Amer Physiological Society. 1997:C810–C815. doi: 10.1152/ajpcell.1997.273.3.C810. [DOI] [PubMed] [Google Scholar]
- 27.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 Promotes Colon Cancer Cell Growth Through a Gs-Axin-β-Catenin Signaling Axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 28.Xia X, Batra N, Shi Q, Bonewald LF, Sprague E, Jiang JX. Prostaglandin promotion of osteocyte gap junction function through transcriptional regulation of connexin 43 by glycogen synthase kinase 3/beta-catenin signaling. Mol Cell Biol. 2010;30:206–219. doi: 10.1128/MCB.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ajubi NE, Klein-Nulend J, Nijweide PJ, Vrijheid-Lammers T, Alblas MJ, Burger EH. Pulsating Fluid Flow Increases Prostaglandin Production by Cultured Chicken Osteocytes - A Cytoskeleton - Dependent Process. Biochem Biophy Res Comm. 1996;225:62–68. doi: 10.1006/bbrc.1996.1131. [DOI] [PubMed] [Google Scholar]
- 30.Forwood MR. Inducible Cyclo-oxygenase (COX-2) Mediates the Induction of Bone Formation by Mechanical Loading In Vivo. J Bone Miner Res. 1996;11:1688–1693. doi: 10.1002/jbmr.5650111112. [DOI] [PubMed] [Google Scholar]
- 31.Pead MJ, Lanyon LE. Indomethacin Modulation of Loading-Related Stimulation of New Bone Formation in vivo. Calcif Tissue Int. 1989;45:34–40. doi: 10.1007/BF02556658. [DOI] [PubMed] [Google Scholar]
- 32.Chow JW, Chambers TJ. Indomethacin has Early and Late Actions on Bone Formation Induced by Mechanical Stimulation. Am J Physiol. 1994;267:E287–E292. doi: 10.1152/ajpendo.1994.267.2.E287. [DOI] [PubMed] [Google Scholar]
- 33.Klein-Nulend J, Semeins CM, Ajubi NE, Nijweide PJ, Burger EH. Pulsating Fluid Flow Increases Nitric Oxide (NO) Synthesis by Osteocytes but not Periosteal Fibroblasts - Correlations with Prostaglandin Upregulation. Biochem Biophys Res Commun. 1995;217:640–648. doi: 10.1006/bbrc.1995.2822. [DOI] [PubMed] [Google Scholar]
- 34.Cherian PP, Wang X, Gu S, Bonewald LF, Sprague E, Jiang JX. Mechanical Strain Opens Connexin 43 Hemichannels in Osteocytes: A Novel Mechanism for the Release of Prostaglandin Molec Biol Cell. 2005;16:3100–3106. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL. Fluid Shear-Induced ATP Secretion Mediates Prostaglandin Release in MC3T3-E1 Osteoblasts. J Bone Min Res. 2005;20:41–49. doi: 10.1359/JBMR.041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saunders MM, You J, Zhou J, Li Z, Yellowley CE, Kunze EL, Jacobs CR, Donahue H. Fluid Flow-induced Prostaglandin E2 Response of Osteoblastic ROS 17/2.8 Cells is Gap Junction-mediated and Independent of Cytosolic Calcium. Bone. 2003;32:350–356. doi: 10.1016/s8756-3282(03)00025-5. [DOI] [PubMed] [Google Scholar]
- 37.Kunnel JG, Igarashi K, Gilbert JL, Stern PH. Bone anabolic responses to mechanical load in vitro involve COX-2 and constitutive NOS. Connect Tissue Res. 2004;45:40–49. doi: 10.1080/03008200490278133. [DOI] [PubMed] [Google Scholar]
- 38.Wadhwa S, Choudhary S, Voznesensky M, Epstein M, Raisz L, Pilbeam C. Fluid flow induces COX-2 expression in MC3T3-E1 osteoblasts via a PKA signaling pathway. Biochemical and Biophysical Research Communications. 2002;297:46–51. doi: 10.1016/s0006-291x(02)02124-1. [DOI] [PubMed] [Google Scholar]
- 39.Wadhwa S, Godwin SL, Peterson DR, Epstein MA, Raisz LG, Pilbeam CC. Fluid Flow Induction of Cyclo-Oxygenase 2 Gene Expression in Osteoblasts Is Dependent on an Extracellular Signal-Regulated Kinase Signaling Pathway. Journal of Bone and Mineral Research. 2002;17:266–274. doi: 10.1359/jbmr.2002.17.2.266. [DOI] [PubMed] [Google Scholar]
- 40.Akhter MP, Cullen DM, Gong G, Recker RR. Bone Biomechanical Properties in Prostaglandin EP1 and EP2 Knock-out Mice. Bone. 2001;29:121–125. doi: 10.1016/s8756-3282(01)00486-0. [DOI] [PubMed] [Google Scholar]
- 41.Alam I, Warden SJ, Robling AG, Turner CH. Mechanotransduction in bone does not require a functional cyclooxygenase-2 (COX-2) gene. J Bone Miner Res. 2005;20:438–446. doi: 10.1359/JBMR.041124. [DOI] [PubMed] [Google Scholar]
- 42.Murray DW, Rushton N. The Effect of Strain on Bone Cell Prostaglandin E2 Release: A New Experimental Method. Calcif Tissue Intl. 1990;47:35–39. doi: 10.1007/BF02555863. [DOI] [PubMed] [Google Scholar]
- 43.Reich KM, Gay CV, Frangos JA. Fluid Shear Stress as a Mediator of Osteoblast Cyclic Adenosine Monophosphate Production. J Cell Physiol. 1990;143:100–104. doi: 10.1002/jcp.1041430113. [DOI] [PubMed] [Google Scholar]
- 44.Searby ND, Steele CR, Globus RK. Influence of Increased Mechanical Loading by Hypergravity on the Microtubule Cytoskeleton and Prostaglandin E2 Release in Primary Osteoblasts. Am J Physiol Cell Physiol. 2005;289:C148–C158. doi: 10.1152/ajpcell.00524.2003. [DOI] [PubMed] [Google Scholar]
- 45.Taylor AF, Saunders M, Shingle DL, Cimbala JM, Zhou Z, Donahue HJ. Mechanically Stimulated Osteocytes Regulate Osteoblastic Activity via Gap Junctions. Am J Physiol Cell Physiol. 2007;292:C545–C552. doi: 10.1152/ajpcell.00611.2005. [DOI] [PubMed] [Google Scholar]
- 46.Yang W, Harris MA, Heinrich JG, Guo D, Bonewald LF, Harris SE. Gene expression signatures of a fibroblastoid preosteoblast and cuboidal osteoblast cell model compared to the MLO-Y4 osteocyte cell model. Bone. 2009;44:32–45. doi: 10.1016/j.bone.2008.08.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paic F, Igwe JC, Nori R, Kronenberg MS, Franceschetti T, Harrington P, Kuo L, Shin D-G, Rowe DW, Harris SE, Kalajzic I. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone. 2009;45:682–692. doi: 10.1016/j.bone.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27:339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]