Summary
Host integration and performance of engineered tissues have been severely limited by the lack of robust strategies to generate patent vascularization and tissue perfusion. This review highlights a selection of exciting developments in vascularization approaches for tissue engineering research. Current strategies for vascularization in tissue engineering are related to growth factor signaling and delivery, cell transplantation, bioactive smart matrix materials, and directed fabrication. Application of these techniques to in vivo models has resulted in a number of robust host vascular responses, especially with synergistic and engineered bioactive systems. The future outlook of the field includes refinement and development of new technologies for vascularization and combining these techniques with functional repair models for metabolically active tissues and relevant disease states.
Introduction
Tissue engineering quickly grew as a field through the 1990's on its promise to deliver manufactured organs and tissue constructs to address the organ transplantation shortage [1]. As we progress into the second decade of the 21st century, the organ shortage still persists and progression of tissue engineering approaches from mere combinations of cells and materials to true living and integrated tissues have stalled due in part to the lack of robust strategies to generate patent vascularization and integration with host vasculature. To provide sufficient oxygen tension for survival, metabolically active tissues must reside within 150 to 200 μm of a capillary lumen [2]. Without a perfusing blood supply as a central component of any tissue engineering design, engineered tissue scalability, survival, and integration are extremely limited. With the exception of a few poorly vascularized tissues such as skin epidermis and cartilage, many of the initial targets of tissue engineering such as artificial pancreas as well as cardiac and hepatic tissues have yet to be realized. Advancement to engineering these higher-order levels of tissue architecture has required a deeper understanding of the mechanisms of vascular development and the patterns of interaction between multiple cell types on molecular, cellular, and tissue scales [3]. Functional tissues are permeated with hierarchical blood vessel, nerve, and lymphatic networks and may additionally contain epithelial ductwork. Much like the wiring, plumbing, and ventilation systems which are central to the function of a modern building, these supportive infrastructures are critical to native tissue survival and integration with organism-level physiology. Engineered tissues on any meaningful scale or complexity must incorporate aspects of functional infrastructure, with vasculature being the most immediately critical for survival and adequate function of transplanted cells. It is little wonder then, that a great impetus has been placed on vascularization as an integral aspect of the regenerative medicine paradigm.
Current Tissue Engineering Approaches to Vascularization
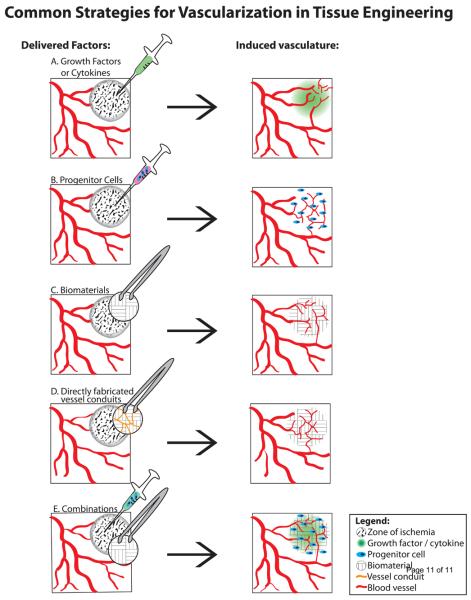
In its fundamental definition, tissue engineering or regenerative medicine is not limited to building artificial tissue in the lab, but ultimately encompasses treatments to enhance or restore function to diseased and damaged tissue [1]. Driving many of the cell and growth-factor based strategies to vascularize engineered tissue constructs in vitro is the clinical research aimed at restoring circulation to ischemic tissue in a variety of pathologies. With the development of recombinant angiogenic and vasculogenic growth factors, initial clinical studies were undertaken to deliver growth factors such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) in myocardial and peripheral limb ischemia (Fig. 1A). The prohibitive cost and complexity of maintaining high levels of recombinant protein over a sufficient time frame in the zone of ischemia quickly led to the experimental evaluation of gene therapy and delivery of regenerative cell types as well as combination therapies. A large body of evidence with more than 1000 patients enrolled in placebo-controlled trials established the relative safety of angiogenic gene therapy, [4] and ongoing studies have generally established the safety of cell-based therapies. Advances in the clinic such as evidence of improved cardiac output and reduced incidence of non-healing ulcers are overshadowed by the fact that rigorous phase II and III trials have failed to unequivocally demonstrate improvements in high level benchmarks such as exercise stress testing.
Figure 1.
Effective strategies for inducing vascularization in engineered tissues include delivery of:
A. Growth factors such as VEGF and bFGF as recombinant proteins or gene vectors. EPC-mobilizing cytokines such as G-CSF
B. Progenitor cells such as EPC and MSC
C. Biomaterials such as bioactive PEG hydrogels
D. Vessel conduits or endothelium-lined channels directly fabricated into an implant.
E. Combination therapies such as growth factor binding scaffolds with cells
Promising results from combination therapies co-delivering [5] or time-release staggering multiple growth factors [6] support the idea that a robust vascular healing response requires coordination of the correct signaling factors, dosing, and exposure time points. Incorporation of biomaterial delivery vehicles aims to solve some of the complex pharmacokinetics, but this approach is ultimately hindered by a lack of knowledge of optimal dosage and timing and the inability of delivered signals to override the “background noise” of a pro-inflammatory environment. Research into “master switch” upstream activators such as HIF-1α [7-8] that activate an entire pro-vascular signaling cascade are an exciting direction to the growth factor delivery field. But even if regenerative medicine can one day fully recapitulate the pharmacokinetics of an endogenous healing or developmental vascularization response, ultimately the question arises as to why the natural healing mechanisms failed to begin with or in the case of myocardial infarction, why no native regenerative repair process occurs at all. Various disease states, aging, and scar-tissue formation are obvious blockades to natural endothelial repair mechanisms, and growth factor signaling alone may be an intrinsically limited strategy when delivered in the context of diseased or non-healing tissue [9].
New approaches to treating ischemia are focusing on delivery of regenerative cells alone (Fig. 1B) and in combination with growth factors and biomaterial scaffolds (Fig. 1E). For cell therapy, we are beginning to understand the importance of delivering multiple progenitor cell-types in creating functional tissues. Advances in gene and cell delivery techniques have yielded astonishing results in animal models such as polymeric nanoparticle gene delivery vehicles combined with human embryonic (hEST) and mesenchymal stem cells (MSC) that showed significant vascularization and engraftment [10].
While current tissue engineering approaches aim to overcome the native tissue dysfunction by delivering effective regenerative cells in conjunction with the appropriate matrix and signaling molecules, the monumental challenge of integrating engineered tissues with the host vasculature remains significant. The challenge of clinically delivering functional and vascularized large-scale tissue substitutes creates a ‘chicken-or-the-egg’ paradox. Is a functional vasculature required before regenerative cells can be transplanted, or are the regenerative cells needed to give rise to the new vasculature simultaneously as functional tissue develops? Researchers must attempt to either: connect and perfuse a pre-fabricated functional critical-sized tissue, form a pre-vascularized site and subsequently add in functional tissue, or simultaneously form vasculature alongside functional tissue.
From the standpoint of in situ tissue formation, tissue engineering research has progressed to the point of predictably and repeatedly producing patent, stable vasculature in a variety of animal models through transplantation of a combination of endothelial and mesenchymal cells or progenitors encapsulated in biological extracellular matrix (ECM) [11] and Matrigel™ implants [12]. The driving force behind this remarkable advancement is a mimicry of embryonic vasculogenesis where angioblasts and mesenchymal stem cells organize into a network to form a pericyte-stabilized capillary bed [13]. The ability to recapitulate this capillary network formation using adult cells obtained in routine sampling procedures of the blood and bone marrow represents a useful and feasible pool from which to further develop clinically relevant vascularized tissue constructs. An alternative source of vasculogenic cells in adult patients are endothelial progenitor cells (EPC) which can be mobilized to circulation from the bone marrow by administration of granulocyte colony stimulating factor (G-CSF) and home to sites of ischemia, inflammation, and biomaterials with artificial EPC capturing motifs [14]. EPC are an intriguing cell type for treating ischemic conditions that have shown promising functional recovery in animal models [15] and human trials [16-17].
Matrigel™, a decellularized matrix derived from mouse sarcoma cells, has been a common component for both in vitro endothelial tube formation and in vivo 3D network vascularization. However, Matrigel™ is a poorly controlled and highly uncharacterized environment from an engineering perspective, containing a mélange of growth factors and matrix-associated bioactive signals. Unfortunately, because of its tumoral and xenogenic origin, Matrigel™ is ultimately a sub-optimal choice for development of clinically-relevant therapies. Major effort is being concentrated on development of fully synthetic or well-defined biological matrices with potent pro-angiogenic properties manifested either through encapsulated co-culture systems of endothelial and mesenchymal cells or cell-free smart materials (Fig. 1C) directly recruiting vascular ingrowth from the surrounding host tissue.
Novel vascular-inductive biomaterial systems include schemes for directly conjugating growth factor to a degradable matrix and releasing it in a cell-demanded manner. One such system that has shown promising results incorporates bioactive ligands into a synthetic polyethylene glycol (PEG) hydrogel. PEG hydrogels for vascularization have been developed with different crosslinking reaction schemes. Popular renditions include 4-arm PEG-vinyl sulfone (PEG-VS) crosslinked by Michael-type addition [18] and PEG-diacrylate (PEGDA) crosslinked by photoinitiated free-radical polymerization [19]. Both systems are functionalized with protease (MMP)-cleavable peptide sequences, domains for cell adhesion (RGD peptide), and tethered growth factors. These PEG-based matrices have been used to promote both in vitro [20] and in vivo [19,21] vascular network formation from encapsulated cells or vessel ingrowth from the surrounding tissue. Engineered natural fibrin-based matrices offer an alternative to the PEG systems, with recombinant variants of growth factors designed to directly bind fibrin, and are remarkably potent inducers of vessel ingrowth from surrounding tissue [22]. These engineered matrices that directly bind growth factor and release it in a proteolytically-dependent or “ondemand” manner induce more stabilized and longer-lasting vasculature compared to diffusive growth factor release. However, even these induced stabilized vessels are reported to regress in time in the absence of true physiological demand [18]. Yet another promising artificial matrix idea utilizes self assembled peptide amphiphile nanofibril matrices with heparin sulfate binding sites to present bioactive ligands and growth factors to promote de novo subcutaneous vascularization [23]. Research in engineered matrices has progressed for tissue engineering models including cardiac progenitor differentiation [24], pancreatic islet encapsulation [25], and epithelial morphogenesis [26]. We can expect future research to combine engineered vascular-inductive matrices with repair or replacement of metabolically active tissues in vivo.
An alternative strategy to inducing vascular organization into a scaffold is to fabricate vascular conduits directly prior to implantation (Fig. 1D). Several clever engineering techniques to generate endothelial-lined channels in tissue engineered constructs have emerged. One simple yet effective technique involves close-packed modular cylindrical collagen matrices coated in endothelial cells to generate endothelial lined channels in a random packed array. These channels remodel in vivo to generate a vascularized graft [27]. Another self-assembly technique uses microtissue building blocks made from human artery-derived fibroblasts coated with human umbilical vein endothelial cells (HUVEC) to mold a small diameter vascular graft with high levels of ECM deposition [28]. In theory, such a system could also be used for inducing vascularization of a tissue-engineered construct. Cell sheet technology is another vascular design technique that employs a process of alternatively stacked monolayers of HUVEC and myoblasts to create highly vascularized implants of myoblasts in vivo with robust endothelial networks [29].
Developing along-side the effort to create clinically-useful and well-characterized pro-vascular matrices are approaches to merge this technology with relevant tissue-specific replacement models. For example, pancreatic islets are highly vascularized spherical clusters of endocrine cells in the pancreas which include the insulin producing β-cells. Islet transplantation is a promising therapeutic option with freedom from exogenous insulin injection for type-1 diabetes, yet current transplantation techniques are severely limited due to high islet morbidity associated with poor engraftment and reperfusion. Current efforts to improve islet transplantation therapy include gene therapy to overexpress angiogenic growth factors [30-31] in transplanted islets and seeding of islets into pre-vascularized Matrigel™ [32] and collagen [33] implants.
Engineering mechanically sound and functional cardiac tissue for the repair of myocardial infarction and associated ischemic heart disease, the leading cause of death in developed countries, is one of the most promising and grand targets for tissue engineering. Progress in development of engineered cardiac tissue has not always addressed the need for vascularization and engineered tissues suffer from necrotic cores and little or no integration with the host tissue [34]. Incorporation of HUVEC and mouse embryonic fibroblasts (MEF) in cardiac patches leads to a strong vascular network formation in vitro and which, if formed preceding implantation in rat heart tissue, shows vastly improved integration and perfusion than patches without HUVEC and MEF [34-35]. Taking the process one step further, neonatal cardiac cell patches containing angiogenic factors pre-vascularized for 1 week in the omentum and subsequently transplanted into infracted heart tissue showed improved structural, electrical, and cardiac output over non-vascularized controls [36]. Alternatively, microvascular segments stabilized in collagen and transplanted into ischemic myocardium formed vascularized cardiac patches with improved left ventricular function [37].
Finally, the most direct approach to providing the necessary cues and allowing cells and tissues to control the ultimate shape of the engineered tissue and associated vasculature is direct fabrication of functioning tissue. Technologies to exert spatial control over the placement and organization of individual cells and tissue microstructures include 3D tissue printing [38-39] and lithographic fabrication [40-41] of vascular-inductive matrices have emerged as viable options. Microfluidic devices are also becoming employed to create controlled in vitro systems for studying underlying mechanisms and testing new ideas [42].
Methods for validation and analysis of vascularization techniques
It is important to measure and validate the architecture and function of induced vasculature. Induced vasculature often has little resemblance to native tissue architecture, and has the potential to look and behave like tumor vasculature [43]. Furthermore, induced vasculature may suffer from poor perfusion and functionality. Several robust analysis strategies have been developed for both in vivo and in vitro models [44].
In vitro analysis techniques
Three dimensional culture of endothelial cell types or co-culture of endothelial and mesenchymal progenitors (MSC or 10T1/2 embryonic fibroblasts [11]) will self-organized to form tubule networks in pro-vascular environments [44]. The addition of a mesenchymal cell type serves to stabilize the endothelial tubes. Furthermore, if these cell types are cultured on the surface of microcarrier beads that are encapsulated in the matrix, endothelial tubes sprout from the beads [44-45]. These bead-initiated sprouts can be easily quantified for number, length, and branching and used as a screening and analysis tool. Three dimensional tissue culture of small sections of aorta from rat or mouse also sprout endothelial tubes from the aortic tissue in pro-vascular environments and are an alternative to single cell culture [46]. Lastly, the chorioallantoic membrane (CAM) is a vascularized membrane on developing chicken embryos that is widely used as a pseudo-in vivo system for testing vascularization effects [44].
In vivo analysis techniques
Traditionally, histological techniques including lectin- and immunostaining have been used to quantify induced vascularization in vivo. Three-dimensional architecture and function (vessel perfusion) are key measures that are difficult to determine from histological sections. One highly effective technique for quantitative analysis of three-dimensional vascular architecture is perfusion with the silicone rubber radio-opaque injection compound Microfil ® (Flow Tech, Inc.) to create a cast of the vasculature. The vascular cast can then be scanned in three dimensions at high resolution (sub 10 μm) with microCT and analyzed with a number of algorithms for density, branching, and connectivity [46-47]. One drawback to the microCT method is that it is a terminal procedure. Development of vasculature over time in live animals can be observed by intravital microscopy with window-models and dorsal skin-fold chambers[44]. Blood flow to ischemic limbs can be observed in live animals over time by laser Doppler perfusion imaging [21] (Moor Instruments) or with GFP-cells [44] and infrared dye tracer studies and in vivo fluorescence imaging (IVIS ®, Caliper Life Sciences, Inc.).
Conclusions / Future Outlook
Current research is tackling the problem of vascularization with four distinct strategies as illustrated in Figure 1:
Direct modulation of existing tissue by growth factor or cytokine signaling
Delivery of endothelial and mesenchymal progenitors to form a self-assembled network
Delivery of vascular-inductive engineered materials
Controlled methods to directly incorporate vessel conduits into the engineered tissue
Combination strategies incorporating materials, cells, and/or growth factors.
It is clear that further undertakings in tissue engineering must consider vascularization as a key design parameter. All of the discussed methods have shown moderate success in animal models. Future directions of tissue engineering research need to apply what has been learned about vascular induction and combine it with the design of functional tissue substitutes to take the field to the next level. In particular, synergistic and coordinated interactions among cells (including stem cells), biomaterials engineered to respond to local and systemic biological stimuli, and bioactive molecules will be required to attain the goal of functional tissue engineered constructs integrated with the host.
Acknowledgements
Funding provided by the JDRF, NIH, AHA predoctoral fellowship support for E.A.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1•.Nerem RM. Tissue engineering: the hope, the hype, and the future. Tissue Eng. 2006;12:1143–1150. doi: 10.1089/ten.2006.12.1143. An excellent perspective on the field offering a historical review, policy implications, and future directions. [DOI] [PubMed] [Google Scholar]
- 2.Kannan RY, Salacinski HJ, Sales K, Butler P, Seifalian AM. The roles of tissue engineering and vascularisation in the development of micro-vascular networks: a review. Biomaterials. 2005;26:1857–1875. doi: 10.1016/j.biomaterials.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 3••.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. An in depth look at the molecular regulators of angiogenesis induction and pathfinding. [DOI] [PubMed] [Google Scholar]
- 4•.Gupta R, Tongers J, Losordo DW. Human studies of angiogenic gene therapy. Circ Res. 2009;105:724–736. doi: 10.1161/CIRCRESAHA.109.200386. Reviews clinical trials of angiogenic gene therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng D, Sefton MV. Dual delivery of placental growth factor and vascular endothelial growth factor from poly(hydroxyethyl methacrylate-co-methyl methacrylate) microcapsules containing doubly transfected luciferase-expressing L929 cells. Tissue Eng Part A. 2009;15:1929–1939. doi: 10.1089/ten.tea.2008.0470. [DOI] [PubMed] [Google Scholar]
- 6.Chen RR, Silva EA, Yuen WW, Mooney DJ. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res. 2007;24:258–264. doi: 10.1007/s11095-006-9173-4. [DOI] [PubMed] [Google Scholar]
- 7•.Sarkar K, Fox-Talbot , Steenbergen C, Bosch-Marce M, Semenza GL. Adenoviral transfer of HIF-1alpha enhances vascular responses to critical limb ischemia in diabetic mice. Proc Natl Acad Sci U S A. 2009;106:18769–18774. doi: 10.1073/pnas.0910561106. Upstream activators of vasculogenic pathways are an exciting addition to the growth factor delivery field. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shyu KG, Wang MT, Wang BW, Chang CC, Leu JG, Kuan P, Chang H. Intramyocardial injection of naked DNA encoding HIF-1alpha/VP16 hybrid to enhance angiogenesis in an acute myocardial infarction model in the rat. Cardiovasc Res. 2002;54:576–583. doi: 10.1016/s0008-6363(02)00259-6. [DOI] [PubMed] [Google Scholar]
- 9.Sun L, Bai Y, Du G. Endothelial dysfunction--an obstacle of therapeutic angiogenesis. Ageing Res Rev. 2009;8:306–313. doi: 10.1016/j.arr.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Yang F, Cho SW, Son SM, Bogatyrev SR, Singh D, Green JJ, Mei Y, Park S, Bhang SH, Kim BS, et al. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc Natl Acad Sci U S A. 2010;107:3317–3322. doi: 10.1073/pnas.0905432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Koike N, Fukumura D, Gralla O, Au P, Schechner JS, Jain RK. Tissue engineering: creation of long-lasting blood vessels. Nature. 2004;428:138–139. doi: 10.1038/428138a. A seminal article on in vivo neovascularization derived from HUVEC / MSC co-culture. [DOI] [PubMed] [Google Scholar]
- 12.Melero-Martin JM, De Obaldia ME, Kang SY, Khan ZA, Yuan L, Oettgen P, Bischoff J. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loffredo F, Lee RT. Therapeutic vasculogenesis: it takes two. Circ Res. 2008;103:128–130. doi: 10.1161/CIRCRESAHA.108.180604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Avci-Adali M, Ziemer G, Wendel HP. Induction of EPC homing on biofunctionalized vascular grafts for rapid in vivo self-endothelialization--a review of current strategies. Biotechnol Adv. 2010;28:119–129. doi: 10.1016/j.biotechadv.2009.10.005. A review of EPC homing mechanisms and biomaterials designed to capture circulating EPC. [DOI] [PubMed] [Google Scholar]
- 15.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, et al. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 16.Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, Poh KK, Weinstein R, Kearney M, Chaudhry M, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 17.Kawamoto A, Katayama M, Handa N, Kinoshita M, Takano H, Horii M, Sadamoto K, Yokoyama A, Yamanaka T, Onodera R, et al. Intramuscular transplantation of G-CSF-mobilized CD34(+) cells in patients with critical limb ischemia: a phase I/IIa, multicenter, single-blinded, dose-escalation clinical trial. Stem Cells. 2009;27:2857–2864. doi: 10.1002/stem.207. [DOI] [PubMed] [Google Scholar]
- 18.Kraehenbuehl TP, Ferreira LS, Zammaretti P, Hubbell JA, Langer R. Cell-responsive hydrogel for encapsulation of vascular cells. Biomaterials. 2009;30:4318–4324. doi: 10.1016/j.biomaterials.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moon JJ, Saik JE, Poche RA, Leslie-Barbick JE, Lee SH, Smith AA, Dickinson ME, West JL. Biomimetic hydrogels with pro-angiogenic properties. Biomaterials. 2010;31:3840–3847. doi: 10.1016/j.biomaterials.2010.01.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leslie-Barbick JE, Moon JJ, West JL. Covalently-immobilized vascular endothelial growth factor promotes endothelial cell tubulogenesis in poly(ethylene glycol) diacrylate hydrogels. J Biomater Sci Polym Ed. 2009;20:1763–1779. doi: 10.1163/156856208X386381. [DOI] [PubMed] [Google Scholar]
- 21•.Phelps EA, Landázuri N, Thule PM, Taylor WR, García AJ. Bioartificial matrices for therapeutic vascularization. Proc Natl Acad Sci USA. 2010;107:3323–3328. doi: 10.1073/pnas.0905447107. Application of engineered bioactive materials to in vivo vascularization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehrbar M, Zeisberger SM, Raeber GP, Hubbell JA, Schnell C, Zisch AH. The role of actively released fibrin-conjugated VEGF for VEGF receptor 2 gene activation and the enhancement of angiogenesis. Biomaterials. 2008;29:1720–1729. doi: 10.1016/j.biomaterials.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Ghanaati S, Webber MJ, Unger RE, Orth C, Hulvat JF, Kiehna SE, Barbeck M, Rasic A, Stupp SI, Kirkpatrick CJ. Dynamic in vivo biocompatibility of angiogenic peptide amphiphile nanofibers. Biomaterials. 2009;30:6202–6212. doi: 10.1016/j.biomaterials.2009.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24••.Kraehenbuehl TP, Zammaretti P, Van der Vlies AJ, Schoenmakers RG, Lutolf MP, Jaconi ME, Hubbell JA. Three-dimensional extracellular matrix-directed cardioprogenitor differentiation: systematic modulation of a synthetic cell-responsive PEG-hydrogel. Biomaterials. 2008;29:2757–2766. doi: 10.1016/j.biomaterials.2008.03.016. A recent application of groundbreaking work involving fully synthetic bioactivematrices. [DOI] [PubMed] [Google Scholar]
- 25.Weber LM, Anseth KS. Hydrogel encapsulation environments functionalized with extracellular matrix interactions increase islet insulin secretion. Matrix Biol. 2008;27:667–673. doi: 10.1016/j.matbio.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung IM, Enemchukwu NO, Khaja SD, Murthy N, Mantalaris A, Garcia AJ. Bioadhesive hydrogel microenvironments to modulate epithelial morphogenesis. Biomaterials. 2008;29:2637–2645. doi: 10.1016/j.biomaterials.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Gupta R, Van Rooijen N, Sefton MV. Fate of endothelialized modular constructs implanted in an omental pouch in nude rats. Tissue Eng Part A. 2009;15:2875–2887. doi: 10.1089/ten.tea.2008.0494. Intriguing design concept for pre-fabricated endothelial conduits in a construct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelm JM, Lorber V, Snedeker JG, Schmidt D, Broggini-Tenzer A, Weisstanner M, Odermatt B, Mol A, Zund G, Hoerstrup SP. A novel concept for scaffold-free vessel tissue engineering: Self-assembly of microtissue building blocks. J Biotechnol. 2010 doi: 10.1016/j.jbiotec.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 29••.Sasagawa T, Shimizu T, Sekiya S, Haraguchi Y, Yamato M, Sawa Y, Okano T. Design of prevascularized three-dimensional cell-dense tissues using a cell sheet stacking manipulation technology. Biomaterials. 2010;31:1646–1654. doi: 10.1016/j.biomaterials.2009.11.036. A good example of a straighforward design concept that is well-executed. [DOI] [PubMed] [Google Scholar]
- 30.Su D, Zhang N, He J, Qu S, Slusher S, Bottino R, Bertera S, Bromberg J, Dong HH. Angiopoietin-1 production in islets improves islet engraftment and protects islets from cytokine-induced apoptosis. Diabetes. 2007;56:2274–2283. doi: 10.2337/db07-0371. [DOI] [PubMed] [Google Scholar]
- 31.Cheng Y, Liu YF, Zhang JL, Li TM, Zhao N. Elevation of vascular endothelial growth factor production and its effect on revascularization and function of graft islets in diabetic rats. World J Gastroenterol. 2007;13:2862–2866. doi: 10.3748/wjg.v13.i20.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hussey AJ, Winardi M, Han XL, Thomas GP, Penington AJ, Morrison WA, Knight KR, Feeney SJ. Seeding of pancreatic islets into prevascularized tissue engineering chambers. Tissue Eng Part A. 2009;15:3823–3833. doi: 10.1089/ten.TEA.2008.0682. [DOI] [PubMed] [Google Scholar]
- 33•.Hiscox AM, Stone AL, Limesand S, Hoying JB, Williams SK. An islet-stabilizing implant constructed using a preformed vasculature. Tissue Eng Part A. 2008;14:433–440. doi: 10.1089/tea.2007.0099. An excellent example of usage of pre-formed vasculature complemented with functional tissue implantation. [DOI] [PubMed] [Google Scholar]
- 34.Stevens KR, Pabon L, Muskheli V, Murry CE. Scaffold-free human cardiac tissue patch created from embryonic stem cells. Tissue Eng Part A. 2009;15:1211–1222. doi: 10.1089/ten.tea.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lesman A, Habib M, Caspi O, Gepstein A, Arbel G, Levenberg S, Gepstein L. Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue Eng Part A. 2010;16:115–125. doi: 10.1089/ten.TEA.2009.0130. [DOI] [PubMed] [Google Scholar]
- 36•.Dvir T, Kedem A, Ruvinov E, Levy O, Freeman I, Landa N, Holbova R, Feinberg MS, Dror S, Etzion Y, et al. Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. Proc Natl Acad Sci U S A. 2009;106:14990–14995. doi: 10.1073/pnas.0812242106. Combination of vascularization strategy with positive cardiac patch functional improvement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shepherd BR, Hoying JB, Williams SK. Microvascular transplantation after acute myocardial infarction. Tissue Eng. 2007;13:2871–2879. doi: 10.1089/ten.2007.0025. [DOI] [PubMed] [Google Scholar]
- 38.Mironov V, Kasyanov V, Drake C, Markwald RR. Organ printing: promises and challenges. Regen Med. 2008;3:93–103. doi: 10.2217/17460751.3.1.93. [DOI] [PubMed] [Google Scholar]
- 39.Visconti RP, Kasyanov V, Gentile C, Zhang J, Markwald RR, Mironov V. Towards organ printing: engineering an intra-organ branched vascular tree. Expert Opin Biol Ther. 2010;10:409–420. doi: 10.1517/14712590903563352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Moon JJ, Hahn MS, Kim I, Nsiah BA, West JL. Micropatterning of Poly(Ethylene Glycol) Diacrylate Hydrogels with Biomolecules to Regulate and Guide Endothelial Morphogenesis. Tissue Engineering Part A. 2009;15:579–585. doi: 10.1089/ten.tea.2008.0196. Exciting functional patterning application with good in vitro results, large potential for future in vivo usage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kloxin AM, Tibbitt MW, Kasko AM, Fairbairn JA, Anseth KS. Tunable Hydrogels for External Manipulation of Cellular Microenvironments through Controlled Photodegradation. Advanced Materials. 2010;22:61–66. doi: 10.1002/adma.200900917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung S, Sudo R, Zervantonakis IK, Rimchala T, Kamm RD. Surface-Treatment-Induced Three-Dimensional Capillary Morphogenesis in a Microfluidic Platform. Advanced Materials. 2009;21:4863–4867. doi: 10.1002/adma.200901727. [DOI] [PubMed] [Google Scholar]
- 43.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 44.Staton CA, Reed MW, Brown NJ. A critical analysis of current in vitro and in vivo angiogenesis assays. Int J Exp Pathol. 2009;90:195–221. doi: 10.1111/j.1365-2613.2008.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dietrich F, Lelkes PI. Fine-tuning of a three-dimensional microcarrier-based angiogenesis assay for the analysis of endothelial-mesenchymal cell co-cultures in fibrin and collagen gels. Angiogenesis. 2006;9:111–125. doi: 10.1007/s10456-006-9037-x. [DOI] [PubMed] [Google Scholar]
- 46.Duvall CL, Weiss D, Robinson ST, Alameddine FM, Guldberg RE, Taylor WR. The role of osteopontin in recovery from hind limb ischemia. Arterioscler Thromb Vasc Biol. 2008;28:290–295. doi: 10.1161/ATVBAHA.107.158485. [DOI] [PubMed] [Google Scholar]
- 47.Duvall CL, Taylor WR, Weiss D, Guldberg RE. Quantitative microcomputed tomography analysis of collateral vessel development after ischemic injury. Am J Physiol Heart Circ Physiol. 2004;287:H302–310. doi: 10.1152/ajpheart.00928.2003. [DOI] [PubMed] [Google Scholar]