Abstract
We studied some aspects of the quantitative and qualitative features of heterologous recombinant (re) virus-vector-induced, antigen-specific CD8+ T cells against Trypanosoma cruzi. We used three different, highly-attenuated re-viruses, i.e., influenza virus, adenovirus and vaccinia virus, which all expressed a single, T. cruzi antigen-derived CD8+ T cell epitope. The use of two out of three vectors or the triple virus vector vaccination regimen not only confirmed that the re-vaccinia virus, which was placed last in order for sequential immunization, was an effective booster for the CD8+ T cell immunity in terms of the number of antigen-specific CD8+ T cells, but also demonstrated that i) the majority of cells exhibit the effector memory (TEM) phenotype, ii) robustly secrete IFN-γ, iii) express higher intensity of the CD122 molecule and iv) present protective activity against T. cruzi infection. In contrast, placing the re-influenza virus last in sequential immunization had a detrimental effect on the quantitative and qualitative features of CD8+ T cells. The triple virus vector vaccination was more effective at inducing a stronger CD8+ T cell immunity than using two re-viruses. The different quantitative and qualitative features of CD8+ T cells induced by different immunization regimens support the notion that the refinement of the best choice of multiple virus vector combinations is indispensable for the induction of a maximum number of CD8+ T cells of high quality.
Keywords: CD8+ T cells, Trypanosoma cruzi, Recombinant adenovirus, Recombinant influenza virus, Recombinant vaccinia virus
1. Introduction
Development of a CD8+ T cell vaccine strategy has been intensely investigated in recent years (Masopust et al., 2007; Masopust, 2009) not only due to the emergence of neutralizing antibody (Ab)-resistant pathogens (Appay et al., 2008; Overstreet et al., 2008) but due to the obvious recognition of imminent pandemic infection and bioterrorism (McMurry et al., 2007; Doherty and Kelso, 2008; Stambas et al., 2008). The rationality of this approach is supported by observations which demonstrated that CD8+ T cells could suppress the proliferation of several infectious pathogens (Goulder and Watkins, 2004; Masopust et al., 2007; Appay et al., 2008).
Trypanosoma cruzi is the etiological agent of Chagas' disease in Central and South America (Chagas, 1909). Since T cell-mediated immunity is critical for resolving the infection (Tarleton et al., 1992; Miyahira, 2008), a T. cruzi infection model is useful for defining a CD8+ T cell-dependent vaccine strategy. We previously identified a major epitope of trans-sialidase surface antigen (TSSA) recognized by CD8+ T cells in T. cruzi-infected C57BL/6 (B6) mice and have demonstrated that vaccination, either with plasmid DNA encoding TSSA (Katae et al., 2002; Miyahira et al., 2003a, b, c) or with recombinant (re) viruses expressing a single CD8+ T cell-inducing epitope (Miyahira et al., 2005), could confer CD8+ T cell-mediated protective immunity against lethal T. cruzi infection.
Vaccination using heterologous re-virus vectors (Stephenson, 2001) has been effective for inducing protective immunity against several infectious pathogens such as HIV (Shiver and Emini, 2004), malaria (Oliveira-Ferreira et al., 2000; Bruna-Romero et al., 2001; Zavala et al., 2001) and T. cruzi (Miyahira et al., 1999, 2005). A heterologous primary/booster vaccine strategy (Miyahira et al., 1998, 1999, 2005; Oliveira-Ferreira et al., 2000) demonstrated that CD8+ T cell immunity can be boosted, offering potential for future clinical applications. Despite its dramatic success demonstrated in animal studies, its efficacy in humans was rather discouraging (Bejon et al., 2007; Sekaly, 2008), and needs to be improved for human application. By considering the limited physiological and spatial capacity for hosts' immunity (Vezys et al., 2009), the improvement of not only the quantitative attribute but the qualitative features of CD8+ T cell immunity must be achieved (Masopust et al., 2006, 2007, 2009; Araki et al., 2009).
Since there is still limited information regarding the qualitative features of CD8+ T cells against infectious pathogens (Masopust et al., 2006; Araki et al., 2009; Masopust, 2009), we elucidated, in the present study, some aspects of the phenotypic and immunological features of antigen-specific CD8+ T cells which were induced by the use of three different, highly-attenuated re-viruses, all expressing a single T. cruzi antigen-derived CD8+ T cell epitope, ANYNFTLV. During the course of our investigation, we verified the remarkable boosting capacity of vaccinia virus for the induction of higher numbers of CD8+ T cells, which exhibit CD122dull, CD44+, CD62L-, IFN-γ+ phenotype, when it was placed last for sequential vaccination. We also verified the superior efficacy of a triple virus vector vaccination regimen for the induction of CD8+ T cell immunity compared with the combination of two viruses. Our study supports the importance and need to refine the choice of multiple virus vector combinations to achieve the induction of a maximum number of CD8+ T cells of high quality.
2. Materials and methods
2.1. Animals and parasites
Female B6 (H-2b) mice, 5-8 weeks of age, were purchased from Japan SLC Inc. (Hamamatsu, Shizuoka, Japan). Blood-form trypomastigotes of T. cruzi Tulahuen strain (Miyahira and Dvorak, 1994) were maintained in BALB/c mice by i.m. inoculation of 5,000 trypomastigotes into naïve mice every 2 weeks. An institutional review committee at the National Defense Medical College, Saitama, Japan, approved the animal studies described here.
2.2. Cells and culture
The B6 mouse-derived thymoma cell line EL-4 was used as antigen-presenting cells for CD8+ T cell cultures and assays. The BHK-21 cell line (ATCC, Manassas, VA, USA) was used for growing a highly-attenuated vaccinia virus strain called modified vaccinia virus Ankara (MVA) (ATCC) (Sutter and Moss, 1992) -derived re-virus. The transformed human embryonic kidney cell line 293 (ATCC) was used for growing replication-deficient re-adenoviruses. These cells were cultured in medium as described previously (Miyahira et al., 2005). The medium used for immunological assays and the culture of lymphocytes was supplemented with phorbol myristate acetate-stimulated EL-4 cell culture supernatant as a source of 30 U/ml IL-2 (Miyahira et al., 1995).
2.3. Peptide
An H-2Kb-restricted CD8+ T cell epitope peptide, ANYNFTLV, derived from TSSA (Katae et al., 2002; Miyahira et al., 2003a, b, c, 2005) was synthesized and used for immunological assays.
2.4. Generation of re-viruses
Re-influenza viruses were generated using an established eight-plasmid influenza reverse genetics system (Hoffmann et al., 2000; Quinlivan et al., 2005). The plasmids containing the segments of A/Puerto Rico/8/34 influenza strain (PR8) were co-transfected in 293T cells and 24 h later cells were harvested and inoculated into 7-8 days-old embryonated eggs. The allantoic fluids containing the virus preparations were collected 48 h post-inoculation. The CD8+-T-cell epitope, ANYNFTLV, was inserted into the neuraminidase (NA) of A/PR/8/34 (H1N1) at residue 42 by PCR mutagenesis using the following primers: 5′-CAATTCAAACTGGAAGTGCCAACTACAACTTCACCCTGGTGCAAAACATCATTAC -3′ and 5′-GTAATGATGTTTTGCACCAGGGTGAAGTTGTAGTTGGCACTTCCAGTTTGAATTG -3′. A corresponding number of coding amino acids were eliminated from the NA segment in order to maintain the length equal to the wild-type NA. To generate NS1 of the PR8 strain containing only the first 126 aminoacids (NS1-D126), we used a strategy described previously (Solorzano et al., 2005). NS1-D126 was constructed by ligation into pDZ vector (Quinlivan et al., 2005) of two PCR fragments obtained using two sets of primers. The 5′end portion of the segment was obtained with the following primers: 5′-GCGCTTAATTAAGAGGGAGCAATTGTTGGCG-3′ (NS1-153) and 5′-CATCGCTCTTCTATTAGTAGAAACAAGGGTGTTTTTTATTATTAAATAAG-3′. The 3′ end portion containing the first 126 amino acids of NS1 was obtained using the following primers: 5′-GCGCTTAATTAATCAAGATCTACTTATCCATGATCGCCTGG-3′ (NS1-126) together with 5′-GATCGCTCTTCTGGGAGCAAAAGCAGGGTGACAAAGAC-3′. NS1 truncations did not affect the sequence of NEP/NS2. The highly-attenuated re-influenza virus expressing an ANYNFTLV epitope is designated as re-FLU.
The re-adenovirus and re-vaccinia viruses expressing an ANYNFTLV epitope (Miyahira et al., 2005) are designated as re-Ad or re-MVA, respectively. The genetically-modified viruses which do not express an ANYNFTLV epitope were used as control re-virus vectors, which were described as FLU, Ad (Miyahira et al., 2005) or MVA (Miyahira et al., 2005).
2.5. Quantification of antigen-specific T cells by ELISPOT assay
The frequency of antigen-specific T cells was determined by the IFN-γ enzyme-linked immunospot (ELISPOT) assay for IFN-γ-secreting cells essentially as described previously (Miyahira et al., 1995).
2.6. Monoclonal antibodies (mAbs)
The following mAbs were used: FITC-conjugated anti-mouse CD62L / L-selectin /LECAM-1 /Ly-22 mAb (MEL-14), peridinin chlorophyll-a protein (PerCP)-Cy5.5-conjugated anti-mouse CD8α mAb (53-6.7), allophycocyanin (APC)-conjugated anti-mouse CD44 /Pgp-1 /Ly-24 mAb (IM7), phycoerythrin (PE)-conjugated anti-mouse IFN-γ mAb (XMG1.2), PerCP-Cy5.5-conjugated anti-CD4 mAb (RM4-5), PE-conjugated anti-CD122 mAb (TM-β1), biotin-conjugated anti-CD25 mAb (7D4), APC-labeled streptavidin, PE-conjugated anti-NK1.1 mAb (PK136), FITC-conjugated anti-T cell receptor (TCR)β chain mAb (H57-597) (BD Biosciences Mountain View, CA, USA), APC-conjugated anti-mouse CD8α mAb (53-6.7) (eBioscience, San Diego, CA, USA), PE-labeled H-2Kb/ANYNFTLV pentamer (Proimmune, Oxford, United Kingdom).
2.7. Flowcytometric analyses
Splenocytes (2 × 106) were stained with each fluorescent dye-conjugated mAb. After washing with 2% FCS-supplemented PBS containing with 1 mM Na2EDTA and 0.1 % sodium azide (washing buffer), the specimens were analyzed on a FACSCalibur™ (BD Biosciences). To quantify the ANYNFTLV-specific, IFN-γ-producing CD8+ T cells, splenocytes (2 × 106) were co-cultured with 1 × 105 of irradiated, 5 mM ANYNFTLV-pre-pulsed EL-4 cells in medium containing 10 mg/ml Brefeldin A for 16 h. They were then stained with APC-conjugated anti-mouse CD8α mAb, fixed and permeabilized with BD FACS Lysing Solution together with FACS Permeabilizing Solution 2 (BD Biosciences), further stained with the PE-conjugated anti-mouse IFN-γ mAb, and were finally analyzed on FACSCalibur. The data were processed using CellQuest software (BD Biosciences).
2.8. Vaccination schedule, dosages, and challenge infection
All of the vaccination schedules, dosages and the protocols for T. cruzi challenge infection are described in each figure legend.
2.9. Statistical analyses
Statistical analyses were performed using the StatView software (SAS Institute, Cary, NC, USA). An unpaired Student's t test was applied to determine the significant differences between two groups for the ELISPOT assays, FACS analyses and for the parasitemia. Significant differences in survival data were calculated by the Kaplan-Meier method with Mantel-Cox logrank. P values less than 0.05 were considered significant.
3. Results
3.1. Phenotypic features of immune responses induced by the use of re-Ad and re-MVA
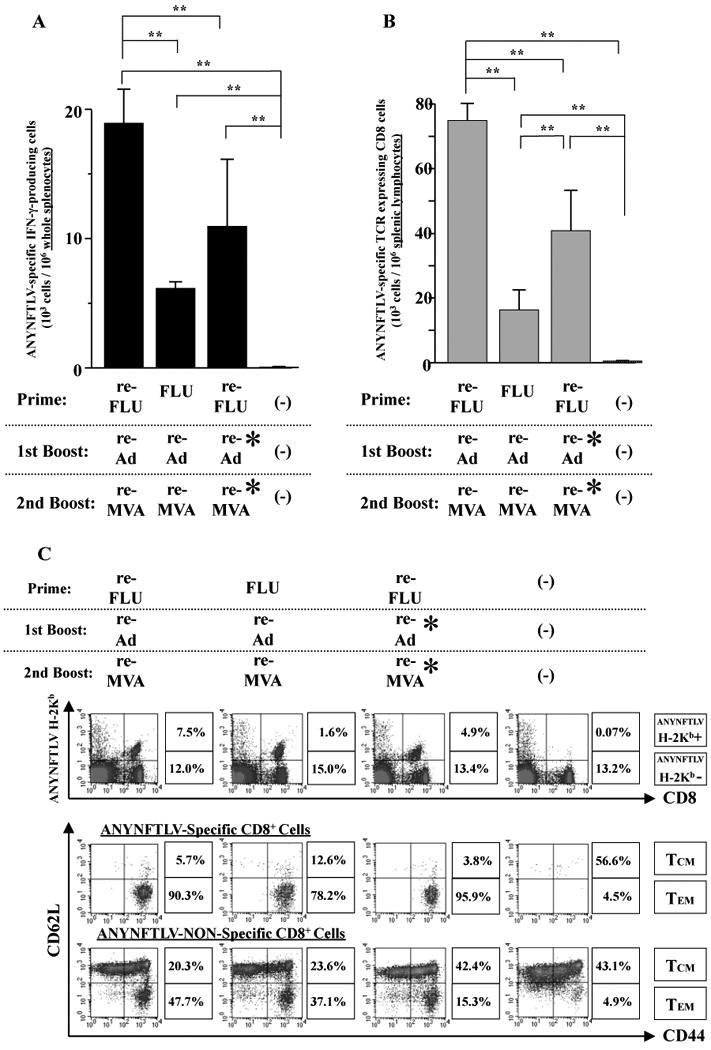
The ELISPOT assay (Fig. 1A) and the Kb/ANYNFTLV pentamer technology (Fig. 1B) confirmed the superior induction of antigen-specific CD8+ T cells by the heterologous re-Ad/re-MVA to the homologous re-Ad immunization. In addition, we found that those induced by the heterologous immunization consisted of higher portions of ANYNFTLV-specific CD44+CD62L-CD8+ T cells (TEM phenotype) (98.2%) than those induced by the homologous one (83.6%) (Fig. 1C). A similar trend in cell type composition was observed in the ANYNFTLV-non-specific CD8+ T cells (13.8% vs. 5.0%) (Fig. 1C). The composition of “regulatory” cell populations such as the CD4+CD25+ T cells, NK1.1+αβTCR+ T (NKT) cells and CD8+CD122+ T cells did not show dramatic differences between experimental groups (Fig. 1D) except that we noticed the increase of CD8+CD122dull cell populations in the heterologous re-Ad/re-MVA-immunized mice (arrow in Fig. 1D). Since the expression of a CD122 molecule on CD8+ T cells is viewed as one of the indications for the maturation of these cells (Khanolkar et al., 2004; Mbitikon-Kobo et al., 2009), we compared its expression between the CD8+ T cells derived from different immunization protocols (Fig. 1E). The CD122 expression on ANYNFTLV-specific CD8+ T cells induced by either of these double re-virus vector immunization regimens exhibit similar levels of flow cytometric mean fluorescence intensity (MFI) (62.5 in heterologous immunization versus 61.5 in the homologous one) (Fig. 1E).
Fig. 1.
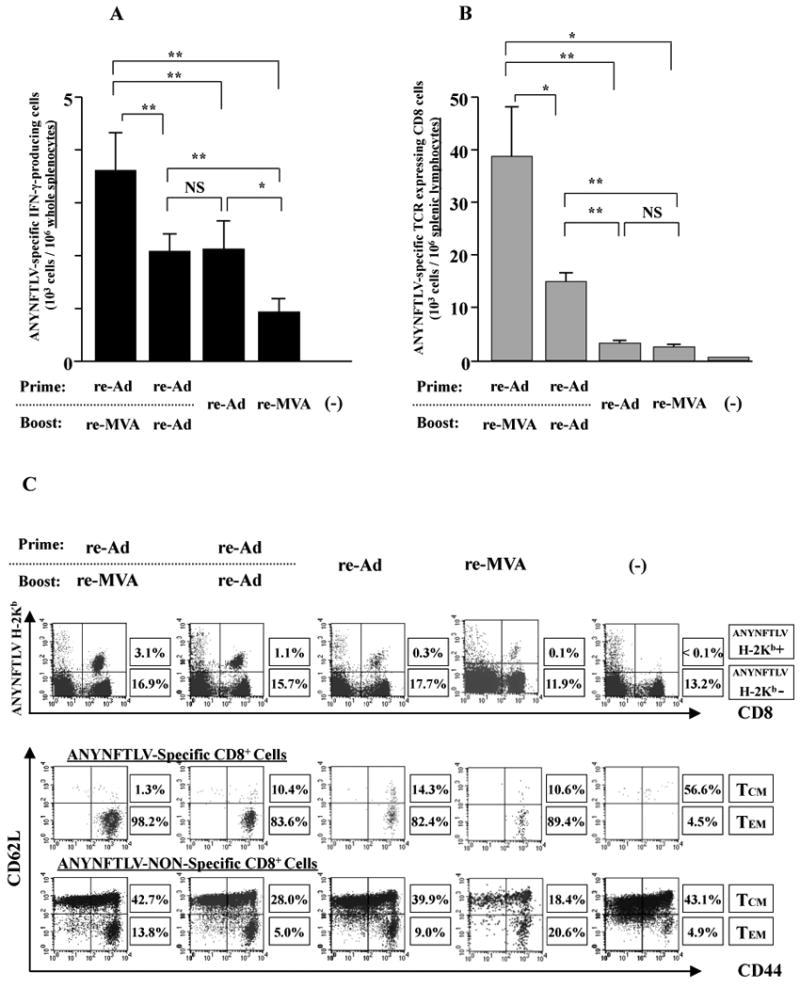
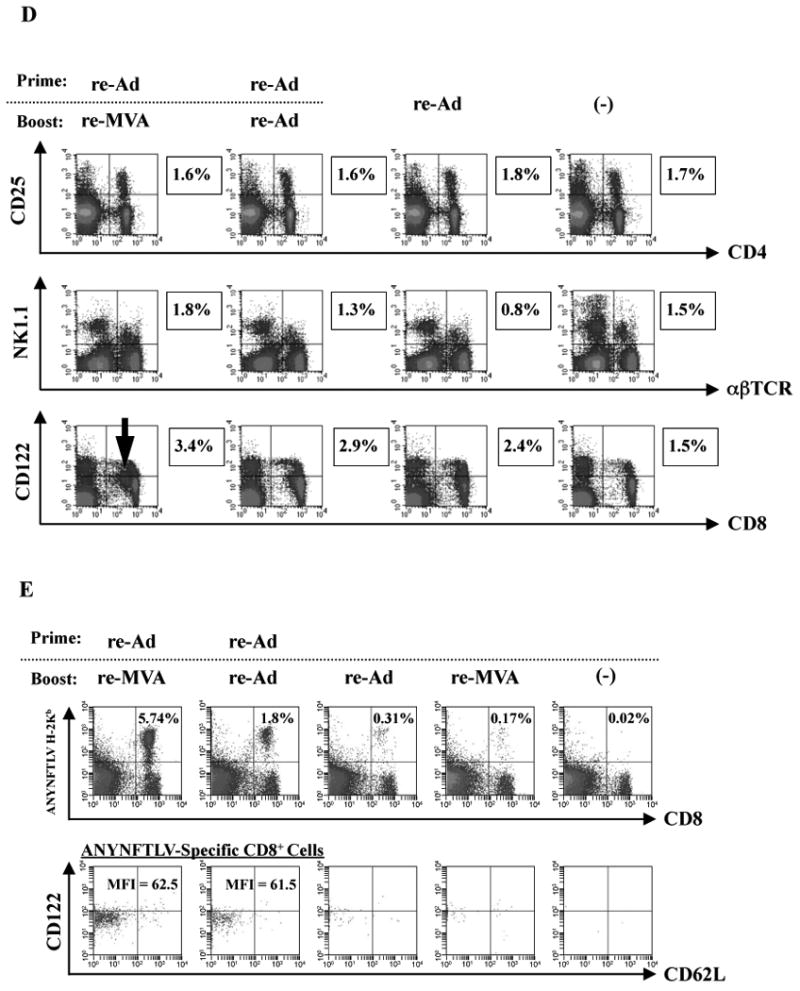
Phenotypic features of ANYNFTLV, a peptide derived from Trypanosoma cruzi surface antigen-specific CD8+ T cells induced by the use of recombinant adenoma virus (re-Ad) and recombinant vaccinia virus (re-MVA). B6 mice were first primed with 5 × 107 plaque forming units (PFU) of re-Ad and then were given a booster immunization 14 days later with 5 × 107 PFU of either re-Ad or re-MVA. The single re-Ad immunized and non-immunized mice were included as controls. The mice were sacrificed 12 days after the last immunization and spleens were removed. The freshly-isolated splenocytes were subjected to immunological assays. (A) The splenocytes were subjected to the ELISPOT assay for IFN-γ-producing cells in response to ANYNFTLV peptide-pulsed EL-4 cells. The number of IFN-γ-secreting cells per 106 splenocytes was counted 22 h later. The number of IFN-γ-secreting cells that appeared against peptide-unpulsed EL-4 was subtracted from the number of IFN-γ-secreting cells that appeared against peptide-pulsed EL-4. Data represent the mean ± S.D. of three to nine mice in each group. **, P < 0.01 determined by the Student's t-test. “NS” means “not statistically significant”. (B) The splenocytes were subjected to flow cytometory (FCM) analyses for detecting Kb/ANYNFTLV pentamer-reactive CD8+ T cells. The T-cell receptor (TCR) was stained by the phycoerythrin (PE)-conjugated Kb/ANYNFTLV pentamer, and the CD8 was stained by the PerCP-Cy5.5-conjugated anti-CD8 monoclonal antibody (mAb). The number of ANYNFTLV+, CD8+ T cells per 106 splenic lymphocytes was calculated. Data represent the mean ± standard deviation of three to nine mice in each group. *, P < 0.05 and **, P < 0.01 determined by the Student's t-test. (C) The representative data of the FACS analyses for detecting Kb/ANYNFTLV pentamer-reactive or non-reactive CD8+ T cells. The ANYNFTLV+, CD8+ T cells or ANYNFTLV-, CD8+ T cells were stained with the APC-conjugated anti-CD44 mAb and the FITC-conjugated anti-CD62L mAb. TCM: T cells of central memory phenotype, TEM: T cells of effector memory phenotype. (D) The representative data of FACS analyses for detection of “regulatory” cell populations. After collecting immune splenocytes, the FITC-conjugated anti-TCRb chain mAb, the PE-conjugated anti-NK1.1 mAb, the PE-conjugated anti-CD122 mAb, the PerCP-Cy5.5-conjugated anti-CD4 mAb and the PerCP-Cy5.5-conjugated anti-CD8 mAb were used to stain immune cells for the identification of CD4+CD25+ T cells, natural killier (NK) T cells and CD8+CD122+ T cells. (E) The representative data of FACS analyses for detection of a Kb/ANYNFTLV pentamer-reactive, CD122dull cell population. The TCR was stained with the PE-conjugated Kb/ANYNFTLV pentamer, the PerCP-Cy5.5-conjugated anti-CD8 mAb, the APC-conjugated anti-CD122 mAb and the FITC-conjugated anti-CD62L mAb. The mean fluorescence intensity (MFI) of CD122 staining was calculated by gating the ANYNFTLV+, CD8+ cells. The single re-MVA immunized mice were also included for this experiment. All of the experiments were repeated at least twice for the confirmation of reproducibility. The use of control Ad and control MVA which are not expressing the epitope did not induce the ANYNFTLV-specific immune responses (data not shown).
3.2. Efficient induction of IFN-γ-secreting, ANYNFTLV-specific CD8+ T cells by the heterologous re-Ad/re-MVA immunization
We next elucidated the capacity for IFN-γ secretion by the ANYNFTLV-specific CD8+ T cells which were induced by either the heterologous or homologous immunizations. The re-Ad/re-MVA immunization could induce a significantly higher portion of IFN-γ-secreting, ANYNFTLV-specific CD8+ T cells than the homologous re-Ad immunization (4.8% ± 0.4 versus 2.7% ± 0.8) (Fig. 2A, B). In addition, the CD8+ T cells induced by the re-Ad/re-MVA immunization exhibited significantly higher MFI of IFN-γ content than those induced by the homologous immunization (217 versus 128) (Fig. 2C), indicating that the IFN-γ content per CD8+ T cell is significantly different depending on the vaccination regimen used for its induction.
Fig. 2.
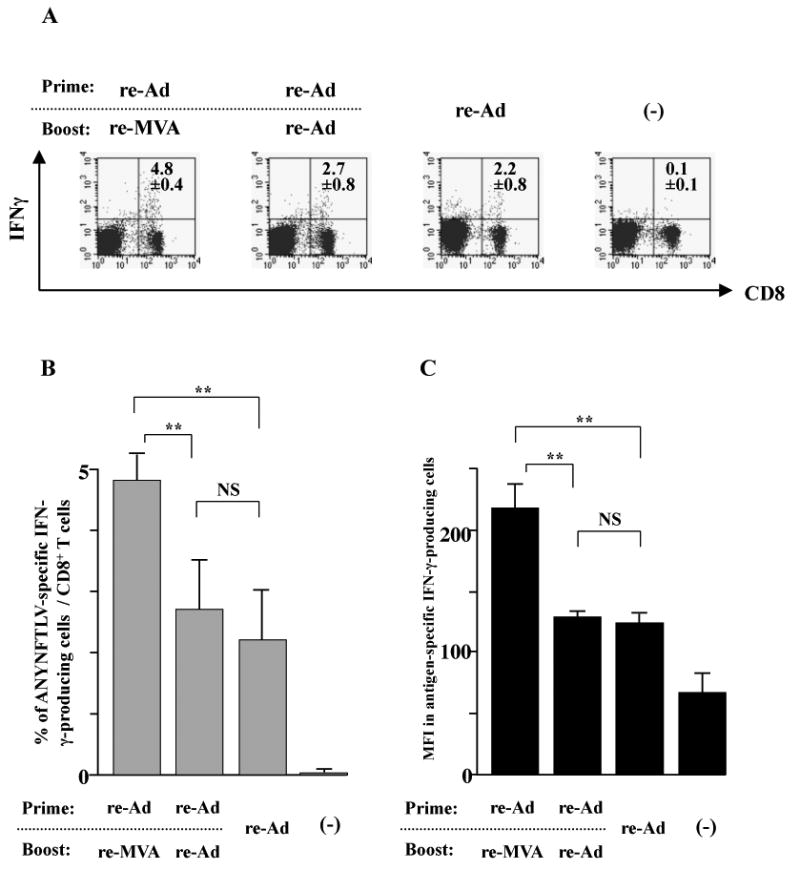
The intracellular IFN-γ staining of the ANYNFTLV, a peptide derived from Trypanosoma cruzi surface antigen-specific CD8+ T cells induced by the use of recombinant adenovirus (re-Ad) and/or recombinant vaccinia virus (re-MVA) for vaccination. (A) The representative data of intracellular IFN-γ staining of ANYNFTLV-specific CD8+ T cells derived from the re-Ad and / or re-MVA-immunized mice. B6 mice were first primed with 5 × 107 plaque forming units (PFU) of re-Ad and then were given a booster immunization 14 days later with 5 × 107 PFU of either re-Ad or re-MVA. The single re-Ad immunized and non-immunized mice were included as controls. The mice were sacrificed 12 days after the last immunization, and spleens were removed. The freshly-isolated splenocytes were subjected to the FACS analyses for the intracellular IFN-γ+ staining in response to the ANYNFTLV-pulsed EL-4 cells. The cell culture condition for the assays was described in the Materials and methods. The number of IFN-γ+ cells that appeared against peptide-unpulsed EL-4 was subtracted from the number of IFN-γ+ cells that appeared against peptide-pulsed EL-4. (B) The percentage of IFN-γ+ cells among total CD8+ T cells was calculated. Data represent the mean ± S.D. of three mice in each group. **, P < 0.01 determined by the Student's t-test. “NS” means “not statistically significant”. (C) The mean fluorescence intensity (MFI) of ANYNFTLV-responding IFN-γ+ cells was calibrated by gating cells which were both IFN-γ-positive and CD8-positive. Data represent the mean ± S.D. of three mice in each group. **, P < 0.01 determined by the Student's t-test. “NS” means “not statistically significant”.
3.3. Characterization of re-FLU for the induction of antigen-specific CD8+ T cells
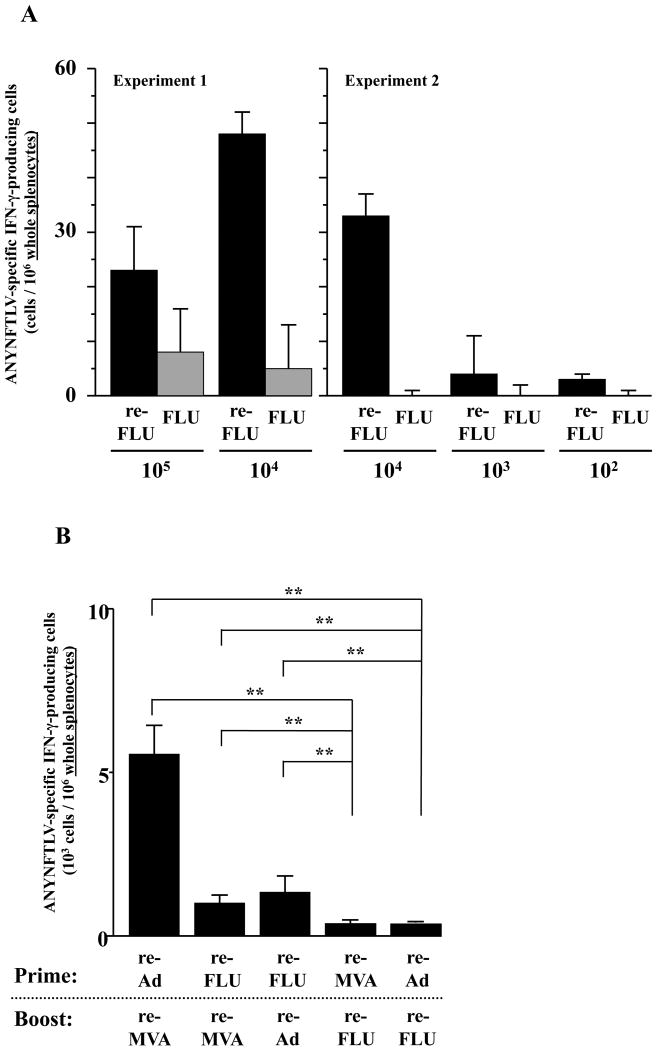
The immunogenicity of the highly-attenuated re-FLU for the induction of ANYNFTLV-specific CD8+ T cells was confirmed by the ELISPOT assay after immunizing B6 mice with different viral doses (Fig. 3A). When we combined the re-FLU with one of the other re-viruses for immunization, both the ELISPOT assay (Fig. 3B) and the pentamer technology (data not shown) presented similar results, showing that the heterologous re-Ad/re-MVA double immunization was better for the induction of CD8+ T cell numbers than any other re-FLU-combined heterologous immunization regimens. The re-FLU-combined heterologous immunization induced a lower percentage of CD8+ T cells of TEM phenotype than those induced by the heterologous re-Ad/re-MVA immunization (Fig. 3C, D). This feature was particularly conspicuous when the re-FLU was used as a booster immunogen, exhibiting TEM phenotype only in 45.9% in cells induced by the re-Ad/re-FLU vaccination (Fig. 3C) and at 39.7% induced by the re-MVA/re-FLU vaccination (Fig. 3D).
Fig. 3.
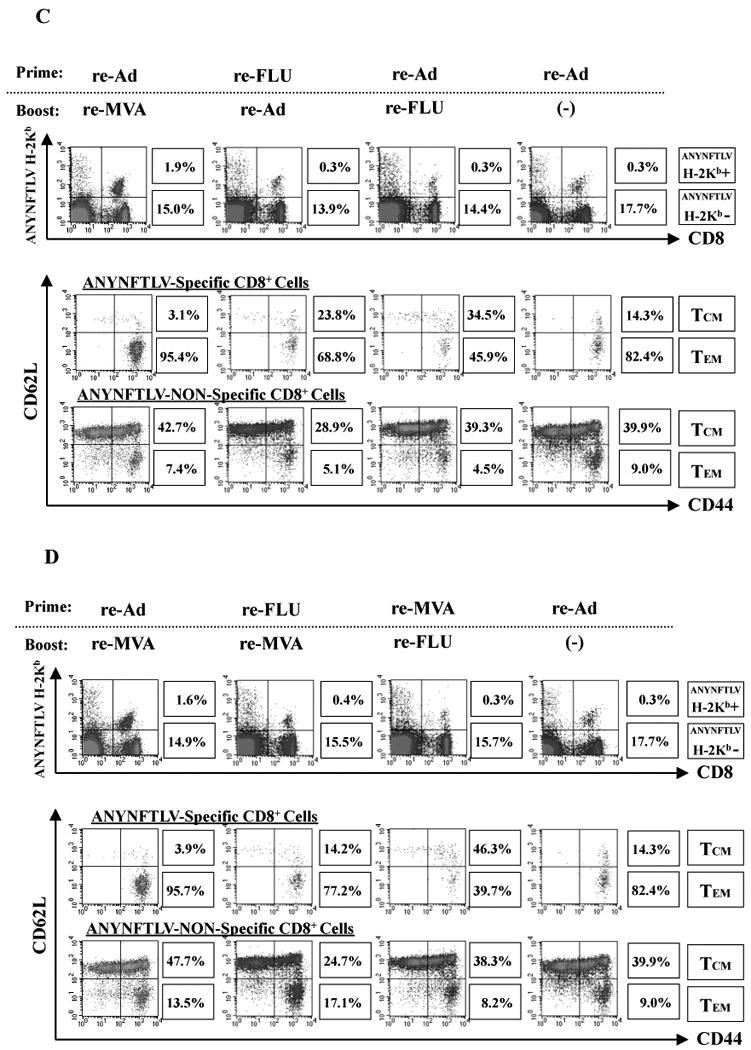
Characterization of recombinant influenza virus (re-FLU) for the induction of antigen-specific CD8+ T cells. (A) B6 mice were immunized with either re-FLU or control FLU at four different immunizing plaque forming unit (PFU) doses. They were sacrificed 12 days (Experiment 1) or 14 days (Experiment 2) after the immunization, and their spleens were removed. The freshly-isolated splenocytes were subjected to the ELISPOT assay for IFN-γ-producing cells in response to ANYNFTLV, a peptide derived from Trypansoma cruzi surface antigen, peptide-pulsed EL-4 cells. The number of IFN-γ-secreting cells × 106 was counted 22 h later. The number of IFN-γ-secreting cells that appeared against peptide-unpulsed EL-4 was subtracted from the number of IFN-γ-secreting cells that appeared against peptide-pulsed EL-4. (B) B6 mice were first primed with 1 × 104 PFU of re-FLU, 5 × 107 PFU of recombinant adenovirus (re-Ad) or 5 × 107 PFU of recombinant vaccinia virus (re-MVA). The same immunization doses of each virus were given to the mice as booster immunizations 14 days later. The mice were sacrificed 12 days after the last immunization and their spleens were removed. The freshly-isolated splenocytes were subjected to the ELISPOT assay for IFN-γ-producing cells in response to ANYNFTLV peptide-pulsed EL-4 cells. The number of IFN-γ-secreting cells that appeared against peptide-unpulsed EL-4 was subtracted from the number of IFN-γ-secreting cells that appeared against peptide-pulsed EL-4. Data represent the mean ± S.D. of three mice in each group. **, P < 0.01 determined by the Student's t-test. (C, D) The representative data of the FACS analyses for detection of Kb/ANYNFTLV pentamer-reactive or non-reactive CD8+ T cells. Immune splenocytes described in (B) were subjected to the flow cytometory analyses for the detection of Kb/ANYNFTLV pentamer-reactive CD8+ T cells. The T-cell receptor (TCR) was stained by the phycoerythrin (PE)-conjugated Kb/ANYNFTLV pentamer, and the CD8 was stained by the PerCP-Cy5.5-conjugated anti-CD8 monoclonal antibody (mAb). The ANYNFTLV+, CD8+ T cells or ANYNFTLV-, CD8+ T cells were stained with the APC-conjugated anti-CD44 mAb and the FITC-conjugated anti-CD62L mAb. TCM: T cells of central memory phenotype, TEM: T cells of effector memory phenotype. The use of control Ad, control MVA and control FLU which do not express the epitope did not induce the ANYNFTLV-specific immune responses (data not shown). All of the experiments were repeated at least twice for the confirmation of reproducibility.
3.4. Phenotypic and immunological features of ANYNFTLV-specific CD8+ T cells induced by heterologous triple virus vector immunization
We then tested whether there are any advantages to using three different re-viruses for the induction of CD8+ T cells. Since the best order for sequential double immunization to induce the maximum number of antigen-specific CD8+ T cells was the heterologous re-Ad/re-MVA (Miyahira et al., 2005), we combined the newly-generated re-FLU with two other re-viruses in different heterologous vaccination orders as shown in Fig. 4A and B. We found that vaccination in the order of re-FLU/re-Ad/re-MVA was the best for maximum induction of ANYNFTLV-specific CD8+ T cell numbers as determined by both the ELISPOT assay (Fig. 4A) and the pentamer technology (Fig. 4B). In contrast, the immunogenicity of re-Ad/re-MVA/re-FLU was significantly impaired compared not only with those demonstrated in two other triple vaccination regimens but with that observed in the re-Ad/re-MVA double vaccination (Fig. 4A, B). Fig. 4C shows the representative data of pentamer analyses demonstrating that the percentage of CD8+ T cells of TEM phenotype was higher when the re-MVA was placed last in the triple sequential vaccination regimen (95.9% and 93.9%). In contrast, the percentage of the TEM phenotypic cell population was the lowest when mice were immunized with re-Ad/re-MVA/re-FLU (81.1%). The percentage of TEM phenotype of ANYNFTLV-non-specific CD8+ T cells showed a similar trend of differences among different regimens (Fig. 4C). Since we found that the expression of CD122 (Fig. 1D) and the capacity for IFN-γ secretion (Fig. 2) by the induced CD8+ T cells were different depending on the vaccination regimens, we therefore characterized these phenotypes among CD8+ T cells induced by each regimen (Fig. 4D). As expected, the quantitative features of the ANYNFTLV-specific CD8+ T cells induced by the re-FLU/re-Ad/re-MVA vaccination (8.18%) and the re-Ad/re-FLU/re-MVA vaccination (6.81%) coincided with the higher expression of CD122 (MFI = 56.9 and 54.0, respectively) and the elevated IFN-γ content per CD8+ T cell (MFI = 55.3 and 53.3, respectively) (Fig. 4D). The inferior induction of ANYNFTLV-specific CD8+ T cells by the re-Ad/re-MVA/re-FLU vaccination (4.86%), on the contrary, coincided with the lower expression of CD122 on the CD8+ T cells (MFI = 42.5) and the lower content of IFN-γ per CD8+ T cell (MFI = 43.5) (Fig. 4D).
Fig. 4.
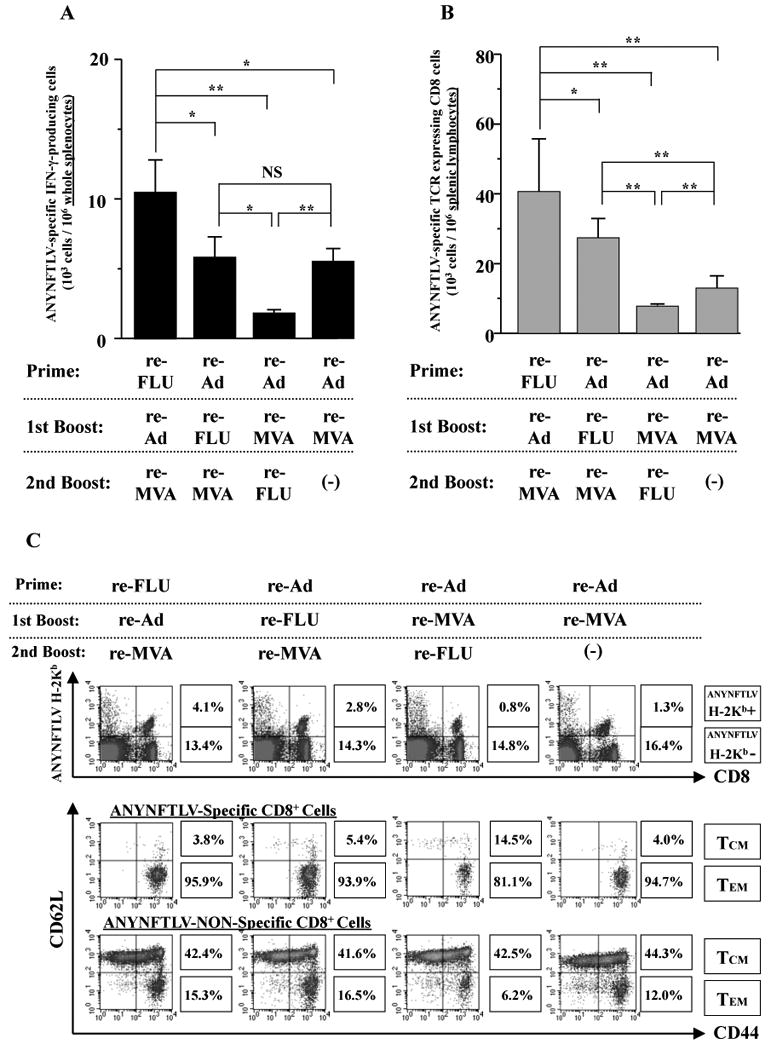
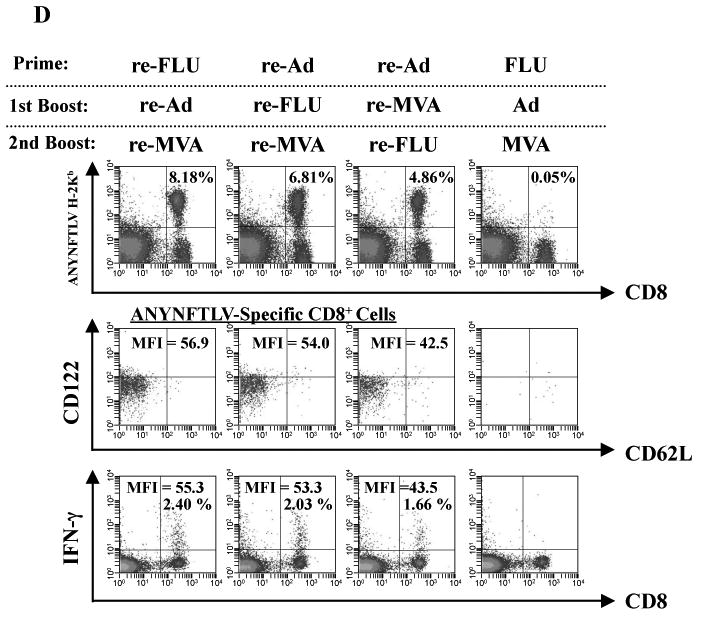
Phenotypical and immunological features of ANYNFTLV, a peptide derived from Trypanosoma cruzi surface antigen-specific CD8+ T cells induced by triple virus vector immunization. B6 mice were first primed with 1 × 104 plaque forming units (PFU) of recombinant influenza virus (re-FLU) or 5 × 107 PFU of recombinant adenovirus (re-Ad), followed by booster immunizations 14 days later with 1 × 104 PFU of re-FLU, 5 × 107 PFU of re-Ad or 5 × 107 PFU of recombinant vaccinia virus (re-MVA). They were further given second booster immunizations 14 days later with 1 × 104 PFU of re-FLU, or 5 × 107 PFU of re-MVA. (A) The mice were sacrificed 12 days after the last immunization, and spleens were removed. The freshly-isolated splenocytes were subjected to the ELISPOT assay for IFN-γ-producing cells in response to ANYNFTLV peptide-pulsed EL-4 cells. The number of IFN-γ-secreting cells × 106 was counted 22 h later. The number of IFN-γ-secreting cells that appeared against peptide-unpulsed EL-4 was subtracted from the number of IFN-γ-secreting cells that appeared against peptide-pulsed EL-4. Data represent the mean ± S.D. of three to nine mice in each group. *, P < 0.05 and **, P < 0.01 determined by the Student's t-test. “NS” means “not statistically significant”. (B) The same immune splenocytes in (A) were subjected to the flow cytometory (FCM) analyses for detection of Kb/ANYNFTLV pentamer-reactive CD8+ T cells. The T-cell receptor (TCR) was stained by the phycoerythrin (PE)-conjugated Kb/ANYNFTLV pentamer, and the CD8 was stained by the PerCP-Cy5.5-conjugated anti-CD8 monoclonal antibody (mAb). The number of ANYNFTLV+, CD8+ T cells per 106 splenic lymphocytes was calculated. Data represent the mean ± S.D. of three to nine mice in each group. *, P < 0.05 and **, P < 0.01 determined by the Student's t-test. (C) The representative data of the FACS analyses for detection of Kb/ANYNFTLV pentamer-reactive or non-reactive CD8+ T cells. The ANYNFTLV+, CD8+ T cells or ANYNFTLV-, CD8+ T cells were stained with the APC-conjugated anti-CD44 mAb and the FITC-conjugated anti-CD62L mAb. TCM: T cells of central memory phenotype, TEM: T cells of effector memory phenotype. (D) The mice were sacrificed 14 days after the last immunization, and spleens were removed. The representative data of the FACS analyses showed the detection of Kb/ANYNFTLV pentamer-reactive, CD122dull cell population and the IFN-γ+, CD8+ T cell population. The TCRs were stained with the PE-conjugated Kb/ANYNFTLV pentamer, the PerCP-Cy5.5-conjugated anti-CD8 mAb, the APC-conjugated anti-CD122 mAb and the FITC-conjugated anti-CD62L mAb. The MFI of CD122 staining was calculated by gating the ANYNFTLV+, CD8+ cells. The same immune splenocytes were also subjected to the FACS analyses for the detection of intracellular IFN-γ+ cells in response to the ANYNFTLV-pulsed EL-4 cells. The cell culture condition for the assays was described in the Materials and methods. The number of IFN-γ+ cells that appeared against peptide-unpulsed EL-4 was subtracted from the number of IFN-γ+ cells that appeared against peptide-pulsed EL-4. The mean fluorescence intensity (MFI) of ANYNFTLV-responding IFN-γ+ cells was calibrated by gating cells which were both IFN-γ-positive and CD8-positive. The use of control Ad, control MVA and control FLU which do not express the epitope did not induce the ANYNFTLV-specific immune responses (data not shown). All the experiments were repeated at least twice for the confirmation of reproducibility.
3.5. Increased immunization doses enhanced the induction of ANYNFTLV-specific CD8+ T cell quantity without changing their memory phenotype
By the 10-fold-increase of the immunizing doses of re-Ad and re-MVA, we found that the induction of ANYNFTLV-specific CD8+ T cells was significantly enhanced as evidenced by both the ELISPOT assay (Fig. 5A) and the pentamer technology (Fig. 5B). This quantitative enhancement of CD8+ T cell immune responses, however, did not coincide with the TEM phenotypic change of the ANYNFTLV-specific CD8+ T cells (90.3% versus 95.9%) (Fig. 5C), implying that the antigenic dose might not influence the qualitative features of induced CD8+ T cells.
Fig. 5.
The impact of the increase in the number of immunizing doses of recombinant adenovirus (re-Ad) and recombinant vaccinia virus (re-MVA) on the induction of ANYNFTLV, a peptide derived from Trypanosoma cruzi surface antigen-specific CD8+ T cells, both the quantity and their memory phenotype. B6 mice were first primed with 1 × 104 plaque forming units (PFU) of either recombinant influenza (re-FLU) or the control FLU, and then were given a booster immunization 14 days later with either 5 × 107 PFU of re-Ad (shown as re-Ad*) or 5 × 108 PFU of re-Ad. The mice were further immunized 14 days later with either 5 × 107 PFU of re-MVA (shown as re-MVA*) or 5 × 108 PFU of re-MVA. The mice were sacrificed 12 days after the last immunization and spleens were removed. (A) The freshly-isolated splenocytes were subjected to the ELISPOT assay for IFN-γ-producing cells in response to ANYNFTLV peptide-pulsed EL-4 cells. The number of IFN-γ-secreting cells × 106 was counted 22 h later. The number of IFN-γ-secreting cells that appeared against peptide-unpulsed EL-4 was subtracted from the number of IFN-γ-secreting cells that appeared against peptide-pulsed EL-4. Data represent the mean ± S.D. of three to nine mice in each group. **, P < 0.01 determined by the Student's t-test. (B) The splenocytes were also subjected to the flow cytometory (FCM) analyses for detection of Kb/ANYNFTLV pentamer-reactive CD8+ T cells. The T-cell receptor (TCR) was stained by the phycoerythrin (PE)-conjugated Kb/ANYNFTLV pentamer, and the CD8 was stained by the PerCP-Cy5.5-conjugated anti-CD8 monoclonal antibody (mAb). The number of ANYNFTLV+, CD8+ T cells per 106 splenic lymphocytes was calculated. Data represent the mean ± S.D. of three to nine mice in each group. **, P < 0.01 determined by the Student's t-test. (C) The representative data of the FACS analyses for detectionn of Kb/ANYNFTLV pentamer-reactive or non-reactive CD8+ T cells. The ANYNFTLV+, CD8+ T cells or ANYNFTLV-, CD8+ T cells were stained with the APC-conjugated anti-CD44 mAb and the FITC-conjugated anti-CD62L mAb. TCM: T cells of central memory phenotype, TEM: T cells of effector memory phenotype. All the experiments were repeated at least twice for the confirmation of reproducibility.
3.6. The heterologous triple virus vector vaccination regimen using three different re-viruses could induce CD8+ T cell-mediated protective immunity
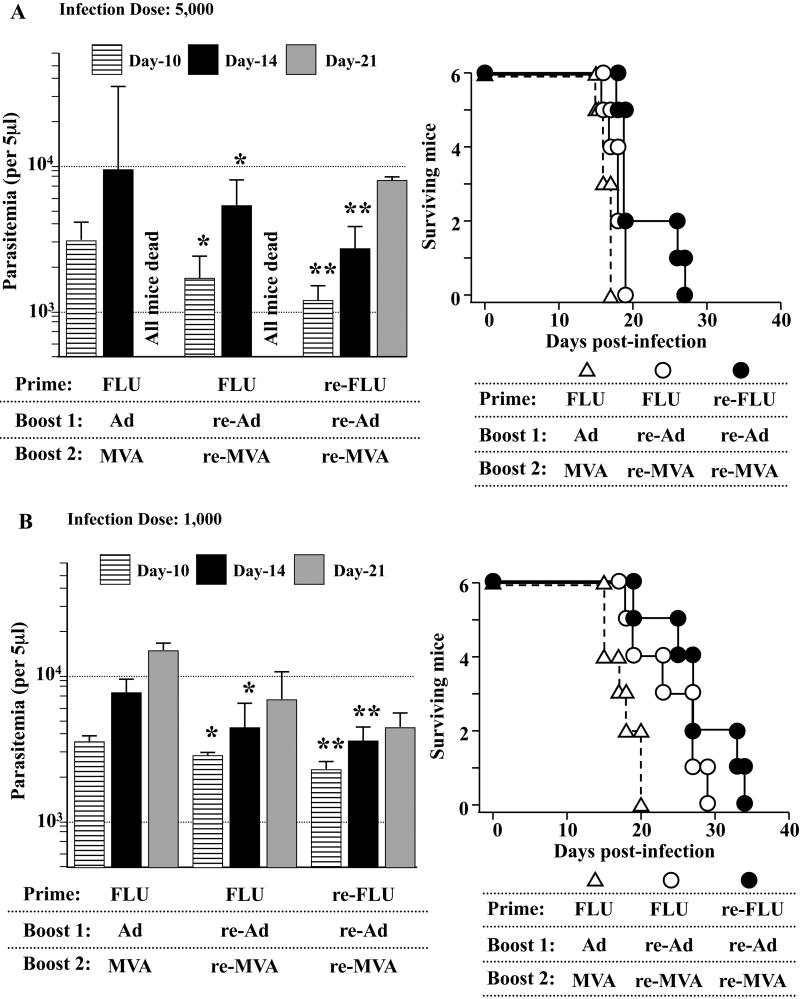
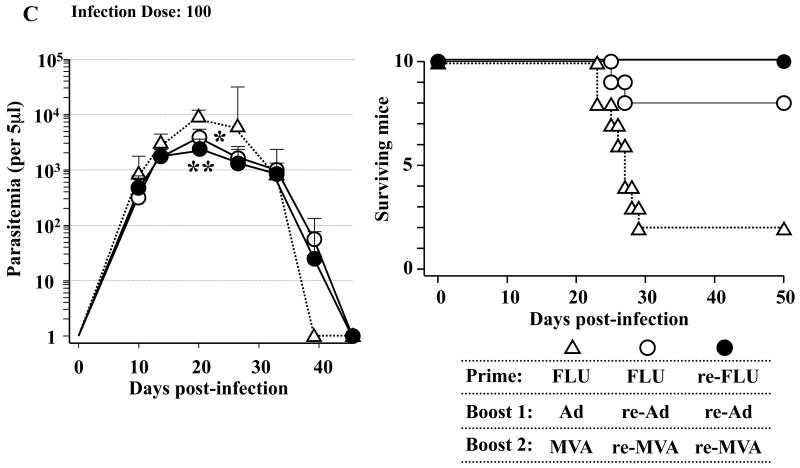
Since the clinical course of T. cruzi infection differs depending on the infection doses, we lastly chose three different infection dose; i.e., 5,000 per mouse (Fig. 6A), 1,000 per mouse (Fig. 6B) and 100 per mouse (Fig. 6C), to assess the immunological efficacy of heterologous triple virus vector vaccination against lethal T. cruzi infection. We found that the protective efficacy of the triple re-FLU/re-Ad/re-MVA vaccination (●) was better, although slightly, than that of the double re-Ad/re-MVA (○) as assessed by both the parasitemia and the survival of infected mice. These protection assays strongly suggest that the heterologous triple virus vector vaccination regimen using three different re-viruses could hold immunological advantages for improving the outcome of lethal T. cruzi infection (Fig. 6).
Fig. 6.
The immunological efficacy of a triple virus vector vaccination regimen using three different recombinant viruses. B6 mice were primed with 4 × 104 plaque forming units (PFU) of either recombinant influenza (re-FLU) or control FLU. Fourteen days later, the mice were immunized with 5 × 108 PFU of either recombinant adenovirus (re-Ad) or control Ad. Then, 14 days after the first booster immunization, mice were given a second booster immunzation with either 5 × 108 PFU of recombinant vaccinia virus (re-MVA) or 5 × 108 PFU of control MVA. Each experimental group includes six to 10 mice. They were infected with 5,000 (A), 1,000 (B) or 100 (C) of Tulahuen strain of Trypanosoma cruzi blood-form trypomastigotes 14 days (A) or 21 days (B, C) after the last immunization. The number of parasites in 5 μl of peripheral blood (parasitemia) was counted at 10, 14, 21, 28, 35, 42 and 49 days p.i. *, P < 0.05 and **, P < 0.01 determined by the Student's t-test compared with the parasitemia of FLU/Ad/MVA-immunized mice. Survival was monitored daily. (A) The survival of re-FLU/re-Ad/re-MVA-immunized mice (●) was significantly different compared with either the survival of FLU/re-Ad/re-MVA-immunized mice (○) (P < 0.05) or the survival of FLU/Ad/MVA-immunized mice (△) (P < 0.01). The survival of FLU/re-Ad/re-MVA-immunized mice (○) was significantly different compared with the survival of FLU/Ad/MVA-immunized mice (△) (P < 0.05). (B) The survival of re-FLU/re-Ad/re-MVA-immunized mice (●) was significantly different compared with the survival of FLU/Ad/MVA-immunized mice (△) (P < 0.01). The survival of FLU/re-Ad/re-MVA-immunized mice (○) was significantly different compared with the survival of FLU/Ad/MVA-immunized mice (△) (P < 0.05). (C) All of the re-FLU/re-Ad/re-MVA-immunized mice (●) survived the T. cruzi infection. The survival of FLU/re-Ad/re-MVA-immunized mice (○) was significantly different compared with the survival of FLU/Ad/MVA-immunized mice (△) (P < 0.01).
4. Discussion
Development of an effective CD8+ T cell vaccine has not yet been clinically achieved despite the epoch-making discovery of a primary/booster vaccination strategy (Vuola et al., 2005; Reyes-Sandoval et al., 2007; Radosević et al., 2009). One of explainations for this difficulty is that the quantitative enhancement of CD8+ T cell immunity might not be sufficient to achieve CD8+ T cell-mediated immunological protection against infectious pathogens. CD8+ T cells consist of an enormously heterogeneous population including cells which might not be capable of controlling infectious diseases (Harari et al., 2006; Kaech and Wherry, 2007; Kedzierska et al., 2008; Hansen et al., 2009). Although hosts' immune cells must countermeasure against, literally, limitless numbers of infectious pathogens in their environment, the capacity for immunity is, on the contrary, not limitless. In this regard, the qualitative considerations for immunity are necessary under the condition that the quantitative enhancement for CD8+ T cells is not achievable in an unrestricted manner due to the physiological and spatial restrictions (Vezys et al., 2009). In the present study, by taking advantage of the T. cruzi infection model, we have aimed to refine a new CD8+ T cell vaccine protocol which should be optimized both in a quantitative and in a qualitative manner for controlling infectious diseases. Although it is worth elucidating the homologous re-virus vector vaccination-induced immune responses, in the current study we have focused on the analyses of heterologous re-virus vector vaccination-induced responses.
The assays employed to assess the CD8+ T cell immune responses in the current study were the ELISPOT assay and pentamer technology. The two assays have fundamental differences which require careful interpretations of the obtained data. The ELISPOT assay detects the functional aspect of activated CD8+ T cells, which is the secretion of IFN-γ. However on the contrary, the pentamer technology detects the CD8+ T cells which could physically bind to the pentamer via TCR. The capacity for physical binding to the TCR does not necessarily mean that the peptide could trigger the activation of CD8+ T cells. It is therefore possible that some of the CD8+ T cell epitopes could physically bind to the TCR on the CD8+ T cells, but could not properly activate those, resulting in the absence of fully-functional, IFN-γ-secreting CD8+ T cells. Although we have no concrete data to explain why there is a discrepancy in the number of cells induced by either the re-Ad/re-Ad homologous vaccination or the single re-Ad one depending on the assays employed for the detection of ANYNFTLV-specific CD8+ T cells (Fig. 1A, B), we speculate that some vaccination regimens tend to induce more antigen-specific CD8+ T cells which exhibit poorer immunological function and secrete lower amounts of IFN-γ.
When analyzing the phenotypic features of CD8+ T cells induced by the re-virus vaccination regimens, we found a strong association between the numerical enhancement of ANYNFTLV-specific CD8+ T cell immunity and the CD8+ T cells of TEM phenotype (Gattinoni et al., 2009; Vezys et al., 2009) upon the heterologous re-Ad/re-MVA sequential immunization (Fig. 1C), re-FLU-combined double vaccinations (Fig. 3C, D) and the heterologous triple virus vector immunizations (Fig. 4C). While examining additional phenotypic markers on cells, we found that the induction of ANYNFTLV-specific CD8+ T cells was associated with the emergence of a CD8+CD122dull T cell population (Fig. 1D). Since the CD122 is an IL-2/IL-15 receptor β-subunit (Létourneau et al., 2009), its critical role for the induction of immunologically functional CD8+ T cell immunity is conceivable. The CD8+ T cells induced by the heterologous re-Ad/re-MVA/re-FLU vaccination unexpectedly exhibited lower MFI of CD122 than those induced by two other triple vaccinations (Fig. 4D), indicating that the CD122 expression would be one of the phenotypic markers of qualitative features of induced CD8+ T cells. CD122 is thought to be correlated with the maturation of CD8+ T cells (Khanolkar et al., 2004; Mbitikon-Kobo et al., 2009), suggesting that the phenotypic changes might possibly result from interference with the maturation of CD8+ T cells. However, since we did not find any differences in CD122 expression between the heterologous re-Ad/re-MVA-induced and the homologous re-Ad/re-Ad-induced CD8+ T cells (Fig. 1E) despite the numerical expansion of CD8+ T cells of both being significantly different (Fig. 1A, B), the expression of CD122 on CD8+ T cells might not be directly linked to their capability for cell proliferation.
Interestingly, the capacity for IFN-γ secretion, which is obviously crucial to combat infectious pathogens (Tsuji et al., 1995), per induced ANYNFTLV-specific CD8+ T cell as predicted by the MFI of IFN-γ staining was higher when the re-MVA was used last in the sequential order both in the double (Fig. 2) and in the triple virus vector vaccination (Fig. 4D). In contrast, the CD8+ T cells induced by the heterologous re-Ad/re-MVA/re-FLU vaccination, again unexpectedly, exhibited lower MFI of IFN-γ staining than those induced by two other triple vaccinations (Fig. 4D). These results indicate that the order of virus vector vaccination affects not only the number of induced antigen-specific CD8+ T cells but their qualitative features. The down-regulation of induced numbers of ANYNFTLV-specific CD8+ T cells, the decreased percentages of TEM phenotype, the down-regulation of CD122 expression and the decreased IFN-γ content in CD8+ T cells induced by the heterologous re-Ad/re-MVA/re-FLU triple vaccination (Fig. 4D) were surprising and unexpected. The precise mechanisms for the induction of these phenotypic changes are not clear. One possible explanation for this qualitative change might be that the re-FLU immunization, which was placed last in the sequence, could induce more CD8+ T cells of TEM phenotype which could accumulate in tissues other than spleen, such as lung and liver (Wherry et al., 2003). The qualitative features of antigen-specific CD8+ T cells in several different tissues must be characterized to clarify this question. Another possible explanation for this is “immunodominance”, an immunological phenomenon by which all of the CD8+ T cell-inducing epitopes compete with one another, leading to the selection of one or a few that actually could induce CD8+ T cell immune responses (Yewdell, 2006). If the re-FLU contains intrinsic antigens which encode stronger CD8+ T cell-inducing epitope(s), it could dominate the CD8+ T cell immunity which would outnumber the induction of ANYNFTLV-specific CD8+ T cells and could suppress the maturation of primed but subdominant CD8+ T cells. In contrast, MVA has been well known both for its outstanding boosting capacity for CD8+ T cell immunity and its safety for use in vaccination (Sutter and Moss, 1992; Hanke et al., 2007), although there has been no concrete evidence to explain its uniqueness in CD8+ T cell immunity (Zavala et al., 2001; Miyahira et al., 2005). The hosts' immune responses against vaccinia virus were recently analyzed in detail (Laouar et al., 2008; Zhao et al., 2009), particularly elucidating phenotypic features of vaccinia virus-specific CD8+ T cells. The coincidence of the enhanced induction of ANYNFTLV-specific CD8+ T cell numbers and their phenotypic changes indicated that the MVA might have an immunological capability which could accelerate the differentiation and maturation of “primed” antigen-specific CD8+ T cells, resulting in the improved protective immunity.
Our results contradicted a previous report (Vuola et al., 2005) which demonstrated that immunization with three vectors showed no improvement over an optimal two vector regimen. We assume that the immunogenicity and immunological efficacy could be different depending on vectors used for sequential heterologous vaccinations. Besides this contradiction, even if the immunological protection against T. cruzi infection induced by the heterologous triple virus vector vaccination was significantly improved, the parasitemia and the survival of these mice exhibited only minimal differences compared with the clinical T. cruzi infection course of double virus vector-vaccinated ones (Fig. 6). This observation might not be surprising, since the infection with T. cruzi itself, which expresses the ANYNFTLV epitope as an intrinsic antigen, could rigorously boost the ANYNFTLV-specific CD8+ T cell immune responses. If the double virus vector vaccination already induced high enough numbers of antigen-specific CD8+ T cells, the T. cruzi infection itself could be sufficient to enhance the antigen-specific CD8+ T cells, enabling the improvement of pathogenic outcome.
Our current study has focused on the elucidation of only some aspects of the quantitative and qualitative features of heterologous re-virus-vector-induced, antigen-specific CD8+ T cells against T. cruzi. There are several other qualitative features such as the expression of multiple cytokines, perforin, granzymes and other T cell phenotypic surface markers (Muller et al., 2003; Bengsch et al., 2007; Precopio et al., 2007). There is an emerging concept that vaccinia virus could induce “polyfunctional” CD8+ T cells (Precopio et al., 2007; Reyes-Sandoval et al., 2010). The addition of a CD4+ T cell epitope for immunizations might change the qualitative features of CD8+ T cells and might improve vaccine efficacy (Machado et al., 2006; Duan et al., 2009; Haolla et al., 2009). In addition, the change of vaccination protocol such as the extension of 2-week vaccination intervals to 8-weeks might also affect both the quantitative and qualitative features of CD8+ T cells (Bruna-Romero et al., 2001). Despite there being many other possible factors which could affect the outcome of heterologous re-virus vaccination, however, our study clearly suggests that the order of multiple virus vector vaccination must be carefully defined to maximize the vaccine efficacy and to avoid an unexpected detrimental outcome.
We are also fully aware that our experimental approach has limitations in achieving the final goal, which is the development of a new vaccine strategy against infectious pathogens. The immune system consists of a variety of immune effector mechanisms. They are closely linked to one another and act as an organized entity to combat invading pathogens. In this regard, ideally vaccines should induce all of the immune effector mechanisms as a whole. We should not lose sight of this, however we believe it is necessary to perform full and thorough analyses on each of the immune effector mechanisms one by one to accumulate incremental knowledge. We believe that this approach will also contribute to the development of innovative future vaccine strategies. Again, we do not consider it effective to use a single peptide for the practical application of virus vector vaccines. To overcome the major histocompatibility complex (MHC) restriction, the inclusion of multiple CD8+ T cell peptides and their combination with CD4+ T cell and B cell peptides will be indispensable to induce maximal and optimal vaccine-induced immune responses. The refinement of optimal induction protocols of each cell; i.e., CD8+ T cells, CD4+ T cells and B cells, will need to be appropriately combined in the future to achieve the development of a new vaccine strategy.
When taking advantage of virus vector vaccination, pre-existing immunity, particularly neutralizing Ab, is known to suppress the induction of antigen-specific CD8+ T cells. Although the use of re-viruses as vaccine vectors is potentially a powerful tool to combat infectious pathogens, its use will require significant precautions to avoid unexpected suppression of immune responses. Our current study suggests that the use of multiple virus vectors for the induction of antigen-specific CD8+ T cells is feasible and that the pre-existing immunity against a specific virus could be overcome by the use of alternative virus vectors. In addition, since the CD8+ T cell immune responses wane over time, the additional booster vaccination will be required by the use of alternative vectors to avoid the pre-existing immunity and to maintain the effective protective immunity for a longer period of time. In these cases, our study will be helpful to consider which vectors must be used when and in which order. Our study could also be beneficial for people who are not able to pay for multiple virus vector immunizations, since they could at least choose which virus vectors should be considered first for the priming vaccination. The selection of priming vectors in critical to avoid possible immune suppression by inappropriate combinations and inappropriate immunization orders of virus vectors.
Overall, the elucidation of immunological mechanisms to induce qualitative differences of CD8+ T cells would be indispensable, since its clarification will complement the physiological and spatial restrictions, which make quantitative enhancement of CD8+ T cell immunity in an unlimited manner impossible, and will lead to the future development of a clinically effective CD8+ T cell vaccine strategy.
Acknowledgments
This study was supported by the Grants-in-Aid for Scientific Research both from the Ministry of Defense and the Ministry of Health, Labor and Welfare of Japan to Y.M. and by the National Institute of Allergy and Infectious Diseases (NIAID), USA grants U01AI70469 to A.G-S. The authors thank Dr. Masaki Sato, Ms. Fumiko Suhara and Dr. Haruko Ota for their help to complete this project. We also thank Richard Cadagan for his excellent technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14:623–628. doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejon P, Ogada E, Mwangi T, Milligan P, Lang T, Fegan G, Gilbert SC, Peshu N, Marsh K, Hill AV. Extended follow-up following a phase 2b randomized trial of the candidate malaria vaccines FP9 ME-TRAP and MVA ME-TRAP among children in Kenya. PLoS ONE. 2007;2:e707. doi: 10.1371/journal.pone.0000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengsch B, Spangenberg HC, Kersting N, Neumann-Haefelin C, Panther E, von Weizsacker F, Blum HE, Pircher H, Thimme R. Analysis of CD127 and KLRG1 expression on hepatitis C virus-specific CD8+ T cells reveals the existence of different memory T-cell subsets in the peripheral blood and liver. J Virol. 2007;81:945–953. doi: 10.1128/JVI.01354-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruna-Romero O, Gonzalez-Aseguinolaza G, Hafalla JC, Tsuji M, Nussenzweig RS. Complete, long-lasting protection against malaria of mice primed and boosted with two distinct viral vectors expressing the same plasmodial antigen. Proc Natl Acad Sci U S A. 2001;98:11491–11496. doi: 10.1073/pnas.191380898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagas C. Nova tripanozomiaze humana: estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., agente etiolojico de nova entidade morbida do homem. Mem Inst Oswaldo Cruz. 1909;1:159–218. [Google Scholar]
- Doherty PC, Kelso A. Toward a broadly protective influenza vaccine. J Clin Invest. 2008;118:3273–3275. doi: 10.1172/JCI37232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Yonemitsu Y, Chou B, Yoshida K, Tanaka S, Hasegawa M, Tetsutani K, Ishida H, Himeno K, Hisaeda H. Efficient protective immunity against Trypanosoma cruzi infection after nasal vaccination with recombinant Sendai virus vector expressing amastigote surface protein-2. Vaccine. 2009;27:6154–6159. doi: 10.1016/j.vaccine.2009.08.026. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, Paulos CM, Muranski P, Restifo NP. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulder PJ, Watkins DI. HIV and SIV CTL escape: implications for vaccine design. Nat Rev Immunol. 2004;4:630–640. doi: 10.1038/nri1417. [DOI] [PubMed] [Google Scholar]
- Hanke T, Goonetilleke N, McMichael AJ, Dorrell L. Clinical experience with plasmid DNA- and modified vaccinia virus Ankara-vectored human immunodeficiency virus type 1 clade A vaccine focusing on T-cell induction. J Gen Virol. 2007;88:1–12. doi: 10.1099/vir.0.82493-0. [DOI] [PubMed] [Google Scholar]
- Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Lifson JD, Nelson JA, Jarvis MA, Picker LJ. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haolla FA, Claser C, de Alencar BC, Tzelepis F, de Vasconcelos JR, de Oliveira G, Silverio JC, Machado AV, Lannes-Vieira J, Bruna-Romero O, Gazzinelli RT, dos Santos RR, Soares MB, Rodrigues MM. Strain-specific protective immunity following vaccination against experimental Trypanosoma cruzi infection. Vaccine. 2009;27:5644–5653. doi: 10.1016/j.vaccine.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Harari A, Dutoit V, Cellerai C, Bart PA, Du Pasquier RA, Pantaleo G. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol Rev. 2006;211:236–254. doi: 10.1111/j.0105-2896.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci U S A. 2000;97:6108–6113. doi: 10.1073/pnas.100133697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katae M, Miyahira Y, Takeda K, Matsuda H, Yagita H, Okumura K, Takeuchi T, Kamiyama T, Ohwada A, Fukuchi Y, Aoki T. Coadministration of an interleukin-12 gene and a Trypanosoma cruzi gene improves vaccine efficacy. Infect Immun. 2002;70:4833–4840. doi: 10.1128/IAI.70.9.4833-4840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierska K, La Gruta NL, Stambas J, Turner SJ, Doherty PC. Tracking phenotypically and functionally distinct T cell subsets via T cell repertoire diversity. Mol Immunol. 2008;45:607–618. doi: 10.1016/j.molimm.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanolkar A, Fuller MJ, Zajac AJ. CD4 T cell-dependent CD8 T cell maturation. J Immunol. 2004;172:2834–2844. doi: 10.4049/jimmunol.172.5.2834. [DOI] [PubMed] [Google Scholar]
- Laouar A, Manocha M, Haridas V, Manjunath N. Concurrent generation of effector and central memory CD8 T cells during vaccinia virus infection. PLoS ONE. 2008;3:e4089. doi: 10.1371/journal.pone.0004089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Létourneau S, Krieg C, Pantaleo G, Boyman O. IL-2- and CD25-dependent immunoregulatory mechanisms in the homeostasis of T-cell subsets. J Allergy Clin Immunol. 2009;123:758–762. doi: 10.1016/j.jaci.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Machado AV, Cardoso JE, Claser C, Rodrigues MM, Gazzinelli RT, Bruna-Romero O. Long-term protective immunity induced against Trypanosoma cruzi infection after vaccination with recombinant adenoviruses encoding amastigote surface protein-2 and trans-sialidase. Hum Gene Ther. 2006;17:898–908. doi: 10.1089/hum.2006.17.898. [DOI] [PubMed] [Google Scholar]
- Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Wherry EJ, Ahmed R. A brief history of CD8 T cells. Eur J Immunol. 2007;37 1:S103–110. doi: 10.1002/eji.200737584. [DOI] [PubMed] [Google Scholar]
- Masopust D. Developing an HIV cytotoxic T-lymphocyte vaccine: issues of CD8 T-cell quantity, quality and location. J Intern Med. 2009;265:125–137. doi: 10.1111/j.1365-2796.2008.02054.x. [DOI] [PubMed] [Google Scholar]
- Mbitikon-Kobo FM, Vocanson M, Michallet MC, Tomkowiak M, Cottalorda A, Angelov GS, Coupet CA, Djebali S, Marçais A, Dubois B, Bonnefoy-Bérard N, Nicolas JF, Arpin C, Marvel J. Characterization of a CD44/CD122int memory CD8 T cell subset generated under sterile inflammatory conditions. J Immunol. 2009;182:3846–3854. doi: 10.4049/jimmunol.0802438. [DOI] [PubMed] [Google Scholar]
- McMurry JA, Moise L, Gregory SH, De Groot AS. Tularemia vaccines - an overview. Medicine and health, Rhode Island. 2007;90:311–314. [PubMed] [Google Scholar]
- Miyahira Y, Dvorak JA. Kinetoplastidae display naturally occurring ancillary DNA-containing structures. Mol Biochem Parasitol. 1994;65:339–349. doi: 10.1016/0166-6851(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Miyahira Y, Murata K, Rodriguez D, Rodriguez JR, Esteban M, Rodrigues MM, Zavala F. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J Immunol Methods. 1995;181:45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- Miyahira Y, García-Sastre A, Rodriguez D, Rodriguez JR, Murata K, Tsuji M, Palese P, Esteban M, Zavala F, Nussenzweig RS. Recombinant viruses expressing a human malaria antigen can elicit potentially protective immune CD8+ responses in mice. Proc Natl Acad Sci U S A. 1998;95:3954–3959. doi: 10.1073/pnas.95.7.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahira Y, Kobayashi S, Takeuchi T, Kamiyama T, Nara T, Nakajima-Shimada J, Aoki T. Induction of CD8+ T cell-mediated protective immunity against Trypanosoma cruzi. Int Immunol. 1999;11:133–141. doi: 10.1093/intimm/11.2.133. [DOI] [PubMed] [Google Scholar]
- Miyahira Y, Akiba H, Katae M, Kubota K, Kobayashi S, Takeuchi T, García-Sastre A, Fukuchi Y, Okumura K, Yagita H, Aoki T. Cutting edge: a potent adjuvant effect of ligand to receptor activator of NF-κ B gene for inducing antigen-specific CD8+ T cell response by DNA and viral vector vaccination. J Immunol. 2003a;171:6344–6348. doi: 10.4049/jimmunol.171.12.6344. [DOI] [PubMed] [Google Scholar]
- Miyahira Y, Katae M, Kobayashi S, Takeuchi T, Fukuchi Y, Abe R, Okumura K, Yagita H, Aoki T. Critical contribution of CD28-CD80/CD86 costimulatory pathway to protection from Trypanosoma cruzi infection. Infect Immun. 2003b;71:3131–3137. doi: 10.1128/IAI.71.6.3131-3137.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahira Y, Katae M, Takeda K, Yagita H, Okumura K, Kobayashi S, Takeuchi T, Kamiyama T, Fukuchi Y, Aoki T. Activation of natural killer T cells by α-galactosylceramide impairs DNA vaccine-induced protective immunity against Trypanosoma cruzi. Infect Immun. 2003c;71:1234–1241. doi: 10.1128/IAI.71.3.1234-1241.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahira Y, Takashima Y, Kobayashi S, Matsumoto Y, Takeuchi T, Ohyanagi-Hara M, Yoshida A, Ohwada A, Akiba H, Yagita H, Okumura K, Ogawa H. Immune responses against a single CD8+-T-cell epitope induced by virus vector vaccination can successfully control Trypanosoma cruzi infection. Infect Immun. 2005;73:7356–7365. doi: 10.1128/IAI.73.11.7356-7365.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahira Y. Trypanosoma cruzi infection from the view of CD8+ T cell immunity - An infection model for developing T cell vaccine. Parasitol Int. 2008;57:38–48. doi: 10.1016/j.parint.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Muller U, Sobek V, Balkow S, Holscher C, Mullbacher A, Museteanu C, Mossmann H, Simon MM. Concerted action of perforin and granzymes is critical for the elimination of Trypanosoma cruzi from mouse tissues, but prevention of early host death is in addition dependent on the FasL/Fas pathway. Eur J Immunol. 2003;33:70–78. doi: 10.1002/immu.200390009. [DOI] [PubMed] [Google Scholar]
- Oliveira-Ferreira J, Miyahira Y, Layton GT, Savage N, Esteban M, Rodriguez D, Rodriguez JR, Nussenzweig RS, Zavala F, Myahira Y. Immunogenicity of Ty-VLP bearing a CD8+ T cell epitope of the CS protein of P. yoelii: enhanced memory response by boosting with recombinant vaccinia virus. Vaccine. 2000;18:1863–1869. doi: 10.1016/s0264-410x(99)00344-8. [DOI] [PubMed] [Google Scholar]
- Overstreet MG, Cockburn IA, Chen YC, Zavala F. Protective CD8+ T cells against Plasmodium liver stages: immunobiology of an ‘unnatural’ immune response. Immunol Rev. 2008;225:272–283. doi: 10.1111/j.1600-065X.2008.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, Asher TE, Douek DC, Harari A, Pantaleo G, Bailer R, Graham BS, Roederer M, Koup RA. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8+ T cell responses. J Exp Med. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlivan M, Zamarin D, Garcia-Sastre A, Cullinane A, Chambers T, Palese P. Attenuation of equine influenza viruses through truncations of the NS1 protein. J Virol. 2005;79:8431–8439. doi: 10.1128/JVI.79.13.8431-8439.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radosević K, Rodriguez A, Lemckert A, Goudsmit J. Heterologous prime-boost vaccinations for poverty-related diseases: advantages and future prospects. Expert Rev Vacc. 2009;8:577–592. doi: 10.1586/erv.09.14. [DOI] [PubMed] [Google Scholar]
- Reyes-Sandoval A, Harty JT, Todryk SM. Viral vector vaccines make memory T cells against malaria. Immunology. 2007;121:158–165. doi: 10.1111/j.1365-2567.2006.02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Sandoval A, Berthoud T, Alder N, Siani L, Gilbert SC, Nicosia A, Colloca S, Cortese R, Hill AV. Prime-boost immunization with adenoviral and modified vaccinia virus Ankara vectors enhances the durability and polyfunctionality of protective malaria CD8+ T-cell responses. Infect Immun. 2010;78:145–153. doi: 10.1128/IAI.00740-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekaly RP. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J Exp Med. 2008;205:7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiver JW, Emini EA. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu Rev Med. 2004;55:355–372. doi: 10.1146/annurev.med.55.091902.104344. [DOI] [PubMed] [Google Scholar]
- Solorzano A, Webby RJ, Lager KM, Janke BH, Garcia-Sastre A, Richt JA. Mutations in the NS1 protein of swine influenza virus impair anti-interferon activity and confer attenuation in pigs. J Virol. 2005;79:7535–7543. doi: 10.1128/JVI.79.12.7535-7543.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambas J, Guillonneau C, Kedzierska K, Mintern JD, Doherty PC, La Gruta NL. Killer T cells in influenza. Pharmacol Ther. 2008;120:186–196. doi: 10.1016/j.pharmthera.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Stephenson JR. Genetically modified viruses: vaccines by design. Curr Pharm Biotechnol. 2001;2:47–76. doi: 10.2174/1389201013378815. [DOI] [PubMed] [Google Scholar]
- Sutter G, Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci U S A. 1992;89:10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarleton RL, Koller BH, Latour A, Postan M. Susceptibility of β 2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature. 1992;356:338–340. doi: 10.1038/356338a0. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Miyahira Y, Nussenzweig RS, Aguet M, Reichel M, Zavala F. Development of antimalaria immunity in mice lacking IFN- receptor. J Immunol. 1995;154:5338–5344. [PubMed] [Google Scholar]
- Vezys V, Yates A, Casey KA, Lanier G, Ahmed R, Antia R, Masopust D. Memory CD8 T-cell compartment grows in size with immunological experience. Nature. 2009;457:196–199. doi: 10.1038/nature07486. [DOI] [PubMed] [Google Scholar]
- Vuola JM, Keating S, Webster DP, Berthoud T, Dunachie S, Gilbert SC, Hill AV. Differential immunogenicity of various heterologous prime-boost vaccine regimens using DNA and viral vectors in healthy volunteers. J Immunol. 2005;174:449–455. doi: 10.4049/jimmunol.174.1.449. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- Yewdell JW. Confronting complexity: real-world immunodominance in antiviral CD8+ T cell responses. Immunity. 2006;25:533–543. doi: 10.1016/j.immuni.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Zavala F, Rodrigues M, Rodriguez D, Rodriguez JR, Nussenzweig RS, Esteban M. A striking property of recombinant poxviruses: efficient inducers of in vivo expansion of primed CD8+ T cells. Virology. 2001;280:155–159. doi: 10.1006/viro.2000.0792. [DOI] [PubMed] [Google Scholar]
- Zhao Y, De Trez C, Flynn R, Ware CF, Croft M, Salek-Ardakani S. The adaptor molecule MyD88 directly promotes CD8 T cell responses to vaccinia virus. J Immunol. 2009;182:6278–6286. doi: 10.4049/jimmunol.0803682. [DOI] [PMC free article] [PubMed] [Google Scholar]