Abstract
Initial studies showed that the anorexigenic peptide oxytocin (OT) regulates gastric motility, responds to stomach distention and to elevated osmolality, and blocks consumption of toxic foods. Most recently, it has been proposed to act as a mediator of general and carbohydrate-specific satiety and regulator of body weight. In the current review, we discuss the function of OT as a homeostatic inhibitor of consumption, capable of mitigating multiple aspects of ingestive behavior and energy metabolism.
Keywords: pituitary, paraventricular nucleus of the hypothalamus, taste aversion, sugar, obesity, anorexia
Feeding behavior depends on the interplay between peripheral and central signals. In the brain, neurons governing this behavior form a complex network (Olszewski et al. , 2008) synthesizing neuropeptides whose general function can be described as orexigens (e.g., ghrelin and neuropeptide Y; NPY) or anorexigens (e.g., corticotrophin releasing hormone; CRH), but whose specific roles in consumption initiation or termination vary significantly. Oxytocin (OT), produced mainly by hypothalamic neurons, belongs to the group of anorexigens. The uniqueness of OT’s hypophagic action arises from its involvement in facilitating peripheral-central and intra-CNS “relay” of feeding-related signals as well as from its role in a wide range of mechanisms, such as those that ensure homeostasis and those that affect reward.
1. General outline on OT and its receptor in the brain
OT and OT-like peptides have been detected in virtually all vertebrates, and the nonapeptide sequence of OT (CYIQNCPLG) is well conserved, which is in line with the involvement of this peptide in the most basic mechanisms (such as osmoregulation, reproduction or feeding) common for organisms regardless of their level of organizational complexity. In fact, OT precursor protein gene is estimated to date back at least 500 million years (Acher et al. , 1995). OT-like precursor polypeptides in invertebrate species also display a high level of homology to the corresponding vertebrate molecule as documented e.g. in the preproannetocin sequence analysis of the earthworm, Eisenia foetida. Interestingly, infusion of OT homologues in invertebrates affects similar processes as those regulated by OT in mammals, e.g., gut motility and reproductive functions (Oumi et al. , 1994, Ukena et al. , 1995).
OT binds to its receptor, a member of the Rhodopsin-type G protein-coupled receptor family (Fredriksson et al. , 2003, Kimura et al. , 1992), and the cyclic rather than linear part of the neuropeptide’s molecule determines binding selectivity (Postina et al. , 1996). It is noteworthy that the OT receptor is relatively unselective since its affinity for OT is just about 10 times higher than for vasopressin (VP) (Postina, Kojro, 1996), and a concentration of VP necessary to induce a response comparable to that elicited by OT is approximately 100 times higher (Chini et al. , 1996, Postina, Kojro, 1996). VP is regarded as a partial agonist at the OT receptor and many studies have revealed that the VP receptor agonists have a dual OT-VP receptor binding profile (Chini, Mouillac, 1996). OT and VP receptors’ affinities for their respective antagonists do not overlap since antagonists target a different and more sequence-specific protein fragment than the one recognized by agonists.
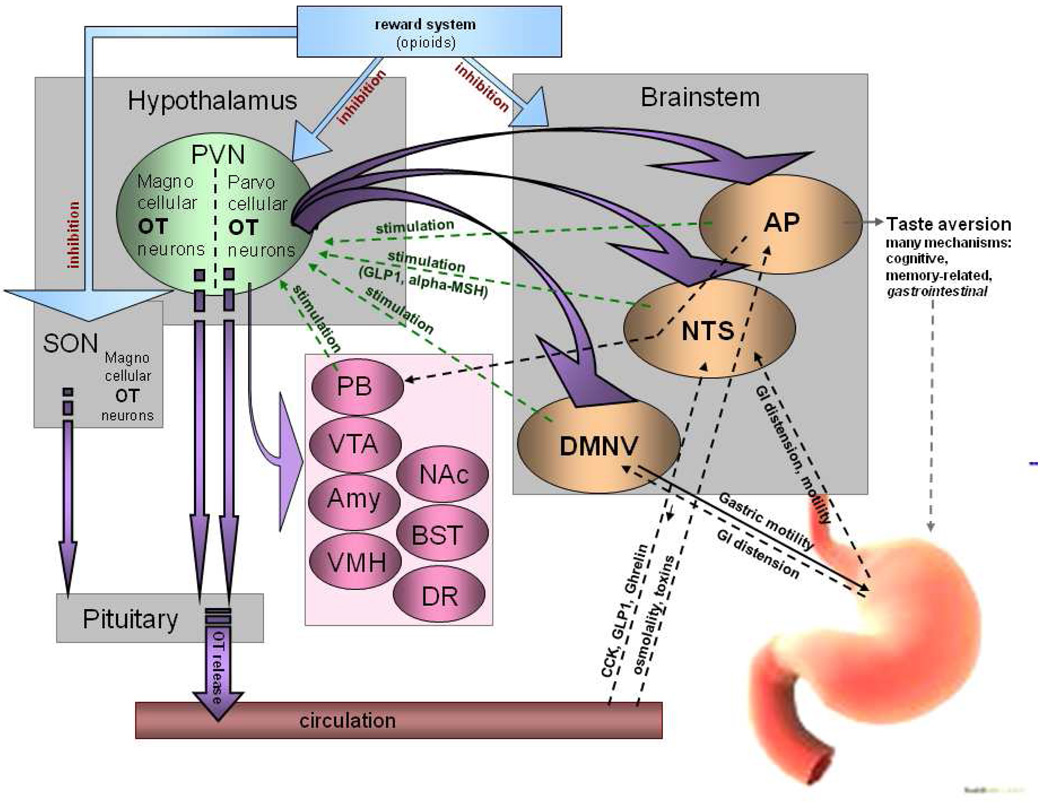
OT is primarily produced in the hypothalamic paraventricular (PVN) and supraoptic (SON) nuclei. Magnocellular OT neurons from the SON and PVN project to the posterior pituitary where OT enters the general circulation. The PVN also contains smaller parvocellular OT neurons. 40% of them terminate in the pituitary, whereas the rest send axons throughout the brain (Gimpl and Fahrenholz, 2001, Pelletier, 1991, Swanson and Sawchenko, 1980). The topography of this central innervation parallels OT’s involvement in food intake. It is estimated that 10% of OT neurons project to three brainstem sites crucial in the exchange of the CNS-periphery feeding-related information, including GI tract motility and chemical plasma profile (Huang et al. , 2000, McCann and Rogers, 1990). Those sites are the nucleus of the solitary tract (NTS), which serves as a ”relay” station for a number of peripheral signals, including those related to gut functioning; the dorsal motor nucleus of the vagus (DMNV) which is part of the efferent and afferent vagal innervation of the stomach; and, finally, the area postrema (AP) which mediates gastric responses triggered by high osmolality or presence of toxins in the blood (Huang, Sved, 2000, Sagar et al. , 1995). Importantly, the OT-brainstem innervation is reciprocal, i.e., brainstem neurons terminate in the proximity of OT perikarya in the hypothalamus and OT terminals are found in the hindbrain, such as in the case of a powerful anorexigen glucagon-like peptide-1 (GLP-1) (Shughrue et al. , 1996). However, OT terminals as well as the OT receptor detected with in situ hybridization or autoradiography are present also outside this basic brainstem-hypothalamus pathway. Recent evidence shows a relationship between OT signaling in the ventromedial hypothalamic nucleus and nutritional state (for a comprehensive review of this and other pathways, refer to (Leng et al. , 2008)). According to histological analyses, sites that integrate OT signaling include components of the reward network that mediates the portion of feeding driven by pleasure (e.g., the ventral tegmental area, nucleus accumbens and bed nucleus of the stria terminalis), areas that alter food intake under stressful conditions (e.g. the amygdala) as well as those that modify affective functions and can consequently change various aspects of eating behavior (the dorsal raphe nucleus) (Ostrowski, 1998, van Leeuwen et al. , 1985, Yoshimura et al. , 1993). In addition, OT fibers terminate at OT perikarya expressing OT receptors, which suggests a positive feedback loop as the OT receptor has excitatory properties (Neumann et al. , 1996). This auto-amplification of OT release plays a role in lactation (Moos et al. , 1989, Neumann et al. , 1993), but it has not been examined in association with other processes. A schematic representation of the OT system in the brain in relation to feeding is presented in Figure 1.
Fig. 1. Topography of central OT pathways involved in food intake regulation with special emphasis on functional significance of the circuits.
OT neurons project from parvocellular PVN neurons in the hypothalamus to brainstem sites known to regulate feeding (NTS, AP, DMNV) and to the pituitary, where OT is released to the general circulation. While the brainstem-hypothalamus pathways have been extensively studied in relation to OT’s involvement in anorexigenic responses stemming from peripheral parameters, such as GI tract distension, osmolality of the blood, etc., many other feeding-related sites that contain OT terminals or OT receptors have not been comprehensively evaluated in relation to anorexigenic action of OT. These include areas involved in reward (ventral tegmental area, VTA; nucleus accumbens, NAc; bed nucleus of the stria terminalis, BST), affect (dorsal raphe nucleus, DR), energy homeostasis (ventromedial hypothalamic nucleus, VMH) and stress (amygdala, Amy; and parabrachial nucleus; PB) (Buijs et al. , 1983, De Vries and Buijs, 1983, Kirchgessner, Sclafani, 1988, Olson et al. , 1993).
2. OT as a feeding regulator: classical concept
Gold and colleagues found that lesions to the PVN resulted in hyperphagia and significant body weight gain in rats (Gold et al. , 1977), which was later confirmed by other groups (e.g. (Shor-Posner et al. , 1985)). Lesions that extended beyond the PVN did not generate a greater effect on ingestive behavior or obese phenotype (Leibowitz et al. , 1983). Knife cuts leading to the disruption of the PVN-hindbrain pathways were shown to be crucial in the development of hyperphagia and obesity (Kirchgessner and Sclafani, 1988, Kirchgessner et al. , 1988). The search was then undertaken for neuropeptides derived from the PVN whose lack precipitated this effect. Injection studies provided preliminary evidence linking OT with feeding termination. Intracerebroventricular (ICV) injections of OT and OT receptor agonists dose-dependently suppressed chow intake in male and female rats stimulated to eat by scheduled feeding or by food deprivation (Arletti et al. , 1990, Benelli et al. , 1991, Olson et al. , 1991a). This effect was reversible by administration of OT receptor antagonists, although OT receptor antagonism by itself did not increase energy intake. (Olson et al. , 1991b) Peripheral administration of only high doses of OT generated hypophagia, which strongly suggested that central OT affects feeding (Arletti, Benelli, 1990, Benelli, Bertolini, 1991). Interestingly, OT homologue injection experiments in several other species have shown that this feeding inhibitory function seems to be as well conserved as the OT molecule itself. For example, ICV infusions of OT in birds caused a dose-dependent decrease in feed intake, feeding time and pecking frequency (Jonaidi et al. , 2003).
The observed hypophagic outcome of central treatment with OT agents produced little explanation as to what mechanisms underlie this effect. Subsequent studies that focused on defining the physiological basis of OT’s effect on feeding generated substantial evidence indicating that OT acts as a “homeostatic” inhibitor of ingestive behavior. As OT is involved in gastric motility, it responds to significant stomach distention and to elevated plasma osmolality that accompanies food intake, and blocks consumption of toxic tastants and promotes avoidance of such tastants upon subsequent presentations, it was concluded that OT prevents the animal from maintaining consumption that could potentially jeopardize the internal milieu (Flanagan et al. , 1992).
2.1. Osmolality
Increases in plasma osmolality signify dehydration, improper fluid homeostasis or ingestion of highly osmotic foods. Changes in the osmotic status activate OT neurons projecting within the CNS and to the pituitary. Hyperosmolality resulting from water deprivation induced expression of an immediate-early gene, c-Fos, in magnocellular OT neurons in the SON and PVN (Fenelon et al. , 1993, Rinaman et al. , 1997, Stricker and Verbalis, 1996). Van Tol reported that prolonged osmotic stimulation through long-term exposure to 2% NaCl instead of drinking water, increased OT mRNA levels of OT-immunoreactive neurons in these sites (Van Tol et al. , 1987). Consequently, intraperitoneal (IP) injections of NaCl solutions as well as hypovolemia due to dehydration produced an elevated plasma OT profile (Ludwig et al. , 1994).
Importantly, dehydration by itself suppresses food intake: mice deprived of both food and water ingested less chow during a 60-minute re-feeding period than animals deprived of only food (Rinaman et al. , 2005). Conversely, administration of hypertonic NaCl inhibited food intake and stimulated concurrent OT release (Chen et al. , 2004, Flynn et al. , 1995, Rinaman, Vollmer, 2005). Puryear and colleagues found that in OT knockout (KO) mice, the hypophagic effect of water restriction was attenuated (Puryear et al. , 2001). The same group of investigators showed that animals deficient in OT displayed an increased consumption of NaCl compared to wild-type controls. Furthermore, genetic deletion of the OT receptor decreased salt appetite (Puryear, Rigatto, 2001, Rigatto et al. , 2003). It is noteworthy that stimulatory effects of intravenous (IV) infusions of NaCl on plasma OT levels were blunted in rats with lesions of the AP, which suggested that AP neurons facilitate OT responses to osmotic challenge. It should be noted, however, that once an IV infusion of 0.5M NaCl was combined with that of 1M mannitol in AP-lesioned animals, plasma OT levels increased normally (Curtis et al. , 1999, Huang, Sved, 2000). Moreover, no effect of AP lesions on the increases of plasma OT levels after plasma volume deficits was observed, indicating that the AP is important only for secretion of OT in response to hypernatremia (Huang, Sved, 2000).
Interestingly, some authors argued that the involvement of OT in the regulation of osmotic balance does not serve as a proof of this peptide’s involvement in the control of consummatory behavior, but rather links OT merely to an improper water balance. Nevertheless, it was later noted that the role of OT as an osmotic regulator in some species (e.g., in sheep) is taken over by VP; in these animals, OT retains its anorexigenic properties (Svennersten-Sjaunja and Olsson, 2005).
2.2. Taste aversion
OT has been proposed to inhibit food intake in order to prevent the organism from ingestion of toxic substances. This notion is supported by conditioned taste aversion (CTA) studies. In natural conditions, aversion develops upon ingestion of food that causes unpleasant gastrointestinal sensation (sickness, malaise and/or nausea) driven by toxicity. The set of behavioral responses follows and it includes termination of consummatory behavior and subsequent avoidance of tastants of a similar flavor recognized as “tainted”. In the laboratory setting, a CTA, as an associative phenomenon, is generated in a paradigm in which presentation of a novel food is paired with an injection of a toxin (Thiele et al. , 1996, Yamamoto et al. , 1992, Yamamoto et al. , 1994). Consumption of this tastant upon subsequent presentations is significantly reduced.
Central mechanisms responsible for the development and maintenance of aversion-based hypophagia are complex. The brainstem sites: the NTS, AP, DMNV and parabrachial nucleus (PBN), take part in the recognition and integration of peripheral aversive signals, including the presence of toxins in the circulation and changes in the gastrointestinal (GI) tract motility parameters. Among these sites, the AP seems to play a particularly crucial role as its lesions prevent animals from acquiring an aversive response. This was shown by Curtis et al. who reported that while sham-operated and non-operated control animals developed a CTA to the novel tastant whose initial presentation was paired with a single LiCl injection, animals with AP lesions failed to show any signs of aversion (Curtis et al. , 1994). In line with those findings, AP as well as the NTS and PBN display an increased Fos immunoreactivity following CTA inducing treatments, such as LiCl or copper sulfate injections (Olszewski et al. , 2000, Sakai and Yamamoto, 1997, Thiele, Roitman, 1996). This signifies the importance of not only the hypothalamic OT system, but also the relay sites that allow the OT neurons to act at central target sites and, in the case of aversion which involves also the pituitary OT release, at peripheral tissues. The CTA-dependent activity of OT neurons most likely diminishes a drive to consume a tastant associated with sickness and favors an abrupt inhibition of consumption that has already been undertaken. One should note however that there is a significant magnocellular (thus, pituitary/peripheral) component of the OT system’s response to aversive stimulation. Since peripheral OT generally does not inhibit consummatory behavior, the role of OT in the periphery in aversion is likely unrelated to consumption, but it may rather be associated with facilitation of mechanism preparing peripheral tissues and organs for consequences of toxicity. In fact, involvement of circulating OT in cardiovascular or natriuretic responses supports this hypothesis (Forsling et al. , 1994, Houshmand et al. , 2009, Loichot et al. , 2002, Ondrejcakova et al. , 2009, Petersson and Uvnas-Moberg, 2008).
OT signaling is observed regardless of the nature of agents that induces sickness; those include chemicals, such as LiCl or copper sulfate, as well as natural or synthetic ligands of central receptors, e.g., high doeses of CCK and naloxone (Flanagan et al. , 1988, Olszewski, Shi, 2000, Olszewski et al. , 2001, Rinaman et al. , 1995). It indicates that OT is the final component of many pathways involved in the mediation of CTAs rather than being limited to treatment-selective mechanisms that activate a specific group of receptors and engage specific mechanisms. Although OT is thought to be the final component of neural circuitry supporting CTA responsiveness, it is not a sole or necessary one: OT administration has not been shown to elicit aversive consequences, whereas LiCl-treated animals, incapable of developing CTAs due to the AP lesion, still display a surge in OT release (Curtis, Sved, 1994). It seems particularly interesting however that, while intraperitoneal LiCl given in rats with AP lesions does not induce a CTA, it produces anorexia accompanied by increased OT levels (Curtis, Sved, 1994). Therefore, OT may play an auxiliary role in the CTA process, being one of the factors ensuring inhibition of consummatory behavior.
One should note that agents that mediate satiation (such as the aforementioned CCK and naloxone), within a certain range of doses, terminate feeding without aversive consequences. However, once injected at higher doses, they precipitate a CTA. This brings about an interesting hypothesis that perhaps extremely robust, uncontrollable eating that could potentially disturb homeostasis has such a profound effect on feeding-related peptide plasma and central profiles that it is eventually curbed by activated aversive feeding termination mechanisms that encompass OT neurons. This could explain temporary avoidance of foods that had been greatly overconsumed during a single meal. Aversion-like eating restrictive behaviors have been observed in humans who interspace binge eating episodes with periods of avoidance of diets that were part of a binging event (Neumark-Sztainer et al. , 2004, Sierra Baigrie and Lemos Giraldez, 2008).
2.4. Changes in feeding during pregnancy and lactation
OT controls parturition and maternal behavior. It is released after dilation of the cervix during labor; in lactating animals, it stimulates milk ejection (Crowley et al. , 1992, Soloff et al. , 1980). Food intake, body weight and adiposity increase during pregnancy and persist throughout lactation to provide for the metabolic demands of the fetus and sustain milk production. At this time mechanisms responsible for the regulation of energy balance require intricate adjustments. It has been suggested that these adjustments are based, at least partially, on a modified responsiveness of the OT system to feeding (Douglas et al. , 2007). For example, meal-induced activity of OT neurons is decreased and central release of OT associated with feeding termination diminishes, which leads to general hyperphagia and results in elevated salt appetite in rats (Brunton et al. , 2008). Concurrent reduced OT responses to high plasma osmolality have been observed (Brunton, Arunachalam, 2008, Koehler et al. , 1994).
Activity of the OT system is elicited by administration of anorexigenic peptides and it reflects a general increase in activation of circuitry that mediates satiation. However, an elevated response of OT cells has been shown also upon injection of powerful orexigens, such as NPY and ghrelin, in both males and females (Brunton et al. , 2006, Olszewski et al. , 2007). It has been speculated that, under basic physiological conditions, activation of the OT system by orexigenic factors, serves as the feedback mechanism that does not allow dangerously excessive food intake to occur. OT neurons in pregnant rats are less sensitive to both orexigenic and anorexigenic peptides. In female virgin rats, OT is released after stimulation with the appetite suppressant, CCK, and stimulant, NPY, and it lessens general hunger and salt craving. In late pregnant rats, no changes in central OT release after CCK or NPY stimulation have been reported and NPY showed no stimulatory effect on OT neurons (Brunton, Bales, 2006). Therefore, not only the apparent diminished output of the satiety related CCK-OT pathway contributes to overfeeding in pregnancy; the lack of NPY’s stimulatory effect on OT neurons also generates hyperphagia as the response of the feedback mechanism is blunted.
3. OT and feeding: novel concepts
Although a general consensus on the role of OT in feeding control as a homeostatic regulator was reached, as the new data were generated - including those derived from molecular studies - it soon became apparent that the function of this peptide in shaping feeding responses that allow homeostasis to be maintained is very complex.
A gene dubbed Single-minded 1 or SIM1 encodes a transcription factor essential, among others, in the development of the PVN. Therefore, generation of mice heterozygous for SIM1 (animals with a complete gene deletion of the gene are not viable) was expected to produce an obese and hyperphagic phenotype similar to that generated by lesioning the PVN (Leibowitz et al. , 1981). Indeed, heterozygous mice developed early-onset obesity and increased linear growth with energy expenditure levels unchanged compared to wild-type controls (Michaud et al. , 2001). As the entire PVN was found to be hypocellular (Kublaoui et al. , 2006a, Michaud, Boucher, 2001), it was therefore anticipated that all neuronal populations within this site were uniformly affected by SIM1 haplosufficiency. In a very well designed set of studies, Kublaoui et al. determined, however, that the impaired development and low activity of the OT system was likely the main culprit behind the observed obese phenotype in this strain. OT mRNA in the PVN was downregulated by 80%, far more than expression of any other satiety-related gene studied, including TRH, CRH, VP and somastotatin (decrease by only 20–40%). Central supplementation of OT reversed hyperphagia and abnormal weight gain precipitated by the heterozygous status (Kublaoui et al. , 2008). These data are of particular interest as they show that OT can single-handedly alleviate consumption-related and obesogenic phenotypic features caused by developmental “silencing” of the PVN. Most likely, feeding and weight gain affected by OT replacement were at least partially unrelated to homeostatic rescue. In support of this assumption, overexpression of human Sim1 in transgenic mice results in resistance to diet-induced obesity (Kublaoui et al. , 2006b); no information on the level of OT mRNA in these animals is available yet.
Studies on the interactions between OT and other feeding-related peptides have provided evidence that the role of the OT system in feeding control appears to be related to the induction of satiety responses. Activation of the melanocortin-4 receptor, whose involvement in the etiology of obesity and satiation has been shown both in humans as well as has been confirmed in animal models, by direct alpha-melanocyte stimulating hormone (alpha-MSH) injections in the PVN reduced feeding stimulated by a variety of factors and causes a concurrent increase in the percentage of c-Fos IR OT neurons (Olszewski, Wirth, 2001). Importantly, this PVN treatment with alpha-MSH did not cause any aversive consequences. In contrast, GLP1 induced satiety and, at higher doses, also a CTA: increase in activity of OT cells was observed in both cases (Larsen et al. , 1997). In addition, other satiety mediators, such as cocaine-amphetamine-regulated transcript (Vrang et al. , 2000) and CCK (Hashimoto et al. , 2005) caused upon injection an increase in OT neuronal activity in the PVN and SON and/or the release of the peptide. It should be emphasized that antagonism of the OT receptor in the brain led to elimination of hypophagia induced by one of the most potent satiety factors known thus far, CRH (Olson et al. , 1991c) and attenuated the hypophagic effect of the adipocyte hormone leptin on consumption (Blevins et al. , 2004). Initial injection studies showed that that the OT receptor antagonist prevented feeding termination by CCK (Olson, Drutarosky, 1991b). Subsequent experiments utilizing peripheral administration of CCK along with OT antagonist infusions to the fourth ventricle showed a diminished anorexigenic response to CCK by 22–23% upon OT receptor blockade (Blevins et al. , 2003). Recent evidence obtained in OT KO mice suggests that activity of the OT-CCK pathway is auxiliary but not mandatory in inducing meal termination by CCK (Mantella et al. , 2003). Finally, an increased activity level of OT neurons coincides with the end of a meal (Olszewski and Levine, 2007), which suggests that these cells are part of the mechanism that supports satiation.
Deletion of the gene encoding OT or OT receptor in mice shed more light on feeding-related mechanisms regulated by the OT system. Nishimori et al. raised mice deficient in the OT receptor gene and found that despite similar food intake as seen in wild-type controls, the KO animals started developing obesity in week 12 after birth (Nishimori et al. , 2008). They had a much higher mass of the white and brown fat. The impairment of the adrenergic receptor system in these animals may also contribute to the elevated adipose tissue weight. Takayanagi and colleagues provided further characterization of the OT receptor −/− obese phenotype (Takayanagi et al. , 2008). They reported an increase in the mass of abdominal fat pads and elevated triglycerides in the blood in the KO strain. This high adiposity was not associated with an altered feeding or locomotor activity profiles, though thermogenesis was impaired in these mice.
Data pertaining to obesity in OT-deficient mice are somewhat conflicting with some authors reporting no body weight differences between KO and wild type animals, whereas others have shown an increase in adiposity whose onset occurs as late as at 4–6 months of age. Camerino found that OT KO mice develop hyperleptinemia, a decreased insulin sensitivity and glucose intolerance as well as lower adrenaline levels (Camerino, 2009), which led to the hypothesis that the metabolic changes accompanying OT deficiency stem from a decreased sympathetic nervous tone. They exhibit altered macronutrient preference profiles and eating patterns. For example, OT KO mice display enhanced preference for carbohydrates, particularly sweet ones (Sclafani et al. , 2007); another study showed that OT may be very effective in reducing intake of sucrose (Amico et al. , 2005). Yet another experiment showed that OT was not involved in the regulation of fat intake, but its main role was limited to inhibiting consumption of carbohydrates (Miedlar et al. , 2007). In line with the KO data, recent real-time PCR studies in wild-type rats showed the effect of scheduled volume-unrestricted consumption of high-sugar versus regular diet on OT gene expression levels in the hypothalamus (Olszewski et al. , 2009).
That feeding inhibitory action of OT appears to be macronutrient-specific does not necessarily mean that it diminishes a rewarding value of food containing this macronutrient. In fact, Lokrantz et al. showed that intracranial injections of OT reduce the intake of the 12.5% glucose solution only in rats deprived of food for 20 hours. No effect was observed in sated animals allowed access to glucose (Lokrantz et al. , 1997). These data support the notion of OT's involvement in satiation (which can be also geared toward a specific macronutrient), but not in reduction of feeding reward. The satiation hypothesis is corroborated by our recent findings showing that rats ingesting daily equal amounts of either high-sugar or high-cornstarch diet display a different level of activity of the OT system at the end of a meal: chronic intake of sucrose without overeating it is associated with a lower percentage of Fos-positive OT neurons in the PVN (Mitra et al. , 2010).
4. OT system in human pathological conditions related to body weight control
Prader-Willi syndrome (PWS), a condition resulting from a loss of paternal genes in region q11–13 on chromosome 15, is characterized by hypotonia, developmental disability, hypogonadism and, importantly, gross obesity and insatiable hunger. Hypophagia during infancy, likely caused by hypotonia, gradually recedes and, around the age of 2 years, individuals start displaying extreme hyperphagia leading to morbid obesity (Fong and De Vries, 2003). Excessive appetite in PWS patients is thought to develop mainly due to impaired perception of satiety. Malfunctioning gut-brain feedback mechanisms related to satiety signaling are speculated to be the underlying cause. PWS has been associated with many abnormalities related to the synthesis and release of peptides at the central and peripheral level; the OT system seems to be affected as well. Martin et al. reported that PWS is associated with altered baseline OT profile in the cerebrospinal fluid in males and females (Martin et al. , 1998). Swaab et al. showed that PWS patients have a reduced number of OT neurons (42% decrease) and smaller OT cell volumes (Swaab et al. , 1995). An undersized OT population seems a developmental rather than transient functional issue, because hypothalamic abnormalities, such as improper cardiovascular parameters, are observed already in the fetus (L'Hermine et al. , 2003). This is supported by SIM1 knock out model data described above in which mice heterozygous for the SIM1 gene had an obese phenotype and showed a lowered number of cells synthesizing OT and insufficiently developed PVN (Kublaoui, Holder, 2006a, Michaud, Boucher, 2001).
Similar symptoms as in PWS were described in the case study of a 9-year-old male with a duplication of chromosome 3p25.3p26.2. This region of chromosome 3 includes genes associated with obesity: ghrelin and peroxisome proliferator-activated receptor gamma (PPARG). Importantly, it also contains the gene for the OT receptor. Quantitative RT-PCR analysis of genomic DNA of this subject revealed that the OT receptor gene expression was two-threefold increased compared to healthy controls (Bittel et al. , 2006).
Patients with anorexia nervosa (AN), among many other psychological and physiological symptoms, display abnormally low threshold for reaching satiety, preoccupation with the adverse consequences of food intake and avoidance of subjectively “dangerous” (high in calories) foods. Therefore, OT was studied as one of the candidate systems whose malfunctioning could underlie eating-related aspects of AN. Initial findings indicate that OT may indeed be involved in AN etiology. Chiodera et. al. found that, in underweight AN patients, estrogen- or insulin-induced hypoglycemia results in an impaired response in plasma OT (Chiodera et al. , 1991). In recovered AN patients, plasma OT responses to stimuli provoking hypoglycemia normalized after weight restoration (Frank et al. , 2000) but OT plasma concentration was reduced in underweight anorectics. It is still unclear however whether the OT system’s malfunctioning could be interpreted as the cause or consequence of an acute phase of AN.
5. Conclusions and perspectives
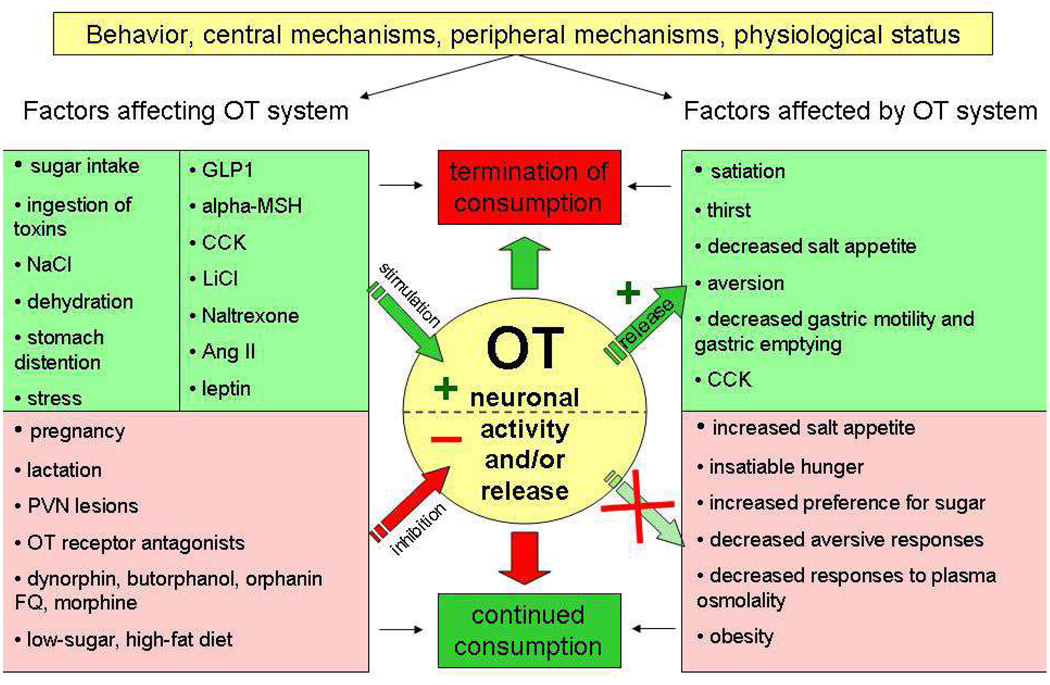
OT responds to alterations in plasma osmolality, stomach distension and presence of toxins in the circulation. OT acts as a mediator of general and macronutrient-specific satiety and, thus, an important factor in the prevention of hyperphagia and obesity (see Fig. 2 for the summary of functional data). Under some circumstances, activity of OT neurons is geared toward protecting the organism from overconsumption of sugar, other carbohydrates and sweet non-carbohydrate tastants. This calls for conducting pharmacological studies to elucidate how to decrease intake of sweet carbohydrates with OT receptor ligands. It is important in light of obesity “epidemic” accelerated by availability and overconsumption of sugars, but also because appetite for dessert (sweet) foods which are often devoid of many essential nutrients, contributes to the paradox of nutritional deficiencies in obesity (Kaidar-Person et al. , 2008a, b).
Fig. 2. A schematic representation of functional relationship between OT neuronal activity/release and feeding-related behaviors, processes and physiological conditions.
Acknowledgements
The studies were supported by the Swedish Research Council (VR), Swedish Brain Research Foundation and the Novo Nordisk Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acher R, Chauvet J, Chauvet MT. Man and the chimaera. Selective versus neutral oxytocin evolution. Adv Exp Med Biol. 1995;395:615–627. [PubMed] [Google Scholar]
- Amico JA, Vollmer RR, Cai HM, Miedlar JA, Rinaman L. Enhanced initial and sustained intake of sucrose solution in mice with an oxytocin gene deletion. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1798–R1806. doi: 10.1152/ajpregu.00558.2005. [DOI] [PubMed] [Google Scholar]
- Arletti R, Benelli A, Bertolini A. Oxytocin inhibits food and fluid intake in rats. Physiol Behav. 1990;48:825–830. doi: 10.1016/0031-9384(90)90234-u. [DOI] [PubMed] [Google Scholar]
- Benelli A, Bertolini A, Arletti R. Oxytocin-induced inhibition of feeding and drinking: no sexual dimorphism in rats. Neuropeptides. 1991;20:57–62. doi: 10.1016/0143-4179(91)90040-p. [DOI] [PubMed] [Google Scholar]
- Bittel DC, Kibiryeva N, Dasouki M, Knoll JH, Butler MG. A 9-year-old male with a duplication of chromosome 3p25.3p26.2: clinical report and gene expression analysis. Am J Med Genet A. 2006;140:573–579. doi: 10.1002/ajmg.a.31132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins JE, Eakin TJ, Murphy JA, Schwartz MW, Baskin DG. Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Res. 2003;993:30–41. doi: 10.1016/j.brainres.2003.08.036. [DOI] [PubMed] [Google Scholar]
- Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol. 2004;287:R87–R96. doi: 10.1152/ajpregu.00604.2003. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Arunachalam S, Russel JA. Control of neurohypophysial hormone secretion, blood osmolality and volume in pregnancy. J Physiol Pharmacol. 2008;59 Suppl 8:27–45. [PubMed] [Google Scholar]
- Brunton PJ, Bales J, Russell JA. Neuroendocrine stress but not feeding responses to centrally administered neuropeptide Y are suppressed in pregnant rats. Endocrinology. 2006;147:3737–3745. doi: 10.1210/en.2006-0048. [DOI] [PubMed] [Google Scholar]
- Buijs RM, De Vries GJ, Van Leeuwen FW, Swaab DF. Vasopressin and oxytocin: distribution and putative functions in the brain. Prog Brain Res. 1983;60:115–122. doi: 10.1016/S0079-6123(08)64379-4. [DOI] [PubMed] [Google Scholar]
- Camerino C. Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity (Silver Spring) 2009;17:980–984. doi: 10.1038/oby.2009.12. [DOI] [PubMed] [Google Scholar]
- Chen H, Morris M, Key MP, Chen Y. Rapid neurosecretory and cardiovascular response to osmotic stimulation in conscious mice. Neuroendocrinology. 2004;80:225–232. doi: 10.1159/000082751. [DOI] [PubMed] [Google Scholar]
- Chini B, Mouillac B, Balestre MN, Trumpp-Kallmeyer S, Hoflack J, Hibert M, et al. Two aromatic residues regulate the response of the human oxytocin receptor to the partial agonist arginine vasopressin. FEBS Lett. 1996;397:201–206. doi: 10.1016/s0014-5793(96)01135-0. [DOI] [PubMed] [Google Scholar]
- Chiodera P, Volpi R, Capretti L, Marchesi C, d'Amato L, De Ferri A, et al. Effect of estrogen or insulin-induced hypoglycemia on plasma oxytocin levels in bulimia and anorexia nervosa. Metabolism. 1991;40:1226–1230. doi: 10.1016/0026-0495(91)90220-q. [DOI] [PubMed] [Google Scholar]
- Crowley WR, Parker SL, Armstrong WE, Spinolo LH, Grosvenor CE. Neurotransmitter and neurohormonal regulation of oxytocin secretion in lactation. Ann N Y Acad Sci. 1992;652:286–302. doi: 10.1111/j.1749-6632.1992.tb34362.x. [DOI] [PubMed] [Google Scholar]
- Curtis KS, Huang W, Sved AF, Verbalis JG, Stricker EM. Impaired osmoregulatory responses in rats with area postrema lesions. Am J Physiol. 1999;277:R209–R219. doi: 10.1152/ajpregu.1999.277.1.R209. [DOI] [PubMed] [Google Scholar]
- Curtis KS, Sved AF, Verbalis JG, Stricker EM. Lithium chloride-induced anorexia, but not conditioned taste aversions, in rats with area postrema lesions. Brain Res. 1994;663:30–37. doi: 10.1016/0006-8993(94)90459-6. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- Douglas AJ, Johnstone LE, Leng G. Neuroendocrine mechanisms of change in food intake during pregnancy: a potential role for brain oxytocin. Physiol Behav. 2007;91:352–365. doi: 10.1016/j.physbeh.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Fenelon VS, Poulain DA, Theodosis DT. Oxytocin neuron activation and Fos expression: a quantitative immunocytochemical analysis of the effect of lactation, parturition, osmotic and cardiovascular stimulation. Neuroscience. 1993;53:77–89. doi: 10.1016/0306-4522(93)90286-o. [DOI] [PubMed] [Google Scholar]
- Flanagan LM, Olson BR, Sved AF, Verbalis JG, Stricker EM. Gastric motility in conscious rats given oxytocin and an oxytocin antagonist centrally. Brain Res. 1992;578:256–260. doi: 10.1016/0006-8993(92)90255-8. [DOI] [PubMed] [Google Scholar]
- Flanagan LM, Verbalis JG, Stricker EM. Naloxone potentiation of effects of cholecystokinin and lithium chloride on oxytocin secretion, gastric motility and feeding. Neuroendocrinology. 1988;48:668–673. doi: 10.1159/000125080. [DOI] [PubMed] [Google Scholar]
- Flynn FW, Curtis KS, Verbalis JG, Stricker EM. Dehydration anorexia in decerebrate rats. Behav Neurosci. 1995;109:1009–1012. [PubMed] [Google Scholar]
- Fong BF, De Vries JI. Obstetric aspects of the Prader-Willi syndrome. Ultrasound Obstet Gynecol. 2003;21:389–392. doi: 10.1002/uog.90. [DOI] [PubMed] [Google Scholar]
- Forsling ML, Judah JM, Windle RJ. The effect of vasopressin and oxytocin on glomerular filtration rate in the conscious rat: contribution to the natriuretic response. J Endocrinol. 1994;141:59–67. doi: 10.1677/joe.0.1410059. [DOI] [PubMed] [Google Scholar]
- Frank GK, Kaye WH, Altemus M, Greeno CG. CSF oxytocin and vasopressin levels after recovery from bulimia nervosa and anorexia nervosa, bulimic subtype. Biol Psychiatry. 2000;48:315–318. doi: 10.1016/s0006-3223(00)00243-2. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Molecular pharmacology. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Gold RM, Ieni JR, Simson EL. Delayed or precocious hyperphagia after symmetrical or asymmetrical hypothalamic knife cuts in male and female weanling rats. Physiol Behav. 1977;18:275–281. doi: 10.1016/0031-9384(77)90133-0. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Onaka T, Kawasaki M, Chen L, Mera T, Soya A, et al. Effects of cholecystokinin (CCK)-8 on hypothalamic oxytocin-secreting neurons in rats lacking CCK-A receptor. Auton Neurosci. 2005;121:16–25. doi: 10.1016/j.autneu.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Houshmand F, Faghihi M, Zahediasl S. Biphasic protective effect of oxytocin on cardiac ischemia/reperfusion injury in anaesthetized rats. Peptides. 2009;30:2301–2308. doi: 10.1016/j.peptides.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Huang W, Sved AF, Stricker EM. Vasopressin and oxytocin release evoked by NaCl loads are selectively blunted by area postrema lesions. Am J Physiol Regul Integr Comp Physiol. 2000;278:R732–R740. doi: 10.1152/ajpregu.2000.278.3.R732. [DOI] [PubMed] [Google Scholar]
- Jonaidi H, Oloumi MM, Denbow DM. Behavioral effects of intracerebroventricular injection of oxytocin in birds. Physiol Behav. 2003;79:725–729. doi: 10.1016/s0031-9384(03)00145-8. [DOI] [PubMed] [Google Scholar]
- Kaidar-Person O, Person B, Szomstein S, Rosenthal RJ. Nutritional deficiencies in morbidly obese patients: a new form of malnutrition? Part A: vitamins. Obes Surg. 2008a;18:870–876. doi: 10.1007/s11695-007-9349-y. [DOI] [PubMed] [Google Scholar]
- Kaidar-Person O, Person B, Szomstein S, Rosenthal RJ. Nutritional deficiencies in morbidly obese patients: a new form of malnutrition? Part B: minerals. Obes Surg. 2008b;18:1028–1034. doi: 10.1007/s11695-007-9350-5. [DOI] [PubMed] [Google Scholar]
- Kimura T, Tanizawa O, Mori K, Brownstein MJ, Okayama H. Structure and expression of a human oxytocin receptor. Nature. 1992;356:526–529. doi: 10.1038/356526a0. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Sclafani A. PVN-hindbrain pathway involved in the hypothalamic hyperphagia-obesity syndrome. Physiol Behav. 1988;42:517–528. doi: 10.1016/0031-9384(88)90153-9. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Sclafani A, Nilaver G. Histochemical identification of a PVN-hindbrain feeding pathway. Physiol Behav. 1988;42:529–543. doi: 10.1016/0031-9384(88)90154-0. [DOI] [PubMed] [Google Scholar]
- Koehler EM, McLemore GL, Martel JK, Summy-Long JY. Response of the magnocellular system in rats to hypovolemia and cholecystokinin during pregnancy and lactation. Am J Physiol. 1994;266:R1327–R1337. doi: 10.1152/ajpregu.1994.266.4.R1327. [DOI] [PubMed] [Google Scholar]
- Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol Endocrinol. 2008;22:1723–1734. doi: 10.1210/me.2008-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kublaoui BM, Holder JL, Jr, Gemelli T, Zinn AR. Sim1 haploinsufficiency impairs melanocortin-mediated anorexia and activation of paraventricular nucleus neurons. Mol Endocrinol. 2006a;20:2483–2492. doi: 10.1210/me.2005-0483. [DOI] [PubMed] [Google Scholar]
- Kublaoui BM, Holder JL, Jr, Tolson KP, Gemelli T, Zinn AR. SIM1 overexpression partially rescues agouti yellow and diet-induced obesity by normalizing food intake. Endocrinology. 2006b;147:4542–4549. doi: 10.1210/en.2006-0453. [DOI] [PubMed] [Google Scholar]
- L'Hermine AC, Aboura A, Brisset S, Cuisset L, Castaigne V, Labrune P, et al. Fetal phenotype of Prader-Willi syndrome due to maternal disomy for chromosome 15. Prenat Diagn. 2003;23:938–943. doi: 10.1002/pd.732. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997;138:4445–4455. doi: 10.1210/endo.138.10.5270. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Hammer NJ, Chang K. Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol Behav. 1981;27:1031–1040. doi: 10.1016/0031-9384(81)90366-8. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Hammer NJ, Chang K. Feeding behavior induced by central norepinephrine injection is attenuated by discrete lesions in the hypothalamic paraventricular nucleus. Pharmacol Biochem Behav. 1983;19:945–950. doi: 10.1016/0091-3057(83)90396-9. [DOI] [PubMed] [Google Scholar]
- Leng G, Onaka T, Caquineau C, Sabatier N, Tobin VA, Takayanagi Y. Oxytocin and appetite. Prog Brain Res. 2008;170:137–151. doi: 10.1016/S0079-6123(08)00413-5. [DOI] [PubMed] [Google Scholar]
- Loichot C, Grima M, De Jong W, Helwig JJ, Imbs JL, Barthelmebs M. Oxytocin-induced renin secretion by denervated kidney in anaesthetized rat. Eur J Pharmacol. 2002;454:241–247. doi: 10.1016/s0014-2999(02)02495-0. [DOI] [PubMed] [Google Scholar]
- Lokrantz CM, Uvnas-Moberg K, Kaplan JM. Effects of central oxytocin administration on intraoral intake of glucose in deprived and nondeprived rats. Physiol Behav. 1997;62:347–352. doi: 10.1016/s0031-9384(97)00021-8. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Callahan MF, Neumann I, Landgraf R, Morris M. Systemic osmotic stimulation increases vasopressin and oxytocin release within the supraoptic nucleus. J Neuroendocrinol. 1994;6:369–373. doi: 10.1111/j.1365-2826.1994.tb00595.x. [DOI] [PubMed] [Google Scholar]
- Mantella RC, Rinaman L, Vollmer RR, Amico JA. Cholecystokinin and D-fenfluramine inhibit food intake in oxytocin-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1037–R1045. doi: 10.1152/ajpregu.00383.2002. [DOI] [PubMed] [Google Scholar]
- Martin A, State M, Anderson GM, Kaye WM, Hanchett JM, McConaha CW, et al. Cerebrospinal fluid levels of oxytocin in Prader-Willi syndrome: a preliminary report. Biol Psychiatry. 1998;44:1349–1352. doi: 10.1016/s0006-3223(98)00190-5. [DOI] [PubMed] [Google Scholar]
- McCann MJ, Rogers RC. Oxytocin excites gastric-related neurones in rat dorsal vagal complex. J Physiol. 1990;428:95–108. doi: 10.1113/jphysiol.1990.sp018202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud JL, Boucher F, Melnyk A, Gauthier F, Goshu E, Levy E, et al. Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Hum Mol Genet. 2001;10:1465–1473. doi: 10.1093/hmg/10.14.1465. [DOI] [PubMed] [Google Scholar]
- Miedlar JA, Rinaman L, Vollmer RR, Amico JA. Oxytocin gene deletion mice overconsume palatable sucrose solution but not palatable lipid emulsions. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1063–R1068. doi: 10.1152/ajpregu.00228.2007. [DOI] [PubMed] [Google Scholar]
- Mitra A, Gosnell BA, Schioth HB, Grace MK, Klockars A, Olszewski PK, et al. Chronic sugar intake dampens feeding-related activity of neurons synthesizing a satiety mediator, oxytocin. Peptides. 2010 doi: 10.1016/j.peptides.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos F, Poulain DA, Rodriguez F, Guerne Y, Vincent JD, Richard P. Release of oxytocin within the supraoptic nucleus during the milk ejection reflex in rats. Exp Brain Res. 1989;76:593–602. doi: 10.1007/BF00248916. [DOI] [PubMed] [Google Scholar]
- Neumann I, Douglas AJ, Pittman QJ, Russell JA, Landgraf R. Oxytocin released within the supraoptic nucleus of the rat brain by positive feedback action is involved in parturition-related events. J Neuroendocrinol. 1996;8:227–233. doi: 10.1046/j.1365-2826.1996.04557.x. [DOI] [PubMed] [Google Scholar]
- Neumann I, Russell JA, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: a microdialysis study. Neuroscience. 1993;53:65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- Neumark-Sztainer D, Wall M, Story M, Fulkerson JA. Are family meal patterns associated with disordered eating behaviors among adolescents? J Adolesc Health. 2004;35:350–359. doi: 10.1016/j.jadohealth.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Nishimori K, Takayanagi Y, Yoshida M, Kasahara Y, Young LJ, Kawamata M. New aspects of oxytocin receptor function revealed by knockout mice: sociosexual behaviour and control of energy balance. Prog Brain Res. 2008;170:79–90. doi: 10.1016/S0079-6123(08)00408-1. [DOI] [PubMed] [Google Scholar]
- Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG. Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides. 1991a;12:113–118. doi: 10.1016/0196-9781(91)90176-p. [DOI] [PubMed] [Google Scholar]
- Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptor antagonism blunts the effects of anorexigenic treatments in rats: evidence for central oxytocin inhibition of food intake. Endocrinology. 1991b;129:785–791. doi: 10.1210/endo-129-2-785. [DOI] [PubMed] [Google Scholar]
- Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptors mediate corticotropin-releasing hormone-induced anorexia. Am J Physiol. 1991c;260:R448–R452. doi: 10.1152/ajpregu.1991.260.2.R448. [DOI] [PubMed] [Google Scholar]
- Olson BR, Freilino M, Hoffman GE, Stricker EM, Sved AF, Verbalis JG. c-Fos Expression in Rat Brain and Brainstem Nuclei in Response to Treatments That Alter Food Intake and Gastric Motility. Mol Cell Neurosci. 1993;4:93–106. doi: 10.1006/mcne.1993.1011. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Bomberg EM, Martell A, Grace MK, Levine AS. Intraventricular ghrelin activates oxytocin neurons: implications in feeding behavior. Neuroreport. 2007;18:499–503. doi: 10.1097/WNR.0b013e328058684e. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Cedernaes J, Olsson F, Levine AS, Schioth HB. Analysis of the network of feeding neuroregulators using the Allen Brain Atlas. Neurosci Biobehav Rev. 2008;32:945–956. doi: 10.1016/j.neubiorev.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski PK, Levine AS. Central opioids and consumption of sweet tastants: when reward outweighs homeostasis. Physiol Behav. 2007;91:506–512. doi: 10.1016/j.physbeh.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Shaw TJ, Grace MK, Hoglund CE, Fredriksson R, Schioth HB, et al. Complexity of neural mechanisms underlying overconsumption of sugar in scheduled feeding: involvement of opioids, orexin, oxytocin and NPY. Peptides. 2009;30:226–233. doi: 10.1016/j.peptides.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski PK, Shi Q, Billington CJ, Levine AS. Opioids affect acquisition of LiCl-induced conditioned taste aversion: involvement of OT and VP systems. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1504–R1511. doi: 10.1152/ajpregu.2000.279.4.R1504. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Wirth MM, Shaw TJ, Grace MK, Billington CJ, Giraudo SQ, et al. Role of alpha-MSH in the regulation of consummatory behavior: immunohistochemical evidence. Am J Physiol Regul Integr Comp Physiol. 2001;281:R673–R680. doi: 10.1152/ajpregu.2001.281.2.R673. [DOI] [PubMed] [Google Scholar]
- Ondrejcakova M, Ravingerova T, Bakos J, Pancza D, Jezova D. Oxytocin exerts protective effects on in vitro myocardial injury induced by ischemia and reperfusion. Can J Physiol Pharmacol. 2009;87:137–142. doi: 10.1139/Y08-108. [DOI] [PubMed] [Google Scholar]
- Ostrowski NL. Oxytocin receptor mRNA expression in rat brain: implications for behavioral integration and reproductive success. Psychoneuroendocrinology. 1998;23:989–1004. doi: 10.1016/s0306-4530(98)00070-5. [DOI] [PubMed] [Google Scholar]
- Oumi T, Ukena K, Matsushima O, Ikeda T, Fujita T, Minakata H, et al. Annetocin: an oxytocin-related peptide isolated from the earthworm, Eisenia foetida. Biochem Biophys Res Commun. 1994;198:393–399. doi: 10.1006/bbrc.1994.1055. [DOI] [PubMed] [Google Scholar]
- Pelletier G. Anatomy of the hypothalamic-pituitary axis. Methods Achiev Exp Pathol. 1991;14:1–22. [PubMed] [Google Scholar]
- Petersson M, Uvnas-Moberg K. Postnatal oxytocin treatment of spontaneously hypertensive male rats decreases blood pressure and body weight in adulthood. Neurosci Lett. 2008;440:166–169. doi: 10.1016/j.neulet.2008.05.091. [DOI] [PubMed] [Google Scholar]
- Postina R, Kojro E, Fahrenholz F. Separate agonist and peptide antagonist binding sites of the oxytocin receptor defined by their transfer into the V2 vasopressin receptor. J Biol Chem. 1996;271:31593–31601. doi: 10.1074/jbc.271.49.31593. [DOI] [PubMed] [Google Scholar]
- Puryear R, Rigatto KV, Amico JA, Morris M. Enhanced salt intake in oxytocin deficient mice. Exp Neurol. 2001;171:323–328. doi: 10.1006/exnr.2001.7776. [DOI] [PubMed] [Google Scholar]
- Rigatto K, Puryear R, Bernatova I, Morris M. Salt appetite and the renin-angiotensin system: effect of oxytocin deficiency. Hypertension. 2003;42:793–797. doi: 10.1161/01.HYP.0000090321.81218.7B. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Hoffman GE, Dohanics J, Le WW, Stricker EM, Verbalis JG. Cholecystokinin activates catecholaminergic neurons in the caudal medulla that innervate the paraventricular nucleus of the hypothalamus in rats. J Comp Neurol. 1995;360:246–256. doi: 10.1002/cne.903600204. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Stricker EM, Hoffman GE, Verbalis JG. Central c-Fos expression in neonatal and adult rats after subcutaneous injection of hypertonic saline. Neuroscience. 1997;79:1165–1175. doi: 10.1016/s0306-4522(97)00022-5. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Vollmer RR, Karam J, Phillips D, Li X, Amico JA. Dehydration anorexia is attenuated in oxytocin-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1791–R1799. doi: 10.1152/ajpregu.00860.2004. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Price KJ, Kasting NW, Sharp FR. Anatomic patterns of Fos immunostaining in rat brain following systemic endotoxin administration. Brain Res Bull. 1995;36:381–392. doi: 10.1016/0361-9230(94)00217-o. [DOI] [PubMed] [Google Scholar]
- Sakai N, Yamamoto T. Conditioned taste aversion and c-fos expression in the rat brainstem after administration of various USs. Neuroreport. 1997;8:2215–2220. doi: 10.1097/00001756-199707070-00025. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Rinaman L, Vollmer RR, Amico JA. Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1828–R1833. doi: 10.1152/ajpregu.00826.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shor-Posner G, Azar AP, Insinga S, Leibowitz SF. Deficits in the control of food intake after hypothalamic paraventricular nucleus lesions. Physiol Behav. 1985;35:883–890. doi: 10.1016/0031-9384(85)90255-0. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Glucagon-like peptide-1 receptor (GLP1-R) mRNA in the rat hypothalamus. Endocrinology. 1996;137:5159–5162. doi: 10.1210/endo.137.11.8895391. [DOI] [PubMed] [Google Scholar]
- Sierra Baigrie S, Lemos Giraldez S. Examining the relationship between binge eating and coping strategies and the definition of binge eating in a sample of Spanish adolescents. Span J Psychol. 2008;11:172–180. doi: 10.1017/s1138741600004212. [DOI] [PubMed] [Google Scholar]
- Soloff MS, Chakraborty J, Sadhukhan P, Senitzer D, Wieder M, Fernstrom MA, et al. Purification and characterization of mammary myoepithelial and secretory cells from the lactating rat. Endocrinology. 1980;106:887–897. doi: 10.1210/endo-106-3-887. [DOI] [PubMed] [Google Scholar]
- Stricker EM, Verbalis JG. Central inhibition of salt appetite by oxytocin in rats. Regul Pept. 1996;66:83–85. doi: 10.1016/0167-0115(96)00058-4. [DOI] [PubMed] [Google Scholar]
- Svennersten-Sjaunja K, Olsson K. Endocrinology of milk production. Domest Anim Endocrinol. 2005;29:241–258. doi: 10.1016/j.domaniend.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Purba JS, Hofman MA. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: a study of five cases. J Clin Endocrinol Metab. 1995;80:573–579. doi: 10.1210/jcem.80.2.7852523. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T, Nishimori K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport. 2008;19:951–955. doi: 10.1097/WNR.0b013e3283021ca9. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Roitman MF, Bernstein IL. c-Fos induction in rat brainstem in response to ethanol-and lithium chloride-induced conditioned taste aversions. Alcohol Clin Exp Res. 1996;20:1023–1028. doi: 10.1111/j.1530-0277.1996.tb01941.x. [DOI] [PubMed] [Google Scholar]
- Ukena K, Oumi T, Matsushima O, Ikeda T, Fujita T, Minakata H, et al. Effects of annetocin, an oxytocin-related peptide isolated from the earthworm Eisenia foetida, and some putative neurotransmitters on gut motility of the earthworm. J Exp Zool. 1995;272:184–193. doi: 10.1002/jez.1402720303. [DOI] [PubMed] [Google Scholar]
- van Leeuwen FW, van Heerikhuize J, van der Meulen G, Wolters P. Light microscopic autoradiographic localization of [3H]oxytocin binding sites in the rat brain, pituitary and mammary gland. Brain Res. 1985;359:320–325. doi: 10.1016/0006-8993(85)91443-x. [DOI] [PubMed] [Google Scholar]
- Van Tol HH, Voorhuis DT, Burbach JP. Oxytocin gene expression in discrete hypothalamic magnocellular cell groups is stimulated by prolonged salt loading. Endocrinology. 1987;120:71–76. doi: 10.1210/endo-120-1-71. [DOI] [PubMed] [Google Scholar]
- Vrang N, Larsen PJ, Kristensen P, Tang-Christensen M. Central administration of cocaine-amphetamine-regulated transcript activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 2000;141:794–801. doi: 10.1210/endo.141.2.7295. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Shimura T, Sako N, Azuma S, Bai WZ, Wakisaka S. C-fos expression in the rat brain after intraperitoneal injection of lithium chloride. Neuroreport. 1992;3:1049–1052. doi: 10.1097/00001756-199212000-00004. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Shimura T, Sako N, Yasoshima Y, Sakai N. Neural substrates for conditioned taste aversion in the rat. Behav Brain Res. 1994;65:123–137. doi: 10.1016/0166-4328(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Yoshimura R, Kiyama H, Kimura T, Araki T, Maeno H, Tanizawa O, et al. Localization of oxytocin receptor messenger ribonucleic acid in the rat brain. Endocrinology. 1993;133:1239–1246. doi: 10.1210/endo.133.3.8396014. [DOI] [PubMed] [Google Scholar]