Abstract
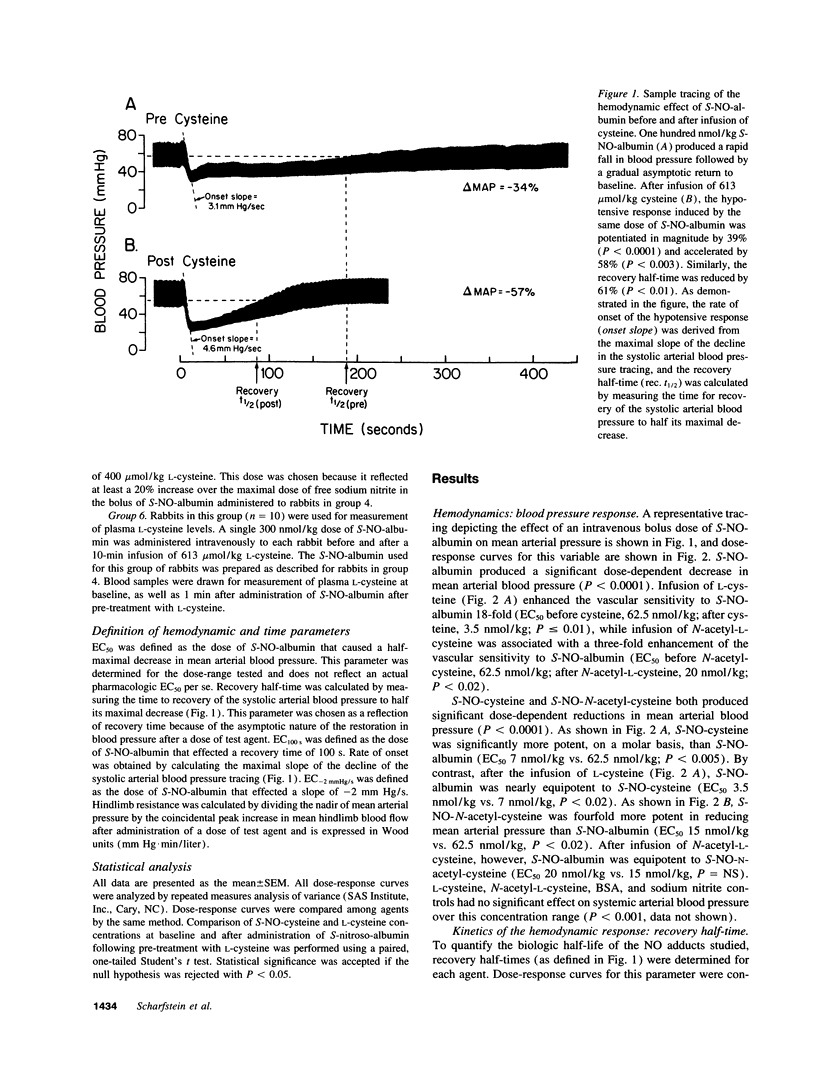
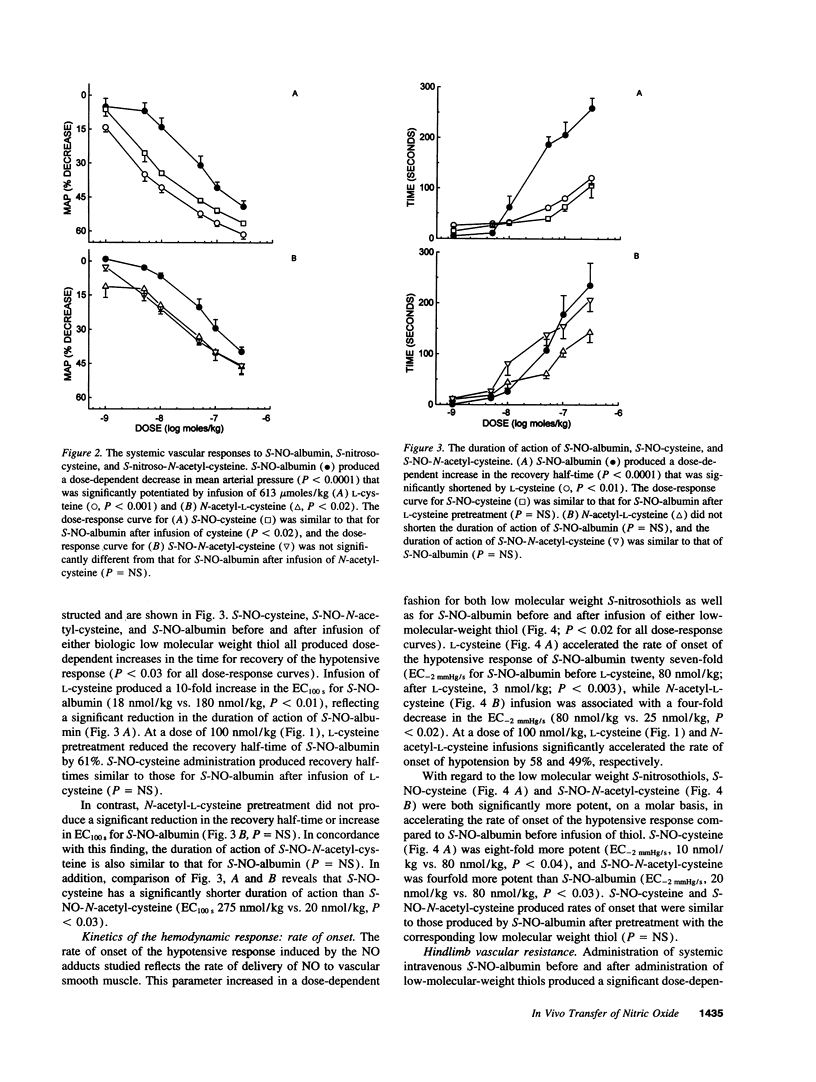
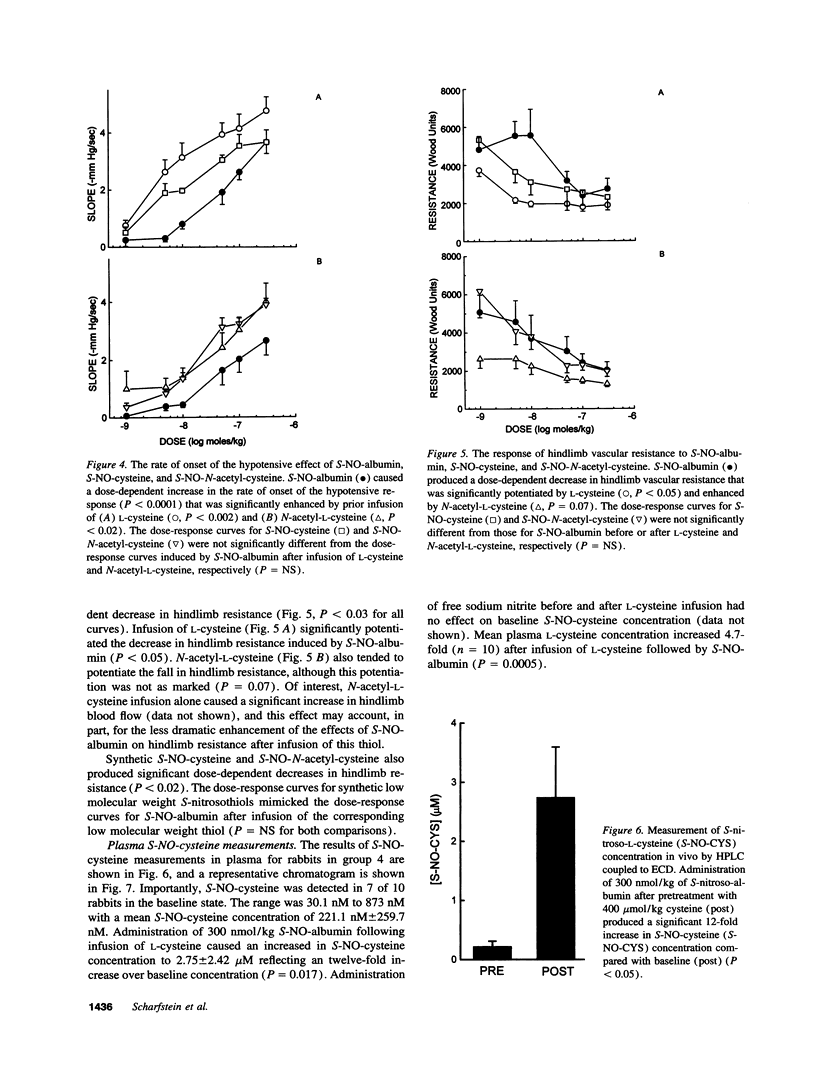
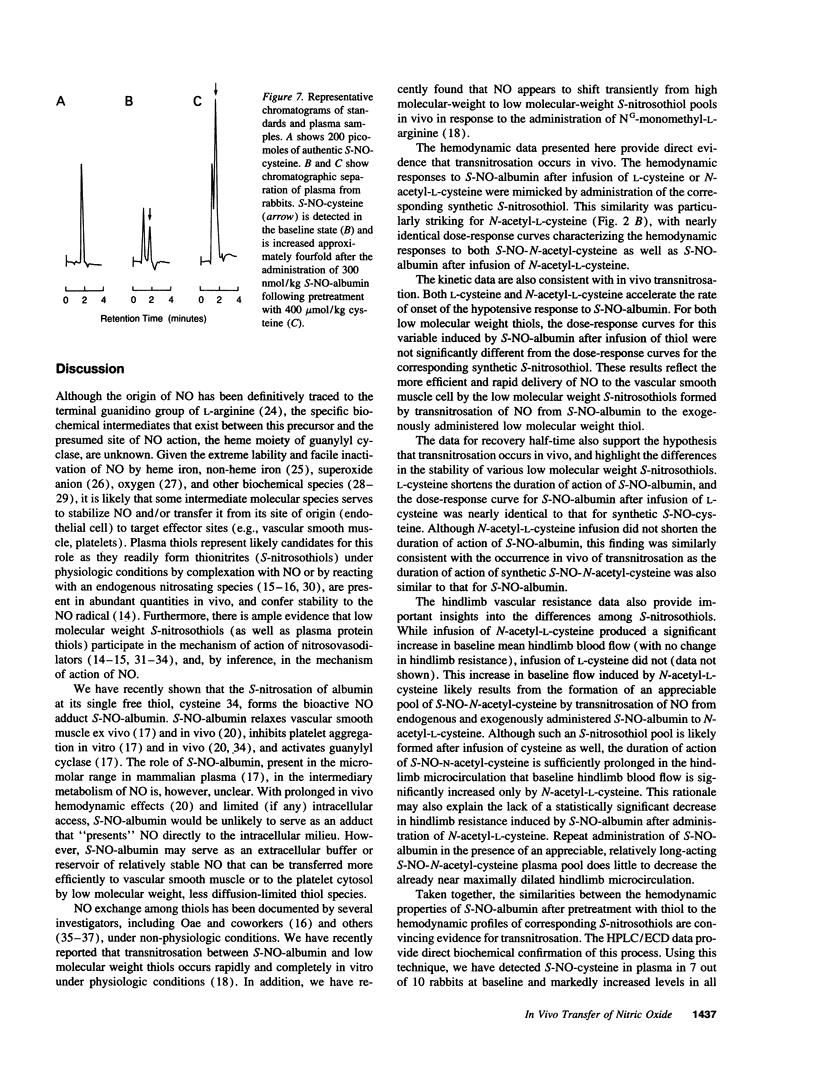
Plasma albumin reacts with nitric oxide (NO) to form the bioactive adduct, S-nitroso-albumin (S-NO-albumin). The limited intracellular access of S-NO-albumin suggests the need for a vascular transfer mechanism of NO from a large plasma S-NO-albumin pool to effect biologic function. To study the role of low molecular weight (LMW) thiols in NO transfer in vivo, we administered intravenous S-NO-albumin (1-300 nmol/kg) to rabbits before and after an intravenous infusion of L-cysteine or N-acetyl-L-cysteine. S-NO-albumin produced dose-dependent hypotension that was significantly augmented by prior infusion of either LMW thiol. LMW thiol infusion significantly accelerated the rate of onset and reduced the duration of action of the hypotension induced by S-NO-albumin. The hemodynamic effects of S-NO-albumin after pretreatment with LMW thiols were mimicked by administration of the corresponding LMW S-nitrosothiol. The transfer of NO from albumin to L-cysteine was directly measured in rabbit plasma using a novel technique that couples high performance liquid chromatography to electrochemical detection. These data demonstrate that NO exchange between plasma protein thiol-bound NO and available LMW thiol pools (transnitrosation) occurs in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett B. M., Kobus S. M., Brien J. F., Nakatsu K., Marks G. S. Requirement for reduced, unliganded hemoprotein for the hemoglobin- and myoglobin-mediated biotransformation of glyceryl trinitrate. J Pharmacol Exp Ther. 1986 May;237(2):629–635. [PubMed] [Google Scholar]
- Buga G. M., Gold M. E., Wood K. S., Chaudhuri G., Ignarro L. J. Endothelium-derived nitric oxide relaxes nonvascular smooth muscle. Eur J Pharmacol. 1989 Feb 14;161(1):61–72. doi: 10.1016/0014-2999(89)90180-5. [DOI] [PubMed] [Google Scholar]
- Chong S., Fung H. L. Thiol-mediated catalysis of nitroglycerin degradation by serum proteins. Increase in metabolism was not accompanied by S-nitrosothiol production. Drug Metab Dispos. 1990 Jan-Feb;18(1):61–67. [PubMed] [Google Scholar]
- Downes M. J., Edwards M. W., Elsey T. S., Walters C. L. Determination of a non-volatile nitrosamine by using denitrosation and a chemiluminescence analyser. Analyst. 1976 Sep;101(1206):742–748. doi: 10.1039/an9760100742. [DOI] [PubMed] [Google Scholar]
- Fung H. L., Chong S., Kowaluk E., Hough K., Kakemi M. Mechanisms for the pharmacologic interaction of organic nitrates with thiols. Existence of an extracellular pathway for the reversal of nitrate vascular tolerance by N-acetylcysteine. J Pharmacol Exp Ther. 1988 May;245(2):524–530. [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gruetter C. A., Gruetter D. Y., Lyon J. E., Kadowitz P. J., Ignarro L. J. Relationship between cyclic guanosine 3':5'-monophosphate formation and relaxation of coronary arterial smooth muscle by glyceryl trinitrate, nitroprusside, nitrite and nitric oxide: effects of methylene blue and methemoglobin. J Pharmacol Exp Ther. 1981 Oct;219(1):181–186. [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Byrns R. E., Buga G. M., Wood K. S. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987 Dec;61(6):866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Edwards J. C., Gruetter D. Y., Barry B. K., Gruetter C. A. Possible involvement of S-nitrosothiols in the activation of guanylate cyclase by nitroso compounds. FEBS Lett. 1980 Feb 11;110(2):275–278. doi: 10.1016/0014-5793(80)80091-3. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Gruetter C. A. Requirement of thiols for activation of coronary arterial guanylate cyclase by glyceryl trinitrate and sodium nitrite: possible involvement of S-nitrosothiols. Biochim Biophys Acta. 1980 Aug 13;631(2):221–231. doi: 10.1016/0304-4165(80)90297-4. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Keaney J. F., Jr, Simon D. I., Stamler J. S., Jaraki O., Scharfstein J., Vita J. A., Loscalzo J. NO forms an adduct with serum albumin that has endothelium-derived relaxing factor-like properties. J Clin Invest. 1993 Apr;91(4):1582–1589. doi: 10.1172/JCI116364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen J. H., Habig W. H., Jakoby W. B. Mechanism for the several activities of the glutathione S-transferases. J Biol Chem. 1976 Oct 25;251(20):6183–6188. [PubMed] [Google Scholar]
- Kelm M., Schrader J. Control of coronary vascular tone by nitric oxide. Circ Res. 1990 Jun;66(6):1561–1575. doi: 10.1161/01.res.66.6.1561. [DOI] [PubMed] [Google Scholar]
- Lancaster J. R., Jr, Hibbs J. B., Jr EPR demonstration of iron-nitrosyl complex formation by cytotoxic activated macrophages. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1223–1227. doi: 10.1073/pnas.87.3.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. D., Misra D. C., Shafer J. A. Determination of interactive thiol ionizations in bovine serum albumin, glutathione, and other thiols by potentiometric difference titration. Biochemistry. 1980 Dec 23;19(26):6129–6137. doi: 10.1021/bi00567a028. [DOI] [PubMed] [Google Scholar]
- Malinski T., Taha Z. Nitric oxide release from a single cell measured in situ by a porphyrinic-based microsensor. Nature. 1992 Aug 20;358(6388):676–678. doi: 10.1038/358676a0. [DOI] [PubMed] [Google Scholar]
- Mellion B. T., Ignarro L. J., Ohlstein E. H., Pontecorvo E. G., Hyman A. L., Kadowitz P. J. Evidence for the inhibitory role of guanosine 3', 5'-monophosphate in ADP-induced human platelet aggregation in the presence of nitric oxide and related vasodilators. Blood. 1981 May;57(5):946–955. [PubMed] [Google Scholar]
- Mordvintsev P. I., Rudneva V. G., Vanin A. F., Shimkevich L. L., Khodorov B. I. Ingibiruiushchee vliianie na agregatsiiu trombotsitov dinitrozil'nykh kompleksov zheleza s nizkomolekuliarnymi ligandami. Biokhimiia. 1986 Nov;51(11):1851–1857. [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pryor W. A., Lightsey J. W. Mechanisms of nitrogen dioxide reactions: initiation of lipid peroxidation and the production of nitrous Acid. Science. 1981 Oct 23;214(4519):435–437. doi: 10.1126/science.214.4519.435. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987 Sep;92(1):181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987 Nov 7;2(8567):1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1482–1489. doi: 10.1016/s0006-291x(87)80299-1. [DOI] [PubMed] [Google Scholar]
- Saran M., Michel C., Bors W. Reaction of NO with O2-. implications for the action of endothelium-derived relaxing factor (EDRF). Free Radic Res Commun. 1990;10(4-5):221–226. doi: 10.3109/10715769009149890. [DOI] [PubMed] [Google Scholar]
- Shikano K., Long C. J., Ohlstein E. H., Berkowitz B. A. Comparative pharmacology of endothelium-derived relaxing factor and nitric oxide. J Pharmacol Exp Ther. 1988 Dec;247(3):873–881. [PubMed] [Google Scholar]
- Stamler J. S., Jaraki O., Osborne J., Simon D. I., Keaney J., Vita J., Singel D., Valeri C. R., Loscalzo J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J. S., Simon D. I., Jaraki O., Osborne J. A., Francis S., Mullins M., Singel D., Loscalzo J. S-nitrosylation of tissue-type plasminogen activator confers vasodilatory and antiplatelet properties on the enzyme. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8087–8091. doi: 10.1073/pnas.89.17.8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J. S., Simon D. I., Osborne J. A., Mullins M. E., Jaraki O., Michel T., Singel D. J., Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J. S., Singel D. J., Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992 Dec 18;258(5090):1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Tannenbaum S. R., Fett D., Young V. R., Land P. D., Bruce W. R. Nitrite and nitrate are formed by endogenous synthesis in the human intestine. Science. 1978 Jun 30;200(4349):1487–1489. doi: 10.1126/science.663630. [DOI] [PubMed] [Google Scholar]
- Vester B., Rasmussen K. High performance liquid chromatography method for rapid and accurate determination of homocysteine in plasma and serum. Eur J Clin Chem Clin Biochem. 1991 Sep;29(9):549–554. doi: 10.1515/cclm.1991.29.9.549. [DOI] [PubMed] [Google Scholar]



