SUMMARY
We have previously shown that selective activation of group I metabotropic glutamate receptors (mGluRs) results in long-lasting enhancement of synchronized network activity in the hippocampal slice. Data herein suggest that activation of group I mGluRs need not result in this potentially epileptogenic effect. (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid (ACPD), a non-selective mGluR agonist, elicits ictaform bursts identical in appearance to those induced by selective agonists, but ACPD-induced bursts do not persist following removal of the agent. Like the bursts induced by selective agonist, the ACPD bursts are blocked with group I mGluR antagonists and are not dependent on activation of either NMDA receptors or protein kinase C. However, they differ from the persistent bursts in that they do not require active protein synthesis and they are not suppressed with L-cysteine sulfinic acid, an agonist at a phospholipase D-coupled metabotropic receptor. These novel findings provide evidence that group I mGluR-induced epileptogenesis may be preventable.
Keywords: ACPD, DHPG, ictogenesis, epileptogenesis, group I mGluRs, hippocampal slice
INTRODUCTION
Activation of group I metabotropic glutamate receptors (mGluRs 1 and 5) causes seizures in vivo (McDonald et al., 1993; Camón et al., 1998) and evokes ictal-length synchronized bursting in vitro (Taylor et al., 1995; Merlin & Wong, 1997). Additionally, the ictal activity induced in hippocampal slices upon exposure to group I mGluR agonist persists for hours after removal of the agonist (Merlin & Wong, 1997), suggesting that activation of group I mGluRs may participate in the initiation of an epileptogenic process. However, this persistent epileptic state is induced only when selective group I agonists are used (e.g., (S)-3,5-dihydroxyphenylglycine [DHPG] or (S)-3-hydroxyphenylglycine [3HPG]). By contrast, when ictaform bursts are elicited with the broad-spectrum mGluR agonist (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid (ACPD), the bursts readily revert to interictal length upon removal of the agonist (Merlin & Wong, 1997). We undertook the studies herein to determine what distinguishes ACPD-induced reversible ictaform activity from DHPG-induced persistent activity. Portions of this work have appeared in abstract form (Fuortes & Merlin, 2009).
METHODS
Hartley guinea pigs 2-4 wks old were administered halothane and decapitated in accordance with protocols approved by our Institutional Animal Care and Use Committee. The brains were removed, then 400 μ transverse hippocampal slices were prepared with a Vibratome (Technical Products International) and placed in an interface chamber maintained at 35.5°C and perfused with artificial CSF containing (in mM) 124 NaCl, 26 NaHCO3, 5 KCl, 1.6 MgCl2, 2.0 CaCl2, and 10 D-glucose. Intracellular recordings were obtained from CA3 stratum pyramidale using 30-80 MΩ tip resistance electrodes. Picrotoxin (Sigma Aldrich, St. Louis, MO) was present throughout all experiments to provide consistent baseline interictal activity and ensure equivalent slice health across experiments. (S)-DHPG, (1S,3R)-ACPD, L-cysteine sulfinic acid, anisomycin, chelerythrine, LY341495, LY367385, (2S)-2-2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP), and D-(-)-2-amino-5-phosphonopentanoic acid (APV) were obtained from Tocris Ellison (Ellisville, MO). All pharmacological agents were bath-applied.
Significance of changes over time was determined using the paired Student’s t-test, with each slice serving as its own control (pre vs. post drug application). ANOVA with Newman-Keuls post hoc test was used to determine significance of differences across groups. P < 0.05 was deemed significant.
RESULTS
Reversible ACPD-induced epileptiform burst prolongation is suppressed by group I mGluR antagonists
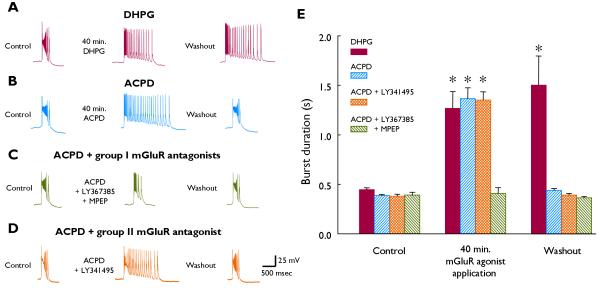
Continuous bath application of 50 μM picrotoxin, an antagonist of GABAA receptor-mediated inhibition, elicited rhythmically-recurring synchronized bursts 385 ± 13 ms in duration in CA3 pyramidal cells. Addition of 100 μM ACPD, an agonist at group I, group II, and PLD-coupled mGluRs, rapidly evoked burst lengthening, achieving 1366 ± 109 ms at 40 min exposure. Removal of the agonist restored the bursts to interictal length (440 ± 18 ms at 1 hr washout, n=7; Fig. 1B). By contrast, when DHPG, a selective group I mGluR agonist, was used to elicit synchronized bursts of similar length, the effect was undiminished at one hour following receptor activation (Fig. 1A; also see Merlin & Wong, 1997). Therefore, to test whether the ACPD-induced bursts were mediated by group I mGluR activation, experiments were performed in which ACPD was applied in the presence of selective mGluR1 and mGluR5 antagonists (LY367385 and MPEP respectively, 25 μM each). In this setting, initial bursts of 394 ± 23 ms achieved a burst duration (BD) of only 410 ± 58 ms at 40 min ACPD exposure (n=3, P>0.05; Fig. 1C).
Fig. 1.
A-D. Representative CA3 intracellular recordings shown for each protocol. Control; picrotoxin-induced interictal activity. Middle traces: DHPG, 75 μM; ACPD, 100 μM; 40 min application time. Washout; one hr following removal of all mGluR-active agents. Calibration bars apply to all traces shown. E. Summary data for protocols shown in A-D; see text for sample sizes. Asterisks denote P<0.05 by paired Student’s t-test; ANOVA confirmed significance of observed differences across groups at each time point.
Neither group II nor PLD-coupled mGluR activation is responsible for reversibility of ACPD effect
To examine whether ACPD-mediated concurrent activation of group II mGluRs prevents the persistence of its group I mGluR-mediated ictogenic effect, we applied ACPD in the presence of the selective group II mGluR antagonist LY341495 (200 nM). Interictal bursts of 381 ± 20 ms achieved a length of 1352 ± 82 ms at 40 min ACPD exposure, then readily reverted to 392 ± 17 ms following 1 hr washout of both agents (n=3; Fig. 1D). These results were not significantly different from those obtained with ACPD in the absence of group II mGluR antagonist (Fig. 1E). ACPD has also been reported to activate PLD-coupled mGluRs (Pellegrini-Giampietro et al., 1996). Nevertheless, ACPD application in the presence of 1 μM PCCG-13, a selective antagonist of PLD-coupled mGluRs, elicited significant and fully-reversible burst prolongation (BD40 min 1342 ± 366 ms; BD1 hr wash 320 ± 26 ms; n=5).
ACPD elicits burst prolongation in the presence of NMDA receptor antagonist
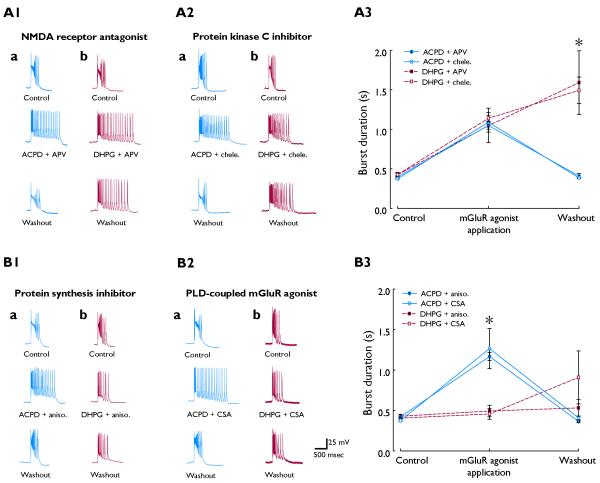
Group I mGluR activation has been reported to potentiate NMDA receptor-mediated responses (O’Connor et al., 1994), yet both induction and expression of persistent prolonged bursts with DHPG are unaffected by NMDA antagonist (Galoyan & Merlin, 2000). Similarly here, in the presence of the NMDA receptor antagonist APV (50 μM), ACPD elicited burst prolongation that was not significantly different from that elicited by ACPD alone (BDcontrol 400 ± 14 ms, BD40 min 1049 ± 218 ms, n=3; Figs. 2A1 and A3).
Fig. 2.
A; Representative recordings (A1, A2) and summary data (A3) showing NMDA antagonist and PKC inhibitor failed to suppress either ACPD- or DHPG-induced burst prolongation. B; Representative recordings (B1, B2) and summary data (B3) revealing suppressive effects of anisomycin and CSA on DHPG-induced activity and lack thereof on ACPD-induced burst prolongation. Traces shown are at times analogous to those in Fig 1. Columns labeled “a” on left of each panel are ACPD (in blue); “b”; DHPG (in red). “Chele,” chelerythrine; “aniso,” anisomycin. Summary data in A3 and B3: n=5 for each DHPG group (red dashed lines, 60-75 μM); see text for ACPD sample sizes (blue solid lines). Asterisks denote significant difference between ACPD and DHPG groups (ANOVA; P<0.05).
ACPD elicits burst prolongation in the presence of protein kinase C (PKC) inhibitor
Group I mGluR activation has numerous effects, one of which is PKC activation. However, we previously reported that chelerythrine, a broad-spectrum inhibitor of protein kinase C, had no effect on bursts induced by DHPG (Cuellar et al., 2005). In an analogous set of experiments, ACPD application in the presence of 10 μM chelerythrine similarly elicited prolonged bursts (BDcontrol 376 ± 10 ms, BD40 min 1090 ± 124 ms, n=6; Figs. 2A2 and A3).
ACPD elicits burst prolongation in the presence of either anisomycin or cysteine sulfinic acid
We have previously shown induction of burst prolongation with selective group I mGluR agonist is prevented by the protein synthesis inhibitor anisomycin (Merlin et al., 1998). By contrast, when burst prolongation was elicited with ACPD, anisomycin (15 μM) was ineffective (BDcontrol 422 ± 11 ms, BD40 min 1167 ± 50 ms, n=7; Figs. 2B1 and B3).
Previous data also showed that induction of persistent ictaform bursts with DHPG is impeded by L-cysteine sulfinic acid (CSA), a compound endogenous to hippocampus that is believed to activate PLD-coupled mGluRs (Rico & Merlin, 2004). However, co-application of 100 μM CSA had no significant effect on ACPD-induced burst prolongation (BDcontrol 376 ± 9 ms; BD40 min 1266 ± 248 ms, n=6, Figs. 2B2 and B3).
DISCUSSION
Mechanisms common to persistent and reversible epileptiform effects of group I mGluR activation
Activation of hippocampal group I mGluRs with either selective or non-selective agonist produces ictaform synchronized bursts in vitro and seizures in vivo. Regardless of whether the resultant ictaform activity is persistent or readily reversible, our data demonstrate three fundamental features in common: (1) interictal burst prolongation is dependent on activation of mGluR1 and mGluR5, the two members of the group I family; (2) burst prolongation is independent of NMDA receptor activation; and (3) burst prolongation does not depend upon activation of protein kinase C. Our previous data reveals a fourth common feature: initiation and expression of mGluR-induced synchronized bursts requires AMPA receptor activation (Merlin 1999; Taylor et al., 1995)
Distinct mechanisms are involved when group I mGluR activation induces persistent changes in network excitability
Although it was difficult to elicit ictaform bursts with selective group I mGluR agonist in the presence of either protein synthesis inhibitor or agonist of PLD-coupled mGluRs (Merlin et al., 1998; Rico & Merlin, 2004), no such difficulty was encountered when ACPD was used to elicit ictaform activity. Our data reveal that the ictogenesis mediated by ACPD-induced group I mGluR activation does not invoke protein synthesis, a pathway necessary for the development of persistent ictaform bursts. Furthermore, we have shown that neither activation nor antagonism of PLD-coupled mGluRs affects the expression of ACPD-mediated ictaform discharges. It therefore seems that although both ACPD and DHPG elicit ictaform discharges by activating group I mGluRs, the mechanisms of induction are truly different, despite the similarities listed above. Distinct intracellular second messenger pathways are invoked, with only the DHPG-mediated pathway leading to epileptogenesis.
Relevance to seizures, epilepsy, and kindling epileptogenesis
One can presume that group I mGluRs are activated during clinical seizures, and excessive excitability of group I mGluRs underlies the seizures and behavioral disturbances in patients with Fragile X syndrome (see Merlin 2009 for review). Thus, any individual with hypersensitive group I mGluRs may be predisposed to develop seizures in the setting of high-intensity glutamatergic stimulation, as may occur during status epilepticus, head trauma, or cytotoxic ischemic injury. These insults may then initiate a kindling-like process that ultimately results in epilepsy if the group I mGluR activation employs the protein synthesis-dependent pathway utilized by DHPG. Alternatively, if the protein synthesis-independent mechanism of mGluR activation utilized by ACPD is invoked, glutamatergic stimulation may cause acute reactive seizures with no long-standing deleterious consequences. As neither group II nor PLD-coupled mGluR activation is responsible for the reversibility of ACPD-induced ictogenesis, it remains unclear whether ACPD activates an additional protective factor or whether DHPG has added epileptogenicity. Further investigations in this area hold promise for determining a means to prevent group I mGluR-induced epileptogenesis.
ACKNOWLEDGEMENTS
Funded in part by NIH grant NS40387 to LRM. The authors thank Brett Cerniglia for assistance with data analysis. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
DISCLOSURE OF CONFLICTS OF INTEREST Neither author has any conflicts of interest to disclose.
REFERENCES
- Camón L, Vives P, de Vera N, Martínez E. Seizures and neuronal damage induced in the rat by activation of group I metabotropic glutamate receptors with their selective agonist 3,5-dihydroxyphenylglycine. J Neurosci Res. 1998;51:339–348. doi: 10.1002/(SICI)1097-4547(19980201)51:3<339::AID-JNR7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Cuellar JC, Griffith EL, Merlin LR. Contrasting roles of protein kinase C in induction versus suppression of group I mGluR-mediated epileptogenesis in vitro. J Neurophysiol. 2005;94:3643–3647. doi: 10.1152/jn.00548.2005. [DOI] [PubMed] [Google Scholar]
- Fuortes MG, Merlin LR. Persistent vs. non-persistent group I mGluR-induced network activity: implications for epileptogenesis. Neurology. 2009;72(Suppl. 3):A381. 2009. [Google Scholar]
- Galoyan SM, Merlin LR. Long-lasting potentiation of epileptiform bursts by group I mGluRs is NMDA receptor independent. J Neurophysiol. 2000;83:2463–2467. doi: 10.1152/jn.2000.83.4.2463. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Fix AS, Tizzano JP, Schoepp DD. Seizures and brain injury in neonatal rats induced by 1S,3R-ACPD, a metabotropic glutamate receptor agonist. J Neurosci. 1993;13:4445–4455. doi: 10.1523/JNEUROSCI.13-10-04445.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin LR, Wong RKS. Role of group I metabotropic glutamate receptors in the patterning of epileptiform activities in vitro. J Neurophysiol. 1997;78:539–544. doi: 10.1152/jn.1997.78.1.539. [DOI] [PubMed] [Google Scholar]
- Merlin LR, Bergold PJ, Wong RKS. Requirement of protein synthesis for group I mGluR-mediated induction of epileptiform discharges. J Neurophysiol. 1998;80:989–993. doi: 10.1152/jn.1998.80.2.989. [DOI] [PubMed] [Google Scholar]
- Merlin LR. Group I mGluR-mediated silent induction of long-lasting epileptiform discharges. J Neurophysiol. 1999;82:1078–1081. doi: 10.1152/jn.1999.82.2.1078. [DOI] [PubMed] [Google Scholar]
- Merlin LR. Differential roles for mGluR1 and mGluR5 in the persistent prolongation of epileptiform bursts. J Neurophysiol. 2002;87:621–625. doi: 10.1152/jn.00579.2001. [DOI] [PubMed] [Google Scholar]
- Merlin LR. The fragile X mental retardation protein: a valuable partner in the battle against epileptogenesis. Epilepsy Curr. 2009;9:116–118. doi: 10.1111/j.1535-7511.2009.01311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JJ, Rowan MJ, Anwyl R. Long-lasting enhancement of NMDA receptor-mediated synaptic transmission by metabotropic glutamate receptor activation. Nature. 1994;367:557–559. doi: 10.1038/367557a0. [DOI] [PubMed] [Google Scholar]
- Pellegrini-Giampietro DE, Torregrossa SA, Moroni F. Pharmacological characterization of metabotropic glutamate receptors coupled to phospholipase D in the rat hippocampus. Br J Pharmacol. 1996;118:1035–1043. doi: 10.1111/j.1476-5381.1996.tb15503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico MJ, Merlin LR. Evidence that phospholipase D activation prevents group I mGluR-induced persistent prolongation of epileptiform bursts. J Neurophysiol. 2004;91:2385–2388. doi: 10.1152/jn.01140.2003. [DOI] [PubMed] [Google Scholar]
- Taylor GW, Merlin LR, Wong RKS. Synchronized oscillations in hippocampal CA3 neurons induced by metabotropic glutamate receptor activation. J Neurosci. 1995;15:8039–8052. doi: 10.1523/JNEUROSCI.15-12-08039.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]