Abstract
Previous research has demonstrated that concurrent systemic administration of CB1 cannabinoid and mu-opioid receptor agonists increases feeding in rats. However, the possible neural loci of this cooperative effect have yet to be identified. These studies tested whether the nucleus accumbens shell may be one site of the interactive effects of opioid and cannabinoid ligands on feeding. Injection of the mu-opioid agonist DAMGO (at 0, .025, .25, or 2.5 μg/0.5 μl/side) directly into the rat nucleus accumbens shell increased feeding on a sweetened fat diet, and this effect was blocked by pretreatment with either the mu-opioid antagonist naltrexone (20 μg/0.5 μl/side) or the CB1 antagonist SR141716 (0.5 μg/0.5 μl/side). Activation of nucleus accumbens shell CB1 receptors with WIN55212-2 alone (at .1 or .5 μg/0.5 μl/side) had no apparent effect on food intake. However, local injections of the low dose of DAMGO (.025 μg/0.5 μl/side) in this region along with WIN55212-2 (at .25 or .50 μg/0.5 μl/side) increased feeding above that induced by DAMGO alone. These data suggest an important modulatory role for cannabinoid receptors in the expression of feeding behaviors in response to mu-opioid receptor activation of the nucleus accumbens shell.
Keywords: Endocannabinoids, Opioids/Opiates, Nucleus accumbens, Feeding/Food intake, Palatable diet, Hedonic properties of food, Neurobiology of Reward, Diet quality, Energy balance
1. Introduction
Obesity is on the rise worldwide, and its occurrence is regularly coupled with serious health complications, including cardiovascular disease, Type II diabetes, and cancer (Guh et al., 2009; James, 2008). Recurrent over-consumption of highly palatable, energy dense foods is believed to be a major contributor to the development of obesity among susceptible individuals (Blundell and Gillett, 2001). Humans and other animals tend to consume high calorie foods even in the absence of any immediate homeostatic need, and current research suggests that brain reward pathways promote feeding beyond the point of satiation when palatable foods are freely available (Avena et al., 2009; Berridge et al., 2009; Berthoud, 2004; Kelley et al., 2005). As the modern milieu is saturated with such foods, researchers interested in targeting diet-induced weight gain have devoted considerable resources to developing a comprehensive understanding of the physiological mechanisms that encourage excessive caloric intake (Hetherington, 2007).
Although many hormonal and neurochemical signaling pathways serve to coordinate consummatory behavior in response to energy need (Berthoud, 2004; Schwartz et al., 2000; Woods et al., 1998), less is known about the pathways that underlie non-homeostatically motivated feeding. Consumption of palatable foods is generally believed to be rewarding even under sated conditions, and both the opioid and cannabinoid systems have been implicated in reward-driven feeding (Matias et al., 2008). In fact, emerging evidence suggests that these systems may interact to synergistically influence eating. For example, several laboratories have shown that systemic co-administration of CB1 cannabinoid and mu-opioid receptor antagonists decreases feeding in the rat (Kirkham and Williams, 2001; Rowland et al., 2001; Tallett et al., 2008). Likewise, food intake elicited by peripheral injections of a CB1 agonist is blocked by concomitant administration of a mu-opioid receptor antagonist (Williams and Kirkham, 2002), while feeding induced by systemic or hypothalamic morphine injection was attenuated by blocking CB1 receptors either systemically or at the level of the hypothalamus (Verty et al., 2003). Furthermore, peripheral administration of the CB1 receptor antagonist SR141716 (Rimonabant®) reduced morphine-induced Fos activation in several brain regions implicated in the homeostatic and hedonic regulation of food intake, suggesting that the joint impact of the cannabinoid and opioid systems may be centrally mediated (Singh et al., 2005). However, the specific neural loci of their combined effects on feeding have yet to be characterized.
The nucleus accumbens shell is widely regarded to be a fundamental component of the distributed neural networks that govern reward-driven consummatory behaviors (Kelley et al., 2005; Smith et al., 2009). Both opioid and cannabinoid receptors in the nucleus accumbens appear to modulate feeding. Specifically, injections of mu-opioid receptor agonists directly into the rat nucleus accumbens increase feeding on both pabulum and palatable foods (Mucha and Iversen, 1986; Pecina and Berridge, 2000; Zhang et al., 1998), while intra-accumbens mu-opioid receptor antagonism decreases feeding (Bodnar et al., 1995). Furthermore, stimulation of mu-opioid receptors in this region significantly increases the number of positive, “liking” reactions exhibited by rats in response to intra-oral sucrose administration, suggesting that activation of the nucleus accumbens opioid system functionally increases the hedonic impact of food (Pecina and Berridge, 2000). Evidence suggests that endogenous cannabinoids also act in this region to modulate food reward. For example, intra-accumbens activation of CB1 receptors has been shown to increase subsequent consumption of both insipid and palatable substances (Kirkham et al., 2002; Mahler et al., 2007; Shinohara et al., 2009; Soria-Gomez et al., 2007), while systemic pre-treatment with a CB1 receptor antagonist abolishes this effect (Kirkham et al., 2002). This cannabinoid-mediated increase in eating may be achieved via augmentation of the perceived palatability of consumed foodstuffs (Mahler et al., 2007). Furthermore, nucleus accumbens CB1 receptor density has been shown to be inversely related to palatable food consumption, indicating that increased ingestion of calorically dense foods may heighten activation of CB1 receptors in this region and lead to their downregulation (Harrold et al., 2002). Taken as a whole, these studies substantiate the role of nucleus accumbens cannabinoid and opioid systems in modulating the rewarding impact of eating.
Although prior reports have identified a role for nucleus accumbens opioid and cannabinoid systems individually on food intake, it is not yet known how these neuromodulators may interact within the ventral striatum to promote feeding. In these experiments, we compared the effects of mu-opioid or CB1 receptor activation within the nucleus accumbens shell upon food consumption following presentation of a sweetened fat diet to rats maintained on ad libitum access to rat chow. In separate groups of rats, we tested the effects of antagonism of opioid or CB1 receptors within the nucleus accumbens on palatable food intake in response to subsequent mu-opioid or CB1-receptor stimulation. Finally, we assessed the effect of co-stimulation of both receptor subtypes within the nucleus accumbens on food intake upon presentation of the palatable sweetened fat diet.
2. Methods
2.1. Subjects
Adult male Sprague-Dawley rats (Harlan, Madison, WI) were acclimated to housing in a colony room maintained at ~21 °C with a 12 hour light-dark cycle. To be consistent with prior reports examining the effects of nucleus accumbens cannabinoid receptor manipulation on food intake (e.g., Kirkham et al., 2002; Soria-Gomez et al., 2007), all experiments were conducted at the beginning of the dark cycle, the period during which rats naturally forage. Animals were dually housed in clear plastic cages, and handled daily following arrival in the laboratory in order to adapt them to experimenter contact. Standard rat chow (Purina Protab RMH 3000) and water were available ad libitum in home cages. All experiments were conducted in accordance with NIH animal care guidelines and were approved by the Wake Forest University Animal Care and Use Committee.
2.2. Surgery
Following one week of acclimation to the housing environment, rats were anesthetized with a Ketamine-Xylazine cocktail (100 mg/kg-10mg/kg). Standard aseptic surgical procedures were used to implant indwelling stainless steel guide cannula (23 gauge) bilaterally above the nucleus accumbens shell (with the nose bar set at 5 mm above interaural zero: 3.1 mm anterior and 1.0 mm lateral to bregma, 5.0 mm ventral to the skull surface). Guide cannulas were affixed to the skull with screws and dental acrylic, and wire stylets were placed in the cannula following surgery to prevent obstruction. Rats were allowed 7 days for recovery prior to behavioral testing; during this time, all animals were handled regularly.
2.3. Apparatus
Food intake was monitored during daily two-hour feeding sessions in an experimental feeding chamber. The chambers were constructed from clear acrylic, with internal dimensions of 42 cm wide, 30.5 cm deep and 33 cm tall. A water bottle was hung at one end of the chamber, and a food intake monitor (Med Associates, St. Albans, VT) was filled with a high fat/high sucrose diet (see below) at the opposite end (head entry at 6.4 cm above the wire floor). Infrared (IR) eyebeams were located along the floor at three locations (5 cm above the wire floor) to measure locomotion; four additional IR beams were placed at a height of 16 cm above the floor to index rearing behavior. IR beam interruption (including at a sensor at the entry to the food intake monitor) was continually recorded by Med-PC software (Med Associates, St. Albans, VT) during feeding sessions. The weights of the food monitors were recorded at 10-sec intervals. A speaker maintained an ambient level of white noise at 65 dB in the experimental room. In order to more closely approximate the animals’ natural foraging environment, lights were off throughout the feeding sessions.
2.4. Feeding Paradigm
Following one week of surgical recovery, ad libitum chow-fed rats were allowed at least six days to habituate to the palatable diet during daily two-hour sessions in the feeding chambers. The high fat/high sucrose diet contained 278.3 g/kg vitamin free casein, 100.0 g/kg sucrose, 4.2 g/kg DL-methionine, 441.2 g/kg shortening, 77.7 g/kg safflower oil, 26.3 g/kg cellulose, 53.3 g/kg mineral mix, 15.2 g/kg vitamin mix and 3.8 g/kg choline chloride (Kilocaloric value of diet = 6.2 kcal/g; Teklad Diets, Madison, WI, USA). Rats freely eat this diet when it is available, and its intake is sensitive to nucleus accumbens injections of opiate and cholinergic drugs (Will et al., 2006). On the final two days of habituation, rats received mock intracranial injections to allow acclimation to microinfusion procedures. On day one, mock injectors were lowered flush to the end of the guide cannula; the second mock injection utilized injectors that were lowered to the infusion site, 2.5 mm below the end of the guides. No solutions were delivered on mock injection days. Experimental treatments began 48 hrs after the last mock injection.
On experimental treatment days, food intake was monitored following the injection of vehicle or drug solutions into the nucleus accumbens shell. Injection cannulas (30 gauge) were lowered bilaterally into the nucleus accumbens shell and 0.5 μl of solution was delivered (at a rate of 0.32 μl per minute) by a Harvard Apparatus (Holliston, MA) microinfusion pump. Injectors remained in place for one minute to allow for diffusion, and rats were immediately placed in the feeding chambers. For each experiment (outlined below), individual animals received all drug treatments across multiple experimental days, the order of which was randomly determined for each rat. All drug treatment days were separated by a minimum of two drug-free days, on which rats received neither injections nor access to the sweetened fat diet. For experiments 3 & 4, the antagonist pretreatment was immediately followed by a separate injection of the agonist. Once the microinfusions were completed, rats were placed immediately into the experimental feeding chambers for the food intake trial.
2.5. Drugs
D-Ala2, N-MePhe4, Gly-ol-enkephalin (DAMGO) and naltrexone hydrochloride were purchased from Sigma-Aldrich; WIN55212-2 was obtained from Tocris BioSciences. SR141716 was obtained from NIDA Research Resources Drug Supply System, Rockville, MD. DAMGO and naltrexone were dissolved in sterile saline; SR141716 and WIN55212-2 were dissolved in 75% and 50% dimethyl sulfoxide (DMSO) in sterile saline, respectively. DAMGO, naltrexone, and SR141716 have reliably been shown to alter feeding in rodents (e.g., Bodnar et al., 1995; Verty et al., 2003; Will et al., 2006). WIN55212-2 was chosen for these experiments based on its selective full agonist activity upon the CB1 receptor, its stability (relative to the endogenous ligands anandamide and 2-AG), and its history of use in previous studies of reinforcement (Maldonado et al, 2006; Solinas et al., 2008). All drug concentrations (outlined below) were chosen based upon behaviorally effective concentrations in previous reports (Nasehi et al., 2009; Soria-Gomez et al., 2007; Will et al., 2006; Zarrindast et al., 2007).
2.6.1. Experiments 1 & 2: Nucleus accumbens mu-opioid or CB1 receptor stimulation on the intake of a sweetened fat diet
The first two experiments directly compared the effects of mu-opioid and CB1 receptor stimulation on the intake of the same sweetened fat palatable diet. Following habituation to the diet, two groups of rats were treated with intra-accumbens infusions of either DAMGO (at 0.0, 0.025, 0.25, and 2.5 μg/side; Experiment 1) or WIN55212-2 (at 0.0, 0.1, and 0.5 μg/side; Experiment 2) across multiple treatment days.
2.6.2 Experiments 3 & 4: Effect of opioid or CB1 receptor antagonist pretreatment on agonist-elicited feeding
The second set of experiments were designed to test the possible effects of nucleus accumbens pretreatment with the opioid receptor antagonist naltrexone or the CB1 receptor antagonist SR141716 prior to stimulation of either mu-opioid receptors with DAMGO or the stimulation of CB1 receptors with WIN55212-2. In experiment 3, rats (n = 8) received intraaccumbens injections of saline vehicle or the mu-opioid receptor antagonist naltrexone (at 20 μg/side, bilaterally), followed by either vehicle, the CB1 agonist WIN55212-2 (at 0.5 μg, bilaterally), or the mu-opioid receptor agonist DAMGO (at 0.25 μg, bilaterally). In experiment 4, rats (n = 6) received intra-accumbens pretreatment with either vehicle or the CB1 receptor antagonist SR141716 (at 1.0 μg, bilaterally), followed by vehicle, the CB1 receptor agonist WIN55212-2 (at 0.5 μg, bilaterally), or the mu-opioid receptor agonist DAMGO (at 0.25 μg, bilaterally). Following each treatment, intake of the sweetened fat diet was monitored for two hours.
2.6.3. Experiment 5: The effect of simultaneous activation of nucleus accumbens mu-opioid and CB1 receptors on sweetened fat diet intake
Experiment 5 examined the response to co-stimulation of mu-opioid receptors and CB1 receptors on sweetened fat diet intake relative to mu-opioid stimulation alone by combining the low dose of DAMGO in a cocktail with either of two doses of WIN55212-2. Rats (n = 8) received four intra-accumbens injections of a 50% DMSO vehicle solution, the mu-opioid receptor agonist DAMGO alone (at 0.025 μg, bilaterally), or a cocktail containing both DAMGO and one of two concentrations of the CB1 receptor agonist WIN55212-2 (at 0.25 or 0.5 μg/bilaterally). Food intake of the high-fat, high-sugar food was measured for two hours following the injection.
2.7. Data Analysis
Dependent measures include the total amount of palatable food eaten across the two-hour period, the number of approaches to the food chamber, ambulation within the chamber (assessed as the number of complete crossings of the chamber from end to end), number of rears recorded, and total water intake during the feeding session. Total food intake for experiments 1, 2, and 5 was analyzed utilizing two-way repeated measures analysis of variance (ANOVA) comparing food intake across drug doses and across each two-hour session (with food intake quantified at 5-min intervals). The SR141716 and naltrexone pre-treatment experiments (3 & 4) were analyzed using three-way, repeated measures factorial ANOVAs, comparing food intake across time and at the levels of antagonist drug pre-treatment and agonist drug treatment. Total ambulation, rearing, water intake, and head entry measures were analyzed using repeated measures ANOVAs with drug dose as the independent variable (one-way or two-way, as appropriate).
2.7. Histology
At the conclusion of each experiment, rats were deeply anesthetized with sodium pentobarbital and perfused through the heart with a 0.9% buffered NaCl solution, followed by 10.0% formalin. Brains were removed and allowed to soak in 10.0% sucrose formalin. The brains were then frozen and sliced into 60-μm sections with a cryostat. Sections were stained with cresyl violet. The location of tips of the cannula were confirmed by light microscopy and charted with reference to Paxinos and Watson (1998). Only those animals whose injectors were bilaterally placed within the medial nucleus accumbens shell were included in the final behavioral analysis.
3. Results
3.1. Experiments 1 & 2: Contrasting the effects of mu-opioid or CB1 receptor stimulation on the intake of a sweetened fat diet
3.1.1. Experiment 1
Consistent with previous reports, stimulation of nucleus accumbens mu-opioid receptors with DAMGO dose-dependently increased consumption of the high fat/sucrose diet. A repeated measures ANOVA on the total amount of palatable food eaten across a two-hour session revealed a significant effect of DAMGO treatment on food intake, F(3, 21) = 24.65, p < .001, as well as a significant interaction of drug X time, F(69, 483) = 6.06, p < .001. As can be seen in Figure 1, increasing doses of intra-accumbens DAMGO resulted in higher food intake, largely by extending the length of time spent feeding during the first hour. DAMGO also increased the number of approaches to the food container across drug conditions, F(3, 21) = 8.12, p = .001; Tukey’s HSD post-hoc testing verified a significant increase in approach behavior at the 2.5 μg/side dose, but not at the lower doses.. The increase in consummatory behavior was directed at the fat diet, as water consumption during the two-hour feeding session was not affected by mu-opioid receptor stimulation, F(3, 21) = 2.76, p = .79 (see Figure 1). Locomotor activity, as assessed by the total number of complete crossings across the length of the feeding chamber, also increased across drug doses, F(3, 21) = 5.49, p = .006, although rearing behaviors were not significantly impacted by drug condition, F(3, 21) = 1.54, p = .23.
Figure 1.
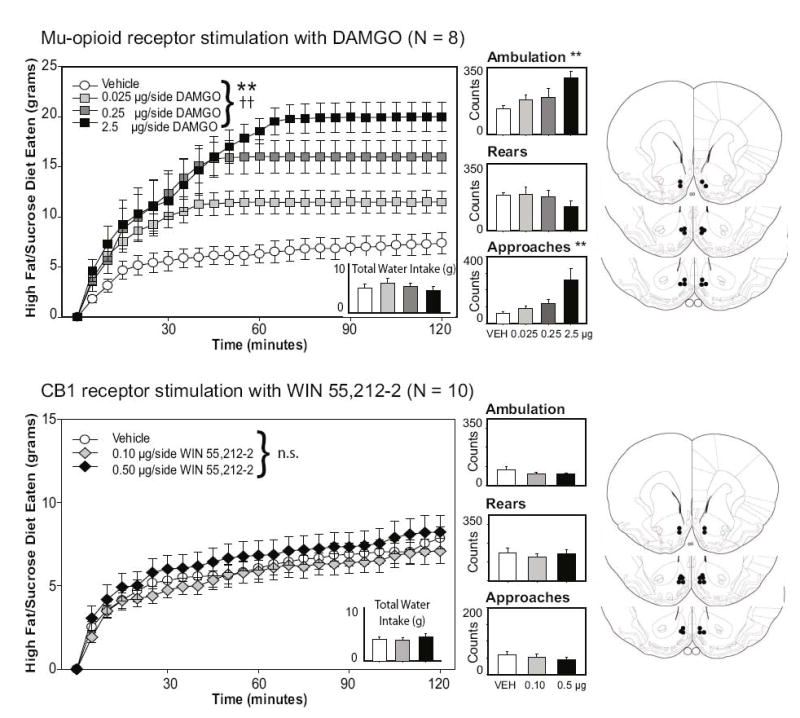
Effects of mu-opioid or CB1 receptor stimulation on feeding and locomotion. Intra-accumbens injections of DAMGO (top panels), but not WIN55212-2 (bottom panels), dose-dependently increased the intake of the sweetened fat diet. Neither drug altered water intake or rearing behavior; ambulatory activity (as measured by complete crossings of the chamber) and the number of approaches to the food hopper increased with higher levels of mu-opioid receptor stimulation. **p < 0.01 for drug effects; double crosses demark p < .01 for the drug x time interaction effect. The right panels represent the placements of the injector tips for each animal for each experiment, adapted from The Rat Brain in Stereotaxic Coordinates, 4th ed., G. Paxinos and C. Watson, Figures 10, 11, and 13, copyright 1998.
3.1.2. Experiment 2
Stimulation of nucleus accumbens CB1 receptors with the potent agonist WIN55212-2 did not alter intake of the sweetened fat diet used here, F(2, 18) = 1.13, p = 0.35, nor was there a significant drug X time interaction on food intake, F(46, 414) = 0.37, p = 1.00 (see bottom panels of Figure 1). The number of approaches to the food chamber was unaffected by CB1 receptor stimulation [F(2, 18) = 1.87, p = .18]. Additionally, there were no detected changes in locomotion following drug treatment [Ambulation: F(2, 18) = 1.08, p = .36; Rears: F(2, 18) = .37, p = .7], nor was water intake significantly impacted by treatment with WIN55212-2 [F(2, 18) = 2.09, p = .88].
3.2. Antagonizing either nucleus accumbens opioid or cannabinoid receptors attenuates food intake elicited by mu-opioid receptor stimulation
3.2.1. Experiment 3
A three-way factorial repeated measures ANOVA, examining the effects of both pretreatment (vehicle or naltrexone) and drug treatment (vehicle, DAMGO, or WIN55212-2) on food intake across time revealed significant effects of pretreatment [F(1,7) = 21.92, p = .002] and drug treatment [F(2,14) = 19.1, p < .001] on food intake, as well as a pretreatment X drug treatment interaction [F(2,14) = 4.51, p = .031]. Two-way interactions examining the effects of pretreatment or drug treatment on food intake across time were also significant (both p-values < .001), and the three way interaction of pretreatment X drug treatment X time did not achieve significance, F(46, 322) = 1.22, p = .166. As can be seen in the top panels of Figure 2, these effects are explained by the prevention of DAMGO-elicited food intake following pretreatment of the nucleus accumbens with naltrexone. Naltrexone alone slightly decreased sweetened fat diet intake during the first 90 minutes of the session. As with experiment 2, infusions of WIN55212-2 into the accumbens had no observable effect on food intake; thus, to simplify visual presentation of the data, figures for both Experiments 3 & 4 have been limited to the treatments that demonstrate the effects of naltrexone or SR141716 pretreatment on the DAMGO-driven feeding increase. Summary intake and locomotor data for the WIN treatment conditions is provided in Table 1.
Figure 2.
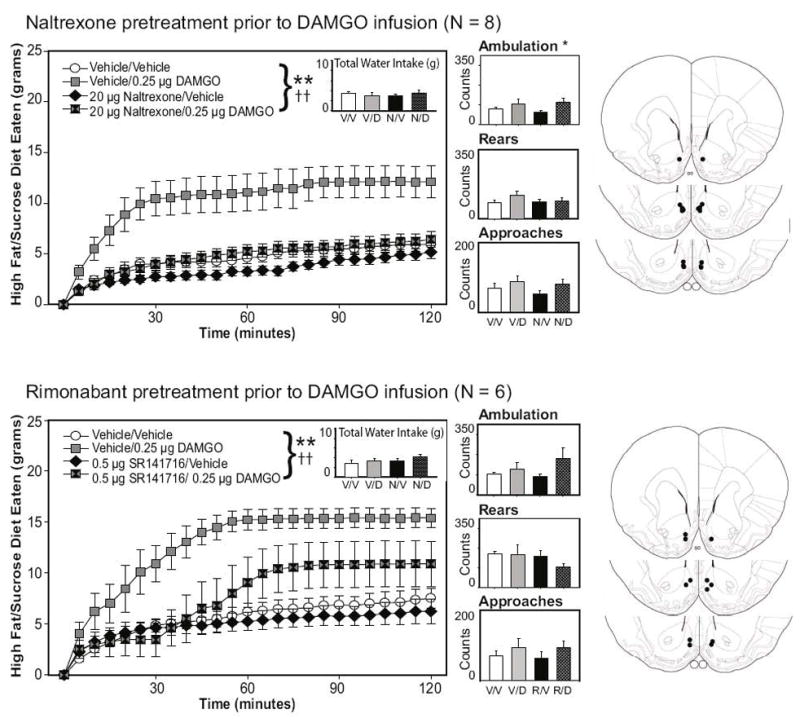
Feeding elicited by mu-opioid receptor stimulation of the nucleus accumbens is attenuated by pretreatments of either the opioid receptor antagonist naltrexone or the CB1 receptor antagonist SR141716. Naltrexone reduced feeding early in the session when given alone, and completely attenuated the impact of DAMGO on the intake of the palatable diet. SR141716 pretreatment delayed and attenuated the food intake elicited by nucleus accumbens mu-opioid receptors. *p < 0.05, **p < 0.01 for drug effects; double crosses demark a significance of p < .01 for at least one secondary or tertiary interaction effect. The right panels represent the placements of the injector tips for each animal for each experiment.
Table 1.
Summary data for WIN55212-2 infusions in Experiments 2 and 3
| Experiment 2 | 30 min intake | 60 min intake | 90 min intake | 120 intake | Ambulation | Rearing | Approaches |
|---|---|---|---|---|---|---|---|
| Veh-Veh | 3.98 ± 0.63 g | 4.61 ± 0.58 g | 5.48 ± 0.59 g | 5.94 ± 0.69 g | 76.8 ± 9.5 | 81.3 ± 11.7 | 69.5 ± 14.8 |
| Veh-WIN | 5.49 ± 0.75 g | 5.78 ± 0.68 g | 6.45 ± 0.56 g | 6.65 ± 0.51 g | 59.9 ± 5.1 | 103.5 ± 13.0 | 69.9 ± 17.9 |
| Nalt-WIN | 3.50 ± 0.89 g | 4.13 ± 0.85 g | 4.52 ± 0.85 g | 4.83 ± 0.77 g | 62.6 ± 8.5 | 89.0 ± 16.9 | 40.6 ± 5.35 |
| Experiment 3 | |||||||
| Veh-Veh | 4.87 ± 0.68 g | 6.18 ± 0.82 g | 6.88 ± 0.89 g | 7.54 ± 0.97 g | 101.5 ± 10.4 | 171.3 ± 13.2 | 70.3 ± 14.0 |
| Veh-WIN | 5.77 ± 0.94 g | 6.89 ± 1.01 g | 7.19 ± 0.94 g | 7.57 ± 1.05 g | 106.3 ± 21.2 | 191.8 ± 27.8 | 77.8 ± 20.4 |
| SR-WIN | 5.86 ± 1.03 g | 6.60 ± 0.98 g | 7.14 ± 1.11 g | 7.32 ± 1.10 g | 116.8 ± 25.7 | 146.8 ± 19.2 | 72.7 ± 13.4 |
Note. Values shown are means plus or minus standard error of the means for food intake and locomotor measures following injections of vehicle solutions or WIN55212-2 (alone or with pretreatment) in Experiments 2 and 3. As in experiment 1, neither food intake nor locomotion was affected by treatments including WIN55212-2 infusions.
Ambulatory activity differed significantly across drug conditions. A two-way factorial analysis of variance revealed a significant effect of drug treatment (Vehicle, DAMGO, WIN; F(2,14) = 4.47, p = .032, but no significant effect of pretreatment (Vehicle, Naltrexone; F(1,7) = 0.0, p = .995) nor was there a pretreatment X treatment interaction, F(2,14) = 1.17, p = .338. As shown in Figure 2, DAMGO treatment increased ambulation relative to vehicle infusion (and WIN55212-2 treatment, although data are not shown); this effect was not attenuated by naltrexone pretreatment. Neither water intake, approaches to the food hopper, nor rearing behavior was significantly affected by any of the drug treatments (all main effects and all interaction p-values > .10).
3.2.2. Experiment 4
Consistent with Experiments 1 & 3, mu-opioid receptor stimulation with 0.25 μg DAMGO substantially increased feeding on the sweetened fat diet; this effect on food consumption was delayed and attenuated by pretreatment of the nucleus accumbens with the CB1 antagonist SR141716 (0.5 μg /side; see figure 2, bottom panels). SR141716, when administered alone into the nucleus accumbens, did not substantially reduce feeding on the fat/high sucrose diet. A three-way factorial repeated measures ANOVA, examining the effects of both pretreatment (vehicle or SR141716) and drug treatment (vehicle, DAMGO, or WIN55212-2) on food intake across time revealed significant effects of pretreatment [F(1, 5) = 39.47, p = .002] and drug treatment on food intake [F(2, 10) = 9.44, p = .005], as well as significant secondary and tertiary interactions [all secondary interactions, p < .01; pretreatment X treatment X time interaction: F(46, 230) = 1.49, p = .032]. Infusions of WIN55212-2 had no overall effect on food intake (see summary data in Table 1).
As in the prior experiments, DAMGO treatment tended to increase ambulation, although for this experiment the effect was not significant F(5, 25) = 1.92, p = .127. Approaches to the food chamber were similar across conditions, and neither rearing nor water intake was significantly affected by the pretreatment or drug treatment conditions (all main effects and all interaction p-values > .10; see figure 2).
3.3. Experiment 5: Co-stimulation of nucleus accumbens CB1 receptors with mu-opioid receptor stimulation augments intake of the sweetened fat diet
Because blocking CB1 receptors with SR141716 in Experiment 4 attenuated the impact of nucleus accumbens mu-opioid receptor stimulation on intake of the sweetened fat diet, it was of interest to determine if CB1 receptor stimulation might augment the food intake elicited by DAMGO infusion. Therefore, we injected either vehicle, the lowest effective dose of DAMGO (0.025 μg/side) or the low dose of DAMGO in a cocktail with one of two doses of WIN55212-2 (0.25 or 0.5 μg/side) into the nucleus accumbens prior to each two-hour feeding session (see Figure 3). A repeated measures ANOVA on the total amount of palatable food eaten across a two-hour session revealed a significant effect of drug treatment on food intake, F(3, 18) = 5.956, p = .005, and a significant interaction effect of drug X time, F(69, 414) = 3.287, p < .001. As can be seen in Figure 3, DAMGO alone increased food intake relative to the vehicle infusion, and combination treatments of DAMGO and WIN55212-2 increased the intake by approximately 50% above that of mu-opioid receptor stimulation alone. To verify this effect, one-way ANOVAs were run on total food intake across all drug treatments at the 60 min and 120 time points. Both of these analysis yielded significant results [60 min: F(3,15) = 9.46, p = .001; 120 min: F(3,15) = 7.69, p = .002], and were then followed up with Fisher LSDs testing for an increase in food intake only with the combination DAMGO/WIN treatments versus the effects of DAMGO alone. At both time points, the DAMGO combined with the 0.25 μg dose of WIN55212-2 significantly increased feeding compared to DAMGO alone (p-values < .05), although the feeding increase following the 0.5 μg dose of WIN combined with DAMGO did not obtain significance (p-values > .10). Neither the low dose of DAMGO alone, nor the combination DAMGO/WIN55212-2 injections, significantly altered the number of approaches to the food hopper [F(3,18) = .870, p = .475], ambulation [F(3, 18) = 1.811, p = .181], rearing behavior [F(3, 18) = .853, p = .483] or water intake [F(3, 18) = 1.521, p = .734].
Figure 3.
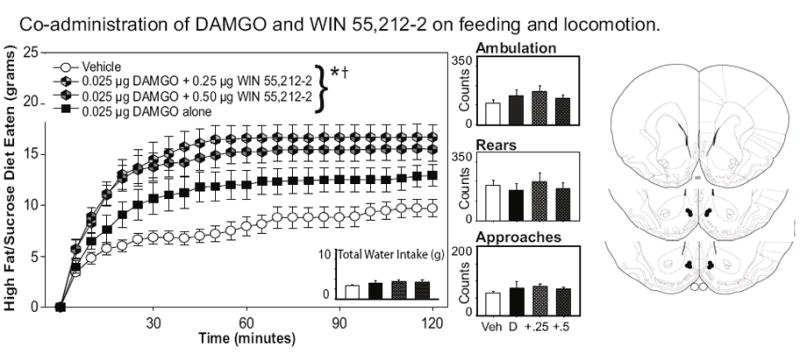
Effects of co-stimulation of mu-opioid and CB1 receptors on sweetened fat diet intake. Nucleus accumbens infusions of the low dose of DAMGO (0.25 μg/side) increased food intake above baseline levels; this effect was enhanced by co-injections of WIN55212-2 (see text for details). There were no significant effects of drug treatment on water intake, approaches to the food hopper, or locomotor activity. *p < 0.05 for drug effects; the single cross demarks a significance of p < .05 for at the drug X time interaction effect. The right panels represent the placements of the injector tips for each animal for each experiment.
4. Discussion
These experiments are the first to directly compare the feeding responses elicited by nucleus accumbens shell stimulation of mu-opioid or CB1 receptors in the same feeding paradigm and on the same sweetened fat diet. Consistent with previous reports, intra-accumbens administration of the potent mu-opioid receptor agonist DAMGO significantly increased palatable food intake, whereas the blockade of opioid receptors with naltrexone decreased feeding on the sweetened fat diet (Bodnar et al., 1995; Levine and Billington, 2004; Pecina and Berridge, 2000; Zhang et al., 1998). Administration of the CB1 receptor agonist WIN55212-2, at the doses used here, had no apparent effect on palatable food intake in rats maintained on ad libitum chow. Interestingly, DAMGO-induced feeding was blocked not only by local pre-treatment with the opioid receptor antagonist naltrexone, but also by pre-administration of the CB1 receptor antagonist SR141716. This suggests that the behavioral effects of nucleus accumbens mu-opioid receptor stimulation may require simultaneous activation of CB1 receptors. Additionally, co-activation of both receptor systems increased intake of the sweetened fat diet relative to a low dose of DAMGO given alone. In no case was there an enhancement of water intake following drug treatments, suggesting that the observed behavioral effects were specific to food-directed behavior. These results are consistent with previous research demonstrating that systemically co-administered cannabinoid and opioid agents interact to modify feeding on both pabulum and palatable foods (Kirkham and Williams, 2001; Rowland et al., 2001; Tallett et al., 2008; Williams and Kirkham, 2002), and suggest that the nucleus accumbens shell may be one site where these two receptor systems cooperatively augment ingestive behavior.
Notably, however, neither SR141716 nor the CB1 agonist WIN55212-2 had any apparent effect on the intake of a sweetened fat diet when administered apart from opioid agents. That SR141716 administration did not affect feeding is consistent with a prior report by Solinas et al. (2003) in which nucleus accumbens CB1 antagonism with AM251 caused no change in food intake when administered alone. However, although intra-cerebral ventricular administration of CB1 ligands have failed to alter food-reinforced behaviors in rats (Gomez et al., 2002; Sink et al., 2009), the current finding that the potent, efficacious agonist WIN55212-2 was unable to elicit a sweetened-fat feeding response in the absence of the mu-opioid stimulation is at odds with other reports in the literature that have shown increased hedonic reactions to food and food intake following stimulation of cannabinoid receptors within the nucleus accumbens. Specifically, Kirkham and colleagues demonstrated a hyperphagic response to intra-accumbens 2-arachidonoylglycerol (2-AG), which was reversible by SR141716 (Kirkham et al., 2002). Similarly, Mahler and colleagues (Mahler et al., 2007) found that anandamide injected into discrete regions within the dorsal medial shell increased the number of hedonic “liking” responses to oral sucrose, as well as increased the fraction of time spent eating during a one-hour trial exposure to rat chow (see also Soria-Gomez et al., 2007). To our knowledge, the present report is the first study to examine nucleus accumbens cannabinoid receptor activation on intake of a sweetened high fat diet in animals maintained on free access to rat chow. It may be that the differing results seen between this and other reports are due to methodological differences involving food quality, hedonic value, or caloric density differences in diet. Prior investigations have also utilized the efficacious but transiently active 2-AG, or else the partial CB1 receptor agonists anandamide or THC, rather than full agonist WIN55212-2 utilized here. Nonetheless, based on the present results, we theorize that an important functional role of the endocannabinoid system may be to augment reward-driven feeding via modulation of striatal mu-opioid signaling pathways.
Although recent neuroanatomical studies strongly suggest the possibility of functional interactions between CB1 and mu-opioid receptors at the level of the nucleus accumbens circuitry, it is not yet clear how the two receptor systems may interact to impact behavior. CB1 and mu-opioid receptors are co-localized on axons and dendrites within the nucleus accumbens, and exhibit trans-synaptic interactions upon nucleus accumbens shell GABAergic neurons (Manzoni and Bockaert, 2001; Pickel et al., 2004; Rodriguez et al., 2001). Prior experiments have shown that inhibition of the nucleus accumbens shell initiates voracious feeding (Stratford and Kelley, 1997). Whole cell recordings from medium spiny neurons in slice preparations of the nucleus accumbens shell suggest that glutamate inputs are inhibited by activation of either mu-opioid receptors or CB1 receptors (with DAMGO or WIN55212-2, respectively). The reduction of glutamate tone following mu-opioid/CB1 receptor activation is likely to reduce neural activity of nucleus accumbens shell GABAergic projection neurons. It is possible, therefore, that the observed synergistic effect of mu-opioid and CB1 receptor stimulation on food intake may result from increased inhibition of nucleus accumbens output, which would increase consumption by disinhibiting ventral pallidal-hypothalamic feeding pathways. However, it should be noted that CB1 receptor activation has also been shown to inhibit GABAergic signaling within striatal tissue, an effect not seen following mu-opioid receptor stimulation (Hoffman and Lupica, 2001). Furthermore, in a recent electrophysiological study examining the decreased reactivity in both striatal glutamatergic and GABAergic post-synaptic potentials following CB1 receptor stimulation, chronic treatment with WIN55212-2 caused tolerance not only to the WIN55212-2, but also reduced the response to the mu-opioid receptor agonist Tyr-D-Ala2, N-CH3-Phe4,Gly-ol-enkephalin (Hoffman et al., 2003), suggesting that a functional CB1 receptor is required for mu-opioid receptor response. If this can be extrapolated to the current study, such an interaction would be consistent with the behavioral finding that CB1 receptor blockade delayed and muted the feeding effect following mu-opioid receptor stimulation with DAMGO.
At the level of the single cell, electron microscopic investigations have shown that CB1 and mu-opioid receptors are co-expressed on dendritic spines of GABAergic medium-spiny neurons within the nucleus accumbens (Pickel et al., 2004), and it could be speculated that these may function as heterodimers (Canals and Milligan, 2008; Rios et al., 2006). However, biochemical studies of cellular signaling suggest that a mutual suppression of activities would be expected by CB1-mu-opioid receptor heteromeric interactions, rather than a synergistic activation as observed in the present studies (Canals and Milligan, 2008; Rios et al., 2006; Shapira et al., 1998; Shapira et al., 2000). Thus, it is unlikely that the present results may be explained as a consequence of heterodimer interactions between the two receptor types.
A recent in vitro investigation found that SR141716 at high concentrations (10 μM) exhibited a low affinity to displace [3H]DAMGO binding to mu-opioid receptors in CHO cell membranes (Cinar et al., 2008). Other reports have suggested that at high concentrations, Δ 9-THC and cannabidiol (which exhibits very poor affinity for CB1 receptors), also non-competitively decrease mu-opioid receptor binding to ligands (Kathmann et al., 2006; Vaysse et al., 1987). Although it is possible that the reduced efficacy of DAMGO to elicit feeding following SR141716 pretreatment may be due to antagonism of binding at the mu-opioid receptor itself, this interpretation is unlikely for two reasons. First, SR141716 did not itself reduce feeding in a manner consistent with the inhibition seen following naltrexone treatment in Experiment 3. Second, the reduced food intake in response to SR141716 pretreatment prior to DAMGO infusion (Experiment 4) differed in the pattern of antagonism compared with the pretreatment of naltrexone (Experiment 3). SR141716 pretreatment delayed and attenuated the feeding response in response to subsequent DAMGO infusion, but it did not completely block it as did naltrexone pretreatment. As such, we conclude that our observed effect is indicative of an endogenous functional interaction between these receptor systems.
Concluding Remarks
The results of these studies support a role for cannabinoid and opioid receptor systems, acting in the nucleus accumbens shell, for impacting palatability-induced feeding. Specifically, the feeding effects elicited by stimulation or blockade of mu-opioid receptors indicate that these receptors are of principal importance for modulating ingestion of a palatable diet in rats maintained on ad libitum access to chow. Although CB1 receptor activation alone was not sufficient to impact reward-driven feeding, these results indicate that mu-opioid induced feeding is modulated by simultaneous activation of CB1 receptors. The findings reported herein strongly suggest that endocannabinoid agonists acting in the nucleus accumbens shell permit the actions of mu-opioid receptors. This modulatory role is consistent with accumulating evidence that cannabinoid receptor activation is important for full expression of the rewarding properties of opioid peptides (Caille and Parsons, 2006; Mas-Nieto et al., 2001; Solinas et al., 2003). The interaction of the cannabinoid and opioid systems may serve to alter the firing of nucleus accumbens shell GABAergic projections to neural structures implicated in the governance of ingestion. Taken as a whole, these studies suggest that the development of pharmacological interventions that target both endocannabinoid and opioid peptide depletion could provide an efficacious treatment strategy for maladaptive eating behaviors, such as those associated with obesity.
Acknowledgments
This research was supported by the Wake Forest Department of Psychology and a cross-campus collaborative grant through Wake Forest University. Additional support was provided to ACH through the NIH (R01-DA-03690). In the previous three years, ACH has served as a consultant for and a member of the Speaker’s Bureau for Sanofi Aventis. We would like to thank Constance B. Polite and Megan E. Connolly for their technical assistance throughout various stages of these projects, and Dr. William C. Gordon for useful comments on the revised version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mary Jane Skelly, Email: mjskelly@gmail.com.
Elizabeth G. Guy, Email: glenea8@wfu.edu.
Allyn C. Howlett, Email: ahowlett@wfubmc.edu.
Wayne E. Pratt, Email: prattwe@wfu.edu.
References
- Avena NM, Rada P, Hoebel BG. Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr. 2009;139:623–628. doi: 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: 'liking', 'wanting', and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR. Neural control of appetite: cross-talk between homeostatic and non-homeostatic systems. Appetite. 2004;43:315–317. doi: 10.1016/j.appet.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Blundell JE, Gillett A. Control of food intake in the obese. Obes Res. 2001;9 (Suppl 4):263S–270S. doi: 10.1038/oby.2001.129. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Glass MJ, Ragnauth A, Cooper ML. General, mu and kappa opioid antagonists in the nucleus accumbens alter food intake under deprivation, glucoprivic and palatable conditions. Brain Res. 1995;700:205–212. doi: 10.1016/0006-8993(95)00957-r. [DOI] [PubMed] [Google Scholar]
- Caille S, Parsons LH. Cannabinoid modulation of opiate reinforcement through the ventral striatopallidal pathway. Neuropsychopharmacology. 2006;31:804–813. doi: 10.1038/sj.npp.1300848. [DOI] [PubMed] [Google Scholar]
- Canals M, Milligan G. Constitutive activity of the cannabinoid CB1 receptor regulates the function of co-expressed Mu opioid receptors. J Biol Chem. 2008;283:11424–11434. doi: 10.1074/jbc.M710300200. [DOI] [PubMed] [Google Scholar]
- Cinar R, Freund TF, Katona I, Mackie K, Szucs M. Reciprocal inhibition of G-protein signaling is induced by CB(1) cannabinoid and GABA(B) receptor interactions in rat hippocampal membranes. Neurochem Int. 2008;52:1402–1409. doi: 10.1016/j.neuint.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Gomez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, Cippitelli A, Nava F, Piomelli D, Rodriguez de Fonseca F. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of comorbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrold JA, Elliott JC, King PJ, Widdowson PS, Williams G. Down-regulation of cannabinoid-1 (CB-1) receptors in specific extrahypothalamic regions of rats with dietary obesity: a role for endogenous cannabinoids in driving appetite for palatable food? Brain Res. 2002;952:232–238. doi: 10.1016/s0006-8993(02)03245-6. [DOI] [PubMed] [Google Scholar]
- Hetherington MM. Cues to overeat: psychological factors influencing overconsumption. Proc Nutr Soc. 2007;66:113–123. doi: 10.1017/S0029665107005344. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Direct actions of cannabinoids on synaptic transmission in the nucleus accumbens: a comparison with opioids. J Neurophysiol. 2001;85:72–83. doi: 10.1152/jn.2001.85.1.72. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Oz M, Caulder T, Lupica CR. Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure. J Neurosci. 2003;23:4815–4820. doi: 10.1523/JNEUROSCI.23-12-04815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James WP. The epidemiology of obesity: the size of the problem. J Intern Med. 2008;263:336–352. doi: 10.1111/j.1365-2796.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- Kathmann M, Flau K, Redmer A, Trankle C, Schlicker E. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:354–361. doi: 10.1007/s00210-006-0033-x. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM. Synergistic efects of opioid and cannabinoid antagonists on food intake. Psychopharmacology (Berl) 2001;153:267–270. doi: 10.1007/s002130000596. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AS, Billington CJ. Opioids as agents of reward-related feeding: a consideration of the evidence. Physiol Behav. 2004;82:57–61. doi: 10.1016/j.physbeh.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances 'liking' of a sweet reward. Neuropsychopharmacology. 2007;32:2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–32. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Manzoni OJ, Bockaert J. Cannabinoids inhibit GABAergic synaptic transmission in mice nucleus accumbens. Eur J Pharmacol. 2001;412:R3–5. doi: 10.1016/s0014-2999(01)00723-3. [DOI] [PubMed] [Google Scholar]
- Mas-Nieto M, Pommier B, Tzavara ET, Caneparo A, Da Nascimento S, Le Fur G, Roques BP, Noble F. Reduction of opioid dependence by the CB(1) antagonist SR141716A in mice: evaluation of the interest in pharmacotherapy of opioid addiction. Br J Pharmacol. 2001;132:1809–1816. doi: 10.1038/sj.bjp.0703990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias I, Cristino L, Di Marzo V. Endocannabinoids: some like it fat (and sweet too) J Neuroendocrinol. 2008;20 (Suppl 1):100–109. doi: 10.1111/j.1365-2826.2008.01678.x. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Iversen SD. Increased food intake after opioid microinjections into nucleus accumbens and ventral tegmental area of rat. Brain Res. 1986;397:214–224. doi: 10.1016/0006-8993(86)90622-0. [DOI] [PubMed] [Google Scholar]
- Nasehi M, Sahebgharani M, Haeri-Rohani A, Zarrindast MR. Effects of cannabinoids infused into the dorsal hippocampus upon memory formation in 3-days apomorphine-treated rats. Neurobiol Learn Mem. 2009;92:391–399. doi: 10.1016/j.nlm.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Pecina S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic 'liking' for food: map based on microinjection Fos plumes. Brain Res. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Kash TL, Rodriguez JJ, MacKie K. Compartment-specific localization of cannabinoid 1 (CB1) and mu-opioid receptors in rat nucleus accumbens. Neuroscience. 2004;127:101–112. doi: 10.1016/j.neuroscience.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Rios C, Gomes I, Devi LA. mu opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Br J Pharmacol. 2006;148:387–395. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. J Neurosci. 2001;21:823–833. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland NE, Mukherjee M, Robertson K. Effects of the cannabinoid receptor antagonist SR 141716, alone and in combination with dexfenfluramine or naloxone, on food intake in rats. Psychopharmacology (Berl) 2001;159:111–116. doi: 10.1007/s002130100910. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Shapira M, Gafni M, Sarne Y. Independence of, and interactions between, cannabinoid and opioid signal transduction pathways in N18TG2 cells. Brain Res. 1998;806:26–35. doi: 10.1016/s0006-8993(98)00697-0. [DOI] [PubMed] [Google Scholar]
- Shapira M, Vogel Z, Sarne Y. Opioid and cannabinoid receptors share a common pool of GTP-binding proteins in cotransfected cells, but not in cells which endogenously coexpress the receptors. Cell Mol Neurobiol. 2000;20:291–304. doi: 10.1023/A:1007058008477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara Y, Inui T, Yamamoto T, Shimura T. Cannabinoid in the nucleus accumbens enhances the intake of palatable solution. Neuroreport. 2009 doi: 10.1097/WNR.0b013e3283318010. [DOI] [PubMed] [Google Scholar]
- Singh ME, McGregor IS, Mallet PE. Repeated exposure to Delta(9)-tetrahydrocannabinol alters heroin-induced locomotor sensitisation and Fos-immunoreactivity. Neuropharmacology. 2005;49:1189–1200. doi: 10.1016/j.neuropharm.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Sink KS, Segovia KN, Nunes EJ, Collins LE, Vemuri VK, Thakur G, Makriyannis A, Salamone JD. Intracerebroventricular administration of cannabinoid CB1 receptor antagonists AM251 and AM4113 fails to alter food-reinforced behavior in rats. Psychopharmacology (Berl) 2009;206:223–232. doi: 10.1007/s00213-009-1602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behav Brain Res. 2009;196:155–167. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR, Piomelli D. The endocannabinoid system in brain reward processes. Br J Pharmacol. 2008;154:369–83. doi: 10.1038/bjp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Antoniou K, Pappas LA, Goldberg SR. The cannabinoid CB1 antagonist N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl) -4-methylpyrazole-3-carboxamide (SR-141716A) differentially alters the reinforcing effects of heroin under continuous reinforcement, fixed ratio, and progressive ratio schedules of drug self-administration in rats. J Pharmacol Exp Ther. 2003;306:93–102. doi: 10.1124/jpet.102.047928. [DOI] [PubMed] [Google Scholar]
- Soria-Gomez E, Matias I, Rueda-Orozco PE, Cisneros M, Petrosino S, Navarro L, Di Marzo V, Prospero-Garcia O. Pharmacological enhancement of the endocannabinoid system in the nucleus accumbens shell stimulates food intake and increases c-Fos expression in the hypothalamus. Br J Pharmacol. 2007;151:1109–1116. doi: 10.1038/sj.bjp.0707313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17:4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallett AJ, Blundell JE, Rodgers RJ. Endogenous opioids and cannabinoids: system interactions in the regulation of appetite, grooming and scratching. Physiol Behav. 2008;94:422–431. doi: 10.1016/j.physbeh.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Vaysse PJ, Gardner EL, Zukin RS. Modulation of rat brain opioid receptors by cannabinoids. J Pharmacol Exp Ther. 1987;241:534–539. [PubMed] [Google Scholar]
- Verty AN, Singh ME, McGregor IS, Mallet PE. The cannabinoid receptor antagonist SR 141716 attenuates overfeeding induced by systemic or intracranial morphine. Psychopharmacology (Berl) 2003;168:314–323. doi: 10.1007/s00213-003-1451-9. [DOI] [PubMed] [Google Scholar]
- Will MJ, Pratt WE, Kelley AE. Pharmacological characterization of high-fat feeding induced by opioid stimulation of the ventral striatum. Physiol Behav. 2006;89:226–234. doi: 10.1016/j.physbeh.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Observational analysis of feeding induced by Delta9-THC and anandamide. Physiol Behav. 2002;76:241–250. doi: 10.1016/s0031-9384(02)00725-4. [DOI] [PubMed] [Google Scholar]
- Woods SC, Seeley RJ, Porte D, Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–1383. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Nouri M, Ahmadi S. Cannabinoid CB1 receptors of the dorsal hippocampus are important for induction of conditioned place preference (CPP) but do not change morphine CPP. Brain Res. 2007;1163:130–137. doi: 10.1016/j.brainres.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther. 1998;285:908–914. [PubMed] [Google Scholar]


