Abstract
Antibodies to malondialdehyde (MDA) modified macromolecules (adducts) have been detected in the serum of patients with atherosclerosis and correlate with the progression of this disease. However, the epitope and its formation have not been characterized. Studies have shown that excess MDA can be degraded to acetaldehyde which combines with proteins to from a stable dihydropyridine adduct. To investigate, mice were immunized with (MDA) adducts in the absence of adjuvant and showed an increase in antibodies to MDA adducts and the carrier protein as the concentration of MDA was increased. In fact, a number of the commercially available antibodies to MDA modified proteins were able to be inhibited by a chemical analogue hexyl-MAA. Also, MDA/MAA adducts were detected in the serum and aortic tissue of JCR diabetic/atherosclerotic rats. These studies determined that commercially available antibodies to MDA were shown to predominantly react with the MAA adduct and are present in the JCR model of atherosclerosis in both the serum and aortic tissue. Therefore, the immune response to MDA modified proteins is most likely to the dihydropyridine structure (predominant epitope in MAA), and suggests that MAA adducts may be playing a role in the development and/or progression of atherosclerosis.
Keywords: Atherosclerosis, modified proteins, acetaldehyde, malondialdehyde, autoantibodies, lipid peroxidation, JCR rats
Introduction
Studies have shown that MDA is formed as a result of lipid peroxidation[1]; and is capable of binding to various macromolecules [2, 3]. Modification of proteins/lipoproteins has been associated with the development and/or progression of atherosclerotic disease [4, 5] Indeed, these modified proteins/lipoproteins have been found in the circulation [6, 7] and atherosclerotic lesions [8–10] of patients with atherosclerotic disease. Within atherosclerotic lesions, a “protein quality disease” develops as the result of protein unfolding and modification of protein and/or macromolecular complex function at the cellular level [10]. Additionally, modified lipoproteins have been proven to be immunogenic, generating autoantibodies against eptiopes within the apolipoprotien B-100 component [11].
The ability of these modified molecules to be highly immunogenic and pro-inflammatory poses a large threat to the vessel tissue if they are not removed, thereby drastically increasing the pathological processes involved in the development and/or progression of atherosclerosis [12]. Additionally the initiation of antibody responses to MDA adducts has been used in the past as a marker of atherosclerosis [6, 13–15]. However, the exact epitope of antibody binding and the role of this adduct in the pathogenesis of this disease remains relatively unknown. It has been demonstrated that MDA can break down to form acetaldehyde (AA)[1], and our group has shown that AA in the presence of MDA forms a unique malondialdehyde-acetaldehyde adduct we have termed MAA [16]. Important to this disease process is the observation that this adduct can initiate pro-inflammatory, pro-fibrotic, and immune responses in the absence of adjuvant resulting in the production of T cell and autoantibody responses [2, 3, 17].
More recently, MAA adducts have proven to be highly immunogenic in the absence of adjuvants [18], they can bind scavenger receptors and elicit both pro-inflammatory and T-cell responses [19, 20]. However, most importantly is the ability of the MAA adduct to generate antibody responses to the carrier protein providing a potential mechanism by which tolerance to self proteins is abrogated resulting in autoimmunity [21]. A concept that has been under investigation involves the lipid peroxidation of membranes to form MDA. This by-product can combine with proteins to modify lysine residues and form MDA adducts, one of which is the highly stable and biological relevant MAA adduct [22].
There are a number of risk factors that predict the development and/or progression of atherosclerosis including; age, gender, obesity, hypertension, diabetes mellitus, serum cholesterol, and smoking increase the oxidative state [15]. Taken into account the number of aldehydes generated from smoking (AA from the cigarette itself) and the oxidative stress detected in obese patients with diabetes, a strong possibility exists for an increased presence of both MDA and AA in these patients which may result in the formation of MAA adducts and the resultant “protein quality disease.” [10] Therefore, it was the purpose of this study to begin examining the relationship between MDA and MAA adducts, and the potential role of adducts in the development and/or progression of atherosclerosis.
Materials and Methods
Animals
Balb/c mice were purchased from the National Cancer Institute and maintained on a Purina rat chow diet. Male JCR (leptin receptor −/−) obese diabetic atherosclerotic rats were purchased from Charles Rivers Laboratories (Wilmington, MA) and maintained on a high cholesterol diet for 6–8 months. Animals were allowed free access to their food and/or water up to 1 hour prior to sacrifice. All procedures were approved by the Animal Subcommittee of The University of Nebraska Medical Center (JCR Rats) and Omaha VA Medical Center (Balb/c mice) in accordance with the National Institutes of Health Guidelines on the Use of Laboratory Animals.
Chemicals and Proteins
Bovine serum albumin (Alb) or rat serum albumin (RSA) was purchased from Cal Biochem (La Jolla, CA). Acetaldehyde (AA) was obtained from Aldrich Chemical Co. (Milwaukee, WI). Malondialdehyde (MDA) was obtained as the sodium salt (MDA~Na) by treatment of tetramethoxypropane (Aldrich Chemical Co.) with NaOH, according to the method of Kikugawa and Ido. [23] Phytic acid (PA), and diethylenetriaminepentaacetic acid (DTPA) were obtained from Sigma Chemical Co. (St. Louis, MO).
Preparation of Modified Proteins
Bovine (Alb) or rat (RSA) serum albumin and LDL were adjusted to 2 mg and modified by reacting increasing concentrations of MDA in the presence or absence of 1.0 mM AA in 0.1 M phosphate buffer pH 7.2, containing 2 mM DTPA and 2mM PA at 37°C for 3 days, followed by dialysis against 3 changes of 0.1 M sodium phosphate buffer for 24 hours at 4°C. [16] MAA-modified albumin was checked for modification by the amount of fluorescent MAA adduct present (excitation 398 nm and emission 460 nm) using a Turner Biosystems (Sunnyvale, CA) LS-5B spectrofluorometer.
Comparison of Anti-MDA and MAA Antibodies
The specificity of the monoclonal and polyclonal anti-MAA antibodies [24] in conjunction with other commercially available anti-MDA antibodies (Mouse 1F83 anti-MDA, Abcam Inc., Cambridge, MA) (Goat anti-MDA, Meridian Life Science, Inc., Saco, ME), (Rabbit anti-MDA, Calbiochem, Damstadt, Germany) were tested against Alb, and MAA-Alb modified at a ratio of 2:1 (2 mM MDA : 1mM AA), or increasing concentrations of MDA with Alb. For these experiments, 96 well Immulon IV (Nunc, Fisher Scientific, St. Louis, MO) microtiter plates were coated with 2 μg/well Alb, Alb modified with increasing concentrations of MDA, or MAA-Alb in bicarbonate buffer (pH 9.6) as previously described [18]. After an overnight incubation at 37°C, the plates were washed three times with phosphate buffered saline containing 0.05% Tween 20 (PBST), the MDA antibodies were diluted as determined by manufacturer directions and incubated at 37°C for 45 minutes. MAA antibodies were diluted 1:1000 for the monoclonal, and 1:2000 for the polyclonal. Plates were washed in PBST and a secondary antibody (HRP rabbit anti-mouse IgG, HRP goat anti-rabbit IgG, or HRP rabbit anti-goat IgG); Sigma Chemical Company (St. Louis, MO), was added and incubated at 37°C for 45 minutes. The plates were washed and TMB substrate was added. Color changes were monitored by an MRX II Microplate Reader (Dynatech, Chantilly, VA) at 450 nm. Standard curves were established using known concentrations of the appropriate mouse, goat, or rabbit IgG (Sigma Chemical Co.), and the concentrations of unknown samples were extrapolated by using Revelations Software (Dynatech, Chantilly, VA). The means +/− SE of the relative concentrations of antibody from individual mice assayed in duplicate were subtracted from activity on the unmodified protein and reported in nanograms per milliliter.
Competitive ELISA demonstrating specificity to the MAA epitope
In order to determine the specificity of the antibodies raised against the MAA epitope, a competitive ELISA was used. Briefly, ELISA plates were coated with MAA-Alb as described above and incubated overnight. A separate (transfer plate) was used to dilute the hexyl-MAA (a synthetic analogue) and the anti-MDA or anti-MAA antibodies. The hexyl-MAA was diluted 2-fold down the plate at a starting concentration of 2,000 pmol/well. The above antibodies were added at a dilution (previously determined) that would result in a 1.0 optical density reading on the spectrophotometer after 30 minutes of incubation. Following an overnight incubation, the plate was washed, blocked, and the contents of the transfer plate moved to the appropriate wells of the ELISA plate. After 1 hour incubation at room temperature, the plate was washed and the secondary antibodies as outlined above were added for 30 minutes. The absorbance was assessed at as described above and the percent inhibition determined using the following formula:
Immunization with MDA/MAA Adducts
In order to examine the immunogenicity of MDA and MAA adducts, Balb/c mice were immunized with increasing concentrations of MDA modified Alb in the presence or absence of 1mM acetaldehyde. Prior to immunization, animals were pre-bleed and serum tested for background levels of anti-MAA antibodies. Modified proteins were immunized at a concentration of 25 μg weekly for 5 weeks in the absence of any adjuvants [18]. Following the immunization schedule, blood was collected using retro-orbital venous puncture and serum frozen at −20°C until processed.
Determination of antibodies to MAA
Serum from animals immunized with MDA or MAA adducts were screened for the presence of anti-MAA and anti-Alb antibodies. For these experiments plates were coated with 2:1 MAA-Alb or Alb as described above and incubated with the anti-serum at a 1:1000 dilution. Following an incubation period, a secondary HRP rabbit anti-mouse antibody was added. Plates were developed, absorbance determined, and concentrations extrapolated as described above. Circulation anti-MAA antibodies from pre-bled serum samples prior to immunization were subtracted from the MAA immunized mice and reported.
Screening of JCR Rat serum for anti-MAA antibodies
Serum from Spraque-Dawley and JCR rats were collected following 6 months on a high cholesterol diet. The JCR rat on this diet has been used as a model of atherosclerosis as it has been shown to induce plaque formation and mimic human disease [25]. These studies were devised to evaluate the relevance of MDA/MAA-modified proteins and anti-MAA/MDA antibodies in an in vivo setting. To determine antibody concentrations, ELISA plates were coated with rat serum albumin (RSA), LDL, oxidized LDL, MAA LDL and aortic tissue that were unmodified or modified with MAA as described above. A Rat IgG standard was also coated on the plate to use as a standard curve. Antiserum was incubated at a 1:50 dilution and a HRP rabbit anti-rat antibody used as the secondary detecting antibody. Plates were developed and concentrations determined as described above. To show specificity to the MAA epitope, RSA-MAA, hexyl-MAA, aortic tissue, and aortic tissue modified with MAA was used as the inhibiting ligand. These experiments were designed in a similar manner as the hexyl-MAA studies described above. However, the proteins (inhibitors) were started at 1000 μg/well, diluted 2-fold down the plate, the antiserum added at 2 × concentrations, and the percent inhibition calculated as described above. Native Alb or RSA (unmodified) were used as negative controls and demonstrated no inhibitory properties of the antibody response.
Determination of MAA antigens in aortic tissue
Aortic tissue from Sprague-Dawley and JCR rats were lysed with PBS-RIPA buffer (PBS, pH 7.4, 0.5% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1 mM Na-EDTA, and 5 ul/ml protease inhibitor cocktail (Sigma Chemical Company) as described previously [26]. Lysates equivalent to 50 ug were resolved under reducing conditions by SDS-PAGE on 10% gels for detection of MAA antigens. Lysates equivalent to 100 ug were resolved under reducing conditions by using an 8% SDS-PAGE. Proteins were transferred to Immuno-Blot™ PVDF membranes (Bio-Rad, Hercules, CA), and blocked 30 minutes in Odyssey blocking buffer (Licor, Lincoln, NE) at 37 degrees. Blots were incubated with anti-MAA mouse monoclonal antibody (1:2000) dilution, followed by an IRDye conjugated anti-mouse antibody (1:15000; Licor, Lincoln, NE). Blots were scanned using an Odyssey IR Scanner (LiCor, Lincoln, NE) and bands were normalized to tubulin by using 1:4000 anti-tubulin mouse monoclonal antibody (Sigma Chemical Co.) and IRDye conjugated anti-mouse antibody as an internal control. Data were expressed as the densitometric volume of MAA relative to the densitometric volume of tubulin for each lane.
Statistical Analysis
Results are expressed as means +/− SEM. Statistical significance was achieved if P values were less than 0.05. All statistical analysis was performed using the SigmaStat (Jandel Scientific, 2002).
Results
Preliminary studies have suggested that the predominant adduct formed when MDA combines with proteins is the MAA epitope. This has been identified as a 1,4 dihyrdopyridine structure possessing strong fluorescence properties at an excitation of 398 nm and emission at 460 nm. Therefore, assays were performed using this characteristic of MAA adducts to determine the amount of MAA modification on proteins modified with different concentrations of MDA. Table 1 shows the amount of MAA fluorescent modification (nm/mg) on MDA modified albumin. By fluorescence assays, MDA alone begins to modify proteins with MAA when using as little as 0.5 to 1.0 mM MDA. At concentrations of 10 to 50 mM MDA modification of the protein with MDA is similar to conditions where MAA modification is performed using 2mM MDA and 1mM AA (standard conditions). The addition of MDA to proteins at concentrations from 0.5 mM to 100 mM demonstrate a dose response with respect to MAA fluorescence (0.18 ± 0.06 to 29.20 ± 3.36 nm/mg) The addition of 1 mM AA to the increasing concentrations of MDA showed a 5–10 fold increase in MAA fluorescence. Also, measurements of the amount of fluorescence showed that 1 mM AA increases the amount of MAA adducts formed as you increased the concentration of MDA. Therefore, these data show that while MDA alone will adduct protein with the MAA epitope, other MDA adducts must also form on proteins.
Table 1. Flourescent modification of MDA adducts.
Fluorescence on MDA or MAA modified albumin. Bovine serum albumin was modified with increasing concentrations of MDA alone or MDA with 1 mM AA (MAA), and assayed fluorescence at 398 nm and 460 nm. Data expressed are the ± S.E.M. of five separate experiments.
| Adduct | nm/mg of MAA Flouresence | |
|---|---|---|
| MDA only | AA 1mM MDA increased | |
| MDA 0.5 | 0.18 ± .06 | 0.03 ± .03 |
| MDA 1.0 | 1.13 ± .52 | 23.06 ± 4.28 |
| MDA 2.0 | 4.87 ± .78 | 51.69 ± 3.34 |
| MDA 5.0 | 15.06 ± 2.76 | 141.86 ± 11.21 |
| MDA 10.0 | 32.82 ± 7.63 | 289.99 ± 23.36 |
| MDA 50.0 | 54.28 ± 9.60 | 504.90 ± 58.43 |
| MDA 100.0 | 29.20 ± 3.36 | 714.64 ± 67.51 |
To further assess whether modification of proteins with MDA results in the modification of proteins with MAA or other MDA adducts; increasing doses of MDA were incubated with a protein and then examined immunologically for MDA and MAA adducts using commercially available anti-MDA antibodies, and previously reported monoclonal (mouse × MAA) or polyclonal (rabbit × MAA) antibodies [24]. In Table 2, the activity of a commercial monoclonal antibody to MDA (Abcam) [3] and a monoclonal antibody prepared at UNMC [27] to the MAA epitope, were compared on proteins modified with different concentrations of MDA (no AA added). As shown, both Abcam and UNMC monoclonal antibodies appear to recognize similar if not the same epitope. The major difference may be in the sensitivity of the assays as the Abcam antibody is an IgG2a and the UNMC antibody is an IgG1.
Table 2. Antibody Concentration to MDA adducts.
Relative antibody concentrations to MDA or MAA adduct using UNMC or Commercially available antibodies. Albumin (Alb), Alb modified with increasing concentrations of MDA, and MAA-Alb (2:1) were coated on ELISA plates and incubated with monoclonal or polyclonal antibodies to MDA/MAA adducts. Data expressed are the ± S.E.M. of five separate experiments.
| Monoclonal Antibodies | Polyclonal Antibodies | ||||
|---|---|---|---|---|---|
| Concentration of MDA | Abcam MS × MDA | UNMC Ms × MAA | Calbiochem Rb × MDA | Biodesign Gt × MDA | UNMC Rb × MAA |
| MDA 0.5 | 2.30 × 105 ± 1.60 × 103 | 0 | 1.39 × 106 ± 3.13 × 105 | 2.75 × 105 ± 1.1 × 104 | 4.05 × 104 ± 1.66 × 103 |
| MDA 1.0 | 2.97 × 105 ± 1.67 × 104 | 0 | 2.83 × 106 ± 3.28 × 105 | 6.25 × 105 ± 2.19 × 104 | 6.67 × 104 ± 5.72 × 103 |
| MDA 2.5 | 8.53 × 105 ± 5.10 × 104 | 9.67 × 103 ± 1.69 × 102 | 4.46 × 106 ± 4.33 × 105 | 1.25 × 106 ± 4.11 × 105 | 2.47 × 105 ± 5.20 × 104 |
| MDA 5.0 | 3.25 × 106 ± 2.99 × 105 | 9.99 × 104 ± 4.79 × 103 | 4.60 × 106 ± 9.49 × 105 | 2.15 × 106 ± 5.61 × 105 | 3.83 × 105 ± 2.68 × 104 |
| MDA 10.0 | 3.16 × 106 ± 3.48 × 105 | 7.51 × 105 ± 2.76 × 104 | 6.35 × 106 ± 1.35 × 105 | 3.47 × 106 ± 8.41 × 105 | 5.83 × 105 ± 5.31 × 104 |
| MDA 50.0 | 2.50 × 106 ± 5.10 × 105 | 1.54 × 106 ± 3.00 × 105 | 1.08 × 107 ± 2.23 × 105 | 5.07 × 106 ± 8.12 × 105 | 7.05 × 105 ± 5.40 × 104 |
| MDA 100.0 | 2.75 × 106 ± 4.46 × 105 | 1.48 × 106 ± 3.02 × 105 | 8.13 × 106 ± 1.75 × 105 | 4.57 × 106 ± 6.10 × 105 | 5.80 × 105 ± 5.41 × 104 |
|
| |||||
| MAA 2:1 | 3.10 × 106 ± 1.95 × 105 | 3.10 × 106 ± 1.95 × 105 | 4.76 × 106 ± 9.84 × 105 | 3.52 × 106 ± 6.18 × 105 | 6.94 × 105 ± 3.09 × 104 |
The activity of the UNMC monoclonal antibody has been shown to be completely inhibited by the addition of the chemical analogue for MAA (hexyl-MAA) [28]. As shown in Figure 1, both the UNMC and Abcam monoclonal antibodies demonstrated similar competitive inhibition curves. The affinity of the two antibodies most likely accounts for the minor differences observed. Thus, the commercially available Abcam monoclonal antibody to MDA appears to actually be specific for the MAA epitope.
Figure 1.
Competitive inhibition of anti-MAA or anti-MDA antibodies binding to MAA modified albumin. Anti-MAA or anti-MDA antibodies were incubated with increasing concentrations hexyl-MAA (chemical analogue of MAA) and used in an ELISA to assess binding to MAA modified albumin. Data is a representative of 5 separate experiments.
With respect to the polyclonal antibodies tested, (Table 2), the UNMC polyclonal antibody reacted to the MDA modified proteins in a dose dependent manner. More importantly, it is an affinity purified antibody to MAA that is completely inhibited by hexyl MAA (Figure 1) in a similar fashion as the two monoclonal antibodies. These data show that the MAA epitope is formed on proteins modified with MDA, dependent upon the concentration of MDA used. In contrast, the two polyclonal antibodies to MDA give variable results. While both react to proteins modified with MDA in a concentration dependent manner, two things are obvious: 1) The Calbiochem antibody reacts at a higher level; (Table 2) however, this antibody is not inhibited by hexyl-MAA (Figure 1). 2) The Biodesign polyclonal was only inhibited with hexyl-MAA by 50% (Figure 1), indicating only partial reactivity to the MAA epitope. Interestingly, the Biodesign polyclonal is prepared by affinity purification on an MDA modified sepharose column, whereas the Calbiochem polyclonal is absorbed against protein carriers. Thus, the differences between these antibodies may be due to the way they were purified. These antibodies show that while the MAA epitope is produced relative to the amount of MDA present, there is/are other MDA adducts that may also form. These data are supported by the findings in Table 1 with respect to MAA fluorescence.
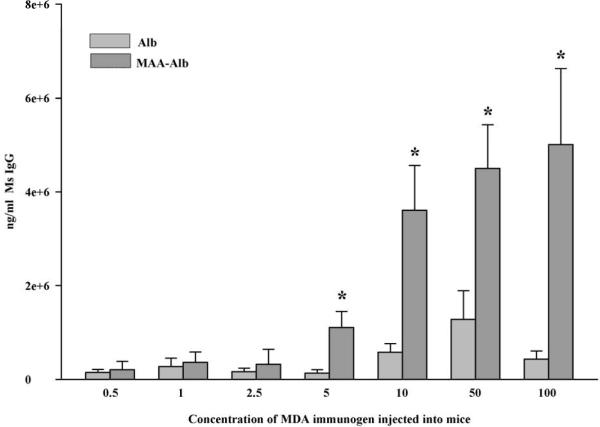
In an effort to determine the concentration of modified MDA that would induce an immune response to the 2:1 MAA adduct and/or the carrier protein, mice were immunized with increasing concentrations of MDA as the immunogen and the serum antibody concentrations tested for activity to Alb and Alb-MAA. Figure 2 shows that there is a small (not significant) antibody response up to concentration of 5 mM where the animals produce antibody to MAA-Alb but little to Alb. However, a concentration above 5 mM increases the amount of anti-MAA antibodies in the serum of these mice. Therefore, the predominant antibody is to MAA and not the carrier. This remains true as the amount of modification of albumin with MDA increased.
Figure 2.
In vivo response to MDA modified albumin. Balb/c mice were immunized with albumin modified with increasing concentrations of MDA, their serum obtained, and screened against 2:1 MAA modified albumin for the presence of anti-MAA antibodies. Data expressed are the ± S.E.M. of five animal experiments. *P ≤ 0.03 significantly increased compared to Alb groups.
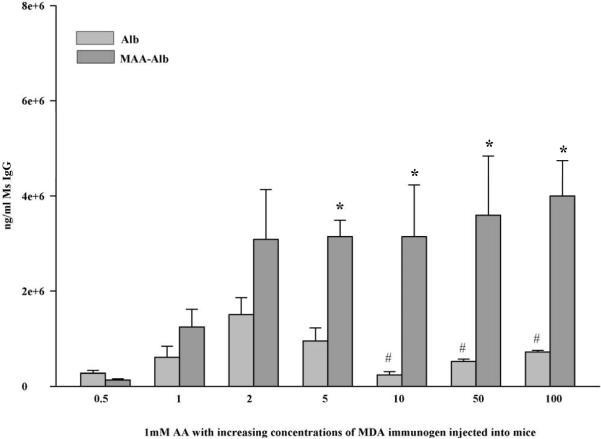
Mice were also injected with increasing concentrations of MDA incubated with 1mM AA and their serum screened for the presence of anti-MAA antibodies. Figure 3 shows that immunization at a ratio of as low as 1:1 (MDA:AA) induced antibody reactivity to the MAA product. This response increased two fold in the 2:1 immunizations and held constant throughout the increased addition of MDA. These antibody titers were not seen in the MDA immunized animals until a concentration of 10 mM was used (Figure 2). These data show that only a small amount of AA incubated with MDA and a protein will cause significant antibody production to the MAA adduct and higher levels of MDA most likely result in the modification of protein with another MDA adduct. One other important observation is the antibody response to the carrier protein Alb. These antibody responses were almost half the concentration of the immunizing agent. Once the concentration of MDA increased above 5 mM the antibody to the carrier protein was significantly decreased leaving only the antibody to the MAA epitope.
Figure 3.
In vivo response to MDA modified albumin in the presence of 1 mM AA. Balb/c mice were immunized with Alb modified with 1 mM AA and increasing concentrations of MDA and their serum was screened against 2:1 MAA modified albumin for the presence of anti-MAA antibodies. Data expressed are the ± S.E.M. of five animal experiments. *P ≤ 0.029 significantly increased compared to Alb groups. # P ≤ 0.03 significantly decreased compared to Alb in the 2:1 immunized mice.
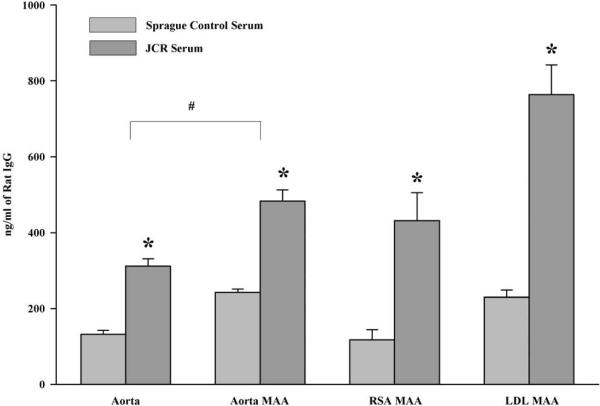
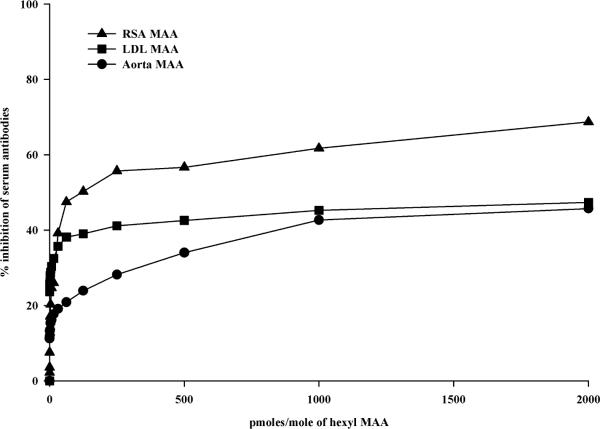
Previous studies have demonstrated the presence of MAA-adducts in atherosclerotic lesions from human subject aortas [29]. Therefore, experiments were performed to confirm the presence of antibodies to MAA in an animal model of atherosclerosis. Serum antibody concentrations of anti-MAA antibodies were determined using the JCR high cholesterol animal model of vascular injury. Figure 4 shows the presence of MAA antibodies in the JCR rat serum as compared to the Sprague-Dawley control rat serum. Reactivity to aortic tissue modified with MAA and rat serum albumin MAA (modified self proteins) were increased ~ 3 fold compared to the control animals, indicating modification of a self protein in vivo. Also, increased was LDL modified with MAA and Aortic tissue compared to the Sprague-Dawley control rat serum. It is interesting to note that JCR rats had significant reactivity to aortic tissue compared to the control group indicating an antibody response to self proteins. Specificity to these antibodies was demonstrated by inhibiting these responses with hexyl MAA (Figure 5). Antibody reactivity was inhibited by 60% for RSA MAA, 40% for LDL MAA and 40% for aortic MAA indicating that the JCR serum had antibody to the self proteins modified with MAA. These antibody responses could not be completely inhibited with hexyl MAA indicating that part of the response could be to the carrier protein conjugate attached to MAA or possible the 1:1 non-fluorescent MAA adduct. No inhibition was demonstrated using unmodified RSA or Alb alone.
Figure 4.
Serum antibody concentrations to MAA modified proteins in JCR rats. JCR rats were fed a high cholesterol diet for 6–8 months. Serum was assayed for the presences of antibody to MAA using aortic tissue, aortic tissue modified with MAA, rat serum albumin modified with MAA and LDL modified with MAA. Results are expressed in ng/ml of Rat IgG using a standard curve. Data are expressed as the ± S.E.M. of six animal experiments. *P ≤ 0.004 significantly increased compared to Sprague control serum. #P ≤ 0.001 significantly different comparing aorta to aorta MAA coating antigens.
Figure 5.
Competitive inhibition of anti-MAA or Aortic serum antibodies in JCR Rats. Serum from JCR rats was incubated with increasing concentrations of hexyl MAA and then incubated with corresponding antigen to look for inhibition binding to the epitope. Data is a representative of three animal experiments.
To further prove the presence of MAA in the JCR model of atherosclerosis, aortic tissue from JCR and Sprague-Dawley control rats was excised and immunoprecipitated using the UNMC monoclonal MAA antibody. Densitometry of a band at 88 kDA showed a 3 fold increase in the amount of MAA modified proteins in the JCR aortic tissue as compared to the control animal aortas (Figure 6). These data in conjunction with the serum antibody levels clearly indicate the presence of both MAA modified proteins and anti-MAA antibodies in the JCR model of atherosclerosis.
Figure 6.
Immunoprecipitation of MAA in aortic tissue from JCR rats. Aortic tissue from JCR or Sprague-Dawley rats was collected and immunoprecipitated using a monoclonal anti-MAA antibody. Presented data is of an 88 kDa band expressed as the ± S.E.M. of three animal experiments. *P = 0.005 significantly different from Sprague control rats.
Discussion
Modifications of proteins or lipoproteins have many deleterious effects in a number of diseases including atherosclerosis. Plasma soluble protein modifications result in their binding to scavenger receptors, stimulating the release of pro-inflammatory cytokines and become immunogenic, elicit autoantibody formation and the generation of T cells responses [15]. The covalent binding of acetaldehyde and malondialdehyde to proteins to form the MAA adduct has been demonstrated to be a major player in both alcoholic liver disease and more recently atherosclerosis [13, 16, 29]. The formation of these modified self-proteins and their biological consequences provide a potential mechanism by which atherosclerotic lesions form. Therefore, it was the purpose of this study to evaluate the relationship of MAA modification of proteins with MDA modification that has been reported by other investigators [1, 16, 17, 23, 30, 31].
Previous studies have demonstrated that following incubation with MAA-albumin and cultured rat heart endothelial cells up-regulate ICAM-1, VCAM, Class I, and Class II molecules on their surface and increase the release of TNF-α [29]. The MAA-adduct in alcoholic liver disease has proven to bind scavenger receptors on liver sinusoidal endothelial cells (SECs), initiate T cell responses, increase the release of pro-inflammatory cytokines, and initiate the fibrogenic or wound healing response [19, 20, 32, 33]. This provides a possible mechanism by which MAA modified proteins or lipoproteins bind scavenger receptors on the surface of endothelial cells of the aorta, increase cell surface markers of inflammation, and initiate macrophage migration into the intimal space. Binding to these scavenger receptors could overload the cells shunting the modified protein into the intima. As well, if dysfunctional protein clearance for these adducts is impaired (i.e. protein chaperones and the Ubiquitin-Proteasome Pathway) and/or the modification of cellular membrane proteins is greater than clearance, then the buildup dysfunctional proteins may result in disease. The resultant accumulation of dysfunctional proteins and protein aggregates (i.e. atherosclerotic amyloid) is hypothesized to play a critical role in vascular disease progression [10].
MDA-modified proteins or lipoproteins have long been thought to play an active role in the onset and/or progression of atherosclerosis [6, 15]. The formation of these MDA adducts has been attributed to the breakdown of unsaturated fatty acids or lipid peroxidation reacting with lysine on proteins. Lipid peroxidation occurs when cells are exposed to reactive oxygen species causing cell walls to rupture and membrane lipids degraded to the end-product malondialdehyde [6]. It has been shown that malondialdehyde is able to spontaneously breakdown and form acetaldehyde [1]. We hypothesize that if only a small concentration of acetaldehyde is present with malondialdehyde and a protein then the stable adduct MAA can be formed as has previously been demonstrated. This is evident as demonstrated in the data above, where MAA modification is present with as little as 0.5 mM MDA in the presence of AA. Many, if not all, anti-MDA antibodies are generated using 10 mM MDA [1, 34–36]. In our hands, while mice immunized with 10 mM MDA resulted in the production of less antibody than mice immunized with MAA (2:1, MDA:AA) ratio, antibody was detected. Also, these data prove that just a small amount of AA can cause MDA to react with proteins to form the MAA adduct and subsequently antibody formation. Some of the commercial antibodies could not be completely inhibited with hexyl-MAA the synthetic analogue which mimics the 4-methyl-1,4-dihydropyridine-3,5-dicarbaldehyde derivative of lysine. One potential reason for this incomplete inhibition would be that some of these antibodies recognize the 1:1 linear MAA adduct which forms when excess AA are present in the reaction [16, 24, 28, 37]. This could explain why when the level of MDA is increased (which can breakdown to form AA) why the antibody response to MAA is decreased.
One question that arises is how these two chemicals (AA and MDA) are present at the same time in the body to make these modified self-proteins. MDA from lipid peroxidation could be attributed to the high fat diet people consume every day, especially as increased fatty liver (alcoholic or non-alcoholic) and incidences of atherosclerosis are on the rise. AA could come from the breakdown of MDA to AA, or the oxidation of alcohol from drinking [38], fermentation of food in the gut [39] metabolism of threonine by threonine aldolase in rodents only [40, 41] and smoking [42]. In fact, cigarette smoke extract has been shown to react with MDA and proteins to make MAA modified proteins [43]. Given the highly oxidative state of smoking, it has been shown that oxidative stress increases the amount of lipid peroxidation in the lungs which increases the plasma and tissue levels of MDA [44–46]. Importantly, this could be one potential mechanism of how cigarette smoking may be a co-factor and increases the risk of cardiovascular disease.
In conclusion, commercially available antibodies to MDA were shown to predominantly react with the MAA adduct. Thus, these data suggest that many of these commercially available antibodies have specificity for the dihydropyridine structure, as confirmed by inhibition studies using an analogue to MAA. MAA modified proteins induce immune responses at physiological modifications better than MDA alone. Proteins modified with MAA are present in the JCR model of atherosclerosis in both the serum and aortic tissue. Modification of carrier proteins with MAA provides a potential mechanism by which self proteins are modified and recognized by the immune system. Therefore, the immune response to MDA modified proteins is most likely to the dihydropyridine structure (predominant epitope in MAA), and suggests that MAA adducts may be playing a role in the development and/or progression of vascular disease such as atherosclerosis.
Acknowledgments
Supported by: National Institutes of Health Grants R01 AA10435, R37 AA07818, and R21 AA15505-01A2. Also supported by the Department of Veterans Affairs National Merit Review Program and the Department of Internal Medicine at the UNMC.
Abbreviations used in this paper
- (MDA)
Malondialdehyde
- (AA)
Acetaldehyde
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Uchida K. Lipofuscin-like fluorophores originated from malondialdehyde. Free Radic Res. 2006;40:1335–1338. doi: 10.1080/10715760600902302. [DOI] [PubMed] [Google Scholar]
- [2].Steinbrecher UP, Fisher M, Witztum JL, Curtiss LK. Immunogenicity of homologous low density lipoprotein after methylation, ethylation, acetylation, or carbamylation: generation of antibodies specific for derivatized lysine. J Lipid Res. 1984;25:1109–1116. [PubMed] [Google Scholar]
- [3].Yamada S, Kumazawa S, Ishii T, Nakayama T, Itakura K, Shibata N, Kobayashi M, Sakai K, Osawa T, Uchida K. Immunochemical detection of a lipofuscin-like fluorophore derived from malondialdehyde and lysine. J Lipid Res. 2001;42:1187–1196. [PubMed] [Google Scholar]
- [4].Fu S, Davies MJ, Stocker R, Dean RT. Evidence for roles of radicals in protein oxidation in advanced human atherosclerotic plaque. Biochem J. 1998;333(Pt 3):519–525. doi: 10.1042/bj3330519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Heinecke JW. Oxidants and antioxidants in the pathogenesis of atherosclerosis: implications for the oxidized low density lipoprotein hypothesis. Atherosclerosis. 1998;141:1–15. doi: 10.1016/s0021-9150(98)00173-7. [DOI] [PubMed] [Google Scholar]
- [6].Holvoet P, Perez G, Zhao Z, Brouwers E, Bernar H, Collen D. Malondialdehyde-modified low density lipoproteins in patients with atherosclerotic disease. J Clin Invest. 1995;95:2611–2619. doi: 10.1172/JCI117963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Palinski W, Horkko S, Miller E, Steinbrecher UP, Powell HC, Curtiss LK, Witztum JL. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest. 1996;98:800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Palinski W, Rosenfeld ME, Yla-Herttuala S, Gurtner GC, Socher SS, Butler SW, Parthasarathy S, Carew TE, Steinberg D, Witztum JL. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989;86:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yla-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, Butler S, Witztum JL, Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84:1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Herrmann J, Soares SM, Lerman LO, Lerman A. Potential role of the ubiquitin-proteasome system in atherosclerosis: aspects of a protein quality disease. J Am Coll Cardiol. 2008;51:2003–2010. doi: 10.1016/j.jacc.2008.02.047. [DOI] [PubMed] [Google Scholar]
- [11].Horkko S, Binder CJ, Shaw PX, Chang MK, Silverman G, Palinski W, Witztum JL. Immunological responses to oxidized LDL. Free Radic Biol Med. 2000;28:1771–1779. doi: 10.1016/s0891-5849(00)00333-6. [DOI] [PubMed] [Google Scholar]
- [12].Matsuura E, Lopez LR. Autoimmune-mediated atherothrombosis. Lupus. 2008;17:878–887. doi: 10.1177/0961203308093553. [DOI] [PubMed] [Google Scholar]
- [13].Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, Choi J, Perkmann T, Backhed F, Miller YI, Horkko S, Corr M, Witztum JL, Binder CJ. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119:1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fredrikson GN, Hedblad B, Berglund G, Alm R, Ares M, Cercek B, Chyu KY, Shah PK, Nilsson J. Identification of immune responses against aldehyde-modified peptide sequences in apoB associated with cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23:872–878. doi: 10.1161/01.ATV.0000067935.02679.B0. [DOI] [PubMed] [Google Scholar]
- [15].Stocker R, Keaney JF., Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- [16].Tuma DJ, Thiele GM, Xu D, Klassen LW, Sorrell MF. Acetaldehyde and malondialdehyde react together to generate distinct protein adducts in the liver during long-term ethanol administration. Hepatology. 1996;23:872–880. doi: 10.1002/hep.510230431. [DOI] [PubMed] [Google Scholar]
- [17].Kikugawa K, Kato T, Iwata A. Determination of malonaldehyde in oxidized lipids by the Hantzsch fluorometric method. Anal Biochem. 1988;174:512–521. doi: 10.1016/0003-2697(88)90051-6. [DOI] [PubMed] [Google Scholar]
- [18].Thiele GM, Tuma DJ, Willis MS, Miller JA, McDonald TL, Sorrell MF, Klassen LW. Soluble proteins modified with acetaldehyde and malondialdehyde are immunogenic in the absence of adjuvant. Alcohol Clin Exp Res. 1998;22:1731–1739. [PubMed] [Google Scholar]
- [19].Duryee MJ, Klassen LW, Freeman TL, Willis MS, Tuma DJ, Thiele GM. Lipopolysaccharide is a cofactor for malondialdehyde-acetaldehyde adduct-mediated cytokine/chemokine release by rat sinusoidal liver endothelial and Kupffer cells. Alcohol Clin Exp Res. 2004;28:1931–1938. doi: 10.1097/01.alc.0000148115.90045.c5. [DOI] [PubMed] [Google Scholar]
- [20].Willis MS, Thiele GM, Tuma DJ, Klassen LW. T cell proliferative responses to malondialdehyde-acetaldehyde haptenated protein are scavenger receptor mediated. Int Immunopharmacol. 2003;3:1381–1399. doi: 10.1016/S1567-5769(03)00136-X. [DOI] [PubMed] [Google Scholar]
- [21].Duryee MJ, Willis MS, Freeman TL, Kuszynski CA, Tuma DJ, Klassen LW, Thiele GM. Mechanisms of alcohol liver damage: aldehydes, scavenger receptors, and autoimmunity. Front Biosci. 2004;9:3145–3155. doi: 10.2741/1467. [DOI] [PubMed] [Google Scholar]
- [22].Uchida K, Sakai K, Itakura K, Osawa T, Toyokuni S. Protein modification by lipid peroxidation products: formation of malondialdehyde-derived N(epsilon)-(2-propenol)lysine in proteins. Arch Biochem Biophys. 1997;346:45–52. doi: 10.1006/abbi.1997.0266. [DOI] [PubMed] [Google Scholar]
- [23].Iwata A, Kikugawa K. Fluorometric determination of malonaldehyde in oxidized lipids. Chem Pharm Bull (Tokyo) 1987;35:5020–5023. doi: 10.1248/cpb.35.5020. [DOI] [PubMed] [Google Scholar]
- [24].Thiele GM, Tuma DJ, Miller JA, Wegter KM, McDonald TL, Klassen LW. Monoclonal and polyclonal antibodies recognizing acetaldehyde-protein adducts. Biochem Pharmacol. 1998;56:1515–1523. doi: 10.1016/s0006-2952(98)00251-2. [DOI] [PubMed] [Google Scholar]
- [25].Vine DF, Takechi R, Russell JC, Proctor SD. Impaired postprandial apolipoprotein-B48 metabolism in the obese, insulin-resistant JCR:LA-cp rat: increased atherogenicity for the metabolic syndrome. Atherosclerosis. 2007;190:282–290. doi: 10.1016/j.atherosclerosis.2006.03.013. [DOI] [PubMed] [Google Scholar]
- [26].Schaffert CS, Sorrell MF, Tuma DJ. Expression and cytoskeletal association of integrin subunits is selectively increased in rat perivenous hepatocytes after chronic ethanol administration. Alcohol Clin Exp Res. 2001;25:1749–1757. [PubMed] [Google Scholar]
- [27].Thiele GM, Klassen LW, Tuma DJ. Formation and immunological properties of aldehyde-derived protein adducts following alcohol consumption. Methods Mol Biol. 2008;447:235–257. doi: 10.1007/978-1-59745-242-7_17. [DOI] [PubMed] [Google Scholar]
- [28].Xu D, Thiele GM, Kearley ML, Haugen MD, Klassen LW, Sorrell MF, Tuma DJ. Epitope characterization of malondialdehyde-acetaldehyde adducts using an enzyme-linked immunosorbent assay. Chem Res Toxicol. 1997;10:978–986. doi: 10.1021/tx970069t. [DOI] [PubMed] [Google Scholar]
- [29].Hill GE, Miller JA, Baxter BT, Klassen LW, Duryee MJ, Tuma DJ, Thiele GM. Association of malondialdehyde-acetaldehyde (MAA) adducted proteins with atherosclerotic-induced vascular inflammatory injury. Atherosclerosis. 1998;141:107–116. doi: 10.1016/s0021-9150(98)00153-1. [DOI] [PubMed] [Google Scholar]
- [30].Mooradian AD, Reinacher D, Li JP, Pinnas JL. Malondialdehyde modification of proteins in vitro is enhanced in the presence of acetaldehyde. Nutrition. 2001;17:619–622. doi: 10.1016/s0899-9007(01)00580-9. [DOI] [PubMed] [Google Scholar]
- [31].Tsai L, Szweda PA, Vinogradova O, Szweda LI. Structural characterization and immunochemical detection of a fluorophore derived from 4-hydroxy-2-nonenal and lysine. Proc Natl Acad Sci U S A. 1998;95:7975–7980. doi: 10.1073/pnas.95.14.7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Duryee MJ, Freeman TL, Willis MS, Hunter CD, Hamilton BC, 3rd, Suzuki H, Tuma DJ, Klassen LW, Thiele GM. Scavenger receptors on sinusoidal liver endothelial cells are involved in the uptake of aldehyde-modified proteins. Mol Pharmacol. 2005;68:1423–1430. doi: 10.1124/mol.105.016121. [DOI] [PubMed] [Google Scholar]
- [33].Thiele GM, Duryee MJ, Freeman TL, Sorrell MF, Willis MS, Tuma DJ, Klassen LW. Rat sinusoidal liver endothelial cells (SECs) produce pro-fibrotic factors in response to adducts formed from the metabolites of ethanol. Biochem Pharmacol. 2005;70:1593–1600. doi: 10.1016/j.bcp.2005.08.014. [DOI] [PubMed] [Google Scholar]
- [34].Bourdel-Marchasson I, Delmas-Beauvieux MC, Peuchant E, Richard-Harston S, Decamps A, Reignier B, Emeriau JP, Rainfray M. Antioxidant defences and oxidative stress markers in erythrocytes and plasma from normally nourished elderly Alzheimer patients. Age Ageing. 2001;30:235–241. doi: 10.1093/ageing/30.3.235. [DOI] [PubMed] [Google Scholar]
- [35].Ishii T, Kumazawa S, Sakurai T, Nakayama T, Uchida K. Mass spectroscopic characterization of protein modification by malondialdehyde. Chem Res Toxicol. 2006;19:122–129. doi: 10.1021/tx050231p. [DOI] [PubMed] [Google Scholar]
- [36].Kinalski M, Sledziewski A, Telejko B, Zarzycki W, Kinalska I. Lipid peroxidation and scavenging enzyme activity in streptozotocin-induced diabetes. Acta Diabetol. 2000;37:179–183. doi: 10.1007/s005920070002. [DOI] [PubMed] [Google Scholar]
- [37].Klassen LW, Thiele GM. Immune reactivity to proteins biotransformed by alcohol metabolites. Alcohol Clin Exp Res. 1998;22:204S–207S. doi: 10.1111/j.1530-0277.1998.tb04002.x. [DOI] [PubMed] [Google Scholar]
- [38].Hawkins RD, Kalant H. The metabolism of ethanol and its metabolic effects. Pharmacol Rev. 1972;24:67–157. [PubMed] [Google Scholar]
- [39].Visapaa JP, Jokelainen K, Nosova T, Salaspuro M. Inhibition of intracolonic acetaldehyde production and alcoholic fermentation in rats by ciprofloxacin. Alcohol Clin Exp Res. 1998;22:1161–1164. [PubMed] [Google Scholar]
- [40].Edgar AJ. Mice have a transcribed L-threonine aldolase/GLY1 gene, but the human GLY1 gene is a non-processed pseudogene. BMC Genomics. 2005;6:32. doi: 10.1186/1471-2164-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ma XL, Baraona E, Hernandez-Munoz R, Lieber CS. High levels of acetaldehyde in nonalcoholic liver injury after threonine or ethanol administration. Hepatology. 1989;10:933–940. doi: 10.1002/hep.1840100607. [DOI] [PubMed] [Google Scholar]
- [42].Seeman JI, Dixon M, Haussmann HJ. Acetaldehyde in mainstream tobacco smoke: formation and occurrence in smoke and bioavailability in the smoker. Chem Res Toxicol. 2002;15:1331–1350. doi: 10.1021/tx020069f. [DOI] [PubMed] [Google Scholar]
- [43].Freeman TL, Haver A, Duryee MJ, Tuma DJ, Klassen LW, Hamel FG, White RL, Rennard SI, Thiele GM. Aldehydes in cigarette smoke react with the lipid peroxidation product malonaldehyde to form fluorescent protein adducts on lysines. Chem Res Toxicol. 2005;18:817–824. doi: 10.1021/tx0500676. [DOI] [PubMed] [Google Scholar]
- [44].Harats D, Ben-Naim M, Dabach Y, Hollander G, Stein O, Stein Y. Cigarette smoking renders LDL susceptible to peroxidative modification and enhanced metabolism by macrophages. Atherosclerosis. 1989;79:245–252. doi: 10.1016/0021-9150(89)90130-5. [DOI] [PubMed] [Google Scholar]
- [45].Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem. 1997;43:1209–1214. [PubMed] [Google Scholar]
- [46].Sandhir R, Subramanian S, Koul A. Long-term smoking and ethanol exposure accentuates oxidative stress in hearts of mice. Cardiovasc Toxicol. 2003;3:135–140. doi: 10.1385/ct:3:2:135. [DOI] [PubMed] [Google Scholar]