Abstract
Contamination of recreational waters with E. coli and Enterococcus sp. is a widespread problem resulting in beach closures and loss of recreational activity. While E. coli is frequently used as an indicator of fecal contamination, and has been extensively measured in waterways, few studies have examined the presence of potentially pathogenic E. coli strains in beach waters. In this study, a combination of high-throughput, robot-assisted colony hybridization and PCR-based analyses were used to determine the genomic composition and frequency of virulence genes present in E. coli isolated from beach water in Avalon Bay, Santa Catalina Island, CA. A total of 24,493 E. coli isolates were collected from two sites at a popular swimming beach between August through September 2007 and from July through August 2008. All isolates were examined for the presence of shiga-like toxins (stx1/stx2), intimin (eaeA), and enterotoxins (ST/LT). Of the 24,493 isolates examined, 3.6% contained the eaeA gene, indicating that these isolates were potential EPEC strains. On five dates, however, greater than 10% of the strains were potential EPEC, suggesting that incidence of virulence genes at this beach has a strong temporal component. No STEC or ETEC isolates were detected, and only eight (<1.0%) of the potential EPEC isolates were found to carry the EAF plasmid. The potential EPEC isolates mainly belonged to E. coli phylogenetic groups B1 or B2, and carried the beta intimin subtype. DNA fingerprint analyses of the potential EPEC strains indicated that the isolates belonged to several genetically diverse groups, although clonal isolates were frequently detected. While the presence of virulence genes alone cannot be used to determine the pathogenicity of strains, results from this study show that potential EPEC strains can be found in marine beach water and their presence needs to be considered as one of the factors used in decisions concerning beach closures.
Keywords: Bacteria, E. coli, Beach water, virulence genes, phylogenetic groups, DNA fingerprinting
1. Introduction
Contamination of recreational waters with Escherichia coli and Enterococcus species is a common problem resulting in beach closures and loss of recreational activity. Because these bacteria are most frequently found in the intestinal tract of warm-blooded animals and are shed with feces, E. coli and Enterococcus have traditionally been used as indicators of fecal contamination. While it has been generally accepted that the presence of indicator organisms in recreational waters suggests the potential presence of fecal pathogens (Cabelli et al., 1982; Dufour, 1984; Prüss, 1998), several studies have shown that these bacteria can persist in the environment in the absence of a host, and can become naturalized to beach sand, soil, and algae (Byappanahalli and Fujioka, 1998; Byappanahalli et al., 2006; Davies et al., 1995; Desmarais et al., 2002; Hardina et al., 1991; Ishii et al., 2006a; Ishii et al., 2006b; Ishii et al., 2007a; Yan and Sadowsky, 2007). Traditionally, E. coli is often characterized as a harmless, commensal, bacterium. Some strains, however, have been shown to be capable of causing human disease (Kaper et al., 2004; Nataro and Kaper, 1998), and fecal material from some animals contains high frequencies of E. coli strains harboring virulence genes (Ishii et al., 2007b). Despite increasing evidence that E. coli strains from several animal hosts contain virulence genes, and some have been shown to cause serious or fatal diseases in humans (Nataro and Kaper, 1998), few studies have determined whether E. coli strains isolated from marine recreational waters contain virulence genes and are potentially pathogenic (Lang et al., 1994).
Pathogenic E. coli generally cause diarrhea and other gastrointestinal disease (Kaper et al., 2004; Nataro and Kaper, 1998), although some strains have been found to cause extraintestinal infections (Dietzman et al., 1974; Manges et al., 2001). Diarrheagenic strains are classified into several groups based on the mechanisms of pathogenesis and on the presence of various virulence factors or determinants. These groups include diffusely adhering (DAEC), enteroaggregative (EAEC), enteroinvasive (EIEC), enteropathogenic (EPEC), enterotoxigenic (ETEC), and shiga-like toxin producing (STEC) E. coli. Detailed information about pathogenic E. coli strains can be found in review articles by Nataro and Kaper (1998) and Kaper et al. (2004). The STEC are defined as E. coli strains expressing either of the shiga-like toxin genes, stx1 or stx2, or other toxins sharing significant homology to the shiga toxin gene originally identified in Shigella dysenteriae (Kaper et al., 2004; Paton and Paton, 1998). The common reservoirs for STEC strains are ruminants and swine (Djordjevic et al., 2004; Fratamico et al., 2004; Gyles, 2007; Ishii et al., 2007b). In contrast, ETEC strains are defined by the presence of at least one of the heat stable (ST) and the heat labile (LT) enterotoxin genes (Levine, 1987). These strains are often associated with infantile diarrhea, particularly in the developing world where the disease is considered endemic (Black, 1990; Kaper et al., 2004; Nataro and Kaper, 1998). ETEC strains are also a common cause of traveler’s diarrhea, where visitors from the developed world may lack immunity to these strains (Black, 1990).
EPEC strains are a common cause of human diarrheal diseases in developing countries, particularly among children less than 2 years of age (Levine and Edelman, 1984; Trabulsi et al., 2002). EPEC strains have been isolated from many animal host species, including humans, cats, cows, dogs, deer, ducks, geese, and horses (Ishii et al., 2007b). These strains are defined by the presence of the LEE pathogenicity island, which encodes several virulence factors, including intimin (eaeA), and the absence of the shiga-like toxin genes (Kaper et al., 2004; Nataro and Kaper, 1998). Strains carrying pEAF are referred to as typical EPEC, while those strains without the plasmid are referred to as atypical EPEC.
Most E. coli strains have been assigned to one of six major phylogenetic groups (A, B1, B2, C, D, and E) based on their evolutionary origins (Clermont et al., 2000; Escobar-Páramo et al., 2004). The association of STEC strains with certain phylogenetic groups has been tentatively established. Escobar et al. (2004) determined the majority of STEC strains they examined from clinical samples belonged to phylogenetic group B1. However, while a tentative a link has been established between intimin subtype and phylogenetic group (Escobar-Páramo et al., 2004, Reid et al., 2000), not all EPEC strains have a specific genetic background, and they may be distributed among several phylogenetic groups (Escobar-Páramo et al., 2004; Ishii et al., 2007b).
Detection of EPEC and STEC strains has previously been done by using multiplex PCR, using primers specific to eaeA and stx1/stx2, respectively (Ahmed et al. 2007, Ishii et al., 2007b; Paton and Paton, 1998, Ram et al. 2007). While these analyses are useful for screening relatively small numbers of isolates, they suffer from the high costs and low throughput associated with PCR.
We previously reported the successful use of high-throughput, semi-automated, robotic technology to detect the presence of host source-specific genes in environmental E. coli isolates (Yan et al., 2007). In the studies presented here, we adapted this technology to quickly screen large numbers of E. coli isolates from marine recreational water for the presence of virulence determinants and confirmed positive hybridization results by using PCR. The objectives of this present study were to: 1) examine the distribution and frequency of potentially pathogenic E. coli stains isolated from beach water at a popular swimming beach in Avalon, CA; 2) characterize the virulence gene determinants; and 3) determine the genetic relatedness of the potentially pathogenic strains by using horizontal fluorophore-enhanced rep-PCR (HFERP) DNA fingerprint analyses.
2. Materials and methods
2.1. Sample Collection
Water samples were obtained in 2007 and 2008, at either 8:00 am or 12:00 pm, from two beach sites at Avalon Bay, Santa Catalina, CA as previously described (Bordner et al., 1978). The two sampling locations are identified as sites B and C in Figure 1. Water samples (4 L) were collected at each time point. The temperature on most sampling days was approximately 29°C and only a few swimmers were in the water during the 8 am sampling time. Bacteria were concentrated by membrane filtration using 142 mm (dia.), 0.45 μm Supor hydrophilic polyethersulfone membranes (Pall Corporation, East Hills, NY). After filtration, membranes were cut into four equal sections and each section was placed in a 50 ml conical tube containing 10 ml of sterile phosphate buffered saline (20 mM sodium phosphate, 15 mM sodium chloride, pH 7.2) amended with 0.1% hydrolyzed gelatin and 10 g of sterile, 3 mm (dia.), glass beads. Bacteria were removed from the filter surface by gentle agitation for 30 min using a wrist action shaker (Burrell Scientific, Pittsburg, PA), filters were removed from solutions, and 50% glycerol was added to obtain a final concentration of 10%. All samples were stored frozen at -80°C until used.
Figure 1.

Sample sites at Avalon Bay, Santa Catalina Island, CA.
2.2. E. coli reference strains
The E. coli reference strains Pig206 (stx1/2+, eaeA-) and Deer090 (stx1/2-, eaeA+) were used as positive and negative controls for colony hybridization with the stx1, stx2, and eaeA probes (Dombek et al., 2000; Johnson et al., 2004). The ETEC strain 1362 and Pig206 were used as positive and negative controls, respectively, for hybridizations with the enterotoxin gene probes. The E. coli strain O157:H7 (ATCC 43895) was used as the positive control for PCR-based assays for virulence genes and for amplifying DNA used as hybridization probes for the eaeA, stx1, and stx2 genes. The ETEC strain 1362 was used as template for amplifying DNA for use as hybridization probes for the STa and LT-I genes. The E. coli strain H120 was used a positive control for PCR reactions to detect the EAF plasmid (Dombek et al., 2000; Ishii et al., 2007b; Johnson et al., 2004), and E. coli strain Pig 294 was used as control for HFERP DNA fingerprint analysis (Dombek et al., 2000; Johnson et al., 2004).
2.3. Isolation and picking of E. coli
E. coli strains were isolated from filter washings as previously described (Yan et al., 2007). One to three ml aliquots of filter washings were spread-plated onto the surface of modified membrane thermotolerant Escherichia coli (mTEC) agar medium in 22-by-22-cm Q-tray bioassay plates (Genetix Boston, MA). Modified mTEC was prepared as described, except that 500 μg of 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid (X-Gluc) per ml was used as the chromogenic indicator (United States Environmental Protection Agency, 2002; Yan and Sadowsky, 2007). Plates were incubated at 35° C for 2 h, and then at 44.5° C for 22 h. After incubation, plates were stored at 4° C overnight to facilitate development of blue pigment in colonies and to differentiate E. coli from other coliform and gram-negative bacteria. Well-isolated blue colonies were picked by hand or by using a Q-Bot robotic system (Genetix, Boston, MA) into 384 well microplates containing Hogness modified freezing medium as previously described (Yan et al., 2007). Microplates were incubated at 37° C overnight, and stored at -80° C until used. A total of 24,493 individual E. coli isolates were obtained in this study. A random sample of 1,024 strains were confirmed as E. coli by using a series of microbiological and biochemical tests including, the indole and methyl red tests, the inability of isolates to grow on citrate, β-d-glucuronidase activity using EC-MUG broth (Difco), and their color reaction on ChromAgar and MacConkey agar (Dombek et al., 2000; Johnson et al., 2004).
2.4. Automated arraying of isolates
Automated arraying of E. coli isolates onto positively charged, 22-by-22-cm, Performa II nylon membranes (Genetix) was done by using a QBot robot system (Genetix) as previously described (Yan et al., 2007). Each membrane was divided into 6 sections, each section contained 384 subunits, and each subunit consisted of 4 individual spots. One spot per subunit contained either a positive or negative control strain as described above. Using this format, each membrane was arrayed with a maximum of 6,912 E. coli isolates obtained from water samples. Arrayed membranes were placed on the surface of LB agar plates, incubated at 37° C for 8-10 h, and stored at 4° C overnight.
2.5. Colony hybridization screening for potentially pathogenic E. coli
Colony hybridizations were performed as previously described (Hamilton et al., 2006; Yan et al., 2007) to determine the reactivity of the E. coli isolates to DNA probes for the stx1, stx2, and eaeA genes. DNA probes for the stx1, stx2, and eaeA genes were obtained from control strains by using the PCR and primers pairs stx1F and stx1R, stx2F and stx2R, and eaeAF and eaeAR, respectively (Paton and Paton, 1998). Primers used in this study are shown in Table S1. Probe DNA for the heat stable (STa) and heat labile (LT-I) toxin genes was amplified using primer pairs JW14/JW7 and LTPr1/JW11, respectively (Stacy-Phipps et al., 1995). Probes were labeled with [32P]dCTP using the Random Primer DNA Labeling system (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Probes for the shiga-like toxin genes and enterotoxin genes were pooled before labeling. Membranes were hybridized overnight at 68° C, and washed under high stringency at 68° C in 0.1× SSC (1× SSC is 0.15 M NaCl and 0.015 M sodium citrate) containing 0.1% sodium dodecyl sulfate (Yan et al., 2007). Membranes were air dried after washing, wrapped in plastic film, and exposed overnight to storage phosphor imaging screens (GE Healthcare, Chalfont St. Giles, UK). All colony hybridizations were done in triplicate. Hybridization images were captured using a STORM 840 densitometer (GE Healthcare) and quantitative image analysis was done using ScanAlyze version 2.51 software (http://rana.lbl.gov/dowloads/scanalyze/scanaylze2_vers_2_51.exe). Positive and negative hybridization signals were determined as previously described (Yan et al., 2007).
2.6. PCR screening for potentially pathogenic E. coli and subtype analysis
The presence of virulence genes in strains showing positive hybridization reactions, on any of the triplicate membranes, was confirmed using a multiplex PCR approach using primer pairs stx1F/stx1R, stx2F/stx2R, eaeAF/eaeAR (Paton and Paton, 1998), and primer pairs JW14/JW7 and LTPr1/JW11 (Stacy-Phipps et al., 1995). Template DNA was extracted from cells grown overnight in LB medium as previously described (Ishii et al., 2006a), diluted 10-fold in distilled H2O, and 1-2 μl of lysate was added to each PCR reaction. Isolates shown to carry the eaeA gene were also tested for the presence of the EAF virulence plasmid using primer pairs (Table S1) EAF1 and EAF25 (Franke et al., 1994)
Subtype analysis of the eaeA gene was done by using a PCR-RFLP technique and primers EaeVF, EaeR, EaeZetaVR, and EaeIotaVR, as previously described (Ramachandran et al., 2003) (Table S1). Amplified products were digested with restriction enzymes AluI, CfoI, or RsaI to distinguish among 14 eae subtypes (Ramachandran et al., 2003). DNA fragments were separated by electrophoresis at 90 V for 2 - 3 h using 2% SeaKem LE agarose gels (FMC, Rockland, ME). Gels were stained with 0.5 μg ethidium bromide per ml and visualized with UV light.
2.7. Phylogenetic group classification and HFERP DNA fingerprinting
E. coli strains were classified into one of four major phylogenetic groups (A, B1, B2, and D/E) using a multiplex PCR protocol as previously described (Clermont et al., 2000). Strains with the Clermont (+, - , +) genotype (phylogenetic group D) were tested for the presence of ibeA to determine if these strains actually belonged to phylogenetic group B2 (Gordon et al., 2008). HFERP DNA fingerprint analyses were done using the BOXA1R primer as previously described (Johnson et al., 2004).
2.8. Statistical analysis
G-tests (likelihood-ratio or maximum likelihood statistical significance tests) were used to determine if E. coli carrying virulence factor genes were evenly distributed among samples and across sites. Statistical analysis of DNA fingerprint data was done using Bionumerics software (version 2.1) (Applied Math, Kortrijk, Belgium). Dendrograms were constructed using the curve-based, Pearson’s product-moment correlation coefficient and clustering was done using the unweighted pair group method with arithmetic means (UPGMA) (Johnson et al., 2004). Multivariate analysis of variance (MANOVA) of HFERP DNA fingerprint data was used to cluster E. coli strains isolated from different sites and in different years (Byappanahalli et al., 2006; Dombek et al., 2000; Ishii et al., 2006a)
3. Results
3.1. Isolation of E. coli from contaminated beach water
A total of 12,000 and 12,493 well isolated individual E. coli colonies were collected from Avalon Bay sites B and C (Figure 1) at Santa Catalina Island, CA in 2007 and 2008, respectively. The frequency of obtaining E. coli from water samples varied considerably by date, and the number of E. coli strains recovered from Avalon Bay was greater at 8 am than at 12 noon (Table S2). Biochemical analyses indicated that 99.8% of the isolates were confirmed to be E. coli as determined by using methyl red and indole tests, their color on ChromAgar and MacConkey agar, citrate utilization reaction, and β-D-glucuronidase activity, respectively (data not shown). These results are consistent with other reports concerning the ability of mTEC medium to selectively isolate E. coli from water samples (United States Environmental Protection Agency, 2002; Yan et al., 2007).
3.2. Screening for potentially pathogenic E. coli
Hybridization analyses were-used to screen environmental E. coli isolates for the presence of virulence genes. Of the 24,493 isolates examined, 875 (3.6%) were positive for eaeA, and thus were considered to be potential EPEC strains. In contrast, none of the isolates carried the STEC stx1 or stx2 toxin genes, or the ETEC toxin genes LT-I or STa. The percentage of eaeA positive isolates present in a given sample varied from 0 to 11.8% (Figure 2), both within and across sampling years. Detailed results for individual samples are shown in Table S2. G-test analysis, done to compare individual samples collected at common dates and times at each of the two sampling sites, indicated that the presence of potential EPEC isolates was not evenly distributed among samples (P <0.001). While potential EPEC strains were found at both sampling locations, isolates carrying the eaeA virulence factor gene were present at a greater frequency (P < 0.001) at site B compared to site C (4.8% and 1.5%, respectively). Since relatively few E. coli strains were obtained from the 12 pm samples, the frequency of eaeA positive strains was not compared across sampling times.
Figure 2.

Frequency of E. coli isolates carrying the eaeA (intimin) gene from water samples collected at Avalon Bay. Samples were collected in 2007 and 2008. All samples were collected at 8:00 AM, except those indicated by * were collected at 12:00 PM. Values above the bars are percentages of isolates containing the eaeA gene.
The 875 potential EPEC isolates, those carrying the eaeA gene, were also examined by PCR (Franke et al., 1994) for the presence of the adherence factor (EAF) plasmid. Of the 875 potential EPEC isolates examined, eight (0.9%) were found to carry the EAF plasmid. Consequently, these isolates were considered to be typical EPEC strains (Ishii et al., 2007b). PCR-RFLP subtype analysis indicated that 87.5% of the 875 potential EPEC strains carried the β intimin subtype (Figure 3, Table 1). While 6.2 and 3.1% of the potential EPEC strains were comprised of eae subtypes κ and ξ, respectively, the intimin subtypes α-2, ζ, η, θ, ι, and ν were found at lower frequencies among the strains examined.
Figure 3.
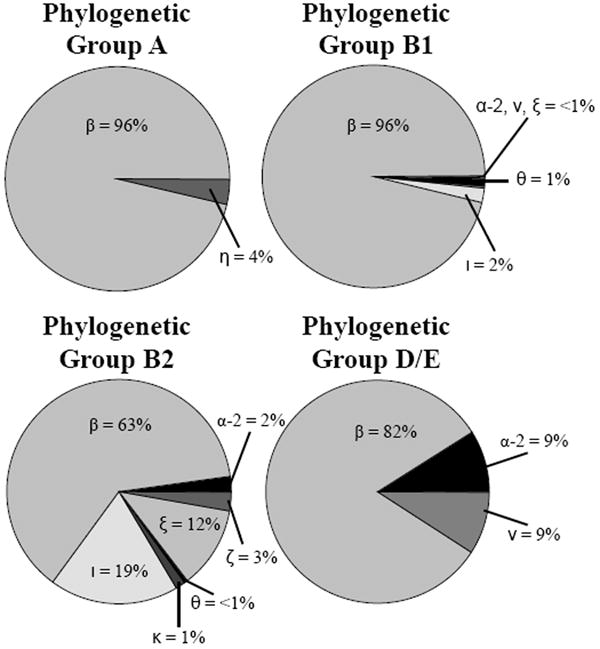
Intimin subtypes among potential EPEC strains separated by E. coli phylogenetic groups A (n = 28), B1 (n = 617), B2 (n = 224), and D/E (n = 6). The percentage of intimin subtypes types α-2, β, ζ, η, θ, ι, κ, ν, and ξ among strains in each phylogenetic group are shown.
Table 1.
Number and frequency of nine intimin (eaeA) subtypes among 875 potential EPEC strains isolated from Avalon Bay, Santa Catalina Island, CA.
| Intimin Subtype | Number and frequency |
|---|---|
| α-2 | 7 (0.8%) |
| β | 766 (87.5%) |
| ζ | 6 (0.7%) |
| η | 1 (0.1%) |
| θ | 8 (0.9%) |
| ι | 54 (6.2%) |
| κ | 3 (0.3%) |
| ν | 3 (0.3%) |
| ξ | 27 (3.1%) |
3.3. Phylogenetic group classification
Phylogenetic grouping analysis was used to investigate the evolutionary origins of the potentially pathogenic EPEC strains. Preliminary assignment into phylogenetic groups A, B1, B2, and D/E was based on multiplex PCR detection of the chuA and yjaA genes, chromosomal TSPE4.C2 DNA (Clermont et al., 2000), and a PCR reaction to detect the presence of ibeA (Gordon et al., 2008). Of the 875 isolates examined, the greatest number (70.5%) belonged to phylogenetic group B1, followed by those in phylogenetic group B2 (25.0%). Strains in phylogenetic groups A and D were represented at lower frequencies, 3.2 and 1.3%, respectively. The relationship between phylogenetic group classification and intimin subtype is shown in Figure 3. While the majority of strains in phylogenetic groups A, B1, and D/E carried the β intimin subtype, those in phylogenetic group B2 carried a greater variety of intimin subtypes. The phylogenetic groups B1 and B2 strains, and β intimin subtypes represented 95.5%, and 63 to 96% of the isolates examined, respectively.
3.4. Population structure of beach water isolates
The genetic relatedness of the potential EPEC isolates was determined by using HFERP DNA fingerprint analysis. Dendrograms created using data from all 875 eaeA+ isolates showed that the strains were distributed into several distinct and genetically diverse groups containing clonal and closely related strains. The relative similarity among these strains ranged from 3.4 to 100% (Figure 4), although the majority of strains were ≥40% similar. The genetic relatedness of isolates from 2007 and 2008 ranged from 6.4 to 100%, and 2.4 to 100%, respectively. The 875 potential EPEC strains could be divided into four large groups based on their overall genetic relatedness. The largest group consisted of 684 strains having ≥60% similarity, the majority of which came from site B in 2007 and 2008. Strains having relative similarity values of ≥92% were considered clones (Johnson et al., 2004). Based on this criterion, 128 different eaeA+ potential EPEC strains were isolated during this study. The two largest groups of clonal isolates, designated as groups I and II, contained 211 and 137 isolates, respectively (Figure 5). Interestingly, group I strains were obtained from sites B and C from several different dates in 2007, and from site B on two dates in 2008. In contrast, group II strains only contained EPEC strains from sites B and C isolated from several dates in 2007, and were not found in 2008 samples. The remaining 126 potential EPEC strains were represented by ≤ 64 isolates. About 64% (82 of 128) strains found in this study were represented by a single isolate.
Figure 4.

Dendrogram of HFERP DNA fingerprint data obtained from potential EPEC isolates obtained from Avalon Bay, CA. The dendrogram is collapsed at 40% similarity due to size constraints.
Figure 5.

Dendrogram of HFERP DNA fingerprint data obtained from strains represented by 10 or more clonal isolates. Strains having ≥92% similarity are considered to be clones. Strain groups I and II are designated.
MANOVA analysis of HFERP DNA fingerprints showed that isolates from 2007 and 2008 generally clustered separately into two large groups (Figure 6). While this suggested that the isolates were generally different in each year of the study, the same clonal potential EPEC isolate was sometimes detected at the same or different sampling site over consecutive years (Figure 5). The first and second discriminants of the MANOVA analysis accounted for ~91% of the variation (Figure 6), indicating the strains clustered well to their respective groups.
Figure 6.

MANOVA analysis of HFERP DNA fingerprint data obtained from all potential EPEC strains isolated in Avalon Bay. Only isolated from site B are shown. Strains isolated in 2007 and 2008 are indicated by ( ) and (●) circles, respectively. Only the first and second discriminants are shown, and together they represent 98% of the variation.
) and (●) circles, respectively. Only the first and second discriminants are shown, and together they represent 98% of the variation.
4. Discussion
The water samples used in this study were collected adjacent to two popular swimming beach sites in Avalon Bay, CA. Both of these sites suffer from fecal contamination due to degraded sewer infrastructure and counts of fecal indicator at these sites frequently exceed state and federal standards. A recent Natural Resources Defense Council report named these sites as 2 of the 10 most highly contaminated beaches in CA, based on the frequency of samples exceeding the single sample standard of 103 enterococi per 100 ml water (Dorfman and Rosselot, 2008).
In this study, we examined Avalon Bay water for the presence of potentially pathogenic E. coli. While E. coli has historically been used as an indicator of fecal contamination in inland, freshwater environments, this bacterium is generally not quantified in marine environments (United States Environmental Protection Agency, 1986), in part, due to its perceived low survival rates (Anderson et al., 1983; Martinez et al., 1989; McCambridge and McMeekin, 1981). This, however, makes this bacterium an ideal candidate to examine recent fecal contamination. In contrast, enterococci species are used as the indicator for fecal contamination in marine systems because Enterococcus counts have been shown to have strong correlation to human disease rates (Cabelli et al., 1982; Dufour, 1984), and this bacterium survives well in marine water (Hanes and Fragala, 1967). Despite this fact, E. coli was readily isolated from marine waters at Avalon, CA and potentially pathogenic strains were detected. This may impact the health of swimmers that ingest contaminated water.
Initial screening of the 24,493 E. coli isolates collected from marine water samples was done using an automated arraying system and methodology which was originally developed to determine sources of fecal bacteria in waterways using host source-specific marker genes (Yan et al., 2007). Results obtained in the study presented here indicate that the high-throughput technology can be adapted to determine the presence of any gene among large populations of culturable bacteria. Of the isolates examined, 875 (3.6%) were found to be potential EPEC strains based on detection of the eaeA gene. Since the majority (> 99%) of the potential EPEC strains described in this study did not harbor the EAF plasmid, these strain were classified as atypical EPEC (aEPEC) (Kaper et al., 2004; Nataro and Kaper, 1998).
There is currently controversy regarding the pathogenicity of aEPEC, and it has been suggested that these strains likely arose from STEC that have lost bacteriophages carrying stx genes, or EPEC strains that have lost the bfpA-encoding EAF plasmid (Bielaszewska et al., 2008, Levine and Edelman, 1984). Recently, Tennant et al. (2009) showed that 80% of the EPEC strains they examined from clinical and water samples were genetically distinct, carried adhesins that may serve as replacements for the lack of bfpA, and suggested that different populations of aEPEC may have varying degrees of pathogenicity. Historically, most cases of EPEC in industrialized countries have been associated with typical strains, but more recently aEPEC strains have been linked to outbreaks of human disease affecting both adults and children around the world (Afset et al., 2003; Afset et al., 2008; Bokete et al., 1997; Hedberg et al., 1997; Nataro, 2006; Nguyen et al., 2006; Yatsuyanagi et al., 2002). More recently, Nguyen et al. (2006) reported that patients infected with aEPEC strains were more likely to experience diarrhea lasting longer than 2 weeks, increasing the risk for serious illness and death. Taken together, these results indicate that aEPEC strains in contaminated recreational water may represent a public health risk to swimmers.
Interestingly, no STEC or ETEC strains were found among the 24,493 isolates screened. This result is similar to that reported by Higgins et al. (2005), where the EPEC virulence gene tir was detected in water samples at a significantly greater frequency than shiga-like toxin genes. The location of the sampling sites may in part explain the lack of STEC isolates, as the common reservoirs for these strains, ruminants and swine (Djordjevic et al., 2004; Fratamico et al., 2004; Gyles, 2007; Ishii et al., 2007b), are likely not contributing to the fecal load in the water we examined. Potential input sources into Avalon Bay include only humans, pets, and wildlife. Also, our lack of detection of ETEC strains was not unexpected, these strains are often a major cause of traveler’s diarrhea and childhood diarrhea in developing countries (Kaper et al., 2004; Nataro and Kaper, 1998).
Potential EPEC strains identified in this study were found in every sample examined, but were present at a greater frequency at site B compared to site C (5.3 and 1.9%, respectively). Statistical analysis indicated the EPEC strains were also not evenly distributed among samples collected at common dates and times at different sites (P <0.001). The reason for the uneven distribution is unknown, but may be related to the proximity of the sampling sites to point sources of fecal contamination, ocean currents present at the sampling sites, and wind and wave action. It is also possible that leaky sewer pipes or run-off originating from humans, pets, and wildlife may have impacted site B to a greater extent than site C.
Results of this study also showed that temporal distribution of the E. coli varied considerably. More than 99% of the E. coli isolates screened in this study were collected from samples obtained at 8 am, and only 3 of the 875 potential aEPEC strains were isolated from the 12 pm samples. Additional isolates from 12:00 pm samples were not collected due to low E. coli counts in samples. Inactivation of fecal indicator bacteria as result of exposure to sunlight has been shown to be a major factor in the persistence of this bacterium in the environment (Davies-Colley et al., 1994), and may help to explain the reduced number of culturable organisms in samples collected at 12 pm.
Phylogenetic analyses, intimin subtyping assays, and HFERP DNA fingerprint analyses revealed a diverse population of potential aEPEC strains. The strains mainly belonged to phylogenetic groups B1 and B2, with frequencies of about 70 and 25%, respectively. In contrast, while strains belonging to phylogenetic groups A and D were also found, they were present at much lower frequencies. Nine intimin subtypes (α-2, β, ζ, η, θ, ι, κ, ν, and ξ) were identified among the potential EPEC isolates by PCR-RFLP analysis. The β, ι , and ζ subtypes were most represented and present in frequencies of 87.5, 6.2, and 3.1% respectively. Strains possessing the β intimin subtype were assigned to all four phylogenetic groups, although the majorityof the strains (77%) were assigned to phylogenetic group B1. This result is consistent with previous reports by Reid et al. (2000) and Ramachandran et al. (2003) who showed that the majority of strains comprising the β intimin subtype belonged to phylogenetic group B1, and that the β intimin subtype was found at higher frequencies than other subtypes in clinical isolates from humans. The ι intimin subtype was found only in B1 and B2 strains, with approximately 75% of the isolates belonging to phylogenetic group B2. The other intimin subtypes were detected at frequencies of <1.0%. Ishii et al. (Ishii et al., 2007b) reported on the presence of the ι subtype in isolates obtained from ducks and geese, and the κ subtype among E. coli from domestic dogs and cats. Humans, pets and birds are likely the main contributors to fecal loading at the sampling sites described in this study and, in light of the studies described above, may explain the prevalence of the β, ι, and κ subtypes. Additional studies are necessary to conclusively determine the sources of the potential aEPEC strains in Avalon Bay.
The HFERP DNA fingerprint data showed that the potential EPEC strains belonged to several groups, consisting of both clonal and closely related strains. While 875 of the potential EPEC isolates were comprised of 128 different genotypes, as defined by Johnson et al. (Johnson et al., 2004), and some were genetically diverse, 650 strains belonged to clonal groups consisting of 10 or more strains. Moreover, 40% of the potential EPEC isolates (348 of 875) belonged to one of the two clonal groups, comprised of strains obtained from both sites and across sampling dates. The remaining 60% of the isolates were comprised of 126 different genotypes which were found only once or in smaller groups, and in most cases, were closely related to other strains. Since some of the clonal potential aEPEC strains were isolated during different years, our results suggest that there is likely a reservoir of E. coli contributing to fecal contamination at Avalon Bay.
Cluster analysis done using MANOVA confirmed results obtained using the correlation analyses represented by dendrograms and showed that isolates cluster relatively well by year into overlapping groups of isolates from each site. Taken together, these results suggest that a few different potential EPEC strains are predominantly isolated from both sites, at least during the summer months, and that most other strains exist transiently. Also, the population of potential EPEC strains may shift in successive years. The reason(s) why some strains were detected at much greater frequencies over a range of dates than other strains is not clear, but may be due to continual deposition as the result of an unknown reservoir or through persistence in the environment.
While the presence and detection of potential EPEC strains in environmental samples has been previously reported, the majority of these studies examined freshwater environments and much fewer isolates were examined (Hamelin et al., 2006; Ishii et al., 2007a; Lauber et al., 2003). To our knowledge, this present study represents the largest examination of virulence genes among E. coli strains obtained from marine recreational water completed to date. Although the presence of virulence determinants alone cannot be used to determine the pathogenicity of strains, results from this study show that potential EPEC strains can be found in marine water at Avalon Bay, adjacent to some of the most highly contaminated beaches in CA. While the ability of the strains described in this study to cause human disease has yet to be determined, potential EPEC strains have previously been implicated in human diarrheal diseases. Screening of the potential EPEC strains for other virulence factor genes, serotype testing, and other assays may provide further evidence to support this hypothesis. A quantitative measure of the health risk associated with exposure to contaminated water containing these strains also needs to be established through epidemiological studies.
5. Conclusions
Potential EPEC strains were readily isolated from contaminated marine recreational water in Avalon Bay, CA, and may represent a public health risk to swimmers and beach users.
Both STEC and ETEC strains were not detected in any of the samples examined.
The frequency of detection of potential EPEC strains varied considerably by sample, suggesting a strong temporal component.
The potential EPEC strains mainly belonged to phylogenetic groups B1 and B2, and PCR-RFLP analysis indicated that the majority of theses strains carried the intimin β subtype.
HFERP DNA fingerprint analyses indicated that potential EPEC strains in Avalon Bay were genetically diverse, although a large number of clonal groups were detected.
Since genotypically identical EPEC strains were detected repeatedly, on successive dates and years, our data suggests that E. coli in Avalon Bay are likely due to continual deposition from an unknown reservoir or through persistence of E. coli in the environment.
Supplementary Material
Acknowledgments
We would like to thank Yiping Cao, Ben Ferraro, and Nick Miller from the Southern California Coastal Water Research Project for their assistance with sample collection and processing. We would also like to thank Nick Hahn and the High-Throughput Biological Analysis facility at the University of Minnesota for assistance with automated arraying, Daniel Norat and Chris Brandsey for help with colony-picking, and John Ferguson for help with cluster analyses.
This work was funded, in part, by a grant from the Minnesota Agricultural Research Station (to MJS), and by training grant 2T32-GM008347 from the National Institutes of Health (to MJH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afset JE, Anderssen E, Bruant G, Harel J, Wieler L, Bergh K. Phylogenetic backgrounds and virulence profiles of atypical enteropathogenic Escherichia coli strains from a case-control study using multilocus sequence typing and DNA microarray analysis. Journal of Clinical Microbiology. 2008;46:2280–2290. doi: 10.1128/JCM.01752-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afset JE, Bergh K, Bevanger L. High prevalence of atypical enteropathogenic Escherichia coli (EPEC) in Norwegian children with diarrhea. Journal of Medical Microbiology. 2003;52:1015–1019. doi: 10.1099/jmm.0.05287-0. [DOI] [PubMed] [Google Scholar]
- Ahmed W, Tucker J, Bettleheim KA, Neller R, Katouli M. Detection of virulence genes in Esherichia coli of an existing metabolic database to predict the sources of pathogenic E. coli in surface waters. Water Research. 2007;41:3785–3791. doi: 10.1016/j.watres.2006.12.026. [DOI] [PubMed] [Google Scholar]
- Anderson IC, Rhodes M, Kator HI. Seasonal variations in survival of Escherichia coli exposed in situ in membrane diffusion chambers containing filtered and nonfiltered estuarine water. Applied and Environmental Microbiology. 1983;45:1877–1883. doi: 10.1128/aem.45.6.1877-1883.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielaszewska M, Middendorf B, Köck R, Friedrich AW, Fruth A, Karch H, Schmidt MA, Mellmann A. Shiga toxin-negative attaching and effacing Escherichia coli: Distinct clinical associations with bacterial phylogeny and virulence traits and inferred in-host pathogen evolution. Clinical Infectious Diseases. 2008;47:208–217. doi: 10.1086/589245. [DOI] [PubMed] [Google Scholar]
- Black RE. Epidemiology of traveler’s diarrhea and relative importance of various pathogens. Reviews of Infectious Diseases. 1990;12:S73–S79. doi: 10.1093/clinids/12.supplement_1.s73. [DOI] [PubMed] [Google Scholar]
- Bokete TN, Wittam TS, Wilson RA, Clausen CR, O’Callahan CM, Moseley SL, Fritsche TR, Tarr PI. Genetic and phenotypic analysis of Escherichia coli with enteropathogenic characteristics isolated from Seattle children. Journal of Infectious Diseases. 1997;175:1382–1389. doi: 10.1086/516470. [DOI] [PubMed] [Google Scholar]
- Bordner R, Winter JA, Scarpino P. Microbiological methods for monitoring the environment, water, and wastes. United States Environmental Protection Agency Washington, D C; Washington, D. C: 1978. p. 338. [Google Scholar]
- Byappanahalli MN, Fujioka RS. Evidence that tropical soil environment can support the growth of Escherichia coli. Water, Science and Technology. 1998;38:171–174. [Google Scholar]
- Byappanahalli MN, Whitman RL, Shively DA, Sadowsky MJ, Ishii S. Population structure, persistence, and seasonality of autochthonous Escherichia coli in temperate, coastal forest soil from a Great Lakes watershed. Environmental Microbiology. 2006;8:504–513. doi: 10.1111/j.1462-2920.2005.00916.x. [DOI] [PubMed] [Google Scholar]
- Cabelli VJ, Dufour AP, McCabe LJ, Levin MA. Swimming-associated gastroenteritis and water quality. American Journal of Epidemiology. 1982;115:606–616. doi: 10.1093/oxfordjournals.aje.a113342. [DOI] [PubMed] [Google Scholar]
- Clermont O, Bonacorsi S, Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. Applied and Environmental Microbiology. 2000;66:4555–4558. doi: 10.1128/aem.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CM, Long JA, Donald M, Ashbolt NJ. Survival of fecal microorganisms in marine and freshwater sediments. Appl Environ Microbiol. 1995;61:1888–1896. doi: 10.1128/aem.61.5.1888-1896.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies-Colley RJ, Bell RG, Donnison AM. Sunlight inactivation of enterococci and fecal coliforms within sewage effluent diluted in seawater. Applied and Environmental Microbiology. 1994:2049–2058. doi: 10.1128/aem.60.6.2049-2058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmarais TR, Solo-Gabriele HM, Palmer CJ. Influence of soil on fecal indicator organisms in a tidally influenced subtropical environment. Applied Environmental Microbiology. 2002;68:1165–1172. doi: 10.1128/AEM.68.3.1165-1172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzman DE, Fischer GW, Schoenknecht FD. Neonatal Escherichia coli septicemia-bacterial counts in blood. Journal of Pediatrics. 1974;85:128–130. doi: 10.1016/s0022-3476(74)80308-2. [DOI] [PubMed] [Google Scholar]
- Djordjevic SP, Ramachandran V, Bettelheim KA, Vanselow BA, Holst P, Bailey G, Hornitzky MA. Serotypes and virulence gene profiles of Shiga toxin-producing Escherichia coli strains isolated from feces of pasture-fed and lot-fed sheep. Applied and Environmental Microbiology. 2004;70:3910–3917. doi: 10.1128/AEM.70.7.3910-3917.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombek PE, Johnson LK, Zimmerley ST, Sadowsky MJ. Use of repetitive DNA sequences and the PCR to differentiate Escherichia coli isolates from human and animal sources. Applied and Environmental Microbiology. 2000;66:2572–2577. doi: 10.1128/aem.66.6.2572-2577.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman M, Rosselot KS. Testing the waters: A guide to water quality at vacation beaches. Natural Resources Defense Council; New York, NY: 2008. [Google Scholar]
- Dufour AP. Health effects criteria for fresh recreational waters. United States Environmental Protection Agency; Washington, D C., Research Triangle Park, NC: 1984. [Google Scholar]
- Escobar-Páramo P, Clermont O, Blanc-Potard AB, Bui H, Bouguenec CL, Denamur E. A specific genetic background is required for acquisition and expression of virulence factors in Escherichia coli. Molecular Biology and Evolution. 2004;21:1085–1094. doi: 10.1093/molbev/msh118. [DOI] [PubMed] [Google Scholar]
- Franke J, Franke S, Schmidt H, Schwarzkopf A, Wieler LH, Baljer G, Beutin L, Karch H. Nucleotide sequence analysis of enteropathogenic Escherichia coli (EPEC) adherence factor probe and development of PCR for rapid detection of EPEC harboring virulence plasmids. Journal of Clinical Microbiology. 1994;32:2460–2463. doi: 10.1128/jcm.32.10.2460-2463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratamico PM, Bagi LK, Bush EJ, Solow BT. Prevalence and characterization of Shiga toxin-producing Escherichia coli in swine feces recovered in the National Animal Health Monitoring System’s Swine 2000 Study. Applied and Environmental Microbiology. 2004;70:7173–7178. doi: 10.1128/AEM.70.12.7173-7178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DM, Clermont O, Tolley H, D E. Assigning Escherichia coli strains to phylogenetic groups: multi-locus sequence typing versus the PCR triplex method. Environmental Microbiology. 2008;10:2484–2496. doi: 10.1111/j.1462-2920.2008.01669.x. [DOI] [PubMed] [Google Scholar]
- Gyles CL. Shiga toxin-producing Escherichia coli: An overview. Journal of Animal Science. 2007;85:E45–E62. doi: 10.2527/jas.2006-508. [DOI] [PubMed] [Google Scholar]
- Hamelin K, Bruant G, El-Shaarawi A, Hill S, Edge TA, Bekal S, Fairbrother MJ, Harel J, Maynard C, Masson L, Brousseau R. A virulence and antimicrobial resistance DNA microarray detects a high frequency of virulence genes in Escherichia coli isolates from Great Lakes recreational waters. Applied and Environmental Microbiology. 2006;72:4200–4206. doi: 10.1128/AEM.00137-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MJ, Yan T, Sadowsky MJ. Development of goose- and duck-specific DNA markers to determine sources of Escherichia coli in waterways. Applied and Environmental Microbiology. 2006;72:4012–4019. doi: 10.1128/AEM.02764-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes B, Fragala C. Effect of seawater concentration on the survival of indicator bacteria. Journal (Water Pollution Control Federation) 1967;39:97–104. [PubMed] [Google Scholar]
- Hardina CM, Fujioka RS. Soil: The environmental source of E. coli and enterococci in Hawaii’s streams. Environmental Toxicology and Water Qualilty. 1991;6:185–195. [Google Scholar]
- Hedberg CW, Savarino SJ, Besser JM, Paulus CJ, Thelen VM, Myers LJ, Cameron DN, Barrett TJ, Kaper JB, Osterholm MT. An outbreak of foodborne illness caused by Escherichia coli O39:NM, an agent not fitting into the existing scheme for classifying diarrheogenic E. coli. Journal of Infectious Diseases. 1997;176:1625–1628. doi: 10.1086/517342. [DOI] [PubMed] [Google Scholar]
- Higgins JA, Belt KT, Karns JS, Russell-Anelli J, Shelton DR. tir- and stx-positive Escherichia coli in stream waters in a metropolitan area. Applied and Environmental Microbiology. 2005;71:2511–2519. doi: 10.1128/AEM.71.5.2511-2519.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S, Hansen DL, Hicks RE, Sadowsky MJ. Beach sand and sediments are temporal sinks and sources of Escherichia coli in Lake Superior. Environmental Science & Technology. 2007a;41:2203–2209. doi: 10.1021/es0623156. [DOI] [PubMed] [Google Scholar]
- Ishii S, Ksoll WB, Hicks RE, Sadowsky MJ. Presence and growth of naturalized Escherichia coli in temperate soils from Lake Superior watersheds. Applied and Environmental Microbiology. 2006a;72:612–621. doi: 10.1128/AEM.72.1.612-621.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S, Meyer KP, Sadowsky MJ. Relationship between phylogenetic groups, genotypic clusters, and virulence gene profiles of Escherichia coli strains from diverse human and animal sources. Applied and Environmental Microbiology. 2007b;73:5703–5710. doi: 10.1128/AEM.00275-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S, Yan T, Shively DA, Byappanahalli MN, Whitman RL, Sadowsky MJ. Cladophora (Chlorophyta) spp. harbor human bacterial pathogens in nearshore water of Lake Michigan. Applied and Environmental Microbiology. 2006b;72:4545–4553. doi: 10.1128/AEM.00131-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LK, Brown MB, Carruthers EA, Ferguson JA, Dombek PE, Sadowsky MJ. Sample size, library composition, and genotypic diversity among natural populations of Escherichia coli from different animals influence accuracy of determining sources of fecal pollution. Applied and Environmental Microbiology. 2004;70:4478–4485. doi: 10.1128/AEM.70.8.4478-4485.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper JB, Nataro JP, Mobley HLT. Pathogenic Escherichia coli. Nature Reviews Microbiology. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- Lang AL, Tsai YL, Mayer CL, Patton KC, Palmer CJ. Multiplex PCR for detection of the heat-labile toxin gene and shiga-like toxin I and II genes in Escherichia coli isolated from natural waters. Applied and Environmental Microbiology. 1994;60:3145–3149. doi: 10.1128/aem.60.9.3145-3149.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber CL, Glatzer L, Sinsabaugh RL. Prevalence of pathogenic Escherichia coli in recreational waters. Journal of Great Lakes Research. 2003;29:301–306. [Google Scholar]
- Levine MM. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemmorrhagic, and enteroadherent. Journal of Infectious Diseases. 1987;155:377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- Levine MM, Edelman R. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: Epidemiology and pathogenesis. Epidemiologic Reviews. 1984;6:31–51. doi: 10.1093/oxfordjournals.epirev.a036274. [DOI] [PubMed] [Google Scholar]
- Manges AR, Johnson JR, Foxman B, O’Bryan TT, Fullerton KE, Riley LW. Widespread distribution of urinary tract infections caused by a multidrug-resistant Escherichia coli clonal group. New England Journal of Medicine. 2001;345:1007–1013. doi: 10.1056/NEJMoa011265. [DOI] [PubMed] [Google Scholar]
- Martinez J, Garcia-Lara J, Vives-Rego J. Estimation of Escherichia coli mortality in seawater by the decrease in 3H-label and electron transport system activity. Microbial Ecology. 1989;17:219–225. doi: 10.1007/BF02012835. [DOI] [PubMed] [Google Scholar]
- McCambridge J, McMeekin TA. Effect of solar radiation and predacious microorganisms on survival of fecal and other bacteria. Environmental Microbiology. 1981;41:1083–1087. doi: 10.1128/aem.41.5.1083-1087.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataro JP. Atypical enteropathogenic Escherichia coli: typical pathogens? Emerging Infectious Diseases. 2006;12:696. doi: 10.3201/eid1204.060125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clinical Microbiology Reviews. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen RN, Taylor LS, Tauschek M, Robins-Browne RM. Atypical enteropathogenic Escherichia coli infection and prolonged diarrhea in children. Emerging Infectious Diseases. 2006;12:597–603. doi: 10.3201/eid1204.051112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton AW, Paton JC. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. Journal of Clinical Microbiology. 1998;36:598–602. doi: 10.1128/jcm.36.2.598-602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prüss A. Review of epidemiological studies on health effects from exposure to recreational water. International Journal of Epidemiology. 1998;27:1–9. doi: 10.1093/ije/27.1.1. [DOI] [PubMed] [Google Scholar]
- Ramachandran V, Brett K, Hornitzky MA, Dowton M, Bettelheim KA, Walker MJ, Djordjevic SP. Distribution of intimin subtypes among Escherichia coli isolates from ruminant and human sources. Journal of Clinical Microbiology. 2003;41:5022–5032. doi: 10.1128/JCM.41.11.5022-5032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram S, Vajpayee P, Shanker R. Prevalence of multi-antimicrobial-agent resistant, shiga toxin and enterotoxin producing Escherichia coli in surface waters of River Ganga. Environmental Science and Technology. 2007;41:7383–7388. doi: 10.1021/es0712266. [DOI] [PubMed] [Google Scholar]
- Reid SD, Herbelin CJ, Bumbaugh AC, Selander RK, Whittam TS. Parallel evolution of virulence in pathogenic Escherichia coli. Nature. 2000;406:64–67. doi: 10.1038/35017546. [DOI] [PubMed] [Google Scholar]
- Stacy-Phipps S, Mecca JJ, Weiss JB. Multiplex PCR assay and simple preparation method for stool specimens to detect enterotoxigenic Escherichia coli DNA during course of infection. Journal of Clinical Microbiology. 1995;33:1054–1059. doi: 10.1128/jcm.33.5.1054-1059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant SM, Tauschek M, Azzopardi K, Bigham A, Bennett-Wood V, Hartland EL, Qi W, Whittam TS, Robins-Browne RM. Characterisation of atypical enteropathogenic E. coli strains of clinical origin. BMC Microbiology. 2009;9:117–128. doi: 10.1186/1471-2180-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabulsi LR, Keller R, Gomes TAT. Typical and Atypical Enteropathogenic Escherichia coli. Emerging Infectious Diseases. 2002;8:508–513. doi: 10.3201/eid0805.010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Environmental Protection Agency. Bacteriological Water Quality Criteria for Marine and Fresh Waters. United States Environmental Protection Administration; Washington, D. C: 1986. [Google Scholar]
- United States Environmental Protection Agency. Method 1603: Escherichia coli (E. coli) in water by membrane filtration using modified membrane-thermotolerant Escherichia coli agar. U. S. Environmental Protection Agency; Washington, D. C., Cincinnati, OH: 2002. [Google Scholar]
- Yan T, Hamilton MJ, Sadowsky MJ. High-Throughput and Quantitative Procedure for Determining Sources of Escherichia coli in Waterways by Using Host-Specific DNA Marker Genes. Applied and Environmental Microbiology. 2007;73:890–896. doi: 10.1128/AEM.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Sadowsky MJ. Determining sources of fecal bacteria in waterways. Environmental Monitoring and Assessment. 2007;129:97–106. doi: 10.1007/s10661-006-9426-z. [DOI] [PubMed] [Google Scholar]
- Yatsuyanagi J, Shioko S, Miyajima Y, Amano K, Enomoto K. Characterization of atypical enteropathogenic Escherichia coli strains harboring the astA gene that were associated with a waterborne outbreak of diarrhea in Japan. Journal of Clinical Microbiology. 2002;40:294–297. doi: 10.1128/JCM.41.5.2033-2039.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


