Abstract
Myoblast fusion is a highly regulated process that is key for forming skeletal muscle during development and regeneration in mammals. Much remains to be understood about the molecular regulation of myoblast fusion. Some molecules that influence mammalian muscle fusion display specific cellular localization during myogenesis. Such molecules can be localized to the contact region between two fusing cells either in both cells or only in one of the cells. How distinct localization of molecules contributes to fusion is not clear. Further complexity exists as other molecules are functionally restricted to myoblasts at later stages of myogenesis to regulate their fusion with multinucleated myotubes. This review examines these three categories of molecules and discusses how spatial and functional restriction may contribute to the formation of a multinucleated cell. Understanding how and why molecules become restricted in location or function is likely to provide further insights into the mechanisms regulating mammalian muscle fusion.
Keywords: myoblast fusion, myogenesis, myotube, muscle regeneration, satellite cells, vertebrate
Myoblast fusion is important not only for skeletal muscle formation during development, but also for post-natal muscle growth and regeneration of skeletal muscle. Satellite cells, the myogenic stem cells responsible for post-natal muscle growth and repair, proliferate in the presence of growth factors and give rise to myoblasts. Myoblast fusion follows an ordered set of cellular events, including elongation, cell migration, recognition/adhesion, and membrane fusion [1]. Upon mitogen withdrawal, myoblasts in culture differentiate and they become elongated, spindle shaped cells that migrate towards other differentiated myoblasts to form groups of aligned cells (Figure 1A). Differentiated myoblasts fuse to form nascent myotubes and later, further fusion of differentiated myoblasts with nascent myotubes gives rise to mature myotubes with many nuclei. A variety of extracellular, cell surface and intracellular molecules act to finely coordinate the cellular and molecular events that influence the ability of mammalian myoblasts to fuse [2].
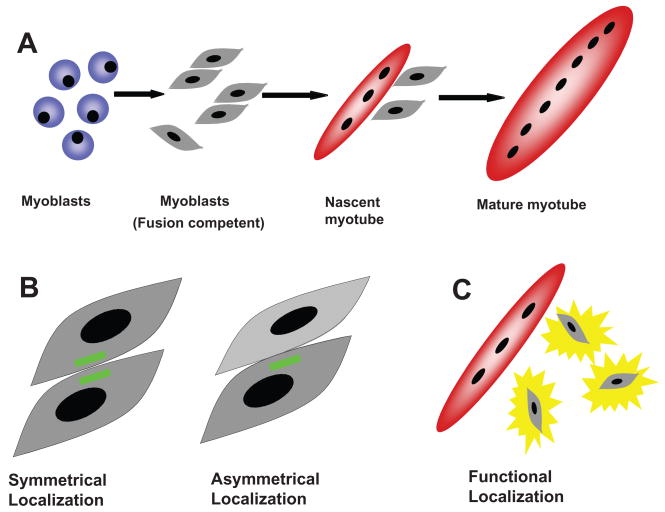
Figure 1. Myoblast fusion is associated with spatial and functional localization of molecules.
A) Myoblasts proliferate and then, in response to environmental cues, differentiate to become fusion-competent. Myoblast fusion occurs in two stages: in the first phase, a subset of differentiated myoblasts fuse together to form a nascent myotube with a limited number of nuclei. Subsequently, additional differentiated myoblasts fuse with a nascent myotube to generate a large, mature myotube with many nuclei. B) During fusion, myogenic cells are closely juxtaposed. Two differentiated myoblasts are shown here. Some molecules that influence fusion are localized to the contact region between the two cells either in both cells (symmetrical localization) or only in one of the cells (asymmetrical localization). C) Some molecules that influence fusion are only functionally required in differentiated myoblasts as they fuse with nascent myotubes. This is an example of functional localization.
Spatial or functional restriction of regulatory molecules during myoblast fusion
During Drosophila developmental myogenesis, small binucleate myotubes form by the fusion of two distinct types of myoblasts, founder myoblasts and fusion-competent myoblasts. In some cases, molecules critical for cell fusion are asymmetrically expressed in founder and fusion-competent myoblasts and are likely to subserve specific roles in each cell type for eventual membrane fusion [3]. No evidence exists for these specialized myoblast types in mammalian myofiber formation and regeneration. However, a number of molecules that influence mammalian myoblast fusion are localized to presumptive sites of fusion in both cells of a pair of fusing cells, whereas other such localized molecules are present only in one of the cells of the pair (Table 1). Furthermore, at later stages of myogenesis other molecules are only functionally required for fusion in differentiated myoblasts not in nascent myotubes (Table 2). In this review, these three categories of molecules are discussed in further detail.
Table 1.
Molecules that are localized to cell-cell contact regions during myoblast fusion
| Molecule | Location | Proposed Function/Activity | References | |
|---|---|---|---|---|
| Symmetrical Localization | Actin | Intracellular | Cytoskeleton, fusion pore | [21] |
| ADAM12 | Cell surface, Intracellular | Cell-cell adhesion | [6] | |
| Alpha3 integrin | Cell surface | Cell-cell adhesion | [4] | |
| Alpha9 integrin | Cell surface | Cell-cell adhesion | [6] | |
| β–catenin | Intracellular | Intracellular signaling | [8] | |
| β1 integrin | Cell surface | Recruitment of CD9 | [5] | |
| Cholesterol | Membrane | Membrane rigidity, accumulation of adhesion molecules | [28] | |
| EB3 | Intracellular | Microtubule regulation at cell cortex | [20] | |
| Kindlin-2 | Intracellular | Integrin-associated cytoplasmic adaptor | [9] | |
| M-cadherin | Cell surface | Cell-cell adhesion | [7] | |
| Myoferlin | Intracellular | Phospholipid binding; membrane repair | [25] | |
| Phosphatidylserine | Membrane | Cell recognition,signaling | [27] | |
| Asymmetrical Localization | Actin | Intracellular | Cytoskeleton, fusion pore | [22] |
| Creatine kinase B | Intracellular | ATP production | [31] | |
| Diacylglycerol kinase ζ | Intracellular | Diacylglycerol metabolism | [23] | |
| N-cadherin | Cell surface | Cell-cell adhesion | [21, 23] | |
| Non-muscle myosin 2A | Intracellular | Associates with actin | [22] | |
| Rac1 | Intracellular | GTPase: WAVE complex formation | [23] | |
| Syntrophin | Intracellular | Scaffold protein; binds diacylglycerol kinase ζ | [23] | |
ADAM 12, a Disintegrin and Metalloprotease 12
WAVE, WASP family Verprolin-homologous protein
Table 2.
Molecules functionally required in myoblasts during myoblast-myotube fusion
| Molecule | Location | Cell type expressed by | Proposed Function/Activity | References |
|---|---|---|---|---|
| Interleukin 4 | Secreted | Mt | Stimulates expression of mannose receptor; migration | [35][[39] |
| Mannose receptor | Cell surface | Mb, Mt | Directed migration; collagen clearance | [36] |
| Nephrin | Cell surface | n.d. | Cell-cell adhesion; signaling | [40] |
| NFATc2 | Intracellular | Mb, Mt1 | Regulates gene expression in nascent myotube | [37] |
Mb, myoblast
Mt, myotube
n.d., not determined
NFATc2, Nuclear Factor of Activated T cells, c2
Only becomes activated and undergoes nuclear translocation in nascent myotubes
Molecules concentrated at the border of contacting myogenic cells: symmetrical localization
Immunofluorescence assays of cultured myoblasts reveal that several types of molecules are localized to the contact region of closely apposed myogenic cells in both cells (Figure 1B, left; Table 1). Cell adhesion molecules as M-cadherin, integrins and a disintegrin and metalloprotease 12 (ADAM12) are commonly found localized in this manner [4–7]. M-cadherin function is required for myotube formation in vitro [8, 9] but not in vivo [10] suggesting other cadherins or cell adhesion molecules may functionally compensate for the lack of M-cadherin. Eliminating the function of β1 integrin, α3 integrin and α9 integrin through genetic ablation or function blocking antibodies decreases myoblast fusion in vitro [4–6] and in vivo [5]. ADAM12 is a transmembrane protein that includes an integrin-binding site and can bind α9β1 integrin in muscle cells [6]. Antisense oligonucleotides to ADAM12 preferentially inhibit myoblast fusion with myotubes [6]. Molecules that associate with the intracellular domains of specific cell adhesion molecules such as β-catenin [11] and kindlin-2 [12] are also localized at cell contact sites. Given the importance of integrin signaling for myogenesis [13], the necessity of kindlin-2, an integrin-associated cytoplasmic adaptor, for myoblast fusion is not unexpected [12]. Kindlin-2 is highly enriched along the plasma membrane between contacting myogenic cells and in the absence of kindlin-2, myoblasts fail to elongate and form myotubes.
Localized transmembrane molecules and their intracellular adaptors most likely activate signal transduction pathways in aligned myogenic cells that ultimately lead to membrane fusion [11, 14]. Extensive cytoskeletal reorganization occurs before and after fusion [15]. A number of studies indicate remodeling of both the actin [16] and microtubule [17] cytoskeletal network are critical for myoblast fusion. The structure of the actin cytoskeleton at the contact site of fusing myoblasts is highly regulated by a complex signal cascade initiated by cell adhesion. The actions of these signaling molecules and actin regulators lead to the recruitment of vinculin and cytoskeletal proteins to contact sites of fusing myoblasts [11], which is hypothesized to be essential to localize Golgi-derived prefusion vesicles [18] and/or fusion pore formation/expansion in Drosphila [19]. Both actin and EB3, a microtubule regulatory protein, are localized near the plasma membrane of both aligned myoblasts [20, 21]; in some studies, actin is localized asymmetrically in pairs of myogenic cells [22, 23] as discussed below although the reasons for this discrepancy are unknown.
Myoferlin, a member of the ferlin family that contain C2 domains with a role in calcium-mediated membrane fusion events, is an intracellular phospholipid binding protein that associates with the plasma membrane [24]. Myoferlin is concentrated at the sites of juxtaposed myoblast-myotube as well as myotube-myotube membranes in both cells [25]. Although myoferlin is expressed throughout myogenesis, myoferlin null myoblasts fuse normally to form nascent myotubes but further nuclear accretion in nascent myotubes is blocked. The muscles of myoferlin null mice are significantly smaller than wild type and fail to fully regenerate after injury [25]. More recently, myoferlin was found to interact with the endocytic recycling protein EHD2 and is hypothesized to help regulate membrane fusion at cell-cell contact sites [26].
The presence of localized molecules at cell-cell contacts is not just limited to proteins. Phosphatidylserine is transiently exposed at the sites of cell-cell contact [27] and is functionally required for myotube formation as inhibition of phosphatidylserine by annexin V inhibits fusion. Multiple roles for this transient exposure of phosphatidylserine are proposed including cell recognition and cell signaling. Additionally, lipid rafts containing cholesterol accumulate at cell contact sites and are required for the accumulation of adhesion molecules at these sites [28]. Soon after cell contact, dynamic redistribution of the lipid rafts occurs and membrane fusion ensues. Cholesterol is proposed to help maintain the proper rigidity of the lipid bilayers necessary for adhesion between the two myogenic cells.
Molecules concentrated at the border of contacting myogenic cells: asymmetrical localization
Molecules in this category localize to the contacting region between two opposing muscle cells, but strikingly in only one of the cells (Figure 1B, right; Table 1). The exact role these asymmetrically localized molecules play during the fusion process is unknown. A greater variety exists among the molecules that are asymmetrically localized than those that are symmetrically localized. In one in vitro study, electron microscopy revealed that actin forms a dense wall structure in one cell, paralleling the long axis of aligned myoblasts [22]. As fusion proceeds, gaps appear in the actin wall at sites of vesicle accumulation, vesicles pair in both cells along the membrane and fusion pores form. Non-muscle myosin 2A associates with the subplasmalemmal actin wall and is required for its formation as well as appearance of the vesicles at the membrane and myoblast fusion. This actin wall may provide membrane rigidity needed for cell fusion or serves as a barrier to temporally impede vesicle movement to the membrane. Although such vesicles are associated with sites of membrane fusion in myoblasts their role is unclear [18].
Time-lapse microscopy reveals that myoblast elongation during differentiation is followed by extension of pseudopodia and filopodia, dynamic cell extensions composed of actin filaments, which make contact with neighboring muscle cells [21, 28–30]. The role of these dynamic cell structures in the fusion process is unknown but they may receive and/or send intercellular signals. Localization of diverse molecules occurs at the distal tip of a pseudopodium in one cell when in close proximity to another myoblast [23]. These molecules include diacylglycerol kinase ζ, an enzyme that generates phosphatidic acid, syntrophin, a scaffold protein that binds diacylglycerol kinase ζ, and the GTPase Rac1. These three molecules may be involved in actin rearrangements required for pseudopodia and filopodia extension. Unknown is whether protein accumulation occurs before a filopodial connection is established or if contact-mediated signaling induces it. Curiously, the adhesion molecule N-cadherin is co-localized with diacylglycerol kinase ζ at nascent intercellular contacts but not at established cell contacts indicating temporal regulation also occurs in these localized molecular components [23].
As myogenic differentiation proceeds the enzyme creatine kinase B becomes prominently localized to the ends of nascent and mature myotubes [31]. This localization is most frequently observed in one myotube of a pair of neighboring myotubes. Cytosolic isoforms of creatine kinase such as creatine kinase B replenish local ATP levels at sites of high ATPase activity by catalyzing the transfer of phosphate from the high-energy intermediate phosphocreatine to ADP thereby preventing ATP depletion [32–34]. These data suggest that ATP-dependent reactions are highly localized at the ends of myotubes. Myonuclear addition to myotubes may be a higher energy process compared to myoblast-myoblast fusion due to the more organized cytoskeletal structure of myotubes. Alternatively, the ends of myotubes may consume ATP too rapidly for replenishment by diffusion due to the larger size and sarcomeric structures of myotubes and therefore, these regions of the myotube may need to rely on localized production of ATP.
Molecules with functional localization during myoblast fusion
Some molecules regulate fusion only at later stages of myogenesis where they are functionally required in differentiated myoblasts and regulate their fusion with nascent myotubes (Figure 1C, Table 2). However, the expression of these molecules is not restricted just to myoblasts. Some molecules are expressed by nascent myotubes only [35], whereas others are expressed by both types of cells [36, 37].
NFATc2 is a calcium-activated transcription factor that plays a key role in orchestrating myoblast-myotube fusion [37, 38]. Loss of NFATc2 results in normal formation of nascent myotubes both in vitro and in vivo but further myonuclear accretion is inhibited due to defects in the recruitment and/or fusion of myoblasts with nascent myotubes. Further studies demonstrated NFATc2 controls myoblast fusion by regulating the expression of IL4 by nascent myotubes [35]. IL4 promotes fusion in part by regulating expression of the mannose receptor (MR), a cell surface endocytic C-type lectin. MR null myoblasts display a general reduction in general motility as well as an impairment of directed migration to unidentified factors released by fusing muscle cells [36]. Collagen uptake is also decreased in MR null muscle cells supporting a role for this endocytic receptor in helping to clear extracellular matrix from the leading edge of migrating cells. Additionally, IL4 may have MR-independent effects on migration [39]. Mutations in either IL4 or mannose MR leads to similar defects in growth of nascent myotubes in vitro and in vivo as observed for NFATc2 mutants [35, 36].
Nephrin is a transmembrane protein of the Ig superfamily whose expression is induced in muscle cells both in vitro during myotube formation and in vivo during development or myofiber regeneration, at times associated with myoblast fusion [40]. In the absence of nephrin, nascent myotubes form normally but further myoblasts are unable to fuse with these myotubes. Nephrin function is required in differentiated myoblasts not in myotubes, although the relative expression pattern between these two cell types is unknown. Nephrin shares structural similarities with the Drosophila transmembrane protein, Sticks and Stones (Sns). During the recognition and adhesion of founder myoblasts and fusion-competent myoblasts, Sns, which is expressed only by fusion-competent myoblasts, interacts with the transmembrane protein kirre/duf on founder myoblasts to initiate intracellular signaling necessary for cell fusion [3]. The mechanism by which nephrin regulates fusion is unknown but it is likely to be part of a larger protein complex that initiates intracellular signaling in myoblasts because of its structural similarity to Sns.
The question arises of why the myoblast-myotube fusion step in myogenesis requires additional unique molecules compared to myoblast-myoblast fusion. Potentially special challenges may be encountered during cell fusion to a multinucleated cell containing sarcomeres. Additionally, these molecules could represent a fine-tuning mechanism for controlling the ultimate number of nuclei within a myotube/myofiber. Furthermore, these molecules may direct myoblast fusion to specific sites along the myotube or with specific myotubes. Finally, since muscle regeneration in vivo is asynchronous, these molecules could specifically control growth of regenerating myofibers rather than allowing new myofibers to form and thus be a means of controlling the number of regenerated myofibers after muscle injury.
Summary
In recent years the number of molecules that regulate mammalian myoblast fusion has greatly expanded and the molecular steps that govern this finely orchestrated process are being revealed in greater detail. An emphasis should be placed on determining where specific molecules are expressed, localized and function in terms of both myoblast-myoblast as well as myoblast-myotube fusion. Understanding how and why such molecules become restricted in location or function is likely to provide further insights into the mechanisms regulating mammalian muscle fusion.
Acknowledgments
I thank Dr. Luciano Apponi for critical reading of the manuscript and helpful suggestions. GKP is supported by grants AR047314, AR051372, AR052730 and NS069234 from the National Institutes of Health.
Abbreviations
- ADAM 12
a Disintegrin and Metalloprotease 12
- IL4
Interleukin 4
- IL13
Interleukin 13
- MR
mannose receptor
- Mb
myoblast
- Mt
myotube
- NFATc2
Nuclear Factor of Activated T cells c2
- Sns
Sticks and Stones
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Knudsen KA, Horwitz AF. Tandem events in myoblast fusion. Dev Biol. 1977;58:328–338. doi: 10.1016/0012-1606(77)90095-1. [DOI] [PubMed] [Google Scholar]
- 2.Jansen KM, Pavlath GK. Molecular control of mammalian myoblast fusion. Methods Mol Biol. 2008;475:115–133. doi: 10.1007/978-1-59745-250-2_7. [DOI] [PubMed] [Google Scholar]
- 3.Abmayr SM, Zhuang S, Geisbrecht ER. Myoblast fusion in Drosophila. Methods Mol Biol. 2008;475:75–97. doi: 10.1007/978-1-59745-250-2_5. [DOI] [PubMed] [Google Scholar]
- 4.Brzoska E, Bello V, Darribere T, Moraczewski J. Integrin alpha3 subunit participates in myoblast adhesion and fusion in vitro. Differentiation. 2006;74:105–118. doi: 10.1111/j.1432-0436.2005.00059.x. [DOI] [PubMed] [Google Scholar]
- 5.Schwander M, Leu M, Stumm M, Dorchies OM, Ruegg UT, Schittny J, Muller U. Beta1 integrins regulate myoblast fusion and sarcomere assembly. Dev Cell. 2003;4:673–685. doi: 10.1016/s1534-5807(03)00118-7. [DOI] [PubMed] [Google Scholar]
- 6.Lafuste P, Sonnet C, Chazaud B, Dreyfus PA, Gherardi RK, Wewer UM, Authier FJ. ADAM12 and alpha9beta1 integrin are instrumental in human myogenic cell differentiation. Mol Biol Cell. 2005;16:861–870. doi: 10.1091/mbc.E04-03-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cifuentes-Diaz C, Nicolet M, Alameddine H, Goudou D, Dehaupas M, Rieger F, Mege RM. M-cadherin localization in developing adult and regenerating mouse skeletal muscle: possible involvement in secondary myogenesis. Mech Dev. 1995;50:85–97. doi: 10.1016/0925-4773(94)00327-j. [DOI] [PubMed] [Google Scholar]
- 8.Zeschnigk M, Kozian D, Kuch C, Schmoll M, Starzinski-Powitz A. Involvement of M-cadherin in terminal differentiation of skeletal muscle cells. J Cell Sci. 1995;108( Pt 9):2973–2981. doi: 10.1242/jcs.108.9.2973. [DOI] [PubMed] [Google Scholar]
- 9.Charrasse S, Comunale F, Grumbach Y, Poulat F, Blangy A, Gauthier-Rouviere C. RhoA GTPase regulates M-cadherin activity and myoblast fusion. Mol Biol Cell. 2006;17:749–759. doi: 10.1091/mbc.E05-04-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollnagel A, Grund C, Franke WW, Arnold HH. The cell adhesion molecule M-cadherin is not essential for muscle development and regeneration. Mol Cell Biol. 2002;22:4760–4770. doi: 10.1128/MCB.22.13.4760-4770.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasyutina E, Martarelli B, Brakebusch C, Wende H, Birchmeier C. The small G-proteins Rac1 and Cdc42 are essential for myoblast fusion in the mouse. Proc Natl Acad Sci U S A. 2009;106:8935–8940. doi: 10.1073/pnas.0902501106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowling JJ, Vreede AP, Kim S, Golden J, Feldman EL. Kindlin-2 is required for myocyte elongation and is essential for myogenesis. BMC Cell Biol. 2008;9:36. doi: 10.1186/1471-2121-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayer U. Integrins: redundant or important players in skeletal muscle? J Biol Chem. 2003;278:14587–14590. doi: 10.1074/jbc.R200022200. [DOI] [PubMed] [Google Scholar]
- 14.Charrasse S, Comunale F, Fortier M, Portales-Casamar E, Debant A, Gauthier-Rouviere C. M-cadherin activates Rac1 GTPase through the Rho-GEF trio during myoblast fusion. Mol Biol Cell. 2007;18:1734–1743. doi: 10.1091/mbc.E06-08-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fulton AB, Prives J, Farmer SR, Penman S. Developmental reorganization of the skeletal framework and its surface lamina in fusing muscle cells. J Cell Biol. 1981;91:103–112. doi: 10.1083/jcb.91.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peckham M. Engineering a multi-nucleated myotube, the role of the actin cytoskeleton. J Microsc. 2008;231:486–493. doi: 10.1111/j.1365-2818.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- 17.Saitoh O, Arai T, Obinata T. Distribution of microtubules and other cytoskeletal filaments during myotube elongation as revealed by fluorescence microscopy. Cell Tissue Res. 1988;252:263–273. doi: 10.1007/BF00214368. [DOI] [PubMed] [Google Scholar]
- 18.Kim S, Shilagardi K, Zhang S, Hong SN, Sens KL, Bo J, Gonzalez GA, Chen EH. A critical function for the actin cytoskeleton in targeted exocytosis of prefusion vesicles during myoblast fusion. Dev Cell. 2007;12:571–586. doi: 10.1016/j.devcel.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 19.Onel SF, Renkawitz-Pohl R. FuRMAS: triggering myoblast fusion in Drosophila. Dev Dyn. 2009;238:1513–1525. doi: 10.1002/dvdy.21961. [DOI] [PubMed] [Google Scholar]
- 20.Straube A, Merdes A. EB3 regulates microtubule dynamics at the cell cortex and is required for myoblast elongation and fusion. Curr Biol. 2007;17:1318–1325. doi: 10.1016/j.cub.2007.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowak SJ, Nahirney PC, Hadjantonakis AK, Baylies MK. Nap1-mediated actin remodeling is essential for mammalian myoblast fusion. J Cell Sci. 2009;122:3282–3293. doi: 10.1242/jcs.047597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan R, Gallagher PJ. Dependence of myoblast fusion on a cortical actin wall and nonmuscle myosin IIA. Dev Biol. 2009;325:374–385. doi: 10.1016/j.ydbio.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abramovici H, Gee SH. Morphological changes and spatial regulation of diacylglycerol kinase-zeta, syntrophins, and Rac1 during myoblast fusion. Cell Motil Cytoskeleton. 2007;64:549–567. doi: 10.1002/cm.20204. [DOI] [PubMed] [Google Scholar]
- 24.Davis DB, Delmonte AJ, Ly CT, McNally EM. Myoferlin, a candidate gene and potential modifier of muscular dystrophy. Hum Mol Genet. 2000;9:217–226. doi: 10.1093/hmg/9.2.217. [DOI] [PubMed] [Google Scholar]
- 25.Doherty KR, Cave A, Davis DB, Delmonte AJ, Posey A, Earley JU, Hadhazy M, McNally EM. Normal myoblast fusion requires myoferlin. Development. 2005;132:5565–5575. doi: 10.1242/dev.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doherty KR, Demonbreun AR, Wallace GQ, Cave A, Posey AD, Heretis K, Pytel P, McNally EM. The endocytic recycling protein EHD2 interacts with myoferlin to regulate myoblast fusion. J Biol Chem. 2008;283:20252–20260. doi: 10.1074/jbc.M802306200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Eijnde SM, van den Hoff MJ, Reutelingsperger CP, van Heerde WL, Henfling ME, Vermeij-Keers C, Schutte B, Borgers M, Ramaekers FC. Transient expression of phosphatidylserine at cell-cell contact areas is required for myotube formation. J Cell Sci. 2001;114:3631–3642. doi: 10.1242/jcs.114.20.3631. [DOI] [PubMed] [Google Scholar]
- 28.Mukai A, Kurisaki T, Sato SB, Kobayashi T, Kondoh G, Hashimoto N. Dynamic clustering and dispersion of lipid rafts contribute to fusion competence of myogenic cells. Exp Cell Res. 2009;315:3052–3063. doi: 10.1016/j.yexcr.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Yoon S, Molloy MJ, Wu MP, Cowan DB, Gussoni E. C6ORF32 is upregulated during muscle cell differentiation and induces the formation of cellular filopodia. Dev Biol. 2007;301:70–81. doi: 10.1016/j.ydbio.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stadler B, Blattler TM, Franco-Obregon A. Time-lapse imaging of in vitro myogenesis using atomic force microscopy. J Microsc. 2010;237:63–69. doi: 10.1111/j.1365-2818.2009.03302.x. [DOI] [PubMed] [Google Scholar]
- 31.O'Connor RS, Steeds CM, Wiseman RW, Pavlath GK. Phosphocreatine as an energy source for actin cytoskeletal rearrangements during myoblast fusion. J Physiol. 2008;586:2841–2853. doi: 10.1113/jphysiol.2008.151027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuiper JW, Pluk H, Oerlemans F, van Leeuwen FN, de Lange F, Fransen J, Wieringa B. Creatine kinase-mediated ATP supply fuels actin-based events in phagocytosis. PLoS Biol. 2008;6:e51. doi: 10.1371/journal.pbio.0060051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuiper JW, van Horssen R, Oerlemans F, Peters W, van Dommelen MM, te Lindert MM, ten Hagen TL, Janssen E, Fransen JA, Wieringa B. Local ATP generation by brain-type creatine kinase (CK-B) facilitates cell motility. PLoS One. 2009;4:e5030. doi: 10.1371/journal.pone.0005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin JB, Streijger F, Beynon A, Peters T, Gadzala L, McMillen D, Bystrom C, Van der Zee CE, Wallimann T, Gillespie PG. Hair bundles are specialized for ATP delivery via creatine kinase. Neuron. 2007;53:371–386. doi: 10.1016/j.neuron.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horsley V, Jansen KM, Mills ST, Pavlath GK. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell. 2003;113:483–494. doi: 10.1016/s0092-8674(03)00319-2. [DOI] [PubMed] [Google Scholar]
- 36.Jansen KM, Pavlath GK. Mannose receptor regulates myoblast motility and muscle growth. J Cell Biol. 2006;174:403–413. doi: 10.1083/jcb.200601102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horsley V, Friday BB, Matteson S, Kegley KM, Gephart J, Pavlath GK. Regulation of the growth of multinucleated muscle cells by an NFATC2-dependent pathway. J Cell Biol. 2001;153:329–338. doi: 10.1083/jcb.153.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pavlath GK, Horsley V. Cell fusion in skeletal muscle--central role of NFATC2 in regulating muscle cell size. Cell Cycle. 2003;2:420–423. [PubMed] [Google Scholar]
- 39.Lafreniere JF, Mills P, Bouchentouf M, Tremblay JP. Interleukin-4 improves the migration of human myogenic precursor cells in vitro and in vivo. Exp Cell Res. 2006;312:1127–1141. doi: 10.1016/j.yexcr.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Sohn RL, Huang P, Kawahara G, Mitchell M, Guyon J, Kalluri R, Kunkel LM, Gussoni E. A role for nephrin, a renal protein, in vertebrate skeletal muscle cell fusion. Proc Natl Acad Sci U S A. 2009;106:9274–9279. doi: 10.1073/pnas.0904398106. [DOI] [PMC free article] [PubMed] [Google Scholar]