Abstract
This communication describes a simple way to improve the sensitivity of spectroscopic ellipsometry, when applied to monitor the adsorption of proteins to solid surfaces. The method described herein is based on the reaction of a commercially available dye (Coomassie brilliant blue G) with the adsorbed proteins and the subsequent analysis by spectroscopic ellipsometry. In order to demonstrate the potential advantages of this method, the adsorption of bovine serum albumin to an antifouling coating was also investigated. According to our results, the modification with the dye significantly affects the optical properties of the adsorbed protein layer, which can be represented using a simple optical model (Lorentz). In general, the proposed modification increases the sensitivity of the detection by 2.5 ± 0.4 fold and enables the analysis of thin layers of adsorbed protein not obtainable by conventional methods. These results particularly reveal the importance of the proposed modification for the evaluation of low adsorbing substrates and antifouling coatings.
Keywords: Spectroscopic ellipsometry; Bovine serum albumin; Protein adsorption; Coomassie brilliant blue G; Lorentz, Antifouling coatings
1. INTRODUCTION
Adsorption of synthetic polymers and proteins to solid surfaces is of interest for many industrial and biomedical applications [1-4]. Aiming to understand the driving forces of such interactions, adsorption processes have been studied with a variety of techniques [5] including reflectometry [6], quartz crystal microbalance [7], atomic force microscopy [8], and fluorescence microscopy [9]. In this regard, spectroscopic ellipsometry (SE) is an attractive alternative for the study of adsorption phenomena because it can provide real-time information regarding the kinetics of the adsorption process as well as the structure of the adsorbed layer for a broad range of adsorbate and substrate materials [10, 11]. Ellipsometry is an optical technique that measures changes in amplitude and phase difference between the parallel (RP) and perpendicular (RS) components of a polarized light beam upon reflection from a surface [12]. The intensity ratio of RP and RS can be related to the ellipsometric angles (Ψ, amplitude and Δ, phase difference) using Equation 1:
| Equation 1 |
Spectroscopic ellipsometry also allows the measurement of the ellipsometric angles as a function of the wavelength of the incident light beam, therefore increasing the accuracy of the measurement. Because ellipsometry measures the ratio of two values originated by the same signal, the data collected are highly accurate and reproducible. One of the drawbacks of ellipsometry is that the interpretation of data requires an optical model describing the sample in terms of refractive index (n), extinction coefficient (k), and thickness (d) [13]. In the specific case of the adsorption of proteins to solid surfaces, the adsorbed layer is typically described by the Cauchy parameterization model (see Equation 2), where λ is the wavelength and A, B, and C are computer generated fitting parameters [14].
| Equation 2 |
Although there are plenty of examples of the use of Cauchy models in literature [11, 15-17], this approach still suffers from fundamental deficiencies; especially when the adsorption process is to be followed under aqueous environments. Among others, it is relevant to mention that at low coverage a) only small changes in the signal (Ψ and Δ as function of time or λ) are obtained, limiting the sensitivity of the experiment; b) the fitting parameters (A, B and C) could be strongly correlated with the thickness; and c) the system cannot distinguish between similar materials (polymers, proteins, etc) competing for the adsorption sites. Such information is crucial for the analysis of the adsorbed layer in many applications such as layer-by-layer deposition [18, 19] or the development of biosensors [20-22], antifouling coatings [15, 23] and biocompatible materials [24, 25]. Although in some cases, the optical constants or thickness of the underlying substrate can be adjusted to maximize the sensitivity of the analysis [26], a simple and broadly applicable procedure is currently not available. In order to address these deficiencies, this study was devoted to the development and characterization of a general method to increase the sensitivity of spectroscopic ellipsometry, when applied to monitor the adsorption process of proteins. To achieve such improvement, a model protein (bovine serum albumin, BSA) was adsorbed and then modified with a protein-specific dye (Coomassie brilliant blue G, BB [27]). Although, a major problem with the assay is the variation in response to different proteins Coomassie blue dye-based protein assays are exceptionally convenient because of their cost, simplicity, sensitivity, speed, and resistance to interfering chemicals, reducing agents, and most buffers [28]. Upon interaction with the proteins, BB changes its color from red to blue, producing an increase in the absorbance that lasts for about 1 h. This change in color, which is proportional to the concentration of proteins, is typically quantified by methods like spectrophotometry. The hypothesis of this work is that adsorbed proteins can be also treated with BB, to turn a transparent protein layer into a light-absorbing one. Despite the capability of spectroscopic ellipsometry to characterize a stained protein layer on a surface, no report has assessed the potential benefits of this method. Particular attention was paid to evaluate this method in terms of sensitivity toward the adsorption of low amounts of proteins. In order to demonstrate the potential advantages of this method, the interaction of BSA with an antifouling polymer-coated surface was also investigated.
2. MATERIALS AND METHODS
2.1 Materials and reagents
Silicon wafers (<111> Si/SiO2, Sumco, Phoenix, AZ, USA) were cleaned in a 3:1 mixture of H2SO4:H2O2 35% for 15 minutes at 80°C, rinsed thoroughly with water, and immersed in ultrapure water until used as substrates. In order to obtain a hydrophobic surface, a wafer was dried at 80°C for 4 h and submerged in a 1% w/v solution of dimethyldichlorosilane (DDS, Aldrich, Saint Louis, MO, USA) in trichloroethylene (AlfaAesar, Ward Hill, MA, USA) for 15 min. Subsequently, the coated wafer was thoroughly rinsed with methanol and ultrapure water (18 MΩ·cm water, NANOpure Diamond, Dubuque, IA, USA) and stored in a clean capped vial until use. This treatment is known to render a hydrophobic surface with a water contact angle around 100° [29]. Pluronic F-127 was purchased from Sigma (Saint Louis, MO, USA) and used to prepare antifouling coatings on hydrophobic substrata. The background electrolyte (10 mM acetate buffer) was prepared by mixing solutions of sodium acetate and acetic acid (Merck, Darmstadt, Germany) in ultrapure water and adjusting the pH to 4.7 (digital pH meter, Orion 420A+, Thermo, Waltham, MA, USA). Bovine serum albumin (BSA), Fraction V (heat-shock treated), was purchased from Fisher Scientific (Fair Lawn, NJ, USA) and used as received. Stock solutions of BSA were prepared at concentrations 1 g·L-1 and diluted in acetate buffer just prior to use. The selected dye (Coomassie brilliant blue G) was obtained from Sigma and diluted in water (5X), according to the protocol provided by the manufacturer.
2.2 Ellipsometric study of adsorbed proteins
All experiments were performed at room temperature (22 ± 1 °C) using a variable angle spectroscopic ellipsometer (WVASE, J.A. Woollam Co; Lincoln, NE, USA). Spectroscopic ellipsometry has proven suitable to study adsorption processes, and provides useful information about the optical constants and thickness the adsorbed film. Ellipsometric measurements were performed in a modified flow cell [30] which was mounted directly on the vertical base of the ellipsometer. In all experiments, the variation of Ψ and Δ was determined at an angle of incidence of 70°, as defined by the inlet/outlet of the UV fused-silica windows. In all cases and prior to the adsorption of proteins, a spectroscopic scan of the cleaned substrate was obtained in the range of 300-900 nm (in 10 nm steps). These measurements allowed a better estimation of the thickness of the oxide layer (SiO2), and improved the accuracy of the measurement. In order to adsorb protein, the substrate was then dipped into a beaker containing BSA solution at the desired concentration, under moderate agitation (100 rpm, Innova 2000 platform shaker, New Brunswick Scientific, Edison, NJ, USA) for 30 min. The substrate was then transferred to the cell and measured by ellipsometry (spectroscopic scan 300-900 nm). Next, the sample was removed from the cell and placed in a second beaker containing the BB solution for 5 min. After a mild rinsing step performed to remove the excess of BB, the substrate was again mounted in the cell for the final spectroscopic scan. The described procedure allowed ellipsometric measurement of adsorbed protein layer before and after BB treatment on a single substrate.
In order to model the collected data, the WVASE software package (J.A. Woollam Co; Lincoln, NE) was used. The mean square error (MSE, performed with a built-in function in WVASE) was used to quantify the difference between the experimental and model generated data.
2.3 Dynamic adsorption of proteins
In order to test the potential advantages of this method to assess the efficacy of antifouling coatings, the adsorption of BSA to a polyethylene oxide brush-coated surface was investigated. For these experiments, a layer of Pluronic F-127 (triblock copolymer of polyethylene oxide (PEO) and polypropylene oxide (PPO) with an average structure of PEO99-PPO65-PEO99) was deposited on hydrophobic Si/SiO2 wafers (DDS-treated). This procedure yields a uniform layer of PEO in a brush-like conformation [31] that is known to efficiently resist protein adsorption [32]. Both the pre-treatment of the substrate with Pluronic and the dynamic BSA adsorption experiments, were performed at room temperature and monitored by real time measurement of Ψ and Δ at three wavelengths (400, 630 and 850 nm) as a function of time. In this case the data acquisition rate was 3.2 Hz which, considering the 3 wavelengths selected, translates into 1 full scan every 56 sec.
The dynamic experiments were started by pumping acetate buffer was onto the surface (in a stagnation point flow setup, Reynolds number = 12) for 5 min in order to set a baseline. Next, the flow was switched to the solution containing the Pluronic (dissolved in buffer at a concentration of 0.2 g·L-1) until saturation was reached (~15 min). Then, the flow was switched back to the solution containing buffer for 35 min in order to remove the excess of Pluronic from the cell. Then, a BSA solution (0.1 mg·L-1) was introduced (~55 min after the start of the experiment) and continued for about 45 min, when buffer was pumped again to remove the excess protein for another 40 min. At ~140 min, the BB solution was pumped onto the surface for 10 min followed by a rinsing step with water. According to preliminary experiments, this procedure allowed staining the adsorbed proteins.
3. RESULTS AND DISCUSSION
Despite the advantages offered by spectroscopic ellipsometry for the investigation of adsorption studies, there are limitations in sensitivity of this technique when characterizing thin layers of different soft materials with similar optical parameters. The method presented in this study enables the measurement of stained proteins adsorbed on a surface as a light absorbing film by spectroscopic ellipsometry. The applicability and advantages of using this method are herein discussed.
3.1 Optical characterization of the substrates
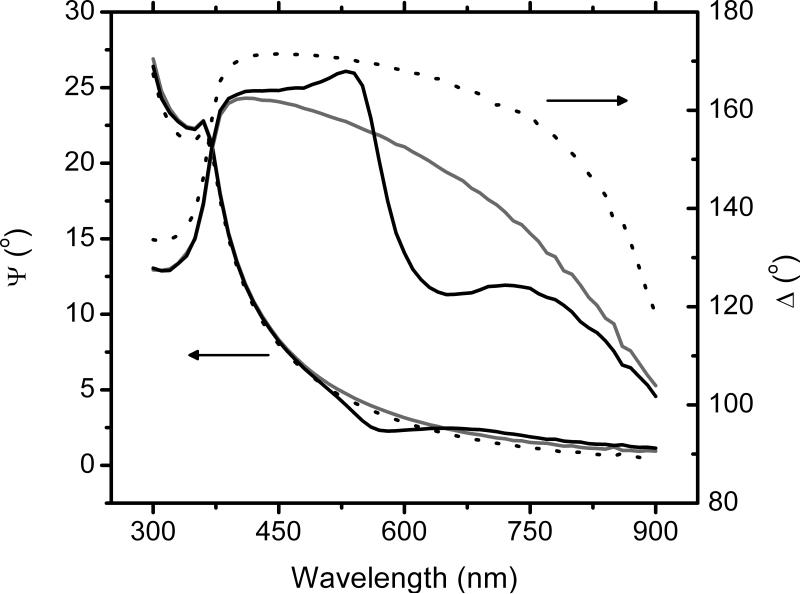
First, and to demonstrate that significant differences in optical constants can be obtained by the proposed method, spectroscopic scans of the substrates (Si/SiO2 wafers), the substrates with a layer of BSA, and with a layer of BSA treated with BB, were performed. Representative examples of the results are shown in Figure 1. As it can be observed, the adsorption of BSA produced considerable shifts in the phase difference (Δ) but did not introduce any new features in the spectroscopic scan, neither produced significant changes in amplitude (Ψ). This response is in a good agreement with previous reports [33], and was interpreted as the adsorption of a thin film of proteins on the surface of the substrate. As it can be also observed, treating the adsorbed protein layer with BB produced rather small changes in Ψ but significant changes in Δ, particularly in the range of 500 to 700 nm. As can be also observed, the maximum difference was obtained at 630 nm, which corresponds to the absorption peak of the BB-protein complex. This finding confirms that BB was able to react with the adsorbed protein layer, and that the change can be detected by ellipsometry. It is also worth mentioning that treating bare Si/SiO2 wafers with BB did not cause any change in the measured ellipsometric angles (data not shown), indicating that, as expected, reaction of BB with the bare substrate, if present, does not lead to significant changes in ellipsometric angles. It is also important to mention that no BSA was detected (spectrophotometrically) in the BB solution after immersing the BSA-coated wafer, indicating that the BB treatment does not induce desorption of the protein layer.
Figure 1.
Spectroscopic scans of the substrate (dotted line), the substrate with a layer of BSA (gray solid line), and with a layer of BSA treated with BB (black solid line) in the range of 300 to 900 nm (10 nm steps)
It has to be realized that although the staining of the adsorbed protein layer with BB is shown to be advantageous, modifications with other dyes might not lead to similar results. In this regard, experiments were performed to assess the applicability of bicinchoninic acid (BCA), another well-established reagent used for protein assays. However, preliminary experiments performed with following the staining protocol described for BCA indicate that the majority of the adsorbed protein layer is desorbed during the staining process. The desorption process was first measured by ellipsometry (spectroscopic scans of the substrate) before and after the staining process and confirmed by measuring (spectrophotometrically) the BCA solution after immersing the BSA-coated wafer (data not shown). It is also worth noting that although many other derivatization procedures are available [34], only few would provide significant advantages (in terms of performance, cost, time, and labor) for the detection of adsorbed proteins by reflection methods (such as ellipsometry or reflectometry), when the signal is originated by a change in ellipticity (at the same wavelength), rather than a change in light intensity or fluorescence emission. For these reasons, the rest of the experiments herein described were performed with BB.
3.2 Adsorption of Proteins
In order to determine how the ellipsometric signal (Δ and Ψ as function of λ) was affected by the amount of protein adsorbed, SiO2 substrates were immersed in solutions containing different concentrations of BSA, within the 1 × 10-4 to 1 g·L-1 range. As previously described, the substrates were then measured, treated with BB and measured again. Although it was observed (see Supplementary Information) that higher concentrations of protein produced the larger changes in both ellipsometric angles, the most significant change was observed in Δ. This observation is in agreement with literature reports [35]. More importantly, the change in Δ was significantly larger when the adsorbed protein layers were treated with BB. It is evident that treating the protein layer with BB induces a significant change in Δ, resulting in a sensitivity enhancement of the ellipsometric measurement of 2.5 ± 0.4 fold (2.3 ± 0.3 103 °·L·g-1 protein after BB treatment versus 0.9 ± 0.1 103 °·L·g-1 before BB treatment, calculated in the concentration region where change in Δ varies linearly with respect to BSA concentration). These results highlight one of the advantages of using BB, which would allow the analysis of small variations in protein amount on solid surfaces, which may otherwise be not detectable by the conventional procedure. This advantage is, among others, of particular importance in the analysis of protein interaction with low adsorbing surfaces.
3.3 Optical Model
Needless to say, it is of interest for many applications to obtain information not only about the optical changes in the system, but also about the physical interpretation of those changes. Therefore, the optical model used to represent the optical properties of the Si/SiO2 substrates was composed by layer of Si (bulk; d=1 mm) and a layer of SiO2 (d=2.5±0.5 nm), with optical axes parallel to the substrate surface. Initially, the adsorption of unmodified proteins was represented by adding an additional layer described using a Cauchy parameterization model (see Equation 1). In such cases, fixing the fitting parameters (A=1.44, B=0.01 and C=0) [30] allowed calculating the thickness of the protein layer. Upon the modification of the proteins with BB, the experimental results were analyzed using a Lorentz model, which is better suited to describe the optical properties of layers that absorb light in a region of the measured spectral range [14, 36]. Equation 3 expresses a general formulation of the Lorentz model,
| Equation 3 |
where ε is the complex dielectric function where E is the photon energy and n is the complex index of refraction. The fit parameters in this expression are the dielectric function at infinite energy (ε(∞)), the amplitude (An), center energy (En), and the broadening (Γn) of each oscillator. In the fitting discussed herein, the number of oscillators was defined as one, in order to reduce the number of fitted parameters to five (four from the optical model plus the thickness). In all cases, a very good agreement was obtained between the experimental and the data generated by the Cauchy (before the modification with BB) and Lorentz (after the modification with BB) models (See Supplementary Information for examples of the experimental and model-generated data for a protein film before and after the modification with BB). Similar MSE values were obtained for other adsorbed layers described in this study supporting the possibility of using a Lorentz model to describe the optical properties of the protein layer after the modification with BB.
In order to further characterize the properties of the BB-treated protein layer, the corresponding optical constants were calculated (See Supplementary Information). It was observed that the refractive index (n) and extinction coefficient (k) did not change significantly in the region from 300 to 450 nm. However, a sharp increase in the extinction coefficient was obtained for the BB-modified layers at λ > 450 nm, showing a well-defined absorption peak at 630 nm. This maximum in k is in close agreement to the absorption peak obtained in solution for the BB-protein complex in solution (595-620 nm [27]). These results not only provide evidence about the formation of the BB-protein complex on the surface and their role in the ellipsometric characterization of the adsorbed film, but also support the use of a simple optical model (like Lorentz) to extract information about the optical properties of the stained protein film.
3.4 Correlation between optical models (Cauchy and Lorentz)
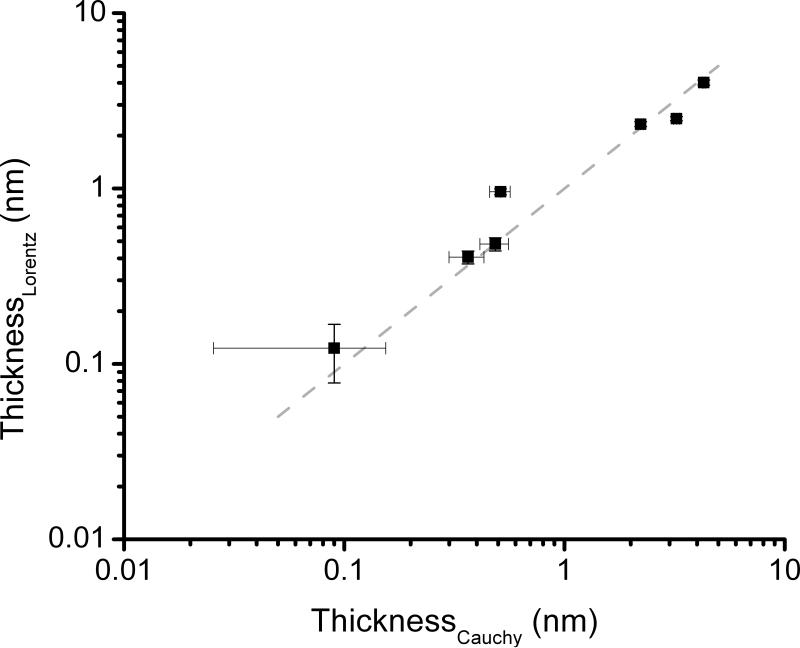
As can be observed in Figure 2, although the thickness calculated using a Cauchy (before the modification) or Lorentz (after the modification) models show a very good correlation (R= 0.98), staining the protein layer produces a significant decrease in the uncertainty, particularly at low coverage values. In agreement with these results, when the thickness of the BSA layer was < 0.51 nm (produced by immersing the wafer in solutions containing < 1 × 10-3 g·L-1 of BSA, see Supplementary Information), only a slight change in Δ (within the noise) was obtained. In such cases, treating the adsorbed proteins with BB improved the limit of detection of the technique from 0.38 mg·m-2 to 0.27 mg·m-2 (as estimated based on the thinnest layer resulting in a change in Δ > 2.6°, that is 3 times the noise). Furthermore, and although the sensitivity values herein reported were calculated using the recommended standard (BSA, 15 % basic residues), the methodology described in this communication can be slightly modified to improve the detection of various proteins [28, 37].
Figure 2.
Thicknesses of the BSA adsorbed layers calculated by Cauchy (before the modification) versus Lorentz (after the modification) models.
3.5 Application of the proposed modification to the detection of proteins adsorbed to a polymer film
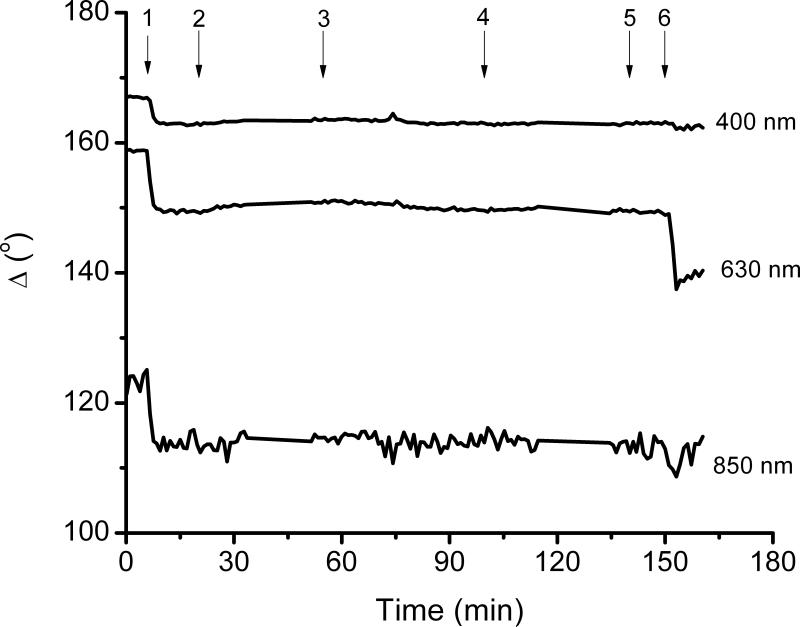
In order to further investigate the advantages of the proposed method in a real application, the adsorption of BSA on a Pluronic F127-coated surface was studied. It has been reported that Pluronic F127 can readily adsorb to hydrophobic surfaces forming brush-like structures of PEO, which have been shown to inhibit protein adsorption and particle adhesion [38, 39]. Figure 3 shows the results obtained in a representative dynamic experiment, monitored in real time by ellipsometry for the adsorption of Pluronic to a hydrophobic SiO2 substrate, and the subsequent interaction with BSA. The quick change in the signal (Δ and Ψ as function of time) upon the introduction of Pluronic F127 indicates that a considerable amount of Pluronic F127 (1.36 mg.m2, as calculated using a Cauchy model) was quickly adsorbed on the hydrophobic surface. In agreement with previous reports, this adsorption process is supported by the interaction of the hydrophobic PPO chain with the hydrophobic surface [40]. Also in agreement with previous reports, the layer of Pluronic F127 was not removed by rinsing the surface with buffer. To demonstrate the advantages of the modification of adsorbed proteins with BB, the antifouling properties of the Pluronic F127 coating were investigated by exposing the films to a solution containing BSA. As can be also observed in Figure 3, only a small change in Ψ and Δ was observed upon introduction of BSA in the cell. Considering the signal/noise ratio, this experiment indicates that the optical properties of the adsorbed layer were not affected by the interaction with BSA and it could be concluded that the amount of BSA adsorbed to the Pluronic film is not significant. However, upon the treatment of the substrate with BB (performed in the 140 to 160 min spam), a significant change in the ellipsometric angles collected at 630 nm was observed. In agreement with Figure 1 (and Supplementary Information), this change cannot be observed if 400 or 850 nm were selected as the wavelength. Modeling the BB-modified proteins layer enabled calculating an adsorbed amount of 0.8 ± 0.1 mg·m2 on the surface of the polymer brush. Once again, although this amount is significantly smaller than the amount of BSA adsorbed to the plain Si/SiO2 surface under similar conditions (2.4 ± 0.1 mg·m2), the adsorbed amount was detectable only after treating the surface with BB.
Figure 3.
A representative dynamic experiment, monitored in real time by ellipsometry for the pre-treatment of the substrate with Pluronic and the subsequent BSA adsorption experiments. The flow had different steps: 1) introduction of Pluronic solution, 2) rinse, 3) flow of BSA solution, 4) rinse, 5) BB solution, 6) rinse.
It is also worth mentioning that although the described method offers several advantages, it does not allow following the modification reaction in real time (with our current experimental set-up). Apparently, having a high concentration of BB in the surroundings of the measuring spot, does not allow enough light to be reflected off the surface. This is the reason why the change in signal displayed in Figure 3 can only be observed after the rinsing step.
4. CONCLUSIONS
This manuscript describes a simple way to improve the sensitivity of spectroscopic ellipsometry, when applied to monitor the adsorption of proteins to solid surfaces. It is based on the reaction of a commercially available dye (brilliant blue) with the adsorbed proteins. The optical properties of the dye-modified protein layer can be represented using a simple Lorentz optical model. In addition to increasing the sensitivity of measurements by a factor 2.5, the proposed modification enables the detection (with a signal/noise ratio > 3) of thin protein layers which represents an improvement of at least 30% with respect to the experiment performed with unmodified (transparent) proteins. These results also reveal the advantage of the proposed modification for the study of protein adsorption on low adsorbing substrates and antifouling surfaces. Furthermore, and because of the specificity of dyes like BB, the proposed method would allow the analysis of more complex systems, with intermixed proteins and polymers. We envision that the use of other dyes would further enhance the capabilities of spectroscopic ellipsometry for the study of biological molecules.
Supplementary Material
5. ACKNOWLEDGMENTS
Financial support for this project was provided by the University of Texas at San Antonio and the National Institute of General Medical Sciences (NIGMS) / National Institutes of Health (1SC3GM081085).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. REFERENCES
- 1.Hanefeld U, Gardossi L, Magner E. Understanding enzyme immobilisation. Chem Soc Rev. 2009;38:453–468. doi: 10.1039/b711564b. [DOI] [PubMed] [Google Scholar]
- 2.Norde W, Gage D. Interaction of bovine serum albumin and human blood plasma with PEO-tethered surfaces: Influence of PEO chain length, grafting density, and temperature. Langmuir. 2004;20:4162–4167. doi: 10.1021/la030417t. [DOI] [PubMed] [Google Scholar]
- 3.Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of biomaterial-cell interactions by adsorbed proteins: A review. Tissue Eng. 2005;11:1–18. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 4.Kallrot N, Dahlqvist M, Linse P. Dynamics of Polymer Adsorption from Bulk Solution onto Planar Surfaces. Macromolecules. 2009;42:3641–3649. [Google Scholar]
- 5.Sapsford KE, Ligler FS. Real-time analysis of protein adsorption to a variety of thin films. Biosens Bioelectron. 2004;19:1045–1055. doi: 10.1016/j.bios.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 6.de Vos WM, Biesheuvel PM, de Keizer A, Kleijn JM, Stuart MAC. Adsorption of Anionic Surfactants in a Nonionic Polymer Brush: Experiments, Comparison with Mean-Field Theory, and Implications for Brush-Particle Interaction. Langmuir. 2009;25:9252–9261. doi: 10.1021/la900791b. [DOI] [PubMed] [Google Scholar]
- 7.Hook F, Kasemo B, Nylander T, Fant C, Sott K, Elwing H. Variations in coupled water, viscoelastic properties, and film thickness of a Mefp-1 protein film during adsorption and cross-linking: a quartz crystal microbalance with dissipation monitoring, ellipsometry, and surface plasmon resonance study. Anal. Chem. 2001;73:5796–5804. doi: 10.1021/ac0106501. [DOI] [PubMed] [Google Scholar]
- 8.Toscano A, Santore MM. Fibrinogen adsorption on three silica-based surfaces: Conformation and kinetics. Langmuir. 2006;22:2588–2597. doi: 10.1021/la051641g. [DOI] [PubMed] [Google Scholar]
- 9.Togashi DM, Ryder AG, Heiss G. Quantifying adsorbed protein on surfaces using confocal fluorescence microscopy. Colloids Surf. B. 2009;72:219–229. doi: 10.1016/j.colsurfb.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Arwin H. Ellipsometry on thin organic layers of biological interest: characterization and applications. Thin Solid Films. 2000;377:48–56. [Google Scholar]
- 11.Mora MF, Wehmeyer J, Synowicki R, Garcia CD. Investigating the Adsorption of Proteins Via Spectroscopic Ellipsometry. In: Bizios R, Puleo D, editors. Biological Interactions on Material Surfaces: Understanding and Controlling Protein, Cell, and Tissue Responses. 2009. [Google Scholar]
- 12.Fujiwara H. Principles and applications. J. Wiley & Sons; West Sussex, England: 2007. Spectroscopic ellipsometry. [Google Scholar]
- 13.Chan R, Chen V. Characterization of protein fouling on membranes: opportunities and challenges. J Membrane Sci. 2004;242:169–188. [Google Scholar]
- 14.Synowicki RA. Spectroscopic ellipsometry characterization of indium tin oxide film microstructure and optical constants. Thin Solid Films. 1998;313:394–397. [Google Scholar]
- 15.Reichelt S, Eichhorn KJ, Aulich D, Hinrichs K, Jain N, Appelhans D, Voit B. Functionalization of solid surfaces with hyperbranched polyesters to control protein adsorption. Colloids Surf. B. 2009;69:169–177. doi: 10.1016/j.colsurfb.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 16.Goyal DK, Subramanian A. In-situ protein adsorption study on biofunctionalized surfaces using spectroscopic ellipsometry. Thin Solid Films. 2010;518:2186–2193. [Google Scholar]
- 17.Berlind T, Poksinski M, Tengvall P, Arwin H. Formation and cross-linking of fibrinogen layers monitored with in situ spectroscopic ellipsometry. Colloids Surf. B. 2010;75:410–417. doi: 10.1016/j.colsurfb.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Jiang K, Zhang HX, Shannon C, Zhan W. Preparation and characterization of polyoxometalate/protein ultrathin films grown on electrode surfaces using layer-by-layer assembly. Langmuir. 2008;24:3584–3589. doi: 10.1021/la704015j. [DOI] [PubMed] [Google Scholar]
- 19.Zhao W, Xu JJ, Chen HY. Electrochemical biosensors based on layer-by-layer assemblies. Electroanal. 2006;18:1737–1748. [Google Scholar]
- 20.Arwin H. Is ellipsometry suitable for sensor applications? Sens. Actuators A. 2001;92:43–51. [Google Scholar]
- 21.Demirel G, Çaglayan MO, Garipcan B, Piskin E. A novel DNA biosensor based on ellipsometry. Surface Science. 2008;602:952–959. [Google Scholar]
- 22.Mora MF, Giacomelli CE, Garcia CD. Interaction of D-Amino Acid Oxidase to Carbon Nanotubes: Implications in the Design of Biosensors. Anal. Chem. 2009;81:1016–1022. doi: 10.1021/ac802068n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brzozowska AM, Hofs B, de Keizer A, Fokkink R, Stuart MAC, Norde W. Reduction of protein adsorption on silica and polystyrene surfaces due to coating with Complex Coacervate Core Micelles. Colloids Surf. A. 2009;347:146–155. [Google Scholar]
- 24.Elwing H. Protein absorption and ellipsometry in biomaterial research. Biomaterials. 1998;19:397–406. doi: 10.1016/s0142-9612(97)00112-9. [DOI] [PubMed] [Google Scholar]
- 25.Wehmeyer J, Bizios R, Garcia CD. Adsorption of Bovine Serum Albumin to Nanostructured Thin-Films of TiO2. Mat. Sci. Eng. C. 2010;30:277–282. doi: 10.1016/j.msec.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Defeijter JA, Benjamins J, Veer FA. Ellipsometry as a Tool to Study Adsorption Behavior of Synthetic and Biopolymers at Air-Water-Interface. Biopolymers. 1978;17:1759–1772. [Google Scholar]
- 27.Sedmak JJ, Grossberg SE. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal. Biochem. 1977;79:544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- 28.Stoscheck CM. Increased uniformity in the response of the Coomassie blue G protein assay to different proteins. Anal. Biochem. 1990;184:111–116. doi: 10.1016/0003-2697(90)90021-z. [DOI] [PubMed] [Google Scholar]
- 29.Boks NP, Kaper HJ, Norde W, van der Mei HC, Busscher HJ. Mobile and immobile adhesion of staphylococcal strains to hydrophilic and hydrophobic surfaces. J Colloid Interf Sci. 2009;331:60–64. doi: 10.1016/j.jcis.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 30.Mora MF, Reza Nejadnik M, Baylon-Cardiel JL, Giacomelli CE, Garcia CD. Determination of a setup correction function to obtain adsorption kinetic data at stagnation point flow conditions. J. Colloid Interface Sci. 2010;346:208–215. doi: 10.1016/j.jcis.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nejadnik MR, Olsson ALJ, Sharma PK, van der Mei HC, Norde W, Busscher HJ. Adsorption of Pluronic F-127 on Surfaces with Different Hydrophobicities Probed by Quartz Crystal Microbalance with Dissipation. Langmuir. 2009;25:6245–6249. doi: 10.1021/la9001169. [DOI] [PubMed] [Google Scholar]
- 32.Schroen CGPH, Stuart MAC, Maarschalk KV, Vanderpadt A, Vantriet K. Influence of Preadsorbed Block-Copolymers on Protein Adsorption - Surface-Properties, Layer Thickness, and Surface Coverage. Langmuir. 1995;11:3068–3074. [Google Scholar]
- 33.Ruths J, Essler F, Decher G, Riegler H. Polyelectrolytes I: Polyanion/polycation multilayers at the air/monolayer/water interface as elements for quantitative polymer adsorption studies and preparation of hetero-superlattices on solid surfaces. Langmuir. 2000;16:8871–8878. [Google Scholar]
- 34.Hermanson GT. Bioconjugate Techniques. Second Edition ed. Academic Press Inc.; 2008. [Google Scholar]
- 35.Herron JN, Muller W, Paudler M, Riegler H, Ringsdorf H, Suci PA. Specific Recognition-Induced Self-Assembly of a Biotin Lipid Streptavidin Fab Fragment Triple Layer at the Air-Water-Interface - Ellipsometric and Fluorescence Microscopy Investigations. Langmuir. 1992;8:1413–1416. [Google Scholar]
- 36.Hilfiker JN, Singh N, Tiwald T, Convey D, Smith SM, Baker JH, Tompkins HG. Survey of methods to characterize thin absorbing films with Spectroscopic Ellipsometry. Thin Solid Films. 2008;516:7979–7989. [Google Scholar]
- 37.Read SM, Northcote DH. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal. Biochem. 1981;116:53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- 38.Green RJ, Davies MC, Roberts CJ, Tendler SJB. A surface plasmon resonance study of albumin adsorption to PEO-PPO-PEO triblock copolymers. J Biomed Mater Res. 1998;42:165–171. doi: 10.1002/(sici)1097-4636(199811)42:2<165::aid-jbm1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 39.Nejadnik MR, van der Mei HC, Norde W, Busscher HJ. Bacterial adhesion and growth on a polymer brush-coating. Biomaterials. 2008;29:4117–4121. doi: 10.1016/j.biomaterials.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Tai YC, Joshi P, McGuire J, Neff JA. Nisin adsorption to hydrophobic surfaces coated with the PEO-PPO-PEO triblock surfactant Pluronic (R) F108. J Colloid Interf Sci. 2008;322:112–118. doi: 10.1016/j.jcis.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.