Abstract
We examined the ability of recombinant guinea pig IL-8 (CXCL8) to activate neutrophils upon infection with virulent M. tuberculosis. Using a Transwell insert culture system, contact-independent cell cultures were studied in which rgpIL-8-treated neutrophils were infected with virulent M. tuberculosis in the upper well, and AM were cultured in the lower well. IL-1β and TNF-α mRNA expression was significantly up-regulated by AM. Neutralizing anti-rgpTNF-α polyclonal antibody abrogated the response of AM to supernatants from the rgpIL-8 treated, infected neutrophils, while an anti-rgpIL-8 polyclonal antibody had no effect. This suggests that TNF-α produced by rgpIL-8 treated, infected neutrophils may play an important role in the activation of AM in the early response of the host against M. tuberculosis infection. Significant induction of apoptosis in M. tuberculosis-infected neutrophils was observed as compared to the uninfected neutrophils. Feeding of infected, apoptotic neutrophils to AM induced a significant up-regulation of TNF-α and IL-1β mRNA compared to AM exposed to staurosporine-treated apoptotic neutrophils. Suppressed intracellular mycobacterial growth was also seen in AM fed with infected, apoptotic neutrophils as compared to the AM infected with M. tuberculosis H37Rv alone. Taken together, these data suggest that neutrophil-macrophage interactions may contribute to host defense against M. tuberculosis infection.
Keywords: Neutrophils, alveolar macrophages, IL-8, tuberculosis, guinea pig
Introduction
Alveolar macrophages (AM) are likely the first cells to encounter M. tuberculosis after inhalation of the bacilli by the host [1, 2]. Interleukin-8 (CXCL8; IL-8) is produced following infection of human AM and THP-1 cells with M. tuberculosis H37Ra, and a significant correlation between the amount of IL-8 protein and the number of neutrophils has also been observed [3]. Significant amounts of IL-8 protein have been found in bronchoalveolar lavage fluid of TB patients, and in supernatants of human monocytes and monocytic cell lines infected with M. tuberculosis [3, 4]. AM from BCG-vaccinated guinea pigs can also produce significant amounts of IL-8 mRNA and protein following infection with M. tuberculosis [5].
IL-8 acts on chemokine receptors that are highly expressed on neutrophils, thereby causing their recruitment and activation [6, 7]. Recombinant human IL-8 (rhIL-8) can prime human peripheral blood neutrophils for enhanced superoxide anion production [8, 9]. Significant synergistic production of IL-1β protein resulted from treatment of human neutrophils by both LPS and rhIL-8 [10]. In vivo studies using anti-IL-8 Ab in rabbits have shown the suppression of the development of a tuberculin skin reaction, i.e., inhibition of accumulation of leukocytes, in rabbit skin [11].
Neutrophils, found in granulomas in humans, guinea pigs and mice [12], are persistently recruited to the site of mycobacterial infection [13]. In vivo neutrophil depletion studies in mice have resulted in increased susceptibility to bacterial growth in an intravenous model of M. avium infection [14] and enhanced growth of M. bovis in the lungs [15]. Neutrophil depletion also impaired the ability of the whole blood to restrict growth of M. bovis BCG and M. tuberculosis [16]. Furthermore, recent studies have shown the entrapment of M. tuberculosis by neutrophil extracellular traps but the mycobacteria were not killed [17]. However, the ability of neutrophils to kill M. tuberculosis is still controversial [18, 19].
Although neutrophils are terminally differentiated, when stimulated with bacteria, LPS or cytokines they can produce an array of cytokines and chemokines such as IL-1β, IL-8, TGF-β, TNF- α, MIP-1α/β, MCP-1 and GRO-α in mice, humans and guinea pigs [5, 20, 21]. In vitro studies have shown that infection of guinea pig neutrophils with M. tuberculosis H37Rv can induce significant amounts of TNF-α and IL-8 protein [22].
Inhibition of spontaneous apoptosis in M. bovis BCG infected human neutrophils has been observed [21], while promotion of apoptosis of neutrophils by infection with M. bovis BCG, M. tuberculosis H37Rv or H37Ra has also been demonstrated [23]. A pro-inflammatory response was induced by human macrophages following uptake of mycobacteria-induced apoptotic neutrophils but not by uninfected apoptotic neutrophils [24]. Uptake of apoptotic neutrophils by human macrophages resulted in decreased survival of M. tuberculosis in macrophages, apparently due to action of neutrophil granules acquired by the macrophages [25].
Taken together, these observations suggest that neutrophils may be important participants in the early pulmonary response to mycobacterial infection. AM are the first cells which encounter mycobacteria after inhalation and they are also a major source of the IL-8 which is responsible for the recruitment of neutrophils to the site of infection [1–3, 5]. In turn, cytokines produced by neutrophils (e.g., TNFα) may have paracrine effects on AM and other lung cells [22]. Therefore, to further evaluate the importance of the interactions between neutrophils and AM in host defense against tuberculosis in the highly relevant guinea pig model of pulmonary disease, the effect of recombinant guinea pig (rgp) IL-8-activated neutrophils on AM infected with M. tuberculosis H37Rv was investigated in non-contact co-cultures. Furthermore, we determined the effect of uptake of infected, apoptotic neutrophils on the anti-mycobacterial functions of AM.
Materials and methods
Animals
Specific-pathogen-free outbred Hartley strain guinea pigs (Charles River Breeding Laboratory Inc., Wilmington, Mass.) were individually housed in an air-filtered environment within polycarbonate cages with stainless steel grid floors. They were provided commercial chow (Ralston Purina, St. Louis, Mo.) in stainless steel feeders and tap water ad libitum. All the procedures were reviewed and approved by the Texas A&M University Laboratory Animal Care Committee.
Mycobacteria
Mycobacterium tuberculosis H37Rv strain (ATCC 27294; American Type Culture Collection, Manassas, Va.) was cultured in Middlebrook 7H9 medium and stocks were prepared and stored at −80°C according to an established procedure [26]. Before use, mycobacterial preparations were rapidly thawed, vortexed and sonicated with an Ultrasonics sonicator (Heat Systems- Ultrasonics, Inc., Plainview, N.Y.) for 45 to 60 s at an output setting of 8.0 to disrupt bacterial clumps.
Production of recombinant guinea pig IL-8
The cloning of gpIL-8 cDNA was previously described [27], and sub-cloning and expression was carried out according to a previously published protocol from our laboratory [28]. The purified protein was assessed for the presence of endotoxin (<18 pg/μg) by the Limulus amebocyte assay (Associates of Cape Cod, Inc., Falmouth, Mass.). The protein concentration was determined by the Bradford protein assay (Bio-Rad, Richmond, Calif.). The purity of the protein was assessed by performing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 10–20% tri-tricine gels (Invitrogen, Carlsbad, CA) and then staining with Coomasie Brilliant Blue R 250. The protein was also sequenced by Edman degradation using the G1000A automated protein sequencer (Dr. L. Dangott, Protein Chemistry Laboratory, Texas A&M University). The bioactivity of rgpIL-8 was confirmed using a chemotaxis assay as published previously [28].
Isolation of neutrophils and alveolar macrophages
Guinea pig neutrophils were isolated according to our previously published protocol [22]. Briefly, the guinea pigs were injected with 20 ml of 9 % casein intra-peritoneally 16–18 h before being given an intramuscular injection of sodium pentobarbital (100 mg/kg - Sleepaway; Fort Dodge Laboratories, Inc., Fort Dodge, Iowa). The peritoneal cavity was washed 3 times with 10–15 ml of ice-cold harvesting solution. (RPMI-1640- L-glutamine without phenol red or antibiotics, plus 2% heat inactivated FBS and 10 U/ml heparin). The cells were pelleted and washed 3 times with media containing 2% heat inactivated FBS and 2- mercaptoethanol (ME). The neutrophils were enriched by Opti-prep density gradients (Axis-Shield, Norway) following the manufacturer’s instructions. The percent of neutrophils was determined using 3% acetic acid and counting cells on a hemocytometer. Cell viability was assessed by the trypan blue exclusion assay (Gibco Life Technologies, NY) and a differential cell count was done by Diff-Quick staining (Dade Behring Inc., Newark, Del.). Cell suspensions were found to contain >95% neutrophils.
The isolation of alveolar macrophages was based on our established protocols [5, 29]. Briefly, the thoracic cavity was opened aseptically and the trachea separated from the surrounding tissue. Bronchoalveolar lavage (BAL) was then performed according to our published protocol [5]. Three lavages were performed on each animal. The pooled lavage cells were then washed once in PBS and the cell viability was determined by Trypan blue exclusion assay (Gibco Life Technologies, N.Y). The cells were then diluted in RPMI-1640 medium (without phenol red) (Gibco Life Technologies, N.Y) supplemented with 2% heat activated FBS and 2-ME.
Recombinant gpIL-8 treatment and mycobacterial infection of neutrophils
Freshly isolated neutrophils were treated with recombinant IL-8 in a 12-well plate (2×106/well) for 2 h at 37°C in 5% CO 2, washed with PBS, and resuspended in media to a final cell concentration of 2×106/ml. For some experiments, the rgpIL-8-treated neutrophils were cultured in a 96-well plate and infected with M. tuberculosis H37Rv at an MOI of 5 according to our previously published protocol [22]. The viability of the neutrophils was measured using the lactate dehydrogenase assay (Promega) and > 80% cells were found to be viable after 24 h. For co-culture experiments, the rgpIL-8-treated, infected neutrophils (2×105/well) were cultured in the upper well (insert) of a 24-well Transwell system (0.4μm pore size) (Corning Costar) while the AM (5×105/well) were cultured in the lower well. The controls used included untreated, infected neutrophils or uninfected, untreated neutrophils in the upper wells. AM were harvested for cytokine mRNA assays after 24h in non-contact co-cultures with infected neutrophils. Supernatants from the lower well which were in contact with AM were plated on 7H9 agar plates and incubated at 37°C in a CO2 incubator. No mycobacterial colonies were found on these plates, indicating that bacteria from infected neutrophils in the insert did not transfer to the lower chamber. In some experiments, neutralizing polyclonal anti-gpTNF and anti-gpIL-8 antibodies were added to the wells at a dilution of 1:600.
Cytokine mRNA and protein analyses
Total RNA from neutrophils or AM was isolated using the RNeasy kit (QIAGEN, Valencia, Calif.) with the addition of RNase-free DNase according to the manufacturer’s instructions [5, 28]. Reverse transcription was performed using MultiScribe reverse transcriptase and random hexamers (Applied Biosystems, Branchburg, N.J.). The real-time primers for guinea pig TNF-α, IL-1β, IL-8 and hypoxanthine ribose transferase (HPRT) mRNA were designed using Primer Express software (Applied Biosystems) as previously published [5, 27, 30]. Fold induction for cytokine mRNA was determined from the threshold cycle (Ct) values and was normalized using HPRT expression and control values (cells cultured in medium alone at each time point) [5, 30]. The supernatants of cell cultures were collected and the concentrations of TNF- α and IL-8 protein were measured using the L929 cytotoxicity assay and ELISA, respectively, as we have published previously [5, 29, 31].
H2O2 production by AM
AM were cultured separately in a 96-well tissue culture plate. Supernatants from rgpIL-8 treated or untreated, infected or uninfected, neutrophils were harvested at 24 h and then added to AM in separate wells after removal of existing medium. Some of the wells also contained a stimulant [phorbol myristate acetate (100 ng/ml PMA)] of H2O2 production or a scavenger [100–200 μM] Catalase, (Sigma, St. Louis, Mo)] of H2O2. Twenty four hours later, medium was removed from the wells and H2O2 production by the AM was measured using the horseradish peroxide-dependent oxidation of phenol red as reported previously by us [31].
Analysis of neutrophil apoptosis
Neutrophils were seeded in Petri dishes and infected with M. tuberculosis H37Rv at an MOI of 5 for 3 h at 37°C. The controls used were uninfected and staurosporine-treated (50 μg/ml) (Sigma Chemical Co., St. Louis, MO) cells as previously described [32]. The cells were centrifuged at 150 × g for 10 min to remove the extracellular bacteria and then washed with sterile, cold PBS for 10 min at 300 × g. They were then cultured in media containing gentamicin (100 μg/ml) for 24 h from the time of neutrophil infection. The cells were centrifuged at 300 × g, washed once with PBS and processed using the Annexin V- FLUOS staining kit (Roche Diagnostics Corp., Indianapolis, IN). Cells were incubated in the incubation buffer for 10 to 15 min at room temperature, then centrifuged and suspended in 0.5 ml of incubation buffer. The infected, stained cells were incubated with 1% formaldehyde for 5 min and finally washed with PBS for 10 min at 300 × g. Apoptosis was measured by flow cytometry (Becton Dickinson, Palo Alto, CA) using Cell Quest software (BD Biosciences, Mountainview, CA). Fluorescence parameters were gated using unstained and single-stained untreated cells. At least 10,000 cells were counted in each sample and the total percentage of apoptotic cells was expressed as the total of both early (Annexin V+) and late (Annexin V+/PI +) apoptosis.
Phagocytosis of apoptotic neutrophils by macrophages
The phagocytic assay used was based on a myeloperoxidase (MPO) staining assay which has been published previously [33]. Guinea pig AM (5×106/ml) were isolated and plated on 8-well fibronectin coated plates (BD Biocoat) for 2 h. The non-adherent cells were washed off with warm PBS and fresh RPMI media was added. The infected, apoptotic neutrophils (2×106/ml) isolated from the same animal were then transferred to the wells containing AMs and incubated for 3 h at 37°C in a CO2 incubator. After 1 h, the un-ingested apoptotic neutrophils were removed, and AM were washed with PBS and fixed overnight with 1% formalin. O-dianisidine (1.25 mg/ml) and 0.05 % hydrogen peroxide were then added to the fixed AM and incubated for 20 min to stain for MPO as a marker of ingested neutrophils. Phagocytosis of apoptotic neutrophils was quantified under a phase contrast microscope by counting at least 500 macrophages in five randomly selected microscopic fields in each well using a 40X objective. Cells containing discrete, round, MPO-positive inclusions were scored as having ingested one or more apoptotic neutrophils. The AM were routinely negative for MPO staining.
Intracellular bacterial survival assays
To determine the effect of secreted neutrophil products on the ability of AM to control intracellular mycobacterial growth, supernatants from rgpIL-8-treated and/or infected neutrophils were centrifuged at 8000 × g for 5 min and then transferred to AM which had been cultured in a 96-well plate. After 6 h, the supernatants were removed and the AM were then infected with live virulent M. tuberculosis H37Rv (MOI of 1) in antibiotic-free RPMI 1640 with 10% FBS. After 3 h of infection, extracellular mycobacteria were washed off, and macrophage cultures were incubated in medium containing gentamicin (50 μg/ml) and 10% FBS for up to 6 days. The cultures were pulsed with 1 μCi of [3H] uracil for the final 24 h of culture and harvested using a FilterMate Cell Harvester. [3H]uracil incorporation was measured in a scintillation counter (Beckman LS-1801) as previously described [31].
To determine the presence of viable mycobacteria in neutrophil apoptotic bodies, the infected cells were cultured in media containing gentamicin in a 24-well plate for 24 h and then pelleted at 8000 × g. The cell pellets were lysed using 0.1% sodium dodecyl sulphate (Sigma Chemical Co., St. Louis, MO) and both the supernatants and the lysates were inoculated onto 7H10 agar plates (Becton Dickinson). The plates were incubated at 37°C for 3–4 weeks and the viable bacterial count was expressed as colony forming units (CFU). The percentage of bacteria phagocytosed was calculated by plating an aliquot of the bacteria initially used to infect the neutrophils.
Statistics
The Analysis of Variance (ANOVA) was used to determine the statistical significance of main treatment effects, and a between-means comparison was performed, using Tukey’s post hoc analysis. In some cases, a two-tailed t-test was used where appropriate. A 95% confidence interval was selected as the criterion for statistical significance. The statistical tests were performed using SAS software (Release 8.01, SAS Institute Inc, NC).
Results
Expression of TNF-α and IL-8 in rgpIL-8 treated, infected neutrophils
Neutrophils were first treated with rgpIL-8 at two doses (10 and 200 ng/ml) and then infected with M. tuberculosis H37Rv. Control cells were untreated and uninfected. Figure 1A shows the significant levels of TNF-α mRNA (p<0.05) induced in the 200ng rgpIL-8 treated, infected neutrophils as compared to the other treatment groups at 6 h. In the case of IL-8 mRNA, the 200 ng treated, infected neutrophils contained significant levels of IL-8 mRNA as compared to the infected neutrophils (p<0.05). Supernatants from these cultures were then assayed for the presence of TNF-α and IL-8 protein. As seen in Figure 1B, the higher dose (200 ng/ml) rgpIL-8-treated and infected neutrophils produced significantly more TNF-α protein compared to the other treatment groups at 18 h after infection (p<0.05). Similarly, rgpIL-8-treated, infected neutrophils also produced significantly more IL-8 protein, but only when compared to the untreated, uninfected cells (p< 0.05).
Figure 1. Expression of TNF-α and IL-8 mRNA and protein in rgpIL-8 treated, infected neutrophils.
Panel A illustrates the expression of TNF-α and IL-8 mRNA in neutrophils after infection with M. tuberculosis H37Rv at an MOI of 5 at 6 h. The treatment groups were infected (In) and rgpIL-8 treated, infected [(10ng +In) and (200ng+In)] neutrophils. The fold induction was determined from Ct values normalized for HPRT expression and then normalized to values from uninfected cells for each respective time point. Panel B illustrates the production of TNF-α and IL-8 protein in supernatant fluids from the same cultures in panel A. TNF-α and IL-8 protein were measured by the L929 cytotoxicity assay and ELISA, respectively. Results are expressed as means ± standard errors of the means of results from three animals. Differences between treatment groups at each time point were analyzed by using ANOVA followed by Duncan’s post hoc analysis (*p < 0.05).
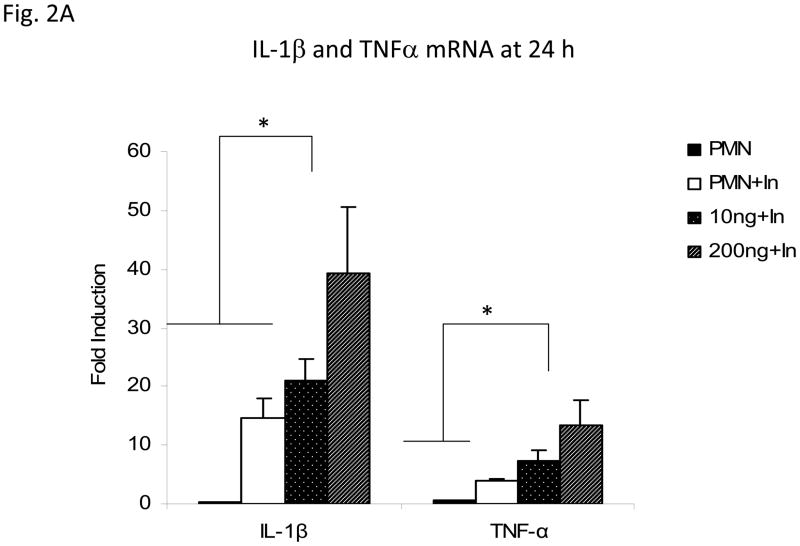
Expression of IL-1β and TNF-α mRNA in AM exposed to supernatants from infected, rgpIL-8-treated neutrophils
The Transwell insert culture system was set up either with rgpIL-8 treated, infected neutrophils, or untreated (either uninfected or infected) neutrophils in the upper well and AM in the lower well. The insert containing neutrophils was removed after 24 h and AM were then harvested 24 h later. The RNA from the AM was reverse transcribed and the levels of IL-1β and TNF-α mRNA were measured by real-time PCR. As seen in Figure 2A, IL-1β mRNA was significantly upregulated (p<0.05) at 24 h in the AM exposed to 200ng/ml rgpIL-8 treated, infected neutrophils as compared to the lower dose (10ng) treated, infected neutrophils or to both of the untreated (uninfected or infected) neutrophils. Soluble products of the rgpIL-8 treated (200ng) and infected neutrophils induced significant levels of TNF-α mRNA (p<0.05) in guinea pig AM, when compared to the soluble products of untreated and infected neutrophils.
Figure 2. Exposure to supernatants from rgpIL-8 treated, infected neutrophils affects the activation status of alveolar macrophages.
Alveolar macrophages (5×105/well) were exposed in a contact-independent manner to supernatants from rgpIL-8 treated, infected neutrophils (2×105/well) (MOI of 5) in a Transwell insert co-culture system for 24 h. The treatment groups were infected (In) and rgpIL-8 treated, infected [(10ng +In) and (200ng+In)] neutrophils. The controls used were infected (PMN+In) or uninfected (PMN) neutrophils. Panel A illustrates the expression of IL-1β and TNF-α mRNA in alveolar macrophages measured by RT-PCR at 24 h and the fold induction was determined from Ct values normalized for HPRT expression and then normalized to values from treated alveolar macrophages at 0 h. Panel B shows the H2O2 production expressed in nanomoles measured by using horseradish peroxide-dependent oxidation of phenol red. Phorbol myristate acetate (PMA) and catalase (Cat) were used as stimulants and inhibitors of H2O2 production by macrophages, respectively. The other controls used were supernatants from infected (In Sup) or uninfected (Un Sup) neutrophils and untreated AM (Un AM). For panel C, supernatants from the different treatment groups of neutrophils were harvested at 18 h and transferred to AM and cultured for 6 h. The supernatants were then removed and AM were infected with M. tuberculosis H37Rv at an MOI of 1. After 3 h, the extracellular bacteria were removed and replaced with media containing gentamicin. [3H] uracil incorporation (cpm) by the viable, intracellular mycobacteria was measured on days 1 and 4. The results are expressed as means ± standard errors of the means of results from 3 animals per group. Differences between the different treatment groups at each time point were compared by using analysis of variance (ANOVA) followed by Duncan’s post hoc analysis (*p < 0.05).
Production of H2O2 in AM treated with supernatants from infected, rgpIL-8 treated neutrophils
To study the effect of soluble mediators from rgpIL-8 treated neutrophils on the activation of AM, the supernatants harvested from different treatment groups were added to the AM seeded in a separate 96 well plate. After 24 h, the amount of H2O2 released by the AM was measured. As can be seen in Figure 2B, AM stimulated with phorbol myristate acetate (PMA) alone responded positively and released a measurable amount of H2O2. AM exposed to supernatants from both rgpIL-8 treated and infected neutrophils [(200ng+In) Sup] produced significantly more H2O2 than that produced by AM exposed to supernatants from all other treatment groups (p< 0.05).
Intracellular bacterial growth in AM exposed to supernatants from rgpIL-8 treated neutrophils
The supernatants from the different treatment groups of rgpIL-8 treated neutrophils were harvested and transferred to AM seeded in a separate 96-well plate as described above. The AM were infected with virulent M. tuberculosis H37Rv at an MOI of 1 and [3H]uracil incorporation by viable, intracellular mycobacteria was measured on days 1 and 4. Figure 2C illustrates the effect of the exposure of AM to the supernatants from rgpIL-8 treated neutrophils on bacterial survival. At day 1, there was no significant effect of either the rgpIL-8 treatment or infection on levels of viable intracellular bacteria in AM. However, by day 4, AM exposed to supernatants from infected neutrophils treated with 200ng/ml of rgpIL-8 showed a significant decrease in intracellular bacterial replication as compared to all other treatment groups.
Effect of anti-TNF-α and anti-IL-8 antibody on cytokine mRNA expression in AM exposed to supernatants from rgpIL-8 treated neutrophils
In order to evaluate the effect of neutralizing gpTNF-α and gpIL-8 on the AM response to neutrophil supernatants, rabbit polyclonal antisera to each recombinant protein were added to the AM in the lower wells of the Transwell insert system during 48 hr of exposure to the soluble products of infected neutrophils in the upper chamber. Both of these antisera have been shown previously to neutralize the biological activity of their respective cytokines [28, 31]. The AM were then harvested from the lower chamber 24 h following removal of the neutrophils, RNA was reverse transcribed, and levels of IL-1β and TNF-α mRNA were measured by real-time PCR. There was a significant (p<0.05) decrease in IL-1β (Fig. 3A) and TNF-α (Fig. 3B) mRNA production in AM treated with the anti-gpTNF Ab as compared to the untreated AM (Fig. 4A & B). There was no significant effect of treatment with the anti-rgpIL-8 Ab on the cytokine mRNA expression in AM exposed to supernatants from the rgpIL-8 treated and infected neutrophils. Normal rabbit IgG was used as a negative control and it had no effect on the cytokine expression in AM exposed to rgpIL-8 treated neutrophils.
Figure 3. Effect of anti-TNF-α and anti-IL-8 antibody on cytokine mRNA expression to AM exposed to supernatants from rgpIL-8 treated, infected neutrophils.
Alveolar macrophages were exposed in a contact-independent manner to supernatants from rgpIL-8 treated, infected neutrophils, (In; MOI of 5) and untreated neutrophils, either infected or uninfected, in a Transwell insert co-culture system for 24 h. Some cultures were treated with anti-TNF-α or anti-IL-8 Ab. The levels of IL-1β (Panel A) and TNF-α (Panel B) mRNA in AM were quantified at 24 h and the fold induction was determined from the Ct values normalized for HPRT expression and then normalized to values from cells at 0 h. Results are expressed as the means ± standard errors of the means of results from 3 animals. Differences between the treatments were compared by using analysis of variance (ANOVA) followed by Duncan’s post hoc analysis (*p < 0.05).
Figure 4. Measurement of apoptosis in infected guinea pig neutrophils and assessment of viable bacteria in infected, apoptotic neutrophils.
The neutrophils were infected (In) with M. tuberculosis H37Rv at an MOI of 5. After 18 and 24 h of infection, apoptosis was measured by using FACS analysis with Annexin V and PI staining (Panel A). Uninfected cells and staurosporine treated neutrophils were used as negative and positive controls, respectively. Results are expressed as the means ± standard errors of the means from four independent experiments. The differences between the treatment groups at each time point were analyzed using ANOVA followed by Tukey’s post hoc analysis. (*p<0.05). Intracellular bacterial survival in apoptotic guinea pig neutrophils was also assessed as shown in Panel B. The neutrophils were infected (In) with M. tuberculosis H37Rv at an MOI of 5. After 3 h of infection, extracellular bacteria were removed and the cells were cultured for 24 h in gentamicin containing media. At 3 and 24 h, cell lysates and supernatants were plated on 7H10 agar plates and incubated at 37°C for 3–4 weeks to determine the number of bacteria surviving within the apoptotic neutrophils. Viable bacterial counts were expressed as mean ± SEM of colony forming units (CFU)/well. The results are the mean of three independent experiments.
Mycobacterial infection induces apoptosis in guinea pig neutrophils and viable bacteria are present in apoptotic neutrophils
To further evaluate the role of neutrophils in host defense in tuberculosis, we first assessed the effect of virulent mycobacterial infection on apoptosis in guinea pig neutrophils. The neutrophils were infected with M. tuberculosis H37Rv at an MOI of 5 and apoptosis was measured by using FACS analysis after 18 and 24 h. Figure 4A illustrates the percentages of total apoptosis (Annexin V-positive + Annexin V/PI double-positive) as mean ± SEM from four independent experiments. After 18 and 24 h of culture, 24.3 ± 0.4 % and 45.9 ± 6.4 % of the uninfected neutrophils were apoptotic, respectively. Of the neutrophils infected with M. tuberculosis, 72. 5 ± 1.2 % and 85.7 ± 0.6% cells were apoptotic after 18 and 24 h or culture, respectively, indicating that infection with virulent M. tuberculosis H37Rv significantly (p<0.05) promoted apoptosis in guinea pig neutrophils. Staurosporine was used as a positive control and > 90 % of staurosporine-treated neutrophils were apoptotic at both time intervals. The proportion of apoptotic neutrophils which were produced by mycobacterial infection or staurosporine treatment were found to be significantly greater than uninfected neutrophils at both 18 and 24 h (p < 0.05).
To assess the presence of viable mycobacteria in the apoptotic neutrophils, the gentamicin protection assay was used [31]. As observed in Figure 4B, viable bacteria were found in neutrophils at 3 and 24 h after infection. There was no significant change in the number of viable bacteria in the neutrophils between 3 and 24 h. Approximately 27 % of the total bacteria added were phagocytosed by the neutrophils after 3 h (data not shown). The supernatants were also plated and the very low number of viable bacteria in the supernatants at both time points indicate successful killing of the extracellular bacteria by gentamicin. These results demonstrate that the apoptotic neutrophils contained significant numbers of viable, intracellular mycobacteria at the time they were fed to AM.
Cytokine mRNA was induced in AM after ingestion of infected, apoptotic neutrophils
Myeloperoxidase (MPO) staining demonstrated the presence of apoptotic neutrophils within AM (data not shown), as identified as brown condensed bodies in the cytoplasm of AM. The percentage of AM which had phagocytosed the apoptotic neutrophils was found to be 32 ± 3.1% in several experiments. The apoptotic neutrophils containing viable mycobacteria were incubated with AM, and the RNA was harvested after 3 h when the un-ingested apoptotic neutrophils were removed. Figure 5 illustrates that IL-1β mRNA expression levels were significantly upregulated in AM after ingestion of infected, apoptotic neutrophils compared to AM taking up staurosporine-treated, apoptotic neutrophils (p<0.05). A similar result was obtained for TNF-α mRNA expression levels measured in these cultures. IL-10 and TGF-β mRNA were also measured but could not be detected (data not shown).
Figure 5. Cytokine mRNA levels in guinea pig AM after ingestion of infected, apoptotic neutrophils.
Expression of IL-1β and TNF-α mRNA in AM after incubation with the infected, apoptotic neutrophils (In PMN). Apoptotic neutrophils were fed to the AM for 3 h. Uninfected or staurosporine-treated neutrophils (St PMN) were used as controls. After removal of un-ingested neutrophils, AM were cultured for 3 h. The fold induction was determined from the Ct values normalized for HPRT expression and then normalized to values from AM incubated with uninfected neutrophils. Results are expressed as the means ± standard errors of the means of results from three animals. The difference between the two treatment groups was determined by using a two-tailed t-test at a 95% confidence interval. (*p<0.05)
Mycobacterial growth in AM was suppressed after ingestion of infected apoptotic neutrophils
Infected, apoptotic neutrophils and M. tuberculosis H37Rv alone were incubated with AM under conditions which were expected to result in the same level of infection of AM. The [3H] uracil incorporation by viable, intracellular mycobacteria was then measured on days 1 and 6 (Figure 6). At Day 1, there was no significant difference between the addition of infected, apoptotic neutrophils or infection with M. tuberculosis H37Rv alone on levels of viable intracellular mycobacteria in AM. However, by Day 6, the AM cultured with infected, apoptotic neutrophils showed a significant decrease (p<0.05) in intracellular bacterial growth as compared to AM exposed to M. tuberculosis H37Rv alone.
Figure 6. Intracellular bacterial survival in guinea pig AM after ingestion of infected apoptotic neutrophils.
Infected, apoptotic neutrophils were fed to AM (open symbols) while some AM were infected with M. tuberculosis alone (closed symbols) at an MOI estimated to result in the same number of ingested bacilli. The [3H] uracil incorporation (cpm) by the viable, intracellular mycobacteria was measured on days 1 and 6. Results are presented as the means ± standard errors of the means of 3 animals per group. The differences between the two treatments at each time point were determined by using a two-tailed t-test at a 95% confidence interval (* p<0.05).
Discussion
In this study, we have demonstrated that rgpIL-8-treated, mycobacteria-infected guinea pig neutrophils produce cytokines (e.g., TNFα) which can activate alveolar macrophages in non-contact co-cultures. Recombinant gpIL-8 was prepared according to our previously published procedure and found to be biologically active in a chemotaxis assay [28]. Guinea pig neutrophils treated with rgpIL-8 and infected with the virulent H37Rv strain of M. tuberculosis produced significant amounts of IL-8 and TNF-α mRNA and protein (Fig. 1A and 1B). Previously, we observed that rgpIL-8 treated guinea pig neutrophils infected with the attenuated H37Ra strain of M. tuberculosis produced more IL-8 mRNA and protein than neutrophils treated with the chemokine alone [28]. Similarly, in the present study, treatment of guinea pig neutrophils with rgpIL-8 in the absence of infection did not induce TNF-α or IL-8 mRNA or protein above that seen in untreated cells (data not shown). However, human peripheral blood neutrophils stimulated with IL-8 alone made more IL-1β and TNFα [10]. Transcriptional profiling studies have also shown that treatment of human neutrophils with IL-8 alone had an effect on many genes encoding signal transducers, membrane receptors and transcriptional factors, thus effecting the response of neutrophils to the external milieu [10]. Moreover, treatment of neutrophils with IL-8 and TNF-α stimulated an increase in respiratory burst activity [34]. Our data show that IL-8 stimulation alone is not sufficient to drive increased cytokine production in guinea pig neutrophils, but does prime the neutrophils to respond to mycobacterial infection with increased IL-8 and TNFα mRNA and protein production.
AM exposed to supernatants from rgpIL-8 treated and infected neutrophils in a contact-independent co-culture system produced significant levels of IL-1β and TNF-α mRNA and H2O2 (Fig. 2A and 2B, respectively) compared to untreated AM or AM treated with supernantants from non-treated neurophils. An increase in respiratory burst in AM after treatment with recombinant TNF-α has been reported previously [35]. Since the activation of AM by infected, rgpIL-8-treated neutrophils occurred when the two cell types were separated by a Transwell insert, it is likely that soluble mediators present in the neutrophil supernatants were responsible for the increased production of H2O2 and cytokine mRNA. More importantly, AM exposed to supernatants from rgpIL-8 treated, infected neutrophils significantly controlled mycobacterial growth over 4 days in culture as compared to AM exposed to supernatants from untreated, or uninfected, rgpIL-8 treated neutrophils (Fig 2C). These data suggest the presence of soluble neutrophil mediators which induced effective anti-mycobacterial mechanisms in AM.
The use of TNF-α and IL-8 polyclonal antibody in neutralization experiments in Fig 3 showed that TNF-α is one of the soluble mediators produced by neutrophils which is responsible for the activation of AM. TNF-α present in the supernatants to which the AM are exposed may have been produced by either AM or neutrophils or both. TNF-α produced by neutrophils may have a paracrine effect on AM to induce its own production [36]. The anti-TNF-α polyclonal antiserum would have neutralized TNF-α produced by either cell type. Future studies will determine the relative contributions of each cell type to the total TNF-α being produced in the non-contact co-cultures. Neutralization of TNF-α production by human PBMC after stimulation with mycobacterial lipoarabinomannan decreased the production of CCL2, CXCL8 and CCL4, emphasizing the importance of TNF-α in regulating chemokine production during mycobacterial infection [37]. Our study demonstrates that the stimulation of infected neutrophils by exogenous IL-8 enhances the production of TNF-α in our Transwell insert culture system and contributes significantly to the overall activation of AM. As the necessary reagents for the guinea pig become available, future studies will examine the roles of other soluble neutrophil products which may play a role in activation of AM. In addition, treatment of infected guinea pigs with anti-rgpIL-8 antiserum will reveal the effect of neutralization of IL-8 in vivo on granuloma formation and disease resistance and will contribute to a better understanding of the role of neutrophils and their products in the pathogenesis of pulmonary tuberculosis in this biologically relevant model.
In this study, the uptake of infected, apoptotic guinea pig neutrophils by AM had a significant effect on cytokine mRNA levels and intracellular mycobacterial survival in macrophages. Our results (Fig. 4A) demonstrate the promotion of apoptosis in guinea pig neutrophils following infection with M. tuberculosis which is in accordance with various studies showing the stimulation of apoptosis in M. tuberculosis-infected neutrophils from other species [20, 24]. The infection of neutrophils with M. tuberculosis consistently induced apoptosis in approximately 85% of neutrophils. Figure 4B indicates the presence of viable bacteria in apoptotic neutrophils by the gentamicin protection assay. Thus, infected, apoptotic neutrophils may act as agents to transfer mycobacteria or antigenic material to guinea pig macrophages after they have been ingested.
The feeding of M. tuberculosis-infected, apoptotic guinea pig neutrophils to AM stimulated the production of pro-inflammatory IL-1β and TNFα mRNA compared to the feeding of uninfected, staurosporine-treated apoptotic neutrophils (Fig. 5). Other studies have shown increased production of TNF-α by human macrophages which ingested infected, apoptotic neutrophils [24]. Uptake of bacteria-infected apoptotic neutrophils stimulated of human macrophages compared to uptake of UV-induced apoptotic neutrophils [38]. Activation of mouse peritoneal macrophages following ingestion of neutrophils containing mycobacteria was also observed [13]. These latter studies imply that ingestion of infected neutrophils by macrophages may contribute to host defense.
In contrast, feeding of BCG-induced apoptotic neutrophils to macrophages led to TGF-β production [23]. Studies with Leishmania have also demonstrated that the uptake of infected, apoptotic neutrophils by human macrophages led to release of anti-inflammatory cytokines like TGF-β and increased intracellular multiplication and survival of Leishmania in those macrophages as compared to direct infection with parasites alone [39]. Other studies have also reported the induction of an anti-inflammatory response in macrophages after ingestion of apoptotic neutrophils [33]. This anti-inflammatory response may be a pathogenic strategy for microbes to suppress the immune response. We did not detect IL-10 or TGFβ mRNA in our culture system (data not shown).
The decreased intracellular survival of the mycobacteria in guinea pig AM fed infected, apoptotic neutrophils as compared to AM infected directly with mycobacteria (Fig. 6) suggests one of two mechanisms. First, the anti-mycobacterial functions or activation status of the AM may be enhanced by the presence of neutrophil granules which are known to have microbicidal properties. Transfer of these granules from neutrophils to macrophages via uptake of apoptotic neutrophils may result in acquisition of additional microbicidal properties [25]. It has also been suggested that neutrophils shed “pseudoplatelets” or “cell-like” particles, containing cytoplasmic contents and nuclear debris which can be ingested by macrophages [40]. These particles may contain myeloperoxidase [41], lactoferrin [13], defensins [42] and other antimicrobial peptides like LL-37 and HNP-1 [16] which might influence the survival of mycobacteria in AM fed infected, apoptotic neutrophils. Recently, Hsp72 produced by M. tuberculosis induced apoptotic neutrophils has been shown to enhance proinflammatory response [24].
Conversely, the mycobacteria may have been prevented by their presence within neutrophil apoptotic bodies from subverting macrophage anti-microbial functions and avoiding destruction within AM. The uptake of M. tuberculosis within apoptotic neutrophils may allow successful activation of anti-mycobacterial mechanisms within AM, resulting in control of intracellular growth. Our results suggest that the uptake of M. tuberculosis-infected, apoptotic neutrophils by guinea pig AM may actually be beneficial for the host and may represent an alternate pathway for AM to gain anti-mycobacterial functions. As the necessary reagents become available for the guinea pig, future studies will involve characterizing the molecular mechanisms involved in this activation of AM.
Acknowledgments
This work was supported by the National Institutes of Health Grant RO1 AI-15495 to Dr. David N. McMurray at Texas A&M College of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sibille Y, Reynolds HY. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis. 1990;141:471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- 2.Wallis RS, Ellner JJ. Cytokines and tuberculosis. J Leukoc Biol. 1994;55:676–681. doi: 10.1002/jlb.55.5.676. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Broser M, Cohen H, Bodkin M, Law K, Reibman J, Rom WN. Enhanced interleukin-8 release and gene expression in macrophages after exposure to Mycobacterium tuberculosis and its components. J Clin Invest. 1995;95:586–592. doi: 10.1172/JCI117702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedland JS, Remick DG, Shattock R, Griffin GE. Secretion of interleukin-8 following phagocytosis of Mycobacterium tuberculosis by human monocyte cell lines. Eur J Immunol. 1992;22:1373–1378. doi: 10.1002/eji.1830220607. [DOI] [PubMed] [Google Scholar]
- 5.Lyons MJ, Yoshimura T, McMurray DN. Mycobacterium bovis BCG vaccination augments interleukin-8 mRNA expression and protein production in guinea pig alveolar macrophages infected with Mycobacterium tuberculosis. Infect Immun. 2002;70:5471–5478. doi: 10.1128/IAI.70.10.5471-5478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peveri P, Walz A, Dewald B, Baggiolini M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 1988;167:1547–1559. doi: 10.1084/jem.167.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thelen M, Peveri P, Kernen P, von Tscharner V, Walz A, Baggiolini M. Mechanism of neutrophil activation by NAF, a novel monocyte-derived peptide agonist. FASEB J. 1988;2:2702–2706. [PubMed] [Google Scholar]
- 8.Daniels RH, Finnen MJ, Hill ME, Lackie JM. Recombinant human monocyte IL-8 primes NADPH-oxidase and phospholipase A2 activation in human neutrophils. Immunology. 1992;75:157–163. [PMC free article] [PubMed] [Google Scholar]
- 9.Wozniak A, Betts WH, Murphy GA, Rokicinski M. Interleukin-8 primes human neutrophils for enhanced superoxide anion production. Immunology. 1993;79:608–615. [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez FO, Sironi M, Vecchi A, Colotta F, Mantovani A, Locati M. IL-8 induces a specific transcriptional profile in human neutrophils: synergism with LPS for IL-1 production. Eur J Immunol. 2004;34:2286–2292. doi: 10.1002/eji.200324481. [DOI] [PubMed] [Google Scholar]
- 11.Larsen CG, Thomsen MK, Gesser B, Thomsen PD, Deleuran BW, Nowak J, Skodt V, Thomsen HK, Deleuran M, Thestrup-Pedersen K, et al. The delayed-type hypersensitivity reaction is dependent on IL-8. Inhibition of a tuberculin skin reaction by an anti-IL-8 monoclonal antibody. J Immunol. 1995;155:2151–2157. [PubMed] [Google Scholar]
- 12.Orme IM. The immunopathogenesis of tuberculosis: a new working hypothesis. Trends Microbiol. 1998;6:94–97. doi: 10.1016/s0966-842x(98)01209-8. [DOI] [PubMed] [Google Scholar]
- 13.Silva MT, Silva MN, Appelberg R. Neutrophil-macrophage cooperation in the host defence against mycobacterial infections. Microb Pathog. 1989;6:369–380. doi: 10.1016/0882-4010(89)90079-x. [DOI] [PubMed] [Google Scholar]
- 14.Appelberg R, Castro AG, Gomes S, Pedrosa J, Silva MT. Susceptibility of beige mice to Mycobacterium avium: role of neutrophils. Infect Immun. 1995;63:3381–3387. doi: 10.1128/iai.63.9.3381-3387.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fulton SA, Reba SM, Martin TD, Boom WH. Neutrophil-mediated mycobacteriocidal immunity in the lung during Mycobacterium bovis BCG infection in C57BL/6 mice. Infect Immun. 2002;70:5322–5327. doi: 10.1128/IAI.70.9.5322-5327.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martineau AR, Newton SM, Wilkinson KA, Kampmann B, Hall BM, Nawroly N, Packe GE, Davidson RN, Griffiths CJ, Wilkinson RJ. Neutrophil-mediated innate immune resistance to mycobacteria. J Clin Invest. 2007;117:1988–1994. doi: 10.1172/JCI31097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos-Kichik V, Mondragon-Flores R, Mondragon-Castelan M, Gonzalez-Pozos S, Muniz-Hernandez S, Rojas-Espinosa O, Chacon-Salinas R, Estrada-Parra S, Estrada-Garcia I. Neutrophil extracellular traps are induced by Mycobacterium tuberculosis. Tuberculosis (Edinb) 2009;89:29–37. doi: 10.1016/j.tube.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Pedrosa J, Saunders BM, Appelberg R, Orme IM, Silva MT, Cooper AM. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect Immun. 2000;68:577–583. doi: 10.1128/iai.68.2.577-583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seiler P, Aichele P, Raupach B, Odermatt B, Steinhoff U, Kaufmann SH. Rapid neutrophil response controls fast-replicating intracellular bacteria but not slow-replicating Mycobacterium tuberculosis. J Infect Dis. 2000;181:671–680. doi: 10.1086/315278. [DOI] [PubMed] [Google Scholar]
- 20.Kasahara K, Sato I, Ogura K, Takeuchi H, Kobayashi K, Adachi M. Expression of chemokines and induction of rapid cell death in human blood neutrophils by Mycobacterium tuberculosis. J Infect Dis. 1998;178:127–137. doi: 10.1086/515585. [DOI] [PubMed] [Google Scholar]
- 21.Suttmann H, Lehan N, Bohle A, Brandau S. Stimulation of neutrophil granulocytes with Mycobacterium bovis bacillus Calmette-Guerin induces changes in phenotype and gene expression and inhibits spontaneous apoptosis. Infect Immun. 2003;71:4647–4656. doi: 10.1128/IAI.71.8.4647-4656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawant KV, McMurray DN. Guinea pig neutrophils infected with Mycobacterium tuberculosis produce cytokines which activate alveolar macrophages in noncontact cultures. Infect Immun. 2007;75:1870–1877. doi: 10.1128/IAI.00858-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Avila H, Roque NR, Cardoso RM, Castro-Faria-Neto HC, Melo RC, Bozza PT. Neutrophils recruited to the site of Mycobacterium bovis BCG infection undergo apoptosis and modulate lipid body biogenesis and prostaglandin E production by macrophages. Cell Microbiol. 2008;10:2589–2604. doi: 10.1111/j.1462-5822.2008.01233.x. [DOI] [PubMed] [Google Scholar]
- 24.Persson YA, Blomgran-Julinder R, Rahman S, Zheng L, Stendahl O. Mycobacterium tuberculosis-induced apoptotic neutrophils trigger a pro-inflammatory response in macrophages through release of heat shock protein 72, acting in synergy with the bacteria. Microbes Infect. 2008;10:233–240. doi: 10.1016/j.micinf.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Tan BH, Meinken C, Bastian M, Bruns H, Legaspi A, Ochoa MT, Krutzik SR, Bloom BR, Ganz T, Modlin RL, Stenger S. Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J Immunol. 2006;177:1864–1871. doi: 10.4049/jimmunol.177.3.1864. [DOI] [PubMed] [Google Scholar]
- 26.Grover AA, Kim HK, Wiegeshaus EH, Smith DW. Host-parasite relationships in experimental airborne tuberculosis. II. Reproducible infection by means of an inoculum preserved at −70 C. J Bacteriol. 1967;94:832–835. doi: 10.1128/jb.94.4.832-835.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshimura T, Johnson DG. cDNA cloning and expression of guinea pig neutrophil attractant protein-1 (NAP-1). NAP-1 is highly conserved in guinea pig. J Immunol. 1993;151:6225–6236. [PubMed] [Google Scholar]
- 28.Lyons MJ, Yoshimura T, McMurray DN. Interleukin (IL)-8 (CXCL8) induces cytokine expression and superoxide formation by guinea pig neutrophils infected with Mycobacterium tuberculosis. Tuberculosis (Edinb) 2004;84:283–292. doi: 10.1016/j.tube.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Lasco TM, Yamamoto T, Yoshimura T, Allen SS, Cassone L, McMurray DN. Effect of Mycobacterium bovis BCG vaccination on Mycobacterium-specific cellular proliferation and tumor necrosis factor alpha production from distinct guinea pig leukocyte populations. Infect Immun. 2003;71:7035–7042. doi: 10.1128/IAI.71.12.7035-7042.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen SS, McMurray DN. Coordinate cytokine gene expression in vivo following induction of tuberculous pleurisy in guinea pigs. Infect Immun. 2003;71:4271–4277. doi: 10.1128/IAI.71.8.4271-4277.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho H, Lasco TM, Allen SS, Yoshimura T, McMurray DN. Recombinant guinea pig tumor necrosis factor alpha stimulates the expression of interleukin-12 and the inhibition of Mycobacterium tuberculosis growth in macrophages. Infect Immun. 2005;73:1367–1376. doi: 10.1128/IAI.73.3.1367-1376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee WD, Flynn AN, LeBlanc JM, Merrill JK, Dick P, Morck DW, Buret AG. Tilmicosin-induced bovine neutrophil apoptosis is cell-specific and downregulates spontaneous LTB4 synthesis without increasing Fas expression. Vet Res. 2004;35:213–224. doi: 10.1051/vetres:2004004. [DOI] [PubMed] [Google Scholar]
- 33.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Dervort AL, Lam C, Culpepper S, Tuschil AF, Wesley RA, Danner RL. Interleukin-8 priming of human neutrophils is not associated with persistently altered calcium fluxes but is additive with lipopolysaccharide. J Leukoc Biol. 1998;64:511–518. doi: 10.1002/jlb.64.4.511. [DOI] [PubMed] [Google Scholar]
- 35.Cho H, de Haas R, Jeevan A, McMurray DN. Differential activation of alveolar and peritoneal macrophages from BCG-vaccinated guinea pigs. Tuberculosis (Edinb) 2008;88:307–316. doi: 10.1016/j.tube.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 37.Algood HM, Chan J, Flynn JL. Chemokines and tuberculosis. Cytokine Growth Factor Rev. 2003;14:467–477. doi: 10.1016/s1359-6101(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 38.Zheng L, He M, Long M, Blomgran R, Stendahl O. Pathogen-induced apoptotic neutrophils express heat shock proteins and elicit activation of human macrophages. J Immunol. 2004;173:6319–6326. doi: 10.4049/jimmunol.173.10.6319. [DOI] [PubMed] [Google Scholar]
- 39.van Zandbergen G, Klinger M, Mueller A, Dannenberg S, Gebert A, Solbach W, Laskay T. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J Immunol. 2004;173:6521–6525. doi: 10.4049/jimmunol.173.11.6521. [DOI] [PubMed] [Google Scholar]
- 40.Hanker JS, Giammara BL. Neutrophil pseudoplatelets: their discrimination by myeloperoxidase demonstration. Science. 1983;220:415–417. doi: 10.1126/science.6301006. [DOI] [PubMed] [Google Scholar]
- 41.Leung KP, Goren MB. Uptake and utilization of human polymorphonuclear leukocyte granule myeloperoxidase by mouse peritoneal macrophages. Cell Tissue Res. 1989;257:653–656. doi: 10.1007/BF00221477. [DOI] [PubMed] [Google Scholar]
- 42.Sharma S, Verma I, Khuller GK. Antibacterial activity of human neutrophil peptide-1 against Mycobacterium tuberculosis H37Rv: in vitro and ex vivo study. Eur Respir J. 2000;16:112–117. doi: 10.1034/j.1399-3003.2000.16a20.x. [DOI] [PubMed] [Google Scholar]