Abstract
We examined crosstalk between the insulin receptor and G-protein-coupled receptor (GPCR) signaling pathways in individual human pancreatic cancer PANC-1 cells. Treatment of cells with insulin (10 ng/ml) for 5 min markedly enhanced the proportion of cells that display an increase in intracellular [Ca2+] induced by picomolar concentrations of the GPCR agonist neurotensin. Interestingly, insulin increased the proportion of a sub-population of cells that exhibit intracellular [Ca2+] oscillations in response to neurotensin at concentrations as low as 50–200 pM. Insulin enhanced GPCR-induced Ca2+ signaling in a time- and dose-dependent manner; a marked potentiation was obtained after an exposure to a concentration of 10 ng/ml for 5 min. Treatment with the mTORC1 inhibitor rapamycin abrogated the increase in GPCR-induced [Ca2+]i oscillations produced by insulin. Our results identify a novel aspect in the crosstalk between insulin receptor and GPCR signaling systems in pancreatic cancer cells, namely that insulin increases the number of [Ca2+]i oscillating cells induced by physiological concentrations of GPCR agonists through an mTORC1-dependent pathway.
Keywords: Pancreatic cancer, Ca2+ oscillations, signaling crosstalk, rapamycin
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is a devastating disease, with overall 5-year survival rate of only 3–5%. The incidence of this disease in the US has increased recently to more than 42,000 new cases each year and is now the fourth leading cause of cancer mortality in both men and women. As the current therapies offer very limited survival benefits, novel targets for therapeutic intervention are urgently needed and will most likely arise from a more detailed understanding of the signaling pathways and crosstalk mechanisms that regulate the behavior of these aggressive cancer cells [1].
G protein–coupled receptors (GPCRs) and their cognate agonists are increasingly implicated as autocrine/paracrine growth factors for multiple solid tumors, including small cell lung cancer, colon, prostate, breast and pancreas [2–9]. We showed that PDAC cell lines express multiple functional GPCRs using a Ca2+ mobilization assay as indicator of productive ligand-receptor interactions [10]. A variety of GPCR agonists, including neurotensin, stimulated DNA synthesis and proliferation in PDAC cells, such as PANC-1 cells [10–14]. Furthermore, a broad-spectrum GPCR antagonist [15, 16], inhibited the growth of PDAC cells either in vitro or xenografted into nu/nu mice [17]. Other studies demonstrated increased expression of agonists and their cognate GPCRs in PDAC tissues [18–21]. More recently, we identified a novel crosstalk between insulin/IGFI receptors and GPCR signaling systems in PDAC cells, leading to enhancement of GPCR-induced signaling [14, 22, 23], including Ins(1,4,5)P3 generation and increase in the intracellular Ca2+ concentration ([Ca2+]i). Insulin-induced potentiation of GPCR signaling was mediated through the phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR signaling module [14, 22], a key pathway in insulin/IGF action [24]. These findings assume an added importance in view of the large number of epidemiological studies linking obesity and long standing type-2 diabetes, characterized by peripheral insulin resistance and compensatory overproduction of insulin, with increased risk for developing PDAC [see [25] for review]. Given the complexity of the pancreatic microcirculation [26] and the close topographical relationship between the islets, small ducts and centroacinar cells [27], locally overproduced insulin is thought to act directly on ductal pancreatic cancer cells. Disruption of crosstalk between insulin receptor and GPCR signaling pathways is emerging as a potential new strategy in the treatment of this devastating disease [23].
Despite its potential clinical implications, the mechanism(s) by which insulin receptor signaling enhances responsiveness of PDAC cells to GPCR agonists remains incompletely understood. Here, we examined crosstalk between insulin receptor and GPCR signaling systems by monitoring changes in [Ca2+]i in single PANC-1 cells, an extensively used model of PDAC cells [11, 12, 14, 28, 29]. Our results demonstrate, for the first time, that brief exposure to insulin increases the proportion of PANC-1 cells that respond to picomolar concentrations of the GPCR agonist neurotensin. Interestingly, insulin enhanced a sub-population of PANC-1 cells that exhibited [Ca2+]i oscillations in response to neurotensin at concentrations as low as 50–200 pM. Given that [Ca2+]i oscillations play a key role in signal transduction by encoding specific biological responses, a novel aspect of the crosstalk between insulin receptor and GPCR signaling systems revealed by this study is that insulin signaling increases [Ca2+]i oscillations induced by physiological concentrations of the GPCR agonist neurotensin in PDAC PANC-1 cells.
MATERIALS and METHODS
Cell Culture
PANC-1, obtained from American Type Culture Collection, is a less well-differentiated line established from human PDAC. PANC-1 cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma, D5796) supplemented with 10% fetal bovine serum, penicillin (10 U/ml), streptomycin (10 µg/ml), and amphotericin B (25 ng/ml), and maintained in a humidified incubator under 10% CO2 and 90% air at 37°C. Cells were plated onto 18 mm diameter circular glass coverslips which were placed inside 35 mm plastic petri dishes filled with growth media and placed in the incubator.
Solutions
Standard saline consisted of Hanks Buffered Salt Solution (HBSS, Invitrogen) without phenol red supplemented with 0.5 mM CaCl2, and 20 mM HEPES buffer. Final concentrations (in mM) were: 138 NaCl, 4 NaHCO3, 0.3 Na2HPO4, 5 KCl, 0.3 KH2PO4, 1.8 CaCl2 , 0.5 MgCl2, 0.4 MgSO4, 5.6 D-Glucose, 20 HEPES, pH 7.4.
Measurement of [Ca2+]i
[Ca2+]i was measured in single cells loaded with the calcium indicator fura-2 as previously described [30]. Briefly, cells were removed from the incubator, washed once with HBSS, then incubated in HBSS containing 5 µM Fura-2 AM (Invitrogen) for 45 min at 37°C. The cells were then washed and placed in an experimental chamber that was perfused with saline solution at 1.5 ml/min at 37°C (Warner Instrument Corp.) The chamber in turn was placed on the stage of an inverted microscope (Zeiss 100 TV; Carl Zeiss, Inc.) connected to a digital imaging system (Attofluor, Atto Instruments). Ratios of images (340 nm excitation/ 380 nm excitation, with emission filter 520 nm) were obtained at 1.5 sec intervals. A region of interest covering 15 µm × 15 µm was defined over each cell, and the average ratio intensity over the region was converted to [Ca2+]i using an standard curve constructed with a series of calibrated buffered calcium solutions (Calcium Calibration Buffer Kit, Invitrogen Corp.).
Statistics
Unless specified, all data presented represent at least three separate slides (n = 3) with an average of 43 cells analyzed in each slide. Data are expressed as mean ± standard error. The statistical significance of the differences was evaluated using Student’s t-test, with P > 0.05 considered as not significant. In the figures, * is used for P < 0.05, ** for P<0.01, and *** for P < 0.001.
RESULTS and DISCUSSION
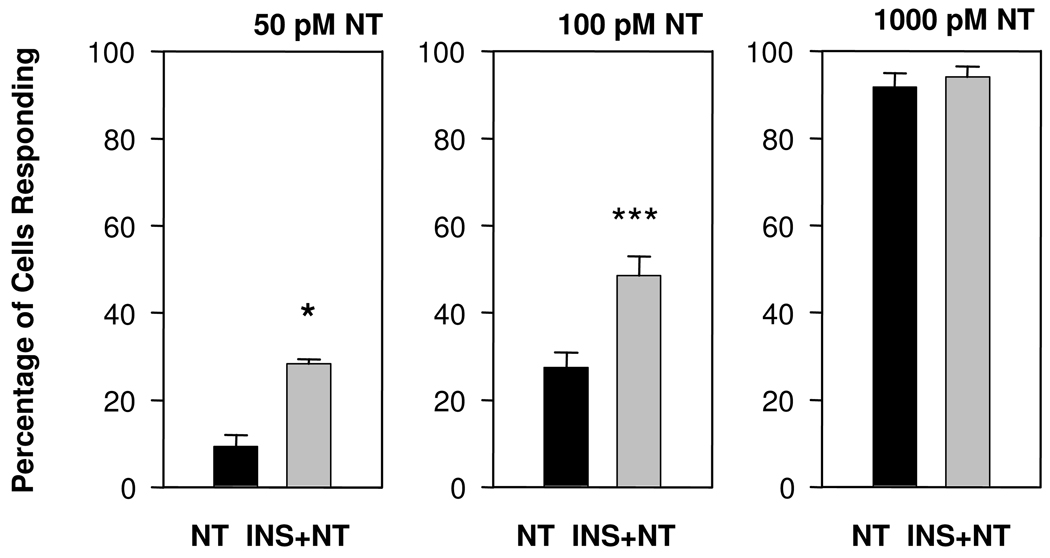
In order to examine crosstalk between insulin receptor and GPCR signaling systems on [Ca2+]i in single cells, human pancreatic cancer PANC-1 cells were loaded with the fluorescent Ca2+ indicator fura 2, and incubated with or without insulin at 10 ng/ml. Intracellular Ca2+ imaging revealed that insulin did not exert any detectable effect on [Ca2+]i in PANC-1 cells and that all cells exhibited a stable [Ca2+]i without any spontaneous oscillatory activity. After 5 min of incubation in the presence of insulin, the cells were challenged with the GPCR agonist neurotensin at increasing concentrations (50, 100 or 1000 pM). As shown in Fig. 1, exposure to insulin strikingly increased the proportion of PANC-1 cells that responded to neurotensin either at 50 pM (from 9.3 ± 3.8% to 28.2 ± 1.2 %) or 100 pM (from 27.7 ± 3.4% to 48.4 ± 4.4%). When neurotensin was applied at 1000 pM, most cells responded to the GPCR agonist and pre-treatment with insulin did not exert any detectable effect (91.7 ± 3.2% and 94.0 ± 2.4% in cultures without or with insulin treatment, respectively). These results demonstrate that a brief exposure to insulin enhances the proportion of PANC-1 cells that respond to low, picomolar, concentrations of neurotensin.
Figure 1.
Increasing neurotensin (NT) concentration increases the proportion of PANC-1 cells responding with increase in [Ca2+]i. PANC-1 cells were pretreated with insulin (INS, 10 ng/ml for 5 min) and then challenged with neurotensin (NT), at 50 pM (n = 3), 100 pM (n = 35) or 1000 pM (n = 12). Significance levels (*) are explained in Methods.
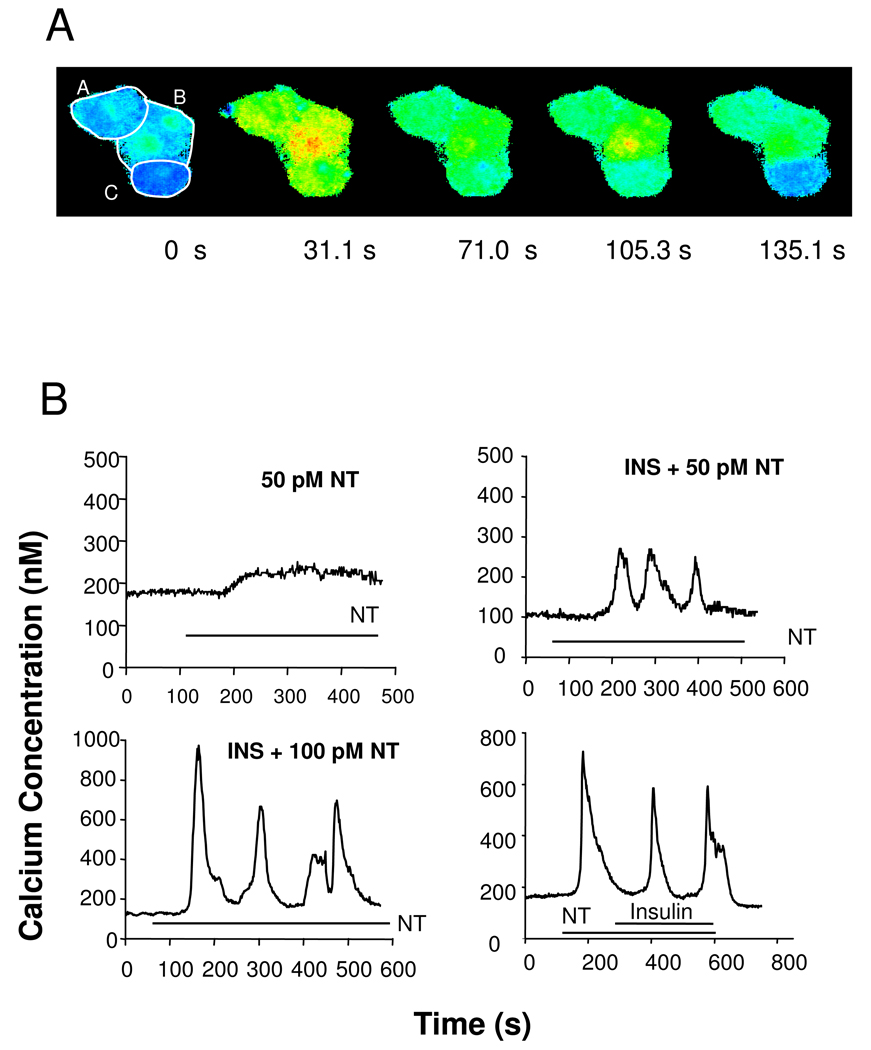
Inspection of Ca2+ signaling in single PANC-1 cells stimulated with neurotensin at 1000 pM identified cell subpopulations characterized by at least three different patterns of [Ca2+]i response: sustained, oscillatory, and transient. Fig 2A shows a pseudo-colored image of a cluster of three PANC-1 cells at rest, and at various times after stimulation with neurotensin which illustrates these classes of response. Cell A displays a rapid increase in [Ca2+]i which is maintained for at least 135 s (sustained response). In contrast, cell B shows a large increase in [Ca2+]i which declines (71 s), and then increases again at 105 s (oscillatory response) while cell C exhibits a rapid increase in [Ca2+]i which then returns to baseline by 135 s (transient response). These results prompted us to determine whether exposure to insulin has any preferential effect on the pattern of Ca2+ signaling by single PANC-1 cells challenged with low (50–100 pM) concentrations of neurotensin.
Figure 2.
Neurotensin stimulates different patterns of [Ca2+]i increase (sustained, oscillatory, transient) in single PANC-1 cells. A time series of three cells in contact are shown in pseudocolor indicating [Ca2+]i increasing from blue to green to yellow to red after stimulation with 1000 pM neurotensin. Cell boundaries are delimited with white overlay. Time is labeled below each image. Cell A shows a rapid increase in [Ca2+]i which is sustained for at least 135 s. Cell B shows a large increase (yellow-red) which declines, and then increases again at 135 s. Cell C shows a transient response with an initial rapid increase in [Ca2+]i which then returns to baseline by 135 s. B. Insulin pretreatment increases the oscillatory responsiveness of PANC-1 cells to neurotensin (NT). Upper: Cells were pretreated for 5 min either without (50 pM NT) or with 10 ng/ml insulin (INS + 50 pM NT) as indicated prior to the addition of neurotensin (50 pM). Time of neurotensin exposure is marked by horizontal bars, marked NT. Lower: Insulin did not affect neurotensin-induced [Ca2+]i oscillations in PANC-1 cells when added after the GPCR agonist. Neurotensin was applied to the cells at the times marked by the bar labeled (NT, 200 pM). After [Ca2+]i oscillations started by stimulation with NT, insulin (1000 ng/ml) was added (marked by bar labeled insulin).
Oscillatory changes in [Ca2+]i in response to receptor stimulation is a fundamental mechanism of cell signaling that can protect cells from the cytotoxic effects of prolonged increases in [Ca2+]i. In many cell types, GPCR agonists applied at low concentrations elicit [Ca2+]i oscillations [31]. In contrast, neurotensin applied at 50 pM did not induce [Ca2+]i oscillations in any of 64 individual PANC-1 cells analyzed (0/64 = 0% oscillating cells) though some cells responded with sustained or transient patterns (a typical sustained trace in Fig. 2 B, upper left). Strikingly, exposure to insulin (10 ng/ml for 5 min) induced the appearance of PANC-1 cells that exhibited [Ca2+]i oscillations in response to stimulation with 50 pM neurotensin (7 out of 68 cells; 10.2%). A typical trace of an insulin-treated PANC-1 cell showing [Ca2+]i oscillations in response to 50 pM neurotensin is shown in Fig. 2 B (upper right). These results demonstrate that treatment with insulin induces a new pattern of response to picomolar concentrations of neurotensin in PANC-1 cells, namely the emergence of PANC-1 cells displaying [Ca2+]i oscillations.
When PANC-1 cells were challenged with neurotensin at 100 pM, we detected PANC-1 cells that exhibited [Ca2+]i oscillations either with or without prior treatment with insulin. A typical trace of a single PANC-1 exposed to insulin and subsequently stimulated with 100 pM neurotensin shown in Fig. 2 B (lower left), illustrates that the amplitude of the[Ca2+]i spikes elicited by 100 pM neurotensin was substantially higher than the amplitude of the[Ca2+]i spikes evoked by neurotensin at 50 pM. When insulin, at concentrations as high as 1000 ng/ml, was applied after [Ca2+]i oscillations were initiated by neurotensin, the oscillations continue to occur with similar amplitude and frequency (Fig. 2 B, lower right).
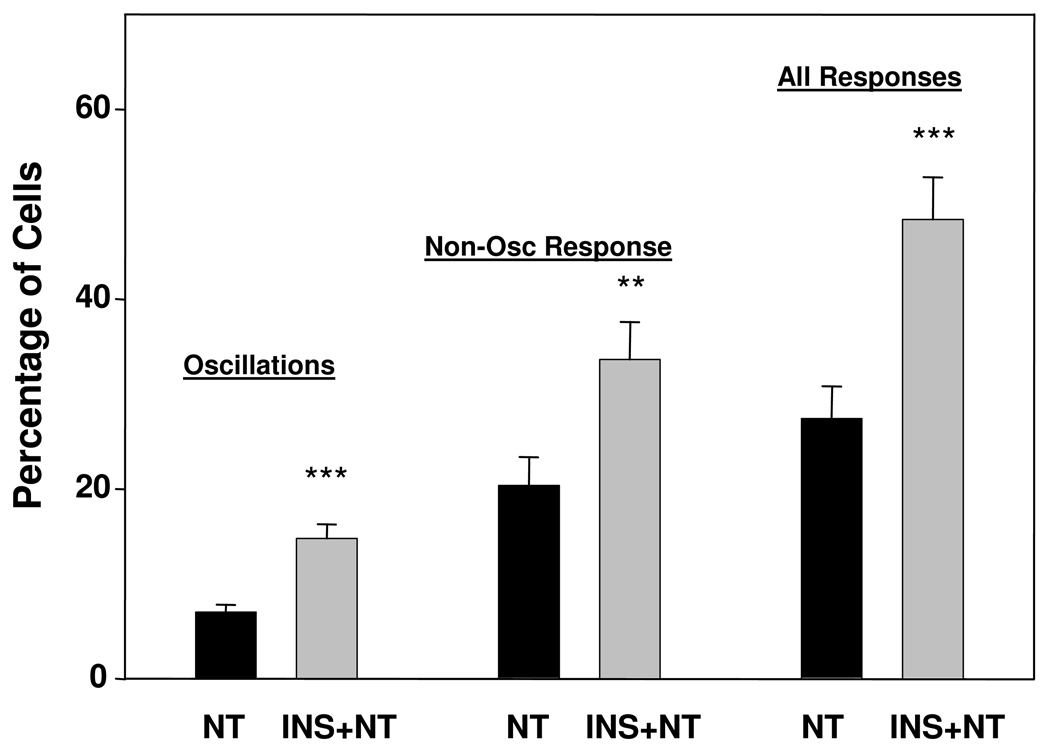
We examined crosstalk between insulin and neurotensin at 100 pM in detail (Fig 3). Combining all studies of cells stimulated with 100 pM neurotensin with and without insulin pretreatment at 10 ng/ml for 5 min, we found 7.0 ± 0.8% of the cells in 35 separate slides (totaling ~1,500 cells) displayed [Ca2+]i oscillations in response to 100 pM neurotensin. After insulin pretreatment (10 ng/ml for 5 min), 14.8 + 1.5% of the cells in 33 separate slides (~1,400 cells) produced oscillations in response to 100 pM neurotensin, a doubling in the proportion of cells that respond to the agonist with [Ca2+]i oscillations. In this same study, the percentage of cells showing other patterns of [Ca2+]i responses (sustained or transient) increased from 20.4 ± 3.0 to 33.6 ± 3.9 after insulin pretreatment (Fig 3). The percentage of cells showing any response increased from 27.4 ± 3.4 to 48.4 ± 4.4. Collectively, the results indicate that prior exposure to insulin increases preferentially the subpopulation of PANC-1 cells that exhibit [Ca2+]i oscillations in response to picomolar concentrations of neurotensin
Figure 3.
Insulin increases the percentage of PANC-1 cells which respond to 100 pM neurotensin with [Ca2+]i oscillations, as well as increasing the percentage of cells that show other pattern of responses. PANC-1 cells were treated in the absence or in the presence of insulin (INS) at a concentration of 10 ng/ml for 5 min prior to stimulation with 100 pM neurotensin (NT), as indicated (NT and INS+NT, respectively).
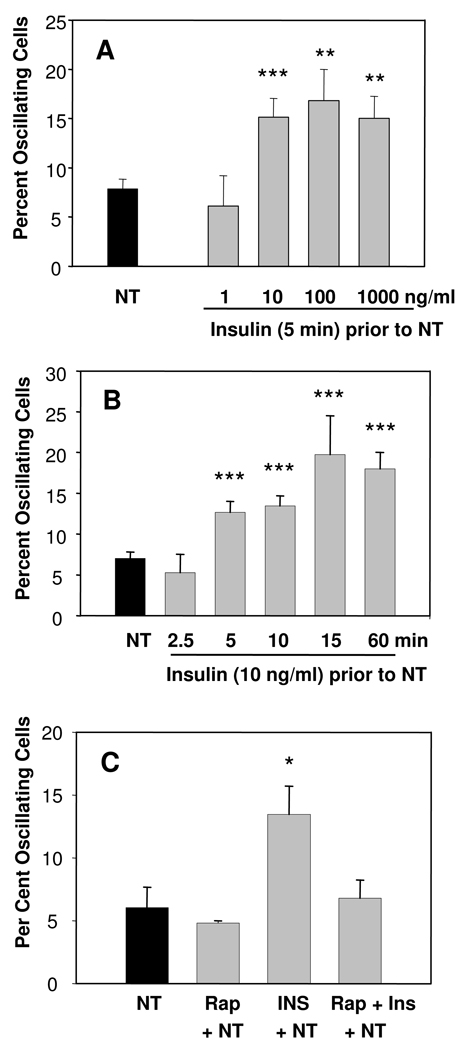
Having established that insulin increases the percentage of PANC-1 cells that display [Ca2+]i oscillations in response to low concentrations of neurotensin, we decided to further characterize the effects of the insulin pre-treatment. Specifically, we determined the effect of exposure to increasing concentrations of insulin ranging from 1 ng/ml to 1000 ng/ml for 5 min on the ability of 100 pM neurotensin to produce [Ca2+]i oscillations. As shown in Fig. 4A, insulin at 1 ng/ml has no effect but exposure to insulin at 10 ng/ml approximately doubled the percentage of PANC-1 cells that responded to neurotensin with [Ca2+]i oscillations (from 7.8 ± 1.0% to 15.1 ± 1.9%), in agreement with the results shown in Fig. 3. Increasing the insulin concentration to 100 ng/ml or 1000 ng/ml did not produce a significant further increase in the number of PANC-1 cells that responded with [Ca2+]i oscillations in the population.
Figure 4.
A). Insulin increased the proportion of PANC-1 cells that exhibit [Ca2+]i oscillations in a dose-dependent manner. PANC-1 cells were pretreated for 5 min with increasing concentrations of insulin (1–1000 ng/ml) as indicated prior to the addition of neurotensin (100 nM). The number of independent slides (n) analyzed was 3–4, except in the group treated with insulin at 10 ng/ml (n = 15) or with neurotensin alone (n = 22). B). Insulin exposure (10 ng/ml) increased the proportion of PANC-1 displaying [Ca2+]i oscillations in a time-dependent manner. The number of independent slides (n) analyzed was: n = 6 at 2.5 min; n = 7 at 5 min; n = 33 at 10 min; n = 5 at 15 min; n = 3 at 60 min. In the absence of insulin pretreatment, n = 35. C). Rapamycin blocked insulin-induced increase in cells exhibiting oscillations in response to 100 pM neurotensin (NT). Cells were incubated with rapamycin (10 nM, Rap) for 60 min prior to the addition of 100 pM NT and/or insulin pretreatment (INS, 10 ng/ml for 5 min). The number of independent slides (n) analyzed was: neurotensin alone (NT), n = 6; rapamycin pre-treatment and then neurotensin, n = 3 (Rap+ NT); insulin pre-treatment and then neurotensin (INS+NT), n=7; rapamycin prior to insulin followed by neurotensin (Rap+INS+NT), n = 6.
Given that the order of addition of the stimuli is critical to elicit crosstalk, we next determined the length of time of exposure to insulin necessary to enhance the response to neurotensin using insulin at a fixed concentration (10 ng/ml). Cells were pre-treated with insulin for 2.5, 5, 10, 15, or 60 min. The percentage of cells showing [Ca2+]i oscillations in response to 100 pM neurotensin were 12.7 ± 1.3, 14.8 ± 1.5, 19.8 ± 4.7, and 18.0 ± 3.0 after 5, 10, 15 or 60 min of exposure to insulin, respectively . There was no significant difference between 5 min and 15 min exposure or between 5 min and 60 min. The results in Fig. 4 verified that insulin increased the proportion of PANC-1 cells exhibiting [Ca2+]i oscillations in response to neurotensin after a treatment as short as 5 min and a concentration of 10 ng/ml (1.7 nM), a concentration in the physiological range within the pancreas.
The rapamycin-sensitive PI3K/Akt/mTORC1 signaling module is a key pathway in insulin receptor signaling in most cell types [24], including PDAC cells[23]. Consequently, we next investigated whether rapamycin has any effect on the insulin-induced increase in [Ca2+]i oscillations in PANC-1 cells challenged with neurotensin. Treatment of PANC-1 cells with rapamycin alone (10 nM for 1 hr) did not change the percentage of cells with [Ca2+]i oscillations in response to 100 pM neurotensin but completely prevented the insulin-induced increase in PANC-1 cells displaying [Ca2+]i oscillations (Fig. 4 C).
CONCLUSIONS and IMPLICATIONS
The spatial organization, amplitude, and frequency of the changes in [Ca2+]i have been the subject of intense interest. Here, we examined the effect of insulin on neurotensin-induced Ca2+ signaling in individual PANC-1 cells, an extensively used model of PDAC cells. In these cancer cells, as in most other cell types, insulin did not induce any significant change in [Ca2+]i even in a minority of cells in the population. However, our results show, for the first time, that brief exposure to physiological concentrations of insulin rapidly and strikingly augmented the proportion of PANC-1 cells that exhibit Ca2+ signaling in response to picomolar concentration of neurotensin, a potent mitogen that acts through endogenously expressed Gq-coupled receptors in these cells [11, 12, 14, 28, 29].
Our results show that exposure to insulin influenced the pattern of [Ca2+]i response to low concentrations of neurotensin in PANC-1 cells. In particular, we identified a subpopulation of cells that exhibit [Ca2+]i oscillations. The frequency and pattern of [Ca2+]i oscillations are recognized to play a key role in signal transduction, regulating the activity of protein kinases, mitochodrial metabolism, and nuclear transcriptional activity leading to differential gene expression [32, 33]. Importantly, insulin-induced potentiation of Ca2+ signaling, including the increase in cells that exhibit [Ca2+]i oscillations, was prevented by exposure to rapamycin, a specific inhibitor of mTORC1. These findings indicated that in addition to its well established role in the regulation of protein synthesis, the rapamycin-sensitive mTORC1 pathway mediates crosstalk between insulin receptor on GPCR signaling systems leading to enhanced proportion of PANC-1 cells displaying [Ca2+]i oscillations in response to low concentrations of a mitogenic GPCR agonist.
Bulleted Points
Crosstalk between insulin receptor and GPCR signaling in pancreatic cancer cells
Insulin increased the proportion of PANC-1 cells that responded to neurotensin.
Insulin enhanced [Ca2+]i oscillations induced by neurotensin as as low as 50–200 pM.
Treatment with the mTORC1 inhibitor rapamycin abrogated insulin induced effect.
[Ca2+]i oscillations novel aspect in crosstalk between insulin receptor and GPCR
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supported by National Institutes of Health Grants R01-DK55003, R0-1DK56930, R21CA137292 and P30-DK41301. E.R. holds the Ronald S. Hirshberg Chair of Pancreatic Cancer Research
REFERENCES
- 1.Brand RE, Tempero MA. Pancreatic cancer. Curr. Opin. Oncol. 1998;10:362–366. doi: 10.1097/00001622-199807000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Woll PJ, Rozengurt E. Multiple neuropeptides mobilise calcium in small cell lung cancer: effects of vasopressin, bradykinin, cholecystokinin, galanin and neurotensin. Biochem. Biophys. Res. Commun. 1989;164:66–73. doi: 10.1016/0006-291x(89)91683-5. [DOI] [PubMed] [Google Scholar]
- 3.Langdon S, Sethi T, Ritchie A, Muir M, Smyth J, Rozengurt E. Broad spectrum neuropeptide antagonists inhibit the growth of small cell lung cancer in vivo. Cancer Res. 1992;52:4554–4557. [PubMed] [Google Scholar]
- 4.Rozengurt E, Walsh JH. Gastrin, CCK, signaling, and cancer. Annu. Rev. Physiol. 2001;63:49–76. doi: 10.1146/annurev.physiol.63.1.49. [DOI] [PubMed] [Google Scholar]
- 5.Rozengurt E. Neuropeptides as growth factors for normal and cancer cells. Trends Endocrinol Metabol. 2002;13:128–134. doi: 10.1016/s1043-2760(01)00544-6. [DOI] [PubMed] [Google Scholar]
- 6.Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol. 2007;213:589–602. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]
- 7.Heasley LE. Autocrine and paracrine signaling through neuropeptide receptors in human cancer. Oncogene. 2001;20:1563–1569. doi: 10.1038/sj.onc.1204183. [DOI] [PubMed] [Google Scholar]
- 8.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 9.Kan Z, Jaiswal BS, Stinson J, Janakiraman V, Bhatt D, Stern HM, Yue P, Haverty PM, Bourgon R, Zheng J, Moorhead M, Chaudhuri S, Tomsho LP, Peters BA, Pujara K, Cordes S, Davis DP, Carlton VEH, Yuan W, Li L, Wang W, Eigenbrot C, Kaminker JS, Eberhard DA, Waring P, Schuster SC, Modrusan Z, Zhang Z, Stokoe D, de Sauvage FJ, Faham M, Seshagiri S. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature advance online publication. 2010 doi: 10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- 10.Ryder NM, Guha S, Hines OJ, Reber HA, Rozengurt E. G protein-coupled receptor signaling in human ductal pancreatic cancer cells: Neurotensin responsiveness and mitogenic stimulation. J. Cell. Physiol. 2001;186:53–64. doi: 10.1002/1097-4652(200101)186:1<53::AID-JCP1004>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 11.Guha S, Rey O, Rozengurt E. Neurotensin Induces Protein Kinase C-dependent Protein Kinase D Activation and DNA Synthesis in Human Pancreatic Carcinoma Cell Line PANC-1. Cancer Res. 2002;62:1632–1640. [PubMed] [Google Scholar]
- 12.Guha S, Lunn JA, Santiskulvong C, Rozengurt E. Neurotensin Stimulates Protein Kinase C-dependent Mitogenic Signaling in Human Pancreatic Carcinoma Cell Line PANC-1. Cancer Res. 2003;63:2379–2387. [PubMed] [Google Scholar]
- 13.Kisfalvi K, Guha S, Rozengurt E. Neurotensin and EGF induce synergistic stimulation of DNA synthesis by increasing the duration of ERK signaling in ductal pancreatic cancer cells. J Cell Physiol. 2005;202:880–890. doi: 10.1002/jcp.20187. [DOI] [PubMed] [Google Scholar]
- 14.Kisfalvi K, Eibl G, Sinnett-Smith J, Rozengurt E. Metformin disrupts crosstalk between G protein-coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth. Cancer Res. 2009;69:6539–6545. doi: 10.1158/0008-5472.CAN-09-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seckl MJ, Higgins T, Widmer F, Rozengurt E. [D-Arg1,D-Trp5,7,9, Leu11]substance P: a novel potent inhibitor of signal transduction and growth in vitro and in vivo in small cell lung cancer cells. Cancer Res. 1997;57:51–54. [PubMed] [Google Scholar]
- 16.Sinnett-Smith J, Santiskulvong C, Duque J, Rozengurt E. [D-Arg(1),D-Trp(5,7,9),Leu(11)]substance P inhibits bombesin-induced mitogenic signal transduction mediated by both G(q) and G(12) in Swiss 3T3 cells] J. Biol. Chem. 2000;275:30644–30652. doi: 10.1074/jbc.M003702200. [DOI] [PubMed] [Google Scholar]
- 17.Guha S, Eibl G, Kisfalvi K, Fan RS, Burdick M, Reber H, Hines OJ, Strieter R, Rozengurt E. Broad-spectrum G protein-coupled receptor antagonist, [D-Arg1, D-Trp5,7,9,Leu11]SP: a dual inhibitor of growth and angiogenesis in pancreatic cancer. Cancer Res. 2005;65:2738–2745. doi: 10.1158/0008-5472.CAN-04-3197. [DOI] [PubMed] [Google Scholar]
- 18.Elek J, Pinzon W, Park KH, Narayanan R. Relevant genomics of neurotensin receptor in cancer. Anticancer Res. 2000;20:53–58. [PubMed] [Google Scholar]
- 19.Wang L, Friess H, Zhu Z, Graber H, Zimmermann A, Korc M, Reubi JC, Bèuchler MW. Neurotensin receptor-1 mRNA analysis in normal pancreas and pancreatic disease. Clin Cancer Res. 2000;6:566–571. [PubMed] [Google Scholar]
- 20.Reubi JC, Waser B, Friess H, Bèuchler M, Laissue J. Neurotensin receptors: a new marker for human ductal pancreatic adenocarcinoma. Gut. 1998;42:546–550. doi: 10.1136/gut.42.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arafat HA, Gong Q, Chipitsyna G, Rizvi A, Saa CT, Yeo CJ. Antihypertensives as novel antineoplastics: angiotensin-I-converting enzyme inhibitors and angiotensin II type 1 receptor blockers in pancreatic ductal adenocarcinoma. J Am Coll Surg. 2007;204:996–1005. doi: 10.1016/j.jamcollsurg.2007.01.067. [DOI] [PubMed] [Google Scholar]
- 22.Kisfalvi K, Rey O, Young SH, Sinnett-Smith J, Rozengurt E. Insulin Potentiates Ca2+ Signaling and Phosphatidylinositol 4,5-Bisphosphate Hydrolysis Induced by Gq Protein-Coupled Receptor Agonists through an mTOR-Dependent Pathway. Endocrinology. 2007;148:3246–3257. doi: 10.1210/en.2006-1711. [DOI] [PubMed] [Google Scholar]
- 23.Rozengurt E, Sinnett-Smith J, Kisfalvi K. Crosstalk between Insulin/Insulin-like Growth Factor-1 Receptors and G Protein-Coupled Receptor Signaling Systems: A Novel Target for the Antidiabetic Drug Metformin in Pancreatic Cancer. Clin Cancer Res. 2010;16:2505–2511. doi: 10.1158/1078-0432.CCR-09-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 25.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132:2208–2225. doi: 10.1053/j.gastro.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 26.Ballian N, Brunicardi FC. Islet vasculature as a regulator of endocrine pancreas function. World J Surg. 2007;31:705–714. doi: 10.1007/s00268-006-0719-8. [DOI] [PubMed] [Google Scholar]
- 27.Bertelli E, Regoli M, Orazioli D, Bendayan M. Association between islets of Langerhans and pancreatic ductal system in adult rat. Where endocrine and exocrine meet together? Diabetologia. 2001;44:575–584. doi: 10.1007/s001250051663. [DOI] [PubMed] [Google Scholar]
- 28.Yuan J, Rozengurt E. PKD, PKD2, and p38 MAPK mediate Hsp27 serine-82 phosphorylation induced by neurotensin in pancreatic cancer PANC-1 cells. J Cell Biochem. 2008;103:648–662. doi: 10.1002/jcb.21439. [DOI] [PubMed] [Google Scholar]
- 29.Kisfalvi K, Hurd C, Guha S, Rozengurt E. Induced overexpression of protein kinase D1 stimulates mitogenic signaling in human pancreatic carcinoma PANC-1 cells. J Cell Physiol. 2010;223:309–316. doi: 10.1002/jcp.22036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young SH, Rozengurt E. Amino acids and Ca2+ stimulate different patterns of Ca2+ oscillations through the Ca2+-sensing receptor. Amer J Physiol-Cell Physiol. 2002;282:C1414–C1422. doi: 10.1152/ajpcell.00432.2001. [DOI] [PubMed] [Google Scholar]
- 31.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 32.Clapham DE. Calcium Signaling. Cell. 2007;131:1047. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 33.Brandman O, Meyer T. Feedback Loops Shape Cellular Signals in Space and Time. Science. 2008;322:390–395. doi: 10.1126/science.1160617. [DOI] [PMC free article] [PubMed] [Google Scholar]