Abstract
Objectives
Epothilone B (EpoB), like Taxol, stabilizes microtubules resulting in an inhibition of microtubule dynamic instability. The drug is being evaluated in phase III clinical trials. An EpoB analog, Ixabepilone, was approved by the FDA for the treatment of taxane-resistant metastatic breast cancer. Epithelial cell adhesion antigen (EpCAM) expression is significantly higher in epithelial ovarian cancer cells compared to normal cells. The effects of EpoB and other microtubule-interacting agents on surface EpCAM expression were studied.
Methods
Biochemical methods, immunofluorescence and flow cytometry were used to identify EpCAM expression on the surface of the ovarian cancer cell line, Hey, after exposure to EpoB. The relationship between EpoB-mediated surface EpCAM expression and EpoB-induced α-tubulin acetylation, a surrogate marker for stable microtubules, in Hey cells also were investiaged.
Results
Nanomolar concentrations of EpoB, Taxol, discodermolide or vinblastine caused a marked increase in surface EpCAM expression in Hey cells. Alpha-tubulin acetylation was increased following treatment with Taxol, EpoB and discodermolide, but not with vinblastine, indicating that drug-enhanced surface EpCAM expression does not correlate with tubulin acetylation or stabilization. Unexpectedly, EpoB did not have a significant effect on EpCAM mRNA expression, nor did it alter the level of total cellular EpCAM in Hey cells.
Conclusions
The results indicate that disruption of the microtubule cytoskeleton is associated with the redistribution of cell surface antigens in ovarian cancer cells. The increase in cell surface EpCAM antigen density may facilitate the antibody targeting of EpCAM-positive ovarian cancer cells.
Keywords: EpCAM, Taxol, Epothilone B, acetylated tubulin, Hey cells
Introduction
Epithelial ovarian cancer is a very aggressive disease, for which standard treatment following surgery is a combination of a taxane and a platinum compound. The majority of ovarian cancers ultimately recur and in many cases this is related to the emergence of drug resistance [1].
Epothilone B (EpoB), like Taxol, binds to β-tubulin and stabilizes microtubules, thereby repressing dynamic instability of spindle microtubules and inhibiting mitosis [2]. It has been demonstrated that EpoB is active in Taxol resistant cell lines and tumors [3, 4]. The drug causes arrest at the G2-M phase of the cell cycle leading to cell death [5]. An EpoB analog, Ixabepilone, has been approved by the FDA for the treatment of taxane-resistant metastatic breast cancer [6–8].
Cell adhesion molecules (CAMs) are receptors that are actively involved in regulating growth, differentiation, and cell death. Epithelial cell adhesion molecule (EpCAM) has been identified as a marker of epithelial lineage [9] and cancer stem cells [10]. Increased surface EpCAM expression (2-10-fold) has been reported in several adenocarcinoma cell lines following Taxol treatment [11]. EpCAM is a 40 kD (314 amino acid residues) type I transmembrane glycoprotein, not structurally related to the other CAM families, and functions as a homophilic intercellular adhesion molecule. Its extracellular domain contains two epidermal growth factor-like repeats. The short intracellular domain (EpICD) contains 26 amino acids with two binding sites for α-actinin that link EpCAM to the actin cytoskeleton [12]. EpCAM is an oncogenic signaling protein [13, 14], since it has been demonstrated recently that upon intramembrane proteolytic cleavage, EpICD is released, associates with components of the wnt signaling pathway, and translocates into the nucleus. This multiprotein complex regulates gene transcription and results in cell proliferation and tumor formation in mice [15].
Staining of ovarian epithelial cancer cells revealed about 80% EpCAM positivity [16]. The expression level of EpCAM mRNA in ovarian cancer cells is approximately 400-fold higher than in normal human ovarian cells [17]. Immunotherapy against EpCAM in patients with ovarian cancer is presently being evaluated. A recent study reported the usefulness of EpCAM as a suitable target for HER-2 negative, ER negative and PR negative breast tumors [18].
Studies on posttranslational modifications of microtubules revealed that acetylation of α-tubulin may play a role in the maintenance of stable populations of microtubules [19]. The acetylation occurs on the € amino group of lysine 40. Microtubule stabilizing agents such as Taxol also induce acetylation of α-tubulin at the same site. Acetylated α-tubulin has been considered as a surrogate marker for stable microtubules. In addition, acetylation and deacetylation of α-tubulin have been reported to be coupled to microtubule turnover [20, 21].
In this report, we studied the effects of EpoB and a variety of other microtubule-interacting agents on surface EpCAM expression in an ovarian cancer cell line, Hey. We also investigated the relationship between this expression and the status of tubulin acetylation.
Materials and methods
Material and cells
Epothilone B, Taxol and discodermolide (Disco) were obtained as described [22, 23]. Vinblastine, cis-platinum (II) diamine dichloride (CDDP), carboplatin and 5-fluorouracil (5-FU) were purchased from Sigma. Monoclonal anti-EpCAM antibodies B302(323/A3) and E144 were from Abcam Inc. Monoclonal anti-acetylated tubulin antibody (6-11B-1), and anti-α-tubulin antibody (DM1A), were purchased from Sigma. FITC-conjugated IgG and anti-HEA-(EpCAM)-FITC antibodies were from Chemicon and Milteney Biotec, respectively. The human ovarian cancer cell line, Hey, was grown in RPMI 1640 containing 10% fetal bovine serum. EpoB-resistant Hey. EpoB8 (EpoB8) cells were derived from Hey cells by stepwise selection and were maintained in 8 nM EpoB.
FACS analysis
Live cells (control and treated) were incubated with either FITC-conjugated IgG or anti-HEA-(EpCAM)-FITC, at 4°C in the dark, and analyzed by FACS. Levels of cell surface EpCAM were determined by subtracting the level of fluorescence in the cells incubated with FITC-IgG from those incubated with FITC-EpCAM.
Immunofluorescence
Control and treated cells were fixed with ethanol, incubated with anti-EpCAM antibody, B302(323/A3), followed by incubation with Alexa 488- or Cy3-conjugated secondary antibody, mounted, and viewed with an Olympus IX70 microscope.
Preparation of cell lysates and immunoblot analysis
Denatured cell lysates were prepared as described [24]. Equal amounts of proteins in the lysates were resolved by SDS-PAGE and total EpCAM expression was determined by an antibody that recognizes a C-terminal epitope of EpCAM (E144).
Expression of EpCAM in membrane vesicles of Hey cells following EpoB treatment
Membrane vesicles were prepared from five 100 mm dishes of cells as described [25]. They were dissolved in sample buffer in the absence of β-mercaptoethanol, followed by SDS-PAGE and Western blot analysis using anti-EpCAM antibody, B302(323/A3) that recognizes an N-terminal extracellular epitope of EpCAM [26].
Determination of cell surface biotinylated EpCAM
Cell surface expression of EpCAM was determined using membrane-impermeant biotinylation reagent Sulfo-NHS-SS-biotin (Pierce) as described [27] Biotinylated EpCAM was analyzed by Western blot analysis using anti-EpCAM antibody E144.
Real-time quantitative PCR (RT-PCR)
RNA was prepared using RNeasy Protect Mini Kit from Qiagen and RT-PCR was done using gene-specific primers for EpCAM [17]. Gene expression levels, normalized to the reference (cyclophilin B) and relative to that in untreated cells, were calculated by comparative CT method [28]. CT is the PCR cycle number at which the fluorescence signal grows beyond the value of the threshold setting.
Results
EpoB and other microtubule-interacting agents enhance surface EpCAM expression in Hey Cells
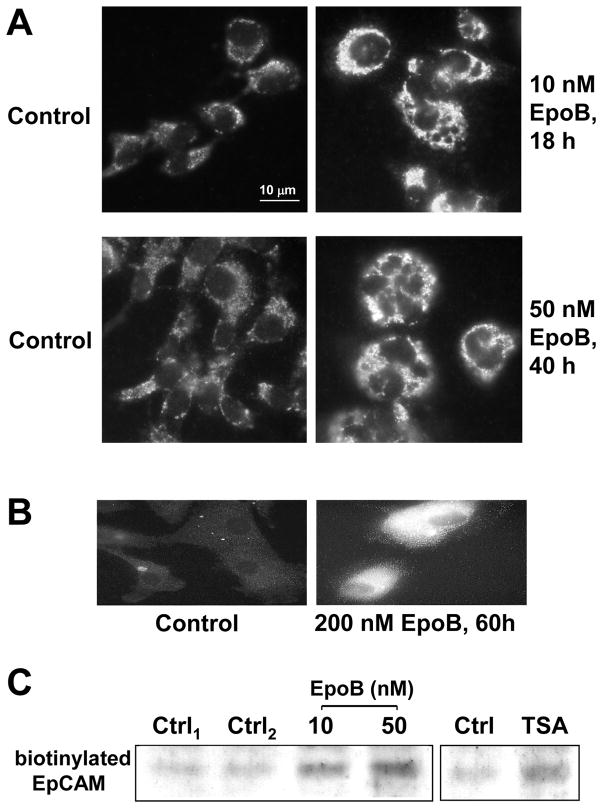
The human ovarian cell line, Hey, has a doubling time of approximately 16 h and the IC50 values for a variety of drugs, based on a 72 h cytotoxicity assay, are shown in Table 1. Immunofluorescence studies indicated that surface EpCAM expression was enhanced by treatment with EpoB in Hey cells (Figure 1A) and in a primary ovarian cancer line, SS4 (Figure 1B). SS4 was developed from the ascites of a patient with ovarian cancer prior to chemotherapy and had a long doubling time. Cell surface biotinylation also was used to demonstrate EpoB-enhanced surface EpCAM expression (Figure 1C, left panel). Since EpoB stabilizes microtubules similarly to Taxol, it would be expected to also increase tubulin acetylation. HDAC6 is a microtubule-associated deacetylase and destabilization of dynamic microtubules is mediated by this enzyme [21, 29]. TSA, an HDAC6 inhibitor, caused a moderate increase in biotinylated EpCAM expression in Hey cells (Figure 1C, right panel), suggesting that inhibition of microtubule deacetylation enhanced surface EpCAM expression. Therefore, tubulin acetylation may be related to the enhanced expression of surface EpCAM.
Table 1.
IC50 values for the Hey cell line
| Drug | IC50 |
|---|---|
| Taxol | 3 nM |
| epothilone B (EpoB) | 1 nM |
| discodermolide (disco) | 30 nM |
| vinblastine (VBL) | 1 nM |
| trichostatin A (TSA) | 40 nM |
| cis-platinum (CDDP) | 10 μM |
| 5-fluorouracil (5-FU) | 1 μM |
| Carboplatin | 700 μM |
IC50 values were determined after 72 hours of incubation at 37°C with the indicated drugs.
Fig. 1.
EpoB enhances surface EpCAM expression in ovarian cancer cells. (A) Hey cells were treated with 10 nM and 50 nM EpoB for 18 h and 40 h, respectively, washed with PBS, and stained with anti-EpCAM antibody, B302(323/A3), and Alexa 488-conjugated secondary antibody as described in Materials and Methods. (B) SS4 primary ovarian cells were treated with 200 nM EpoB for 60 h, followed by indirect immunofluorescence using anti-EpCAM antibody, B302(323/A3), and Cy3-conjugated secondary antibody. (C) Hey cells were treated with 10 and 50 nM EpoB and 0.5 μM TSA for 18 h, followed by extraction of biotinylated proteins and determination of biotinylated EpCAM as described in Materials and Methods.
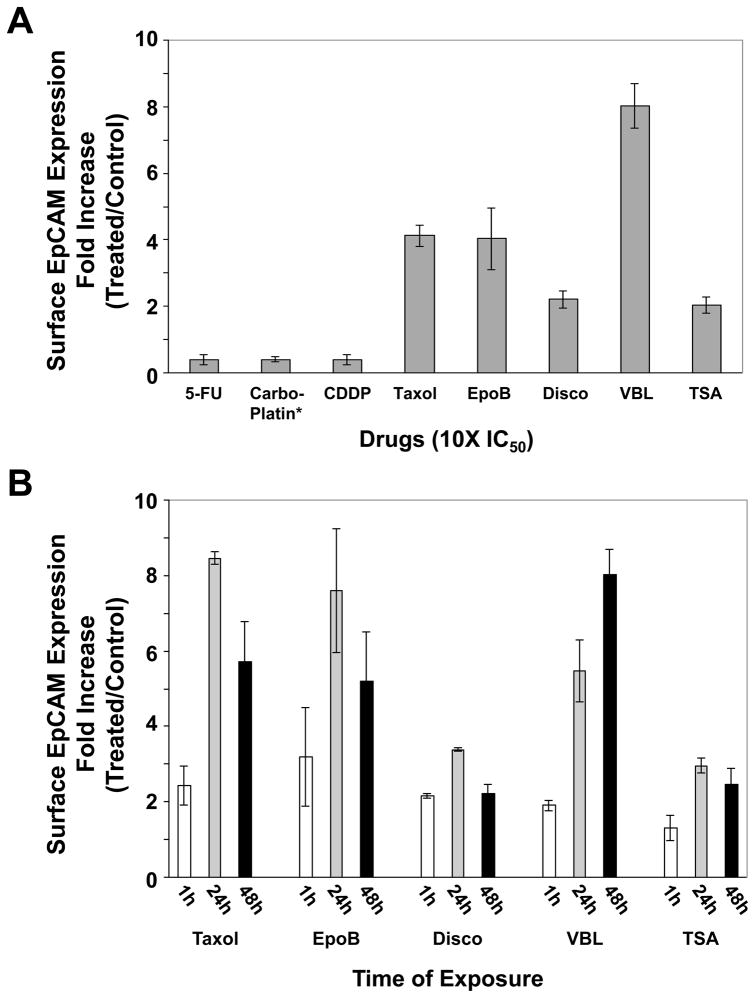
To quantify the levels of drug-induced surface EpCAM expression, Hey cells were treated with 10-fold IC50 concentrations of EpoB and a variety of other drugs for 48 h, and live-cell FACS analysis was performed (Figure 2A). It was found that Taxol, EpoB, Disco, VBL and TSA caused a 4.1-, 4.0-, 2.2-, 8.1 and 2.5-fold increase in surface EpCAM expression, respectively. Carboplatin, 5-FU and CDDP, drugs that do not interact with microtubules, did not have an enhanced effect on surface EpCAM expression. In fact these three drugs appeared to have an inhibitory effect on surface EpCAM expression (Figure 2A). The time course of four microtubule-interacting agents and TSA on surface EpCAM expression was done by treating the cells with 10X IC50 values of these drugs for 1 h, 24 h and 48 h, washing, adding fresh medium, and continuing incubation for a total of 48 h (Figure 2B). The data indicate that, with the exception of VBL, 24 h drug treatment resulted in a greater increase in surface EpCAM expression than 48 h treatment. Taxol, EpoB, Disco, VBL and TSA caused an 8.5-, 7.6-, 3.4-, 5.5- and 3.0-fold increase in surface EpCAM expression, respectively, after 24 h treatment.
Fig. 2.
Microtubule-interacting agents induce surface EpCAM expression in Hey cells. (A) Hey cells were treated with 10X IC50 values of a variety of drugs (see Table 1) for 48 h. (B) Hey cells were treated with 10X IC50 values of Taxol, EpoB, Disco, VBL and TSA for 1 h, 24 h and 48 h, washed, fresh medium added, and incubation continued at 37°C for a total of 48 h. Cells were collected, immunostained with FITC-IgG or anti-HEA- (EpCAM)-FITC, followed by FACS analysis as described in Materials and Methods.
Taxol, EpoB, Disco, TSA, but not VBL, induces tubulin acetylation in Hey cells
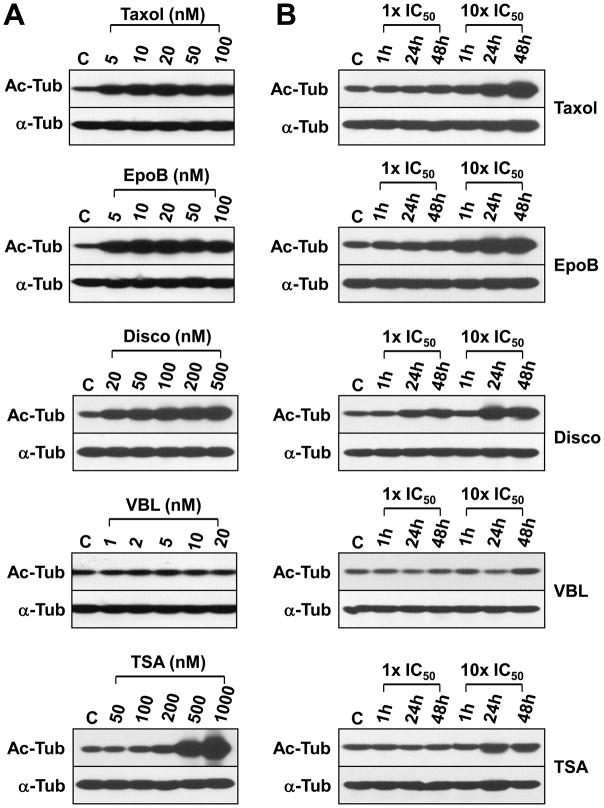
A dose and time course of tubulin acetylation was done following treatment of Hey cells with the microtubule-interacting agents and the HDAC inhibitor TSA (Figure 3). The concentration ranges used in this experiment were determined by the effect of the microtubule-interacting agents on the expression of CENP-E, a mitotic checkpoint protein that is highly expressed during mitosis [30]. Five nM of Taxol and EpoB and 20 nM of Disco were sufficient to induce tubulin acetylation 16 h after drug treatment. TSA-enhanced tubulin aetylation was observed at 200–1000 nM of drug (Figure 3A). VBL has essentially no effect on tubulin acetylation. VBL may even cause a decrease in tubulin acetylation 24 h after drug treatment (Figure 3B). Since VBL induced surface EpCAM expression significantly (see Figure 2) but did not affect tubulin acetylation, it is concluded that increased surface EpCAM expression does not correlate with tubulin acetylation.
Fig. 3.
Taxol, EpoB, Disco, TSA, but not VBL, induce tubulin acetylation in Hey cells. (A) Hey cells were treated with 5–100 nM Taxol or EpoB, 20–500 nM Disco, 1–20 nM VBL, and 50–1000 nM TSA for 16 h. Total denatured cell lysates were prepared and acetylated tubulin (Ac-tub) and α-tubulin (α-tub) levels were determined by Western blot analysis using anti-Ac-tub and anti-α-tub antibodies. C: untreated, control cells. (B) Hey cells were treated with 1X and 10X IC50 values of Taxol, EpoB, Disco, VBL and TSA (see Table 1) for 1h, 24 h and 48 h, washed, fresh medium added, and incubation continued at 37°C for a total of 48 h. Total cell lysates were prepared and Ac-tub and α-tub levels determined as in (A). C: untreated, control cells.
Total cellular EpCAM expression was not altered by EpoB
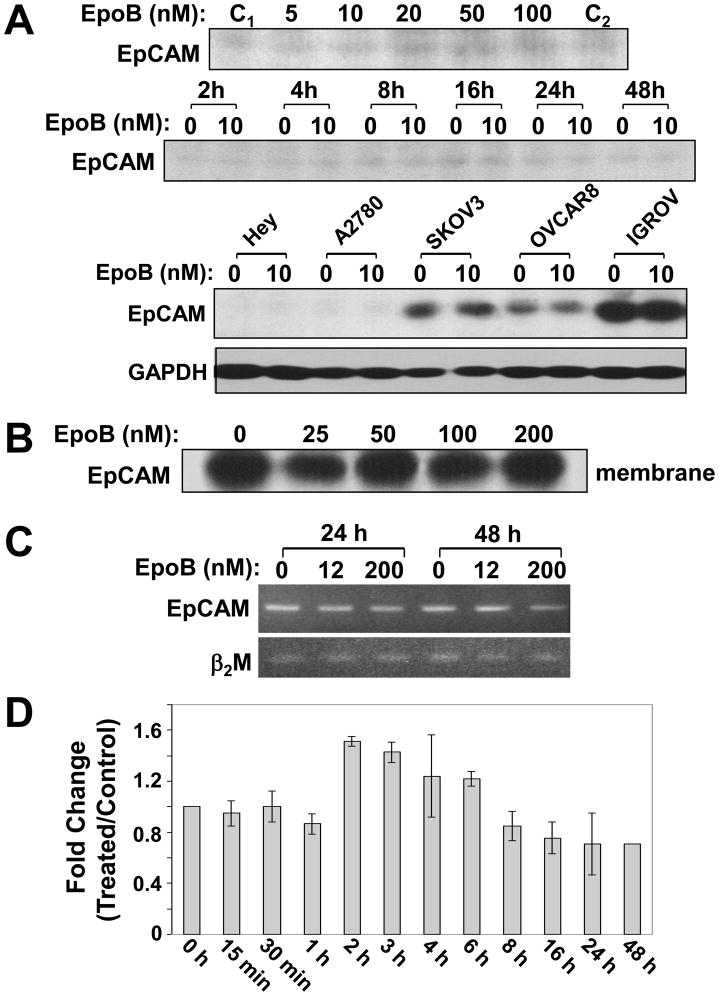
To examine the total EpCAM levels in Hey cells after EpoB treatment, proteins in the denatured lysates were analyzed by an anti-EpCAM antibody E144 that recognizes a C-terminal epitope of EpCAM. EpoB did not alter total EpCAM expression at any concentration (5–100 nM) or at any time point tested (Figure 4A). The effect of 10 nM EpoB on total cellular EpCAM expression was examined in a variety of human ovarian cell lines. The results indicate that 10 nM EpoB had no effect on total cellular EpCAM expression, regardless of the endogenous EpCAM levels (Figure 4A).
Fig. 4.
Total cellular EpCAM levels are not altered by EpoB. (A) Upper panel: Hey cells were treated with 5–100 nM EpoB for 16 h. Middle panel: Hey cells were treated with 10 nM EpoB for the indicated times. Lower panel: A variety of human ovarian cell lines, including Hey, A2780, SKOV3, OVCAR8 and IGROV, were treated with 10 nM EpoB for 16 h. Total denatured lysates were prepared and proteins were resolved by SDS-PAGE. Total cellular EpCAM levels were determined by Western blot analysis using anti-EpCAM antibody E144. C1 and C2: two different preparations of control Hey cells. (B) Hey cells were treated with 25–200 nM EpoB for 16 h and membrane vesicles were prepared as described in Materials and Methods. Eighty μg of membrane vesicles were resolved by SDS-PAGE, and EpCAM levels in the membranes were determined by Western blot analysis using anti-EpCAM antibody, B302(323/A3), under non-reducing conditions. (C) Hey cells were treated with 12 and 200 nM of EpoB for 24 h and 48 h. Expression of EpCAM mRNA was determined by competitive reverse transcription-PCR, using β2 macroglobulin (β2M) as control. (D) Hey cells were treated with 10 nM EpoB for the indicated times. EpCAM mRNA levels were determined by quantitative RT-PCR as described in Material and Methods. Each value is the mean of two independent RNA preparations assayed in triplicate.
Since the total cellular EpCAM level in Hey cells is low, based on Western blot analysis (see Figure 4A), partially purified membrane vesicles from larger quantities of Hey cells treated with 25–200 nM EpoB were prepared and dissolved under non-reducing conditions. EpCAM levels were determined using an antibody that recognizes the N-terminal epitope of EpCAM and the results indicated that there was no increased expression of EpCAM in the membrane preparations following drug treatment (Figure 4B).
To investigate whether EpoB altered EpCAM mRNA synthesis, Hey cells were treated with low (12 nM) and relatively high (200 nM) concentrations of EpoB for 24 and 48 h. EpCAM mRNA was analyzed by competitive reverse transcription-PCR and it was shown that EpoB did not cause an increase in the expression of EpCAM mRNA (Figure 4C).
To further quantify EpCAM mRNA expression, total RNA was isolated from Hey cells treated with 10 nM EpoB for 15 min-48 h and levels of EpCAM were determined by quantitative RT-PCR, using cyclophilin B mRNA as control (Figure 4D). EpCAM mRNA synthesis was not induced 60 min following EpoB treatment. At 2–6 h, its synthesis was a slightly increased (<50%), probably due to indirect effects of EpoB. No change in EpCAM mRNA was observed 8–48 h after drug treatment. Therefore, our results indicated that EpoB did not transcritionally activate EpCAM mRNA synthesis.
Expression of surface of EpCAM was reduced in an EpoB-resistant Hey cell line
An EpoB-resistant Hey cell line, EpoB8, was isolated by stepwise selection. It exhibited an approximately 20- and 4-fold resistance to EpoB and Taxol, respectively (Figure S1A). This cell line expressed a lower level of surface EpCAM compared to the sensitive Hey cells, as determined by indirect immunofluorescence (figure S1B) and surface biotinylation (Figure S1C, upper panel). Total cellular EpCAM levels are the same in the sensitive and the resistant cells (Figure S1C, lower panel). Although total α-tubulin levels in Hey and EpoB8 cells were comparable, EpoB8 expressed a much higher level of acetylated tubulin (Figure S1D) that may be related to the resistant phenotype.
Discussion
Lack of significant progress in the treatment of advanced or refractory ovarian cancer has stimulated the design of targeted therapy, including immunotherapy. EpCAM is one of the most frequently and intensely expressed tumor-associated antigens [31]. The potential utility of EpCAM as a target for the treatment of ovarian cancer is under investigation in phase I, II, and III clinical trials. It is known that patients with EpCAM positive disseminated tumor cells have a poor prognosis indicating a possible role of EpCAM in tumor growth and progression. Treatment with intraperitoneal anti-EpCAM antibody revealed significant antitumor activity in patients with ovarian cancer with established ascites and peritoneal carcinomatosis [32].
Taxol caused an increase in surface EpCAM antigen in adenocarcinoma cells and this increase was proportional to the cells in the G2/M phase of the cell cycle. It has been suggested that increased cell surface EpCAM antigen after Taxol and Navelbine treatment is related to microtubules that prevent receptor internalization and recycling [11]. Changes in the microtubule cytoskeleton by Taxol can alter the dynamics of membrane receptor movement through the endosomal pathway and it has been reported that Taxol disrupted endocytic events and inhibited endosomal-lysosomal membrane trafficking [33]. Other studies revealed that both inward and outward microtubule-dependent vesicle movement changed significantly after Taxol treatment in CV1 cells [34]. Vesicular intracellular membrane traffic may be coordinately regulated with microtubule-dependent motor activity. Our results have indicated that although surface EpCAM was enhanced by EpoB, total cellular EpCAM levels remained the same following EpoB treatment, suggesting that EpCAM was re-distributed in the cell upon drug treatment.
There is evidence that acetylation occurs only on the polymerized tubulin, whereas unpolymerized tubulin is efficiently deacetylated by HDAC6, a microtubule-associated deacetylase [21]. We have shown that the HDAC6 inhibitor TSA increased the expression of acetylated tubulin, and concomitantly enhanced surface EpCAM expression (see Figures 1–3). We also observed a moderate increase in tubulin acetylation 48 hours after exposure of Hey cells to 10 nM vinblastine (see Figure 3B). Since the substrate for tubulin acetyl transferase is polymerized tubulin, our result indicates that 10 nM vinblastine increases the level of stable microtubules, consistent with the previous data that low concentrations of vinblastine suppress microtubule dynamics [35]. Our data suggest that disruption of dynamics of microtubules affect cell trafficking and redistribution of EpCAM antigen at the cell surface. It is also reasonable to believe that other surface antigens and receptors could be increased when cells are exposed to microtubule-interacting agents. In addition, acetylated α-tubulin has been used as a surrogate of therapeutic success or failure of microtubule-stabilizing agents, such as Ixabepilone [36].
As shown in Figure S1, an EpoB-resistant Hey cell line, EpoB8, exhibited a decreased expression of surface EpCAM. This cell line expressed a higher level of acetylated tubulin, compared to the sensitive Hey cells, further indicating that tubulin acetylation does not correlate with surface EpCAM expression. However, we can not rule out the possibility that surface EpCAM expression is not changed or is altered differently in resistant cells that develop in vivo.
Immunotherapy against EpCAM in patients with ovarian cancer is presently being evaluated as a single agent [37]. For example, one of the promising anti-EpCAM immunotherapeutic agents is EpCAM trifunctional antibody catumaxomab. This antibody is directed towards the tumor-associated antigen, EpCAM, and T cell receptor-associated CD3 molecules. The combination of an anti-CD3 and anti tumor-associated antigen antibody is able to facilitate the contact of effector T cells with tumor cells, leading to tumor lysis [32, 38]. However, our finding that surface EpCAM expression was reduced in resistant cells suggests that this combination therapy be used as the first regimen prior to the development of resistance.
Low-dose Taxol has been shown to change the tumor microenvironment and improve the outcome of immunotherapy [39]. Under some circumstances, treatment with low dose chemotherapeutic drugs can be advantageous. This may occur partly through regulation of the antitumor immune response. The immunomodulatory effects of some chemotherapeutics could be further potentiated by combination with selected biological response modifiers or the use of targeted immunotherapy. In this report, we have demonstrated a rationale for the development of a combination of low dose EpoB and EpCAM immunotherapy in ovarian cancer patients.
Supplementary Material
Acknowledgments
The authors thank Dr. Lingling Liu for assistance with immunofluorescence.
Footnotes
Conflict of Interest Statement: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shahabi S, Smith R, Delpriore G. Fast Facts: Gynecology Oncology. 2. Health Press; 2009. [Google Scholar]
- 2.Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, et al. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 1995;55:2325–33. [PubMed] [Google Scholar]
- 3.Kowalski RJ, Giannakakou P, Hamel E. Activities of the microtubule-stabilizing agents epothilones A and B with purified tubulin and in cells resistant to paclitaxel (Taxol(R)) J Biol Chem. 1997;272:2534–41. doi: 10.1074/jbc.272.4.2534. [DOI] [PubMed] [Google Scholar]
- 4.Harrison M, Swanton C. Epothilones and new analogues of the microtubule modulators in taxane-resistant disease. Expert Opin Investig Drugs. 2008;17:523–46. doi: 10.1517/13543784.17.4.523. [DOI] [PubMed] [Google Scholar]
- 5.Donovan D, Vahdat LT. Epothilones: clinical update and future directions. Oncology (Williston Park) 2008;22:408–16. discussion 416, 421, 424 passim. [PubMed] [Google Scholar]
- 6.Fornier MN. Ixabepilone, first in a new class of antineoplastic agents: the natural epothilones and their analogues. Clin Breast Cancer. 2007;7:757–63. [PubMed] [Google Scholar]
- 7.Goodin S. Ixabepilone: a novel microtubule-stabilizing agent for the treatment of metastatic breast cancer. Am J Health Syst Pharm. 2008;65:2017–26. doi: 10.2146/ajhp070628. [DOI] [PubMed] [Google Scholar]
- 8.McDaid HM, Mani S, Shen HJ, Muggia F, Sonnichsen D, Horwitz SB. Validation of the pharmacodynamics of BMS-247550, an analogue of epothilone B, during a phase I clinical study. Clin Cancer Res. 2002;8:2035–43. [PubMed] [Google Scholar]
- 9.Balzar M, Prins FA, Bakker HA, Fleuren GJ, Warnaar SO, Litvinov SV. The structural analysis of adhesions mediated by Ep-CAM. Exp Cell Res. 1999;246:108–21. doi: 10.1006/excr.1998.4263. [DOI] [PubMed] [Google Scholar]
- 10.Gires O, Klein CA, Baeuerle PA. On the abundance of EpCAM on cancer stem cells. Nat Rev Cancer. 2009;9:143. doi: 10.1038/nrc2499-c1. author reply 143. [DOI] [PubMed] [Google Scholar]
- 11.Thurmond LM, Stimmel JB, Ingram AC, Ryan CH, Murray DM, Eberwein DJ, et al. Adenocarcinoma cells exposed in vitro to Navelbine or Taxol increase Ep-CAM expression through a novel mechanism. Cancer Immunol Immunother. 2003;52:429–37. doi: 10.1007/s00262-003-0386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balzar M, Briaire-de Bruijn IH, Rees-Bakker HA, Prins FA, Helfrich W, de Leij L, et al. Epidermal growth factor-like repeats mediate lateral and reciprocal interactions of Ep-CAM molecules in homophilic adhesions. Mol Cell Biol. 2001;21:2570–80. doi: 10.1128/MCB.21.7.2570-2580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baeuerle PA, Gires O. EpCAM (CD326) finding its role in cancer. Br J Cancer. 2007;96:417–23. doi: 10.1038/sj.bjc.6603494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munz M, Baeuerle PA, Gires O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res. 2009;69:5627–9. doi: 10.1158/0008-5472.CAN-09-0654. [DOI] [PubMed] [Google Scholar]
- 15.Maetzel D, Denzel S, Mack B, Canis M, Went P, Benk M, et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol. 2009;11:162–71. doi: 10.1038/ncb1824. [DOI] [PubMed] [Google Scholar]
- 16.Heinzelmann-Schwarz VA, Gardiner-Garden M, Henshall SM, Scurry J, Scolyer RA, Davies MJ, et al. Overexpression of the cell adhesion molecules DDR1, Claudin 3, and Ep-CAM in metaplastic ovarian epithelium and ovarian cancer. Clin Cancer Res. 2004;10:4427–36. doi: 10.1158/1078-0432.CCR-04-0073. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Herlyn D, Wong KK, Park DC, Schorge JO, Lu KH, et al. Identification of epithelial cell adhesion molecule autoantibody in patients with ovarian cancer. Clin Cancer Res. 2003;9:4782–91. [PubMed] [Google Scholar]
- 18.Schmidt M, Hasenclever D, Schaeffer M, Boehm D, Cotarelo C, Steiner E, et al. Prognostic effect of epithelial cell adhesion molecule overexpression in untreated node-negative breast cancer. Clin Cancer Res. 2008;14:5849–55. doi: 10.1158/1078-0432.CCR-08-0669. [DOI] [PubMed] [Google Scholar]
- 19.Blagosklonny MV, Robey R, Sackett DL, Du L, Traganos F, Darzynkiewicz Z, et al. Histone deacetylase inhibitors all induce p21 but differentially cause tubulin acetylation, mitotic arrest, and cytotoxicity. Mol Cancer Ther. 2002;1:937–41. [PubMed] [Google Scholar]
- 20.Zhang Y, Li N, Caron C, Matthias G, Hess D, Khochbin S, et al. HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J. 2003;22:1168–79. doi: 10.1093/emboj/cdg115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, et al. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J. 2002;21:6820–31. doi: 10.1093/emboj/cdf682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He L, Yang CP, Horwitz SB. Mutations in beta-tubulin map to domains involved in regulation of microtubule stability in epothilone-resistant cell lines. Mol Cancer Ther. 2001;1:3–10. [PubMed] [Google Scholar]
- 23.Xia S, Kenesky CS, Rucker PV, Smith AB, 3rd, Orr GA, Horwitz SB. A photoaffinity analogue of discodermolide specifically labels a peptide in beta-tubulin. Biochemistry. 2006;45:11762–75. doi: 10.1021/bi060497a. [DOI] [PubMed] [Google Scholar]
- 24.Yang CP, Horwitz SB. Taxol mediates serine phosphorylation of the 66-kDa Shc isoform. Cancer Res. 2000;60:5171–8. [PubMed] [Google Scholar]
- 25.Lever JE. Active amino acid transport in plasma membrane vesicles from Simian virus 40-transformed mouse fibroblasts. Characteristics of electrochemical Na+ gradient-stimulated uptake. J Biol Chem. 1977;252:1990–7. [PubMed] [Google Scholar]
- 26.Balzar M, Bakker HA, Briaire-de-Bruijn IH, Fleuren GJ, Warnaar SO, Litvinov SV. Cytoplasmic tail regulates the intercellular adhesion function of the epithelial cell adhesion molecule. Mol Cell Biol. 1998;18:4833–43. doi: 10.1128/mcb.18.8.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen JG, Liu-Chen S, Rudnick G. External cysteine residues in the serotonin transporter. Biochemistry. 1997;36:1479–86. doi: 10.1021/bi962256g. [DOI] [PubMed] [Google Scholar]
- 28.Chen JG, Yang CP, Cammer M, Horwitz SB. Gene expression and mitotic exit induced by microtubule-stabilizing drugs. Cancer Res. 2003;63:7891–9. [PubMed] [Google Scholar]
- 29.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–8. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 30.Yang CP, Liu L, Ikui AE, Horwitz SB. The interaction between mitotic checkpoint proteins, CENP-E and BubR1, is diminished in epothilone B-resistant A549 cells. Cell Cycle. 2010;9:1207–13. doi: 10.4161/cc.9.6.11122. [DOI] [PubMed] [Google Scholar]
- 31.Chaudry MA, Sales K, Ruf P, Lindhofer H, Winslet MC. EpCAM an immunotherapeutic target for gastrointestinal malignancy: current experience and future challenges. Br J Cancer. 2007;96:1013–9. doi: 10.1038/sj.bjc.6603505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burges A, Wimberger P, Kumper C, Gorbounova V, Sommer H, Schmalfeldt B, et al. Effective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM x anti-CD3 antibody: a phase I/II study. Clin Cancer Res. 2007;13:3899–905. doi: 10.1158/1078-0432.CCR-06-2769. [DOI] [PubMed] [Google Scholar]
- 33.Sonee M, Barron E, Yarber FA, Hamm-Alvarez SF. Taxol inhibits endosomal-lysosomal membrane trafficking at two distinct steps in CV-1 cells. Am J Physiol. 1998;275:C1630–9. doi: 10.1152/ajpcell.1998.275.6.C1630. [DOI] [PubMed] [Google Scholar]
- 34.Hamm-Alvarez SF, Kim PY, Sheetz MP. Regulation of vesicle transport in CV-1 cells and extracts. J Cell Sci. 1993;106 (Pt 3):955–66. doi: 10.1242/jcs.106.3.955. [DOI] [PubMed] [Google Scholar]
- 35.Dhamodharan R, Jordan MA, Thrower D, Wilson L, Wadsworth P. Vinblastine suppresses dynamics of individual microtubules in living interphase cells. Mol Biol Cell. 1995;6:1215–29. doi: 10.1091/mbc.6.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhuang SH, Hung YE, Hung L, Robey RW, Sackett DL, Linehan WM, et al. Evidence for microtubule target engagement in tumors of patients receiving ixabepilone. Clin Cancer Res. 2007;13:7480–6. doi: 10.1158/1078-0432.CCR-06-2883. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt M, Scheulen ME, Dittrich C, Obrist P, Marschner N, Dirix L, et al. An open-label, randomized phase II study of adecatumumab, a fully human anti-EpCAM antibody, as monotherapy in patients with metastatic breast cancer. Ann Oncol. 2009 doi: 10.1093/annonc/mdp314. [DOI] [PubMed] [Google Scholar]
- 38.Ruf P, Lindhofer H. Induction of a long-lasting antitumor immunity by a trifunctional bispecific antibody. Blood. 2001;98:2526–34. doi: 10.1182/blood.v98.8.2526. [DOI] [PubMed] [Google Scholar]
- 39.Zhong H, Han B, Tourkova IL, Lokshin A, Rosenbloom A, Shurin MR, et al. Low-dose paclitaxel prior to intratumoral dendritic cell vaccine modulates intratumoral cytokine network and lung cancer growth. Clin Cancer Res. 2007;13:5455–62. doi: 10.1158/1078-0432.CCR-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.