Abstract
Aging, which is an independent risk factor for heart disease, alters body fat mass and its function. Epicardial fat plays an important physiological and pathophysiological role on cardiac structure and function. This study investigated if aging altered the abundance of epicardial (EF) and abdominal fat (AF) derived mediators in a sex dependent manner in female and male Fischer 344 × Brown Norway hybrid (FBN) rats. EF and AF were obtained from 48 female and male, young (6 months), aged (26/30 months) and very aged (30/36 months) FBN rats. Adipose derived anti-inflammatory and pro-inflammatory mediators were measured using ELISA, adipokine array and real-time qPCR. No dramatic changes in circulating lipids other than a higher triglyceride and high density lipoprotein in aged females and a significantly increased circulating adiponectin (p< 0.005) in aged rats were observed. Real time PCR results showed that compared to 6mo old female rats, the aged (26mo) and very aged (30mo) rats had significantly lower levels of EF genes: adiponectin (p<0.005), PPARγ (p<0.01, 0.005), IL-6 (p<0.01) and PAI-1 (p<0.01, 0.01), respectively, but not in AF. In contrast, the male rats exhibited an increase in IL-6 in EF (p< 0.005) but a decrease in adiponectin and PPARγ in AF with aging. These changes might be attributed to differences in adipocyte make-up or macrophage infiltration. In conclusion, aging had a more profound impact on EF derived mediators in female rather than male rats, which might help explain the increased risk to cardiovascular disease seen in older women.
Keywords: Aging, adipose tissue, gender, obesity
1. Introduction
The incidence of cardiovascular disease (CVD)1 increases sharply with age, differs with sex and appears to disproportionately affect women older than 65 yrs2. Although both women and men are influenced by similar risk factors for CVD such as aging, smoking, diet/alcohol, and physical inactivity3, CVD and heart disease are more prevalent in older men than women, whereas, stroke is more prevalent in older women4. However, between women and men suffering from CVD, there is higher mortality rates in women compared to men. This health concern has lately reached critical proportions and likely to worsen as the elderly comprise the fastest growing segment of the United States population5. Although not well understood, previous reports have suggested that alterations in adipose derived mediators (adipokines) may play an important role in these disease processes, as these molecules can modulate inflammatory processes6 7. It is thought that different fat depots differ in their expression of adipokines, for example abdominal fat secretes a greater amount of the inflammatory mediators, IL-6 (Interleukin-6) and PAI-1 (Plasminogen activator inhibitor-1) than does other fat depots8. Aging dramatically changes the mass, distribution and function of adipose tissue, and it also results in increased infiltration and redistribution of fat into non-adipose tissues such as muscle, bone, liver and the heart9, 10.
In addition to abdominal fat, studies have suggested that alterations in epicardial fat (EF) may be particularly important in the pathophysiology of CVD due to its close proximity to the heart and coronary vasculature and its capacity to secrete high amount of pro-inflammatory adipokines11–13. To our knowledge, how aging may alter adipose derived mediators and if the abundance of adipokines varies between different fat depots with aging and between sexes has not been investigated. We hypothesized that aging would alter the abundance of abdominal and epicardial fat derived mediators and that age-associated changes in these mediators would differ between the two sexes. Using male and female Fischer 344 × Brown Norway hybrid (FBN) rats at matching age groups, we examined circulating and tissue adipokine mRNA expression in abdominal and epicardial fat depots. Our findings reveal that aging, significantly altered adipose derived mediators in a depot specific manner and that the magnitude and directionality of these changes was influenced by the sex of the animal.
2. Materials and Methods
2.1. Animals
Twenty-four 6-, 26-, and 30- months (mo) old female (n=8/group) and twenty-four 6-, 30-, and 36- months old male (n=8/group), Fischer 344 × Brown Norway hybrid (FBN) rats were obtained from National Institute on Aging, USA. Animal ages were chosen on the basis of previous literature demonstrating that these ages corresponded roughly to humans in their third (6mo rats), sixth (26/30mo rats; female/male, respectively) and eighth (30/36mo rats; female/male, respectively) decade of life14. Animal care and procedures were conducted in accordance with the Animal Use Review Board of Marshall University, using the criteria outlined by the American Association of Laboratory Animal Care as proclaimed in the Animal Welfare Act. Animals were fed with a standard laboratory diet and water ad libitum. Rats were kept under standard conditions: temperature: 21.0±2.0°C, humidity: 55.0±5.0% with a 12:12 hr light/dark cycle (07:00–19:00). After 2 weeks of acclimatization, the animals were anesthetized using ketamine-xylazine (45:5 mg/kg i.p) and euthanized by exsanguinations via a cardiac puncture. Care was taken that all the animals were sacrificed at the same time each day and the same day each month. This was particularly important to synchronize cycle times in female rats. The body (BW) and heart weights (HW) of each animal were recorded. The blood was collected by heart puncture and immediately centrifuged to collect the plasma. The epicardial adipose tissue (EF) and abdominal visceral adipose tissue (AF) (omental fat) was collected from each animal and aliquoted for mRNA isolation in Tri-reagent (Sigma, St. Louis, MO) and stored immediately in −80°C for future use.
2.2. Quantitative Real-Time Reverse Transcriptase Polymerase Chain Reaction (RT-qPCR)
After RNA extraction from the adipose tissue using Tri-reagent (100mg adipose tissue), real-time PCR for various adipokines and macrophage markers was carried out using iQSYBR Green Supermix (Bio-Rad, Hercules, CA), and the respective primers. The following primers were used: Adiponectin (NM_009605): 5’-gcagagatggcactcctgga-3’, 3’-cccttcagctcctgtcattcc-5’. PPARγ (Peroxisome proliferators activated receptor γ) (NM_013124): 5’-catttttcaagggtgccagt-3’, 3’-gaggccagcatggtgtagat-5’. IL-6 (NM_012589): 5’-gcccttcaggaacagctatg-3’, 3’-gtctcctctccggacttgtg-5’. PAI-1 (M33960): 5’-cccacggagatggttttaga-3’, 3’-ccagttttgtcccaaaggaa-5’. CD68 (NM_001031638): 5’-caaaaaggctgccactcttc-3’, 3’-gtgggagaaactgtggcatt-5’. MCP-1 (Monocyte chemotactic protein-1) (AY920404): 5’-ttccttcttggggtcagcacagac-3’, 3’-actgaagccagctctctcttcctc-5’. GAPDH was used as the housekeeping gene. GAPDH (BC145810): 5’-tcggtgtgaacggatttggccgta-3’, 3’- atggactgtggtcatgagcccttc-5’.
qPCR was performed using the Bio-Rad MyiQ Real Time PCR system (Bio-Rad, Hercules, CA). Results were calculated using the Pfaffl method (2−ΔΔCt) and expressed as “mean fold change ±SEM (Standard Error of the mean)” in the experimental gene compared to the housekeeping gene in each group15. The differences in gene expression were analyzed by the levels of expression of any particular gene in aged and very aged rats compared to the younger (6mo control) rats.
2.3. Determination of metabolic parameters in plasma
Blood was collected from all groups of rats (males and females). Isolated plasma was used for the analysis of metabolic parameters such as circulating lipid profile (total cholesterol (TC) and triglycerides (TG) and high density lipoprotein (HDL)) using the Cholestech LDX system (Hayward, CA).
2.4. Enzyme-linked immunosorbent assay (ELISA) and Proteome profiler array for circulating adipokines
Circulating levels of total adiponectin (ADPRT-E01, ALPCO, Salem, NH), TNFα (KRC3012) (Invitrogen, Carlsbad, CA) and IL-6 (KL138035) (Thermo Scientific, Rockford, IL) were measured using commercial ELISA kits. The levels of adipokines in each sample were calculated using the standard curve generated for each assay during the ELISA analysis.
Circulating adipokines was also detected using a commercial adipokine array (ARY-013, R&D systems, Minneapolis, MN) following the manufacturer’s protocol. The Proteome Profiler Adipokine array detects 38 adipokines in duplicates that were captured on nitrocellulose membranes. Following streptavidin-HRP and chemiluminescent detection the proteins bound to each captured antibody was quantified using densitometry (Alpha-Innotech, Santa Clara, CA). The results were calculated as the ratio of density obtained from 30mo/36mo rat samples vs 6mo control and expressed as percent change in each adipokine. Significance was analyzed by one-way ANOVA.
2.5. Histology of abdominal fat
AF tissues were fixed in 10% formaldehyde followed by dehydration and embedded in paraffin. 5 µm paraffin sections were stained with hemotoxylin & eosin (H&E). The adipocytes were recognized and counted using ImageJ PC-based software (National Institute of Health, NIH Version v1.32j). The adipocyte size in approximately five microscopic fields of view was measured by calculating its Feret diameter (µm). The difference between the groups was analyzed using one-way ANOVA followed by Bonferroni post-hoc test.
2.6. Statistics
For the RT-qPCR analysis, all statistics were performed at the level of ΔCt, in order to exclude potential bias due to averaging of data transformed through the Pfaffl equation, 2−(ΔΔCt)16. The differences in genes in each sex, between groups (young versus aged or very aged) were determined by one-way ANOVA using the SPSS statistical package (Chicago, IL, USA). A probability value of less than 0.05 was considered statistically significant. Significance was confirmed using post-hoc analysis using Bonferroni test.
3. Results
3.1. Aging mediated changes in body weight (BW) and heart weight (HW) in both sexes
Aging is accompanied by increasing changes in body mass and fat redistribution9. As shown in Table 1, in the current study, the female FBN rats had increased body weight at ages 26mo (p<0.001) and 30mo (p<0.001) compared to 6mo old rats. In male rats, there was a significant increase in body weight at 30mo (p<0.005) old rats but only minimal increase in 36mo old rats compared to 6mo old male rats.
Table 1. Physical and Biochemical parameters in young and aging FBN rats of both sexes.
Body weight (g) and heart weight (g) were measured for all animals in each group. The biochemical parameters were measured using the plasma collected from all age groups of FBN rats in both sexes. The data is expressed as mean ± SEM. One-way ANOVA followed by Bonferroni test was performed on all age groups compared to the young (6 mo) controls. TC: Total Cholesterol, TG: Triglycerides, HDL: High Density Lipoprotein.
| Female | Male | |||||
|---|---|---|---|---|---|---|
| 6 mo | 26 mo | 30 mo | 6 mo | 30 mo | 36 mo | |
| Body Weight (g) | 230.5±14.1 | 294.5±38.2**** | 320.4±20.6**** | 422.5±42.3 | 560.7±11.3*** | 450.8±35.2 |
| Heart Weight (g) | 0.74±0.03 | 0.98±0.03**** | 1.16±0.03**** | 1.08±0.03 | 1.63±0.09*** | 1.70±0.13** |
| Plasma Lipid Profile | ||||||
| TC (mg/dl) | 96.3±8.01 | 97.1±6.54 | 97.4±7.91 | 102.7±3.15 | 101.2±2.54 | 100.7±2.92 |
| TG (mg/dl) | 56.3±5.09 | 70.4±9.29 | 107.9±7.84*** | 75.2±6.50 | 87.5±7.21 | 101.4±8.97 |
| HDL (mg/dl) | 18.9±0.87 | 28.3±9.17 | 33.4±1.43* | 16.3±1.32 | 17.1±2.12 | 22.7±3.57** |
| Circulating Adipokines | ||||||
| Adiponectin | 7.48±1.34 | 10.09±1.00** | 9.30±0.76 | 9.51±1.32 | 10.26±0.57** | 14.56±3.08*** |
| (pg/ml) | ||||||
p< 0.05;
p< 0.01;
p< 0.005;
p< 0.001.
We also observed changes in heart weight with aging. As shown in Table 1, the heart weight was increased at 26mo (p<0.001) and 30mo (p<0.0005) compared to 6mo old rats. The heart weight increased significantly in 30mo female rats compared to 26mo rats (18.4% (p<0.005)). In male rats the heart weight increased by 50.9% (p<0.005) and 57.5% (p<0.01) at 30mo and 36mo respectively compared to 6mo old rats.
When the body weights and heart weights were compared between genders, the females had lower weights compared to males at all age groups, −46.5% and −31.5% in 6mo old, −47.5% and −39.9% in 26mo old and −28.9% and −31.8% in 30mo, in BW and HW respectively compared to similar age groups in males.
3.2. Aging mediated changes in metabolic parameters in the plasma of both sexes
As shown in Table 1, there were no changes in total cholesterol levels between young (6mo), aged (26/30mo) and very aged (30/36mo) rats, even though the levels were slightly higher in males compared to females. A dramatically higher TG levels were seen only in 30mo female (91.7% (p<0.005)) rats compared to 6mo rats. A higher HDL levels were observed in both 30mo female (p<0.01) and 36mo male (p<0.05) rats compared to 6mo control rats (Table 1).
3.3. Aging mediated changes in abundance of anti- and pro-inflammatory mediators in abdominal fat of both sexes
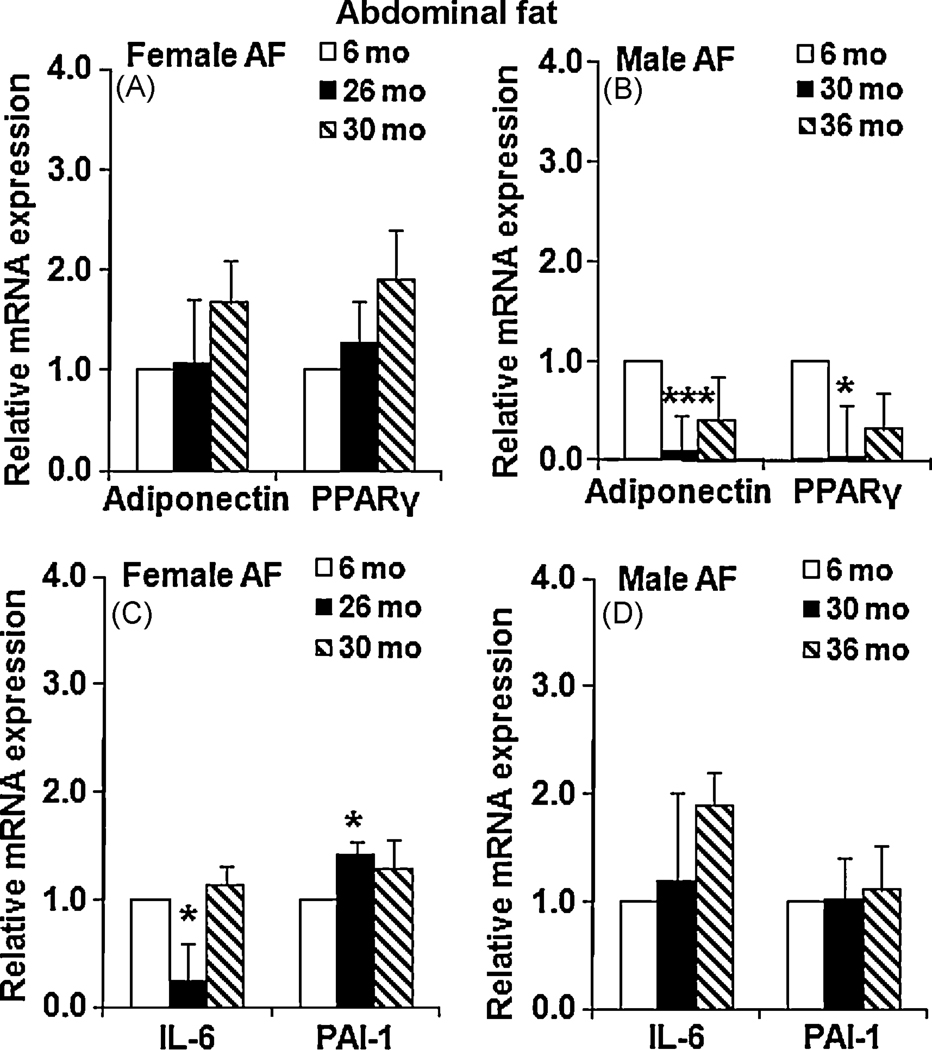
Adipose tissue synthesizes and secretes several mediators with varying functions7. For the purpose of this study only two anti- and pro-inflammatory adipokines were investigated. Adiponectin is anti-inflammatory and has a beneficial role. Its levels are altered during obesity and other disease conditions17. PPARγ is an important regulator of adiponectin levels in abdominal visceral adipose tissue18. In our studies, there were no significant differences in the abundance in adiponectin and PPARγ mRNA in AF in female rats at 26mo and 30mo compared to 6mo old FBN rats (Fig. 1A). However, there was a dramatic down-regulation of adiponectin (p<0.005) and PPARγ (p<0.05) in the abdominal fat obtained from 30mo old male rats (Fig. 1B), but then recovered to half of the baseline levels at a later age (36mo).
Figure 1. Changes in adipokine mRNA levels in abdominal fat (AF) with age.
Age mediated changes in mRNA levels of adipokines in AF were analyzed using RT-qPCR and expressed as fold change ± SEM of the gene expression in 26/30mo female rats and 30/36mo male rats compared to 6 mo rats in each gender (n=8). 1A: adiponectin and PPARγ - female AF; 1B: adiponectin and PPARγ – male AF; 1C: IL-6 and PAI-1 – female AF; 1D: IL-6 and PAI-1 –male AF. One-way ANOVA followed by Bonferroni was performed on all samples. p<0.05 were accepted as significant. *: p< 0.05; **: p< 0.01, ***: p< 0.005.
In obesity, there is also an increase in pro-inflammatory adipokines especially in abdominal fat which results in altered lipid homeostasis19. Aging is accompanied with sustained inflammation and therefore might result in pro-inflammatory changes in adipose tissue20. In abdominal omental fat obtained from female FBN rats, there was a significant down-regulation of IL-6 (p<0.05) but an up-regulation of PAI-1 (p<0.05) at 26mo rats, compared to 6mo controls, Fig. 1C, but not much higher compared to baseline levels at 30 mo. There was no significant variation in IL-6 and PAI-1 in the AF of male rats with aging (Fig. 1D) compared to 6 mo rats, though there was a slight increase albeit not significant in IL-6 levels with aging.
3.4. Aging alters anti- and pro-inflammatory adipose derived factors in epicardial fat of both sexes
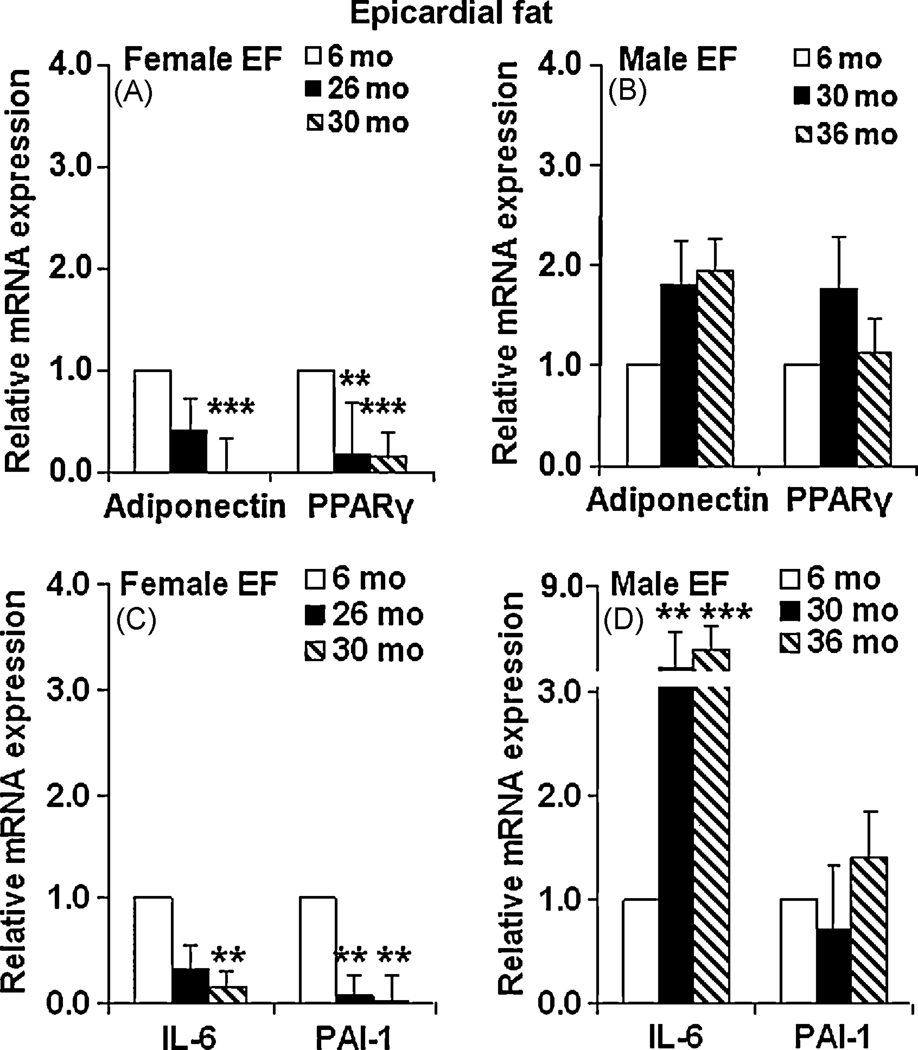
Adiponectin and PPARγ are both expressed in epicardial fat21. Adiponectin in EF is negatively correlated to atherosclerosis22. In aging FBN rats, both adiponectin and PPARγ mRNA levels were found decreased in female EF. As shown in Fig. 2A, in epicardial fat of 26mo old female rats, the mRNA levels of adiponectin and PPARγ (p<0.01) declined significantly, compared to 6mo old rats. At 30mo the levels of these genes were even more dramatically down-regulated compared to 6mo old female rats (p<0.005). In contrast, in male rats, there were fewer changes in levels of adipose derived factors in epicardial fat. As shown in Fig. 2B, there was a slight increase in the levels of adiponectin and PPARγ at both 30mo and 36mo compared to 6mo old FBN rats, but did not reach significance.
Figure 2. Changes in mRNA levels of adipokines in epicardial fat (EF) with age.
Age mediated changes in mRNA levels of epicardial fat (EF) adiponectin, PPARγ, IL-6 and PAI-1 were expressed as fold change ± SEM, of the gene expression in 26/30mo female rats and 30/36 male FBN rats compared to 6mo younger rats in each gender (n=8). 2A: adiponectin and PPARγ – female EF; 2B: adiponectin and PPARγ – male EF; 2C: IL-6 and PAI-1 – female EF; 2D: IL-6 and PAI-1 – male EF. One-way ANOVA followed by Bonferroni test was performed on all samples. p<0.05 were accepted as significant. *: p< 0.05; **: p< 0.01, ***: p< 0.005.
The effect of aging on epicardial fat pro-inflammatory genes is not known. Our results show that the expression of IL-6 and PAI-1 were significantly down-regulated in the epicardial fat obtained from both 26mo and 30mo female FBN rats compared to 6mo old rats (Fig. 2C). In males, there was a significant up-regulation of IL-6 in 30mo (p<0.01) and 36mo (p<0.005) male rats however, the mRNA level of PAI-1 was not altered significantly in the EF with aging compared to 6mo old rats (Fig. 2D).
3.5. Aging alters circulating adipokines in both sexes
Circulating adipokines, such as adiponectin, TNFα and IL-6 were measured using ELISA in the plasma samples obtained from each group of rats. As seen in Table 1, the circulating levels of total adiponectin was approximately 34.7% (p<0.01) higher in 26mo and 24.1% higher in 30mo rats compared to 6mo female FBN rats. A similar increase in circulating adiponectin levels was also observed in male rats, with 7.92% (p<0.01) increase in 30mo and 53.1% (p<0.005), increase in 36 mo rats compared to 6mo old FBN rats. The circulating levels of TNFα and IL-6 were below the detection limit of the ELISA kit (data not shown).
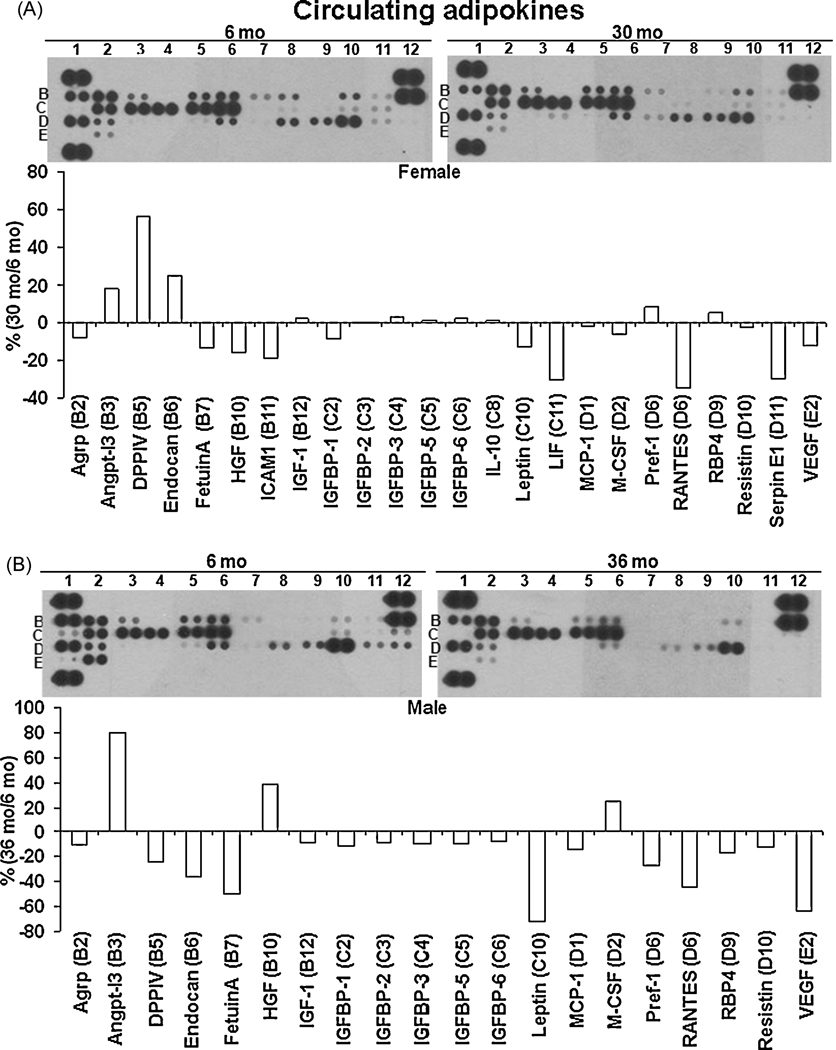
An alternate technique, the Proteome Profiler Adipokine array from R&D systems was used to detect 38 different circulating adipokines. As shown in Fig. 3A and B, both female and male aged rats exhibited a trend towards lower levels of circulating adipokines. Similar to that observed in the ELISA assays, the levels of TNFα (C7), IL-6 (E1) and PAI-1 (D11) were undetectable in the array.
Figure 3. Aging mediated changes in circulating adipokines in both sexes.
Circulating adipokine levels were measured in the plasma of both female and male rats at 6mo and 30/36mo (n=3/gp) using the Proteome Adipokine array. The levels of each adipokine was quantified after densitometry and expressed as the percentage (%) of the ratio of each adipokine in aged rat to 6mo rat. 3A: % change in circulating adipokines in female rats; 3B: % change in circulating adipokines in male rats.
3.6. Sex differences in adipose derived mediators with aging
Our results clearly indicated that aging alters adipose derived mediators in both sexes. However, we also found that these aging mediated changes are different between the two sexes. In abdominal fat the levels of these genes were found to be higher with aging in female rats compared to males of similar age (Fig. 4A). In contrast, aging had more profound effect on the epicardial fat derived mediators in female rats compared to male rats. There were higher levels of adiponectin, PPARγ, IL-6 (p<0.005) and PAI-1 (p< 0.005) in the epicardial fat at the younger aged (6mo old) female FBN rats, compared to 6 mo old male rats. However, the levels of these genes continued to decrease with aging until a significant decline in very aged group in female rats, compared to males (Fig. 4B). This decline in the levels of adipokines in the EF might suggest an altered function in female rats compared to male rats with aging.
Figure 4. Influence of sex in the differences in AF and EF derived mediators with aging.
Sex differences in abundance of adipose mediators, adiponectin, PPARγ, IL-6 and PAI-1 were expressed as fold change ± SEM of the gene expression in each age group (6mo, 26/30mo and 30/36mo) between male and female FBN rats. Male rats from each age group was used as controls (n=8). 4A: Sex differences in mRNA levels in AF; 4B: Sex differences in mRNA levels in EF. *: p< 0.05; ***: p< 0.005.
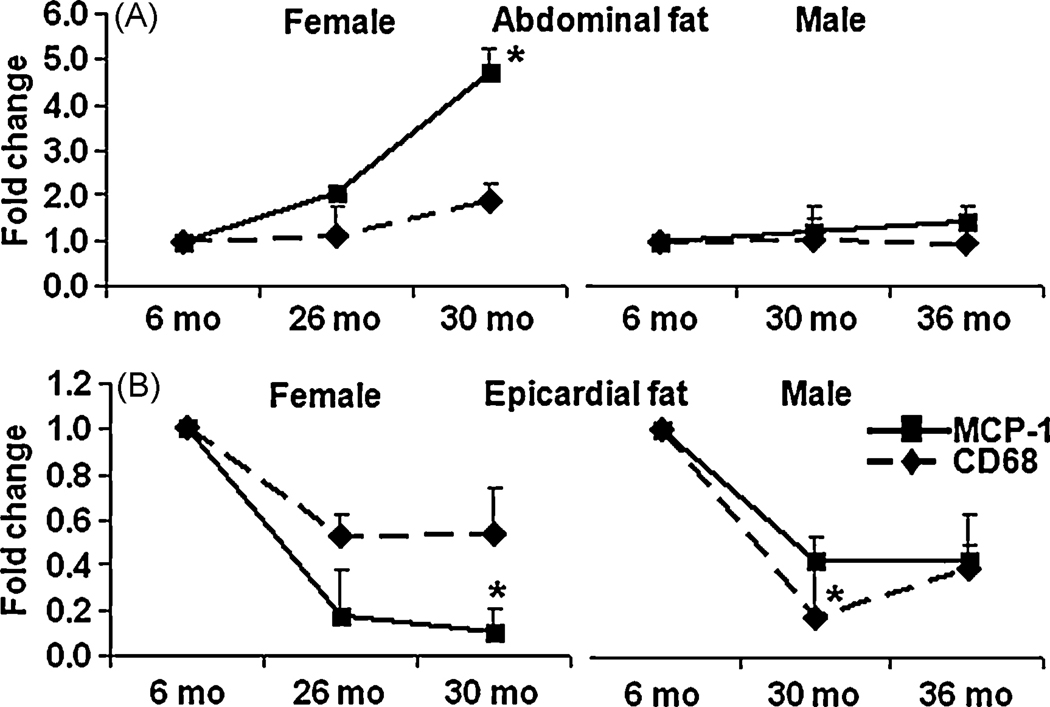
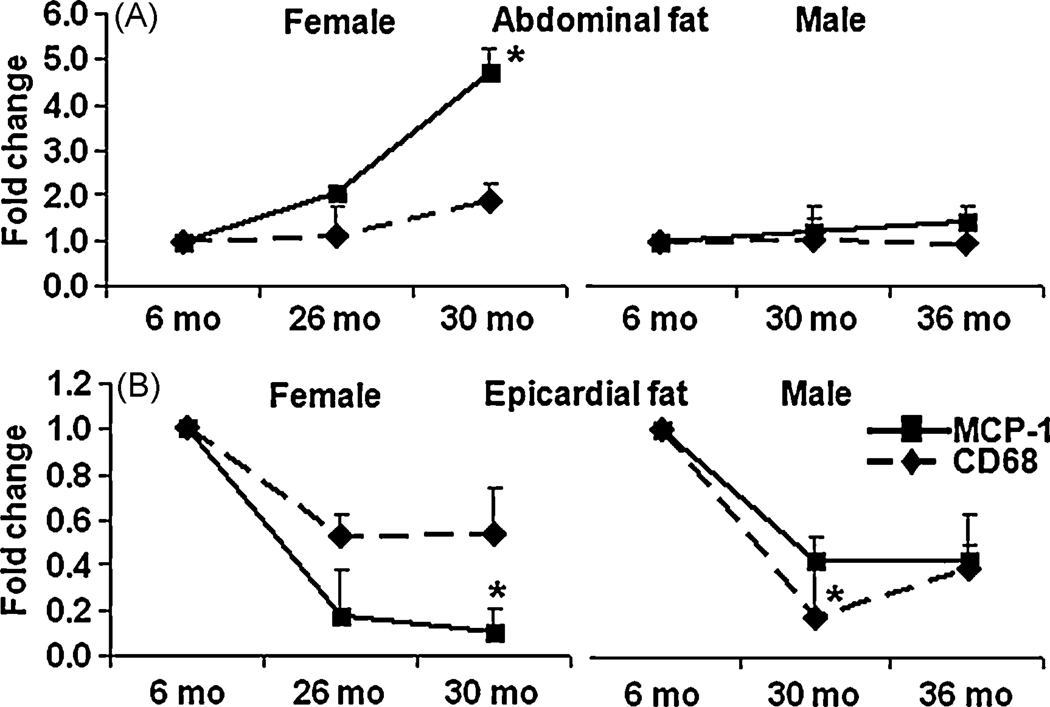
3.7. Age induced differences in macrophage markers
Macrophage infiltration alters adipose tissue function 23 24. To investigate if macrophage contributed to the age mediated differences in adipokines, the mRNA levels of both the surface and secretary markers of macrophages, CD68 and MCP-1 were analyzed in the EF and AF using RT-qPCR. As shown in Fig. 5A, both the macrophage markers increased with aging, in female AF, but MCP-1 was altered significantly however, no significant variations in the levels of these markers were observed with aging in male AF. In contrast, as shown in Fig. 5B, aging significantly decreased macrophage markers in both 26 and 30mo female EF, again, MCP-1 was decreased significantly. Similar decline in macrophage markers was also observed in male EF.
Figure 5. Aging mediated changes in macrophage markers in EF and AF.
Aging mediated changes in mRNA levels of macrophage markers, CD68 and MCP-1were detected using RT-qPCR in both AF and EF. Results were expressed as fold change ± SEM of each gene in 26/30mo female rats and 30/36 male FBN rats compared to 6mo younger rats (n=8). 5A: macrophage markers in AF of aged female and male rats; 5B: macrophage markers in EF of aged female and male rats. *: p< 0.05.
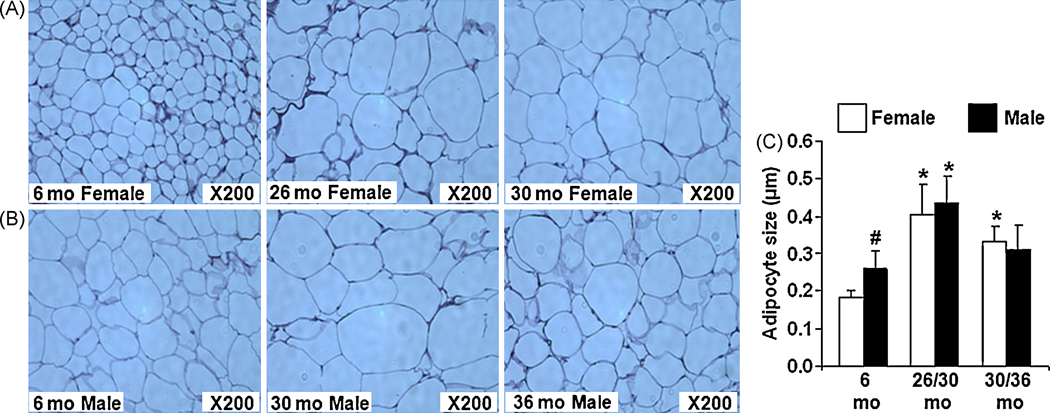
3.8. Aging alters adipocyte size and number
To determine if the differences in the mRNA and circulating levels of adipokines with aging is due to altered adipocyte morphology, we performed histology followed by H&E staining of abdominal fat obtained from all age groups of rats. As shown in Fig. 6A and B, aging significantly increased adipocyte size in AF, especially in the middle age. Although younger (6mo) male rats had significantly larger adipocyte size than female, this difference was reversed with advanced aging (Fig. 6C). Due to very low tissue availability, histology was not performed on EF.
Figure 6. Aging mediated changes in adipocyte size and number in abdominal fat (AF) of both sexes.
H&E staining of paraffin sections of AF from both sexes (n=3/gp) were performed to quantify the size and number of adipocytes using Image J. 6A: Adipocytes from female 6mo, 26mo and 30 mo rats; 6B: Adipocytes from male 6mo, 30mo and 36 mo rats; 6C: One-way ANOVA analysis performed on the data obtained after Image J analysis. *: p< 0.05, old vs young in the same sex; #: p< 0.05, male vs female.
4. Discussion
Risk to cardiovascular disease differs between men and women2. Aging is one of the major independent risk factor for CVD4. There is increased fat redistribution during aging which is more pronounced in women than men9. Whether these differences in fat distribution between the two sexes play a role in susceptibility to CVD is not clear. Recently, the importance of epicardial fat in the pathology of CVD has been recognized12, 25.
Our findings indicated that there are sex differences in the abundance of epicardial and visceral abdominal fat derived factors with aging. Compared to younger animals, aging in the female FBN rats had decreased epicardial fat expression of adiponectin, PPARγ, IL-6 and PAI-1. Conversely, in the male FBN rats the expression of these genes remained unchanged or moderately increased with aging. In addition to these sex-related differences, the regulation of adipokine expression pattern appeared to differ markedly within animals with aging and between the two fat depots. For example, in female animals, the epicardial fat expression of adiponectin and PPARγ diminished with aging while conversely, the levels of these genes remained either unchanged or increased with age in the abdominal fat depot. It is thought that adiponectin is regulated transcriptionally at least in part by PPARγ expression18. Our findings of an age-related decrease in the levels of PPARγ mRNA in the EF of female rats but not in aging male rats support the observed lower levels of adiponectin mRNA in the EF of female rats. In contrast to the tissue levels, there was actually an increase in circulating levels of adiponectin in female rats. This phenomenon has been observed in aging humans. This increase in circulating adiponectin with aging might be due to compromised kidney function and/or differences in androgen levels26.
The abnormal adiposity associated increase in pro-inflammatory mediators has been attributed to an increase in macrophage infiltration into adipose tissue in obese animals and humans19. However, the adipocytes along with inflammatory cells such as macrophages can by themselves initiate inflammatory processes during aging27. An increased expression of IL-6 in epicardial fat compared to other adipose tissues has been observed in patients with coronary artery disease and other vascular disorders25. However, in our study, even though the male rats had higher IL-6 levels, the aged female rats exhibited decreased epicardial IL-6 and PAI-1 expression. We also found non-detectable levels of circulating TNFα, IL-6, and PAI-1 and the Proteome adipokine array also showed a trend towards lower levels of circulating adipokines with aging in both sexes. This might suggest that during normal aging process, both the fat depots might probably be mediating its physiological functions through a paracrine/autocrine manner rather than having a systemic effect.
Our data show that in females there was a decrease in both the pro- and anti-inflammatory mediators with aging in EF, compared to males. This decrease in pro-inflammatory factors in EF with aging might be a protective mechanism; however, a simultaneous decrease in anti-inflammatory factors will actually have a counter-effect. A loss of both these factors might suggest a loss of EF function with aging, which was more prominent in female than in male rats. Since, EF has an immediate paracrine effect on cardiac and coronary function, a loss of function in this fat might increase risk for CVD. Change in epicardial fat function is also a reflection of changes in abdominal visceral fat and is a predictor of CVD25.
The loss of pro-inflammatory genes in EF was accompanied by lower expression of markers of macrophage infiltration such as CD68 and MCP-1. The same phenomenon was also observed in aged male EF even though the levels of CD68 and MCP-1 were much lower. On the contrary, there was an increase in the macrophage markers in AF in female rats with aging but no change in male rats. This suggests a pathological role for adipose infiltrated macrophages during aging process.
The reason for differences in epicardial fat adipokines between the two sexes with aging or between the two fat depots is currently only speculative. One of the reasons might be the differences in body weight with aging between male and female rats9. Our results indicated an increase in body weight and adipocyte size with aging in both the sexes. However, a comparison between the two sexes showed that the females had lower body weight. Secondly, the preadipocyte composition of the fat tissue is thought to strongly influence its function. Preadipocytes isolated from young and old rats are different, with the adipocytes from older rats exhibiting a lower capacity for lipid accumulation, lipogenic enzyme activities, and changes in differentiation-dependent gene expression with aging28. The loss of preadipocyte function with aging might contribute to the progressive exhaustion of release of adipose derived factors from EF. Whether sex differences in preadipocyte composition contributed to the changes observed in our study is yet to be established. We however, did find differences in the size and number of adipocytes in the visceral abdominal fat between young and aging rats in both sexes. There were remarkable differences in size and number of the adipocytes between male and female rats at 6 mos. The size of the adipocyte increased with aging in both sexes.
Even though this study does not include any co-morbidity other than aging or perform any interventions, the knowledge that the normal aging process itself can alter epicardial fat function is an important finding. The reason(s) for differences in adipokines with aging, sex and between fat depots are not clear. Also whether these changes in expression of adipose derived factors have physiological implications will require further experimentation. However, taken together our data suggest that the regulation of adipose derived factors may be much more complex than previously thought. Whether these changes are acting alone, or in concert with other factors, will help explain the observed differences in risk to metabolic syndrome and CVD between the two sexes.
Acknowledgements
The authors acknowledge the grant support from NIH, HL-074239 (NS) and 5P20RR016477. The authors thank Ms. Amy Deborde and Dr. Elsa Mangiarua for their help with proofreading our manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mensah GA, Brown DW. An overview of cardiovascular disease burden in the United States. Health Aff (Millwood) 2007;26:38–48. doi: 10.1377/hlthaff.26.1.38. [DOI] [PubMed] [Google Scholar]
- 2.Regitz-Zagrosek V, Lehmkuhl E, Mahmoodzadeh S. Gender aspects of the role of the metabolic syndrome as a risk factor for cardiovascular disease. Gend Med. 2007;4 Suppl B:S162–S177. doi: 10.1016/s1550-8579(07)80056-8. [DOI] [PubMed] [Google Scholar]
- 3.Mosca L, Banka CL, Benjamin EJ, Berra K, Bushnell C, Dolor RJ, Ganiats TG, Gomes AS, Gornik HL, Gracia C, Gulati M, Haan CK, Judelson DR, Keenan N, Kelepouris E, Michos ED, Newby LK, Oparil S, Ouyang P, Oz MC, Petitti D, Pinn VW, Redberg RF, Scott R, Sherif K, Smith SC, Jr, Sopko G, Steinhorn RH, Stone NJ, Taubert KA, Todd BA, Urbina E, Wenger NK. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. J Am Coll Cardiol. 2007;49:1230–1250. doi: 10.1016/j.jacc.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Arnold AM, Psaty BM, Kuller LH, Burke GL, Manolio TA, Fried LP, Robbins JA, Kronmal RA. Incidence of cardiovascular disease in older Americans: the cardiovascular health study. J Am Geriatr Soc. 2005;53:211–218. doi: 10.1111/j.1532-5415.2005.53105.x. [DOI] [PubMed] [Google Scholar]
- 5.Lunenfeld B. An Aging World--demographics and challenges. Gynecol Endocrinol. 2008;24:1–3. doi: 10.1080/09513590701718364. [DOI] [PubMed] [Google Scholar]
- 6.Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab. 2004;89:2569–2575. doi: 10.1210/jc.2004-0165. [DOI] [PubMed] [Google Scholar]
- 7.Zou C, Shao J. Role of adipocytokines in obesity-associated insulin resistance. J Nutr Biochem. 2008;19:277–286. doi: 10.1016/j.jnutbio.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 9.Perissinotto E, Pisent C, Sergi G, Grigoletto F. Anthropometric measurements in the elderly: age and gender differences. Br J Nutr. 2002;87:177–186. doi: 10.1079/bjn2001487. [DOI] [PubMed] [Google Scholar]
- 10.van Herpen NA, Schrauwen-Hinderling VB. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol Behav. 2008;94:231–241. doi: 10.1016/j.physbeh.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 11.de Vos AM, Prokop M, Roos CJ, Meijs MF, van der Schouw YT, Rutten A, Gorter PM, Cramer MJ, Doevendans PA, Rensing BJ, Bartelink ML, Velthuis BK, Mosterd A, Bots ML. Peri-coronary epicardial adipose tissue is related to cardiovascular risk factors and coronary artery calcification in post-menopausal women. Eur Heart J. 2008;29:777–783. doi: 10.1093/eurheartj/ehm564. [DOI] [PubMed] [Google Scholar]
- 12.Chaowalit N, Lopez-Jimenez F. Epicardial adipose tissue: friendly companion or hazardous neighbour for adjacent coronary arteries? Eur Heart J. 2008;29:695–697. doi: 10.1093/eurheartj/ehm643. [DOI] [PubMed] [Google Scholar]
- 13.Iacobellis G, Barbaro G. The Double Role of Epicardial Adipose Tissue as Pro- and Anti-inflammatory Organ. Horm Metab Res. 2008 doi: 10.1055/s-2008-1062724. [DOI] [PubMed] [Google Scholar]
- 14.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 15.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao X, Hong JY, Dong LQ. The adiponectin signaling pathway as a novel pharmacological target. Mini Rev Med Chem. 2006;6:1331–1340. doi: 10.2174/138955706778992978. [DOI] [PubMed] [Google Scholar]
- 18.Berger JP. Role of PPARgamma, transcriptional cofactors, and adiponectin in the regulation of nutrient metabolism, adipogenesis and insulin action: view from the chair. Int J Obes (Lond) 2005;29 Suppl 1:S3–S4. doi: 10.1038/sj.ijo.0802904. [DOI] [PubMed] [Google Scholar]
- 19.Lago F, Dieguez C, Gomez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol. 2007;3:716–724. doi: 10.1038/ncprheum0674. [DOI] [PubMed] [Google Scholar]
- 20.Vasto S, Candore G, Balistreri CR, Caruso M, Colonna-Romano G, Grimaldi MP, Listi F, Nuzzo D, Lio D, Caruso C. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128:83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Fain JN, Sacks HS, Bahouth SW, Tichansky DS, Madan AK, Cheema PS. Human epicardial adipokine messenger RNAs: comparisons of their expression in substernal, subcutaneous, and omental fat. Metabolism. 2010 doi: 10.1016/j.metabol.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 22.Spiroglou G, Kostopoulos G, Varakis N, Papadaki H. Adipokines in Periaortic and Epicardial Adipose Tissue: Differential Expression and Relation to Atherosclerosis. J Atheroscler Thromb. 2010;17:115–130. doi: 10.5551/jat.1735. [DOI] [PubMed] [Google Scholar]
- 23.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iacobellis G, Sharma AM. Epicardial adipose tissue as new cardio-metabolic risk marker and potential therapeutic target in the metabolic syndrome. Curr Pharm Des. 2007;13:2180–2184. doi: 10.2174/138161207781039670. [DOI] [PubMed] [Google Scholar]
- 26.Isobe T, Saitoh S, Takagi S, Takeuchi H, Chiba Y, Katoh N, Shimamoto K. Influence of gender, age and renal function on plasma adiponectin level: the Tanno and Sobetsu study. Eur J Endocrinol. 2005;153:91–98. doi: 10.1530/eje.1.01930. [DOI] [PubMed] [Google Scholar]
- 27.Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill AH, Meydani SN. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol. 2007;179:4829–4839. doi: 10.4049/jimmunol.179.7.4829. [DOI] [PubMed] [Google Scholar]
- 28.Kirkland JL, Dobson DE. Preadipocyte function and aging: links between age-related changes in cell dynamics and altered fat tissue function. J Am Geriatr Soc. 1997;45:959–967. doi: 10.1111/j.1532-5415.1997.tb02967.x. [DOI] [PubMed] [Google Scholar]