Abstract
Study Objectives:
This 12-month, open-label, flexible-dose study with an extension period evaluated the tolerability and efficacy of armodafinil in patients with excessive sleepiness associated with treated obstructive sleep apnea (OSA), shift work disorder (SWD), or narcolepsy.
Methods:
Armodafinil-naïve, adult patients with excessive sleepiness associated with treated OSA (n = 170), SWD (n = 108), or narcolepsy (n = 50) received armodafinil (100–250 mg) once daily (treated OSA or narcolepsy) or before night shifts (SWD). Patients with OSA were regular users of continuous positive airway pressure (CPAP) therapy. Efficacy measures included the Clinical Global Impression of Improvement (CGI-I) and the Epworth Sleepiness Scale (ESS).
Results:
Across the diagnosis groups, the most commonly occurring adverse event was headache (14%–24%). Forty-three patients (13%) and 13 patients (4%) were withdrawn because of adverse events and insufficient efficacy, respectively. Armodafinil did not adversely affect CPAP therapy. At the final visit, 80% (95% CI: 74.1, 86.7) of patients with treated OSA and 84% (72.7, 94.8) of patients with narcolepsy were rated on the CGI-I as at least minimally improved with regard to overall clinical condition; 98% (95.2, 100.0) of patients with SWD were rated as improved with regard to sleepiness during night shifts, including the commute to and from work. Armodafinil improved ESS total scores in patients with treated OSA (mean [SD] [95% CI] change from baseline, −7.3 [5.6] [−8.39, −6.30]) and patients with narcolepsy (−4.7 [6.0] [−7.41, −1.93]).
Conclusions:
Armodafinil administered for 12 months or more was generally well tolerated and improved wakefulness in patients with excessive sleepiness associated with treated OSA, SWD, or narcolepsy. Armodafinil improved the overall clinical condition of patients with treated OSA or narcolepsy.
Citation:
Schwartz JRL; Khan A; McCall WV; Weintraub J; Tiller J. Tolerability and efficacy of armodafinil in naïve patients with excessive sleepiness associated with obstructive sleep apnea, shift work disorder, or narcolepsy: a 12-month, open-label, flexible-dose study with an extension period. J Clin Sleep Med 2010;6(5):450-457.
Keywords: Armodafinil, excessive sleepiness, wakefulness, obstructive sleep apnea, shift work disorder, narcolepsy
Excessive sleepiness affects millions of Americans. Its prevalence is estimated to be in the range of 24% to 36%, based on objective and subjective assessments of sleepiness in community-based samples.1–3 Excessive sleepiness interferes with daily living by impairing social, cognitive, and physical functioning and contributes to errors and accidents at home,4 in the workplace,4–7 and while driving.4,8–12 The effects of excessive sleepiness on well-being and on individual and public safety highlight the importance of recognizing this symptom, identifying its causes, and implementing strategies and therapies for its appropriate management.
Excessive sleepiness is associated with a variety of medical conditions and is a debilitating symptom of various disorders of sleep and wakefulness, such as obstructive sleep apnea (OSA), a disorder that is characterized by recurrent upper airway collapse, reduction in levels of blood oxygen saturation, and frequent arousals during sleep; shift work disorder (SWD), a condition that arises from a misalignment between internally driven circadian processes and externally determined sleep-wake behavior in individuals who rotate work shifts or work at night; and narcolepsy, a primary disorder of the central nervous system that is characterized by abnormalities of rapid eye movement sleep (i.e., sleep attacks, cataplexy, hypnagogic hallucinations, and sleep paralysis).13 A majority of patients with OSA who are treated with continuous positive airway pressure (CPAP) remain excessively sleepy.14 It is estimated that up to 20% of the workforce in the United States are shift workers and, while the prevalence of SWD is currently unknown, up to 45% of shift workers may be at risk for SWD.10 All patients with narcolepsy report being excessively sleepy.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Previous double-blind studies have shown that armodafinil is effective in treating excessive sleepiness associated with treated OSA, SWD, and narcolepsy and is generally well tolerated for up to 12 weeks. The present open-label study was designed to assess the tolerability and efficacy of armodafinil when administered for 12 months or longer in previously armodafinil-naïve patients with excessive sleepiness associated with treated OSA, SWD, and narcolepsy.
Study Impact: Armodafinil once daily for 12 months or more was associated with improvements in wakefulness and overall clinical condition in patients with excessive sleepiness associated with treated OSA or narcolepsy. Patients with SWD who received armodafinil before night shifts also had improvements in excessive sleepiness during the night shift. Armodafinil was well tolerated during 12 months or more of exposure at dosages ranging from 100 mg/day to 250 mg/day. The tolerability and efficacy results are consistent with those reported in 12-week, double-blind studies of armodafinil in patients with excessive sleepiness associated with treated OSA, SWD, or narcolepsy.
The non-amphetamine, wakefulness-promoting medication armodafinil is the R- and longer-lasting isomer of racemic modafinil. As a single enantiomer, the R-isomer has a longer elimination half-life compared with the S-isomer of modafinil (∼15 vs. ∼4 h) and a 3-fold slower rate of clearance.15–18 Armodafinil is indicated to improve wakefulness in patients with excessive sleepiness associated with treated OSA, SWD, or narcolepsy. In patients with treated OSA, armodafinil is indicated as an adjunct to standard therapies that address the upper airway obstruction (e.g., CPAP therapy).19 Twelve-week, randomized, double-blind, placebo-controlled studies conducted in patients with excessive sleepiness associated with treated OSA, SWD, or narcolepsy have shown armodafinil improved objectively and subjectively determined wakefulness compared with placebo.20–23 Across the studies, armodafinil was generally well tolerated. The present study was designed to assess the tolerability and efficacy of armodafinil when administered for 12 months or longer in previously armodafinil-naïve patients with excessive sleepiness associated with these sleep disorders.
METHODS
Study Design
Previously armodafinil-naïve patients were enrolled in a 12-month, open-label, flexible-dose study that was conducted at 41 centers in the United States (34) and Russia (7). At centers in the United States, patients could participate in an extension, which was planned to be open-ended when patients began enrollment. Clinic visits for study procedures and assessments were scheduled at screening and baseline; at the end of months 1, 3, 6, 9, and 12 of the 12-]−month study; and at 3-month intervals during the extension, with telephone contacts between visits (at the end of week 2 and months 2, 5, 8, and 11 during the 12-month study; monthly between clinic visits during the extension). The protocol was approved by an institutional review board or independent ethics committee at each participating center. The study was conducted in accordance with the E6 Good Clinical Practice: Consolidated Guidance24 and national and local laws and regulations. Participants were informed of the anticipated benefits and potential risks of study medication before providing written informed consent.
Patients
Eligible subjects were men and women, aged 18 to 65 years, who had a complaint of excessive sleepiness associated with a current diagnosis of OSA, SWD, or narcolepsy based on International Classification of Sleep Disorders (first edition) criteria.25 For patients with a diagnosis of OSA, documentation of previous adequate education and intervention efforts to encourage use of CPAP therapy was required. In addition to meeting diagnostic criteria, patients with OSA had to be receiving CPAP therapy that was stable (≥ 4 weeks) and effective (in the opinion of the clinician). Evidence of regular CPAP therapy (i.e., ≥ 4 h/night on ≥ 70% of nights) had to be demonstrated during a 2-week evaluation period. Patients with a diagnosis of SWD had to meet minimal criteria (i.e., a primary complaint of excessive sleepiness or insomnia and temporal association of symptoms with a work period that occurred during the habitual phase of sleep) and had to have experienced excessive sleepiness during night shifts for ≥ 3 months. Patients were required to work a minimum of 5 night shifts each month, with ≥ 6 h of work occurring between 22:00 and 08:00 and with work shifts no longer than 12 h. Patients had to maintain their shift-work schedules for the duration of the study. For patients with a diagnosis of narcolepsy, anticataplectic medications were permitted if these agents did not contribute to their sleepiness. The daily dose of anticataplectic medication had to be stable for ≥ 1 month before baseline and was required to remain unchanged during the study.
All patients had to have a Clinical Global Impression of Severity (CGI-S)26 rating ≥ 4 (i.e., moderately ill or worse). The CGI-S was used by the clinician to assess the overall clinical condition of patients with treated OSA or narcolepsy and to assess sleepiness during the night shift, including the commute to and from work, in patients with SWD. Any patient who had been prescribed modafinil or stimulant therapy to treat a sleep disorder was required to undergo a washout period ≥ 7 days before screening assessments. All female patients of childbearing potential were required to use a medically accepted method of birth control; steroidal contraceptives had to be used together with a barrier method. Main exclusion criteria included any clinically significant, uncontrolled medical conditions, treated or untreated; a probable diagnosis of a current sleep disorder other than the primary diagnosis of OSA, SWD, or narcolepsy; any disorder that could interfere with drug absorption, distribution, metabolism, or excretion; use of prescription drugs disallowed by the protocol or clinically significant use of over-the-counter drugs within 7 days before the baseline visit; history of alcohol, narcotic, or any other drug abuse; positive urine drug screen; pregnancy or lactation; and excessive consumption of caffeine (> 600 mg/day or > 8 cups of coffee/day).
Study Drug
Patients received armodafinil formulated as 50-mg tablets for oral administration. For patients with a diagnosis of OSA or narcolepsy, the starting dosage of 100 mg/day was titrated to 150 mg/day on day 4. Thereafter, the dosage could be increased in 50-mg increments on days 8 and 10 to a maximum of 250 mg/day. The maximum dosage for each patient was determined by individual tolerability. The dosage could be decreased, as appropriate, to a minimum of 100 mg/day. Patients were instructed to take study drug in the morning at approximately 08:00 or, if taken later, immediately upon rising.
For patients with a diagnosis of SWD, armodafinil was to be taken only on nights worked. Armodafinil was initiated at 100 mg/day and titrated to 150 mg/day before night shift 4. Subsequently, the dosage could be increased in 50-mg increments before night shifts 7 and 10 to a maximum of 250 mg/day. The dosage could be decreased to a minimum of 100 mg/day if necessary. Patients were instructed to take study medication 30 minutes to 1 h before the start of the night shift but no later than 23:00.
Assessments
Tolerability was assessed by evaluating adverse events, results of clinical laboratory tests (serum chemistry, hematology, and urinalysis), vital sign measurements (heart rate and blood pressure), 12-lead electrocardiograms (ECG), and physical examination findings. Adverse events were reported throughout the study and recorded at all scheduled visits and telephone contacts. Blood samples for serum chemistry and hematology tests were collected and vital sign measurements and ECG were conducted at screening/baseline; at months 1, 3, 6, 9, and 12; every 3 months thereafter; and at the final visit (last postbaseline visit or termination). Samples for urinalysis were collected at screening; at months 6 and 12; every 6 months thereafter; and at the final visit. Physical examinations were performed at screening/baseline; at month 12; and at the final visit. For patients with OSA, use of CPAP therapy was assessed at screening and at every clinic visit. CPAP devices were provided by the sponsor. Patients were required to use the CPAP device provided by the sponsor or an equivalent device that would record data and be able to print out those data at each clinic visit. During the telephone contact, patients were counseled to continue to use their CPAP devices, but no CPAP evaluation was conducted during the telephone interview.
The clinician-rated Clinical Global Impression of Improvement (CGI-I)26 was used to assess improvement in the overall clinical condition of patients with treated OSA or narcolepsy and to assess changes in sleepiness during the night shift, including the commute to and from work, in patients with SWD compared with the pretreatment Clinical Global Impression of Severity (CGI-S). Rating categories for the CGI-I are assigned an ordinal value ranging from 1 (very much improved) to 7 (very much worse). At screening, the severity of illness was assessed by the CGI-S, which consists of 7 categories: normal, not at all ill; borderline ill; mildly ill; moderately ill; markedly ill; severely ill; and among the most extremely ill.26
The Epworth Sleepiness Scale (ESS)27,28 was used to assess the extent to which sleepiness interfered with daily activities in patients with treated OSA or narcolepsy. The ESS is a self-administered instrument that assesses the likelihood of dozing or falling asleep during 8 situations of daily life (e.g., watching TV; in a car, while stopped for a few minutes in traffic), rated on a scale of 0 to 3, with 3 indicating a high chance of dozing. The total score is the sum of scores for the 8 items. An improvement in wakefulness corresponds to a reduction in the ESS total score; a score of ≥ 1027 was used to define excessive sleepiness.
Statistical Analysis
Continuous and categorical demographic variables were summarized using descriptive statistics. Safety analyses included those patients who received ≥ 1 dose of armodafinil. Safety and tolerability data were summarized using descriptive statistics. Efficacy analyses included all patients who received ≥ 1 dose of armodafinil and had at least 1 postbaseline efficacy assessment. Efficacy data (CGI-I ratings and ESS total scores) were summarized at months 1, 3, 6, 9, and 12 using observed cases and at the final visit using last observed postbaseline data from the 12-month study or the extension. Efficacy variables were the proportion of patients with at least minimal improvement in the CGI-I rating and, for patients with treated OSA or narcolepsy, the change from baseline in ESS total scores. For the CGI-I, the 95% confidence interval (CI) was calculated based on normal approximation; for the ESS, the 95% CI was calculated using a paired t-statistic for the change from baseline in total score.
RESULTS
Patients
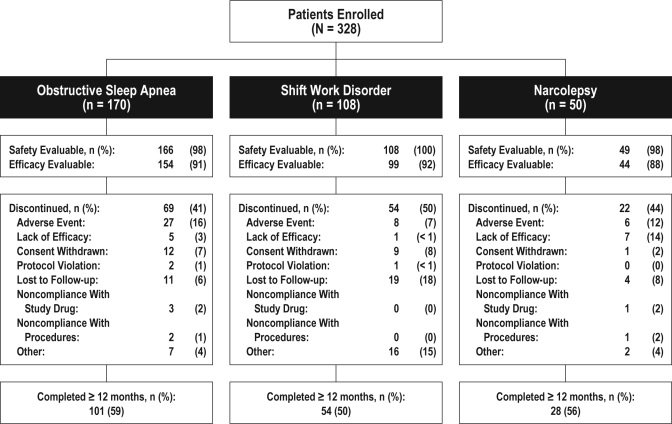
In total, 328 patients were enrolled; 170, 108, and 50 patients were diagnosed with OSA that was being treated, SWD, and narcolepsy, respectively (Figure 1). Overall, 183 patients (56%) had a month 12 visit or ≥ 365 days of study participation. Reasons for not completing the 12-month study included adverse event (13%), loss to follow-up (10%), other (8%), consent withdrawn (7%), lack of efficacy (4%), noncompliance with study medication (1%), noncompliance with study procedures (1%), and protocol violation (1%).
Figure 1.
Disposition by diagnosis group for patients who completed 12 months or more of the study
Demographics and baseline characteristics for patients included in the safety analysis set are shown in Table 1. In the narcolepsy group, 2 patients were older than 65 years but were included as exceptions to the protocol. On the basis of mean ESS total scores, patients with treated OSA or narcolepsy were moderately to severely sleepy at baseline (the overall mean ESS baseline score was 14.5 for the 134 patients with excessive sleepiness associated with narcolepsy or treated OSA who had an ESS score available). Patients with treated OSA were sleepy despite a high nightly duration of CPAP use (mean [SD], 6.7 [1.1] h). Overall, 65% of patients were rated by the clinician as moderately ill on the CGI-S, and 35% of patients were rated as markedly, severely, or among the most extremely ill. A greater proportion of patients with narcolepsy (25/49 [51%]) were considered markedly, severely, or extremely ill than patients with treated OSA (54/166 [33%]) or SWD (35/108 [32%]). One hundred thirty-nine patients (43%) had a medical history of cardiovascular disease (i.e., hypertension, n = 100; chest pain, n = 7; congestive heart failure, n = 6; arrhythmias, n = 5; other, n = 70). Of the 100 patients with a history of hypertension, 79 had a diagnosis of OSA.
Table 1.
Patient demographics and baseline characteristics
| Characteristic | OSA (n = 166)a | SWD (n = 108)a | Narcolepsy (n = 49)a | Overall (N = 323)a |
|---|---|---|---|---|
| Age, y | ||||
| Mean (SD) | 48.9 (8.7) | 39.6 (10.9) | 44.8 (14.5) | 45.2 (11.3) |
| Range | 25–65 | 19–61 | 20–70b | 19–70 |
| Sex, n (%) | ||||
| Men | 119 (72) | 69 (64) | 24 (49) | 212 (66) |
| Women | 47 (28) | 39 (36) | 25 (51) | 111 (34) |
| Race, n (%) | ||||
| White | 150 (90) | 87 (81) | 39 (80) | 276 (85) |
| Black | 7 (4) | 9 (8) | 5 (10) | 21 (7) |
| Asian | 1 (< 1) | 3 (3) | 3 (6) | 7 (2) |
| Other | 8 (5) | 9 (8) | 2 (4) | 19 (6) |
| BMI, kg/m2 Mean (SD) | 35.8 (7.6) | 30.2 (6.8) | 27.7 (5.4) | 32.7 (7.8) |
| ESS total scoresc | ||||
| Mean (SD) | 14.1 (3.8) | – | 16.3 (3.5) | – |
| CGI-S, n (%) | ||||
| Moderately ill | 112 (67) | 73 (68) | 24 (49) | 209 (65) |
| Markedly ill | 37 (22) | 31 (29) | 18 (37) | 86 (27) |
| Severely ill | 16 (10) | 4 (4) | 7 (14) | 27 (8) |
| Extremely ill | 1 (< 1) | 0 (0) | 0 (0) | 1 (< 1) |
BMI, body mass index; CGI-S, Clinical Global Impression of Severity of Illness; ESS, Epworth Sleepiness Scale; OSA, obstructive sleep apnea; SWD, shift work disorder.
Safety-evaluable population;
One patient was 70 and one patient was 68 years old;
OSA, n = 112; Narcolepsy, n = 22.
Forty-six percent of patients overall received armodafinil 250 mg/day as their most commonly used (modal) dosage. Proportionally more patients with narcolepsy (31/49 [63%]) were receiving 250 mg/day as their modal dosage than patients with treated OSA (69/166 [42%]) or SWD (48/108 [44%]); 100 mg/day was the modal dosage for 22%, 16%, and 6% of patients with treated OSA, SWD, and narcolepsy, respectively. The overall mean (SD) duration of exposure was 309 (207) days for patients with treated OSA and 312 (220) days for patients with narcolepsy. The mean (SD) duration of participation in the study was 262 (163) days for patients with SWD.
Tolerability Outcomes
Adverse events reported by ≥ 5% of patients overall included headache, insomnia, upper respiratory tract infection, nausea, dizziness, anxiety, sinusitis, nasopharyngitis, and hypertension (Table 2). Most adverse events were mild to moderate in intensity (92%). Fifteen serious adverse events were reported for 12 patients (treated OSA, n = 7; SWD, n = 5); 4 of these (pulmonary embolism, myocardial infarction, exacerbation of depression, and nonspecific chest pain) were considered by the clinician to be possibly related to armodafinil. Four patients had serious adverse events (pulmonary embolism, myocardial infarction, depression, and multiple sclerosis) that led to discontinuation from the study. Treatment with study drug was interrupted for 3 additional patients as a result of a serious adverse event (noncardiac chest pain, chest pain, or exacerbated chronic obstructive airways disease). No deaths were reported during the study.
Table 2.
Adverse events that occurred in ≥ 5% of patients in any 1 diagnosis group
| Adverse event, n (%) | OSA (n = 166) | SWD (n = 108) | Narcolepsy (n = 49) | Overall (N = 323) |
|---|---|---|---|---|
| Headache | 23 (14) | 20 (19) | 12 (24) | 55 (17) |
| Insomnia | 25 (15) | 19 (18) | 2 (4) | 46 (14) |
| Upper respiratory tract infection | 22 (13) | 7 (6) | 4 (8) | 33 (10) |
| Nausea | 18 (11) | 5 (5) | 4 (8) | 27 (8) |
| Dizziness | 17 (10) | 7 (6) | 3 (6) | 27 (8) |
| Sinusitis | 11 (7) | 2 (2) | 5 (10) | 18 (6) |
| Somnolence | 7 (4) | 0 (0) | 5 (10) | 12 (4) |
| Anxiety | 14 (8) | 3 (3) | 4 (8) | 21 (7) |
| Nasopharyngitis | 9 (5) | 5 (5) | 4 (8) | 18 (6) |
| Hypertension | 14 (8) | 3 (3) | 1 (2) | 18 (6) |
| Fatigue | 8 (5) | 1 (< 1) | 3 (6) | 12 (4) |
| Decreased appetite | 3 (2) | 1 (< 1) | 3 (6) | 7 (2) |
| Feeling jittery | 8 (5) | 0 (0) | 1 (2) | 9 (3) |
| Irritability | 2 (1) | 5 (5) | 1 (2) | 8 (2) |
| Bronchitis | 3 (2) | 5 (5) | 0 (0) | 8 (2) |
OSA, obstructive sleep apnea; SWD, shift work disorder.
Forty-three patients (13%) (treated OSA, n = 27 [16%]; SWD, n = 8 [7%]; and narcolepsy, n = 8 [16%]) were withdrawn from the 12-month study (n = 41) and the extension (n = 2) because of adverse events. Headache (n = 5) and anxiety (n = 5) were the most commonly occurring adverse events associated with early withdrawal. One patient was withdrawn from the study because of rash (moderate, non-serious), 9 patients were withdrawn because of psychiatric events (anxiety, n = 5; nervousness, n = 1; agitation, n = 1; and depression, n = 2), and 5 patients were withdrawn because of cardiovascular events (hypertension, n = 2; increased blood pressure, n = 1; chest pain, n = 1; and myocardial infarction, n = 1).
There were no clinically meaningful changes from baseline to the final visit in laboratory variables, vital sign measurements, ECG, or physical examination findings in any patient population. For patients overall, mean heart rate was slightly higher at the final visit than at baseline, and mean changes from baseline to the final visit in systolic and diastolic blood pressure were minimal (Table 3). Few patients had clinically meaningful ECG findings; 2 patients with treated OSA had ECG findings reported as adverse events (long QT syndrome, n = 1; QRS complex prolonged, n = 1). Both events were considered by the investigator to be mild in intensity and were reported as resolved with no residual effect. Thirteen patients, 11 of whom were diagnosed with OSA (and were being treated with CPAP therapy) and 2 of whom were diagnosed with SWD, had a QTc interval (Fridericia) > 450 msec for at least 1 ECG. One patient with treated OSA had QTc intervals (Fridericia) > 500 msec during administration of study drug. Three patients, all with treated OSA, had changes from baseline in QTc interval (Fridericia) > 60 msec. For each of these patients, subsequent changes from baseline were < 60 msec.
Table 3.
Mean values for vital signs at baseline and the final visit
| Variable | OSA (n = 166) | SWD (n = 108) | Narcolepsy (n = 49) | Overall (N = 323) |
|---|---|---|---|---|
| Heart rate, bpm | ||||
| Mean (SD) at baseline | 72.5 (9.8) | 71.9 (10.8) | 71.0 (10.2) | 72.1 (10.2) |
| Mean (SD) at the final visita | 73.2 (10.1) | 73.1 (9.1) | 73.1 (9.4) | 73.1 (9.6) |
| Mean (SD) change at the final visita | 0.8 (9.9) | 1.3 (10.1) | 2.1 (10.2) | 1.2 (10.0) |
| Systolic blood pressure, mm Hg | ||||
| Mean (SD) at baseline | 128.1 (14.1) | 122.6 (12.2) | 122.9 (16.1) | 125.5 (14.1) |
| Mean (SD) at the final visita | 128.5 (15.3) | 122.1 (13.1) | 124.1 (14.3) | 125.7 (14.7) |
| Mean (SD) change at the final visita | 0.4 (13.8) | −0.2 (11.6) | 1.2 (13.1) | 0.3 (13.0) |
| Diastolic blood pressure, mm Hg | ||||
| Mean (SD) at baseline | 79.0 (8.4) | 79.0 (8.6) | 78.3 (9.8) | 78.9 (8.7) |
| Mean (SD) at the final visita | 80.4 (8.9) | 79.6 (7.8) | 76.6 (9.3) | 79.6 (8.7) |
| Mean (SD) change at the final visita | 1.6 (10.3) | 0.7 (8.5) | −1.7 (10.4) | 0.8 (9.8) |
Bpm, beats per minute; OSA, obstructive sleep apnea; SWD, shift work disorder.
OSA, n = 160; SWD, n = 104; Narcolepsy, n = 48; Overall, n = 312.
For patients with OSA, the mean (SD) nightly duration of CPAP therapy decreased slightly from 6.7 (1.1) h at baseline (average value before the start of study drug) to 6.3 (1.2) h after baseline (average value after the start of study drug). The mean (SD) change from baseline in CPAP therapy usage was −0.4 (0.8) h (24 min; p < 0.0001).
Efficacy Outcomes
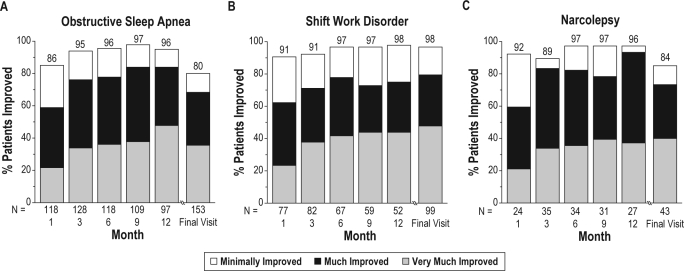
The majority of patients in each diagnosis group were rated by the clinician as at least minimally improved on the CGI-I at each post-baseline visit, beginning with month 1 and continuing through month 12 and the final visit. At the final visit, 80% (95% CI: 74.1, 86.7) of patients with treated OSA and 84% (95% CI: 72.7, 94.8) of patients with narcolepsy were rated as at least minimally improved with regard to overall clinical condition (Figure 2). At the final visit, 98% (95% CI: 95.2, 100.0) of patients with SWD were rated as at least minimally improved with regard to sleepiness during night shifts, including the commute to and from work (Figure 2). A similar pattern of early and sustained improvement was shown for patients rated as much or very much improved on the CGI-I. At the final visit, 68% of patients with treated OSA, 72% of patients with narcolepsy, and 80% of patients with SWD were rated as much or very much improved (Figure 2).
Figure 2.
Percentages of patients who were rated as clinically improved on the Clinical Global Impression of Improvement
The number of patients with an assessment on the CGI-I at months 1, 3, 6, 9, 12, 18, and 24 was 219, 245, 219, 199, 176, 31, and 9, respectively. Patients had to have a baseline assessment and at least 1 postbaseline assessment to be included in the analysis.
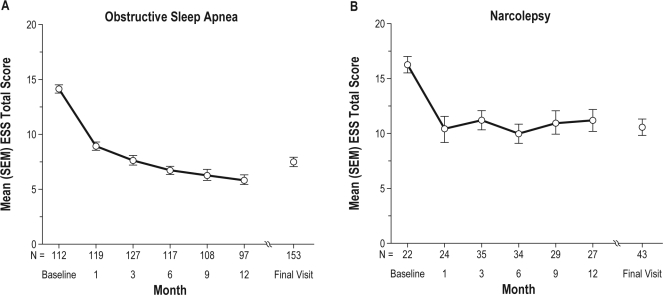
Armodafinil improved patient-reported wakefulness in patients with treated OSA or narcolepsy at each postbaseline visit, starting with month 1 and continuing through month 12 and the final visit, as indicated by reductions in ESS total scores. For patients with treated OSA, the mean (SD) ESS score decreased by 7.3 (5.6) points from baseline to the final visit (95% CI: −8.39, −6.30) (Figure 3). For patients with narcolepsy, the mean ESS score decreased by 4.7 (6.0) points (95% CI: −7.41, −1.93) (Figure 3).
Figure 3.
Mean ± SEM Epworth Sleepiness Scale (ESS) total scores for patients diagnosed with obstructive sleep apnea or narcolepsy
The number of patients with an assessment on the ESS at months 1, 3, 6, 9, 12, 18, and 24 was 143, 162, 151, 137, 124, 25, and 9. Patients had to have a baseline assessment and at least 1 post-baseline assessment to be included in the analysis. The ESS was not used as an assessment in the SWD study.
DISCUSSION
Although OSA, SWD, and narcolepsy have different causes, these disorders all have excessive sleepiness as a cardinal symptom. In the present study, patients with OSA were regular users of CPAP therapy, yet experienced residual excessive sleepiness despite being treated for their airway obstruction. Based on the mean BMI at baseline, patients with OSA were overweight or obese, more so than patients in the other diagnosis groups. In general, patients with OSA tended to have more comorbid conditions than patients with SWD or narcolepsy. There is a known association between OSA and cardiovascular morbidity, particularly hypertension.29,30 Patients with OSA in this study were typical in this regard; nearly one-half of patients in the OSA group (48%) entered the study with a history of hypertension. Use of antihypertensive medications was similar before and during the study and reflected individual medical histories as well as the occurrence of adverse events during the study.
SWD is an under-recognized condition that carries risk for sleepiness-related accidents and medical comorbidities, such as ulcers and depression.10 In the present study, changes in levels of sleepiness were determined for patients with SWD using the clinician-rated CGI-I; these patients did not undergo ESS testing because the ESS asks about sleep propensity during daily activities over the past week. Since patients with excessive sleepiness associated with SWD were required to work 3 or more consecutive shifts a week to be eligible for this study, they may not have been performing shift work for the entire week covered by the ESS.
Narcolepsy, a chronic disorder of sleep-wake regulation, has an estimated prevalence of 0.02% to 0.08% in the general population.31 In the present study, patients with narcolepsy were more severely sleepy at baseline than patients with OSA, based on self-reports. One-half of these patients were substantially burdened by their illness, as evidenced by baseline severity ratings provided by the clinician.
In the present study, adverse event profiles observed for each sleep diagnosis population did not differ qualitatively from those reported in shorter-term studies.21–23 The percentages of patients who discontinued because of adverse events (7%–16% for the sleep diagnosis populations; 13% overall) were comparable to or greater than those reported in 12-week studies of patients with OSA (7.5% [150 mg/day] and 11.5% [250 mg/day] vs. 4% [placebo],22 and 4% [150 mg/day] vs. 5% [placebo]23) or narcolepsy (8% [150 mg/day] and 3% [250 mg/day] vs. 2% [placebo]21). Only 4% of patients overall discontinued the present study because of insufficient efficacy, suggesting that responses to armodafinil were maintained in the majority of patients.
The lack of clinically meaningful changes from baseline to the final visit in vital signs in this study is consistent with findings reported in a 12-week study of armodafinil that was conducted in patients with OSA.23 In another OSA study of 12 weeks' duration, mean changes from baseline in morning diastolic blood pressure and evening heart rate were statistically significant with armodafinil (250 mg alone; 150 and 250 mg combined) compared with placebo (all p < 0.05).22 In a 12-week study conducted in narcolepsy patients, statistically significant differences in the mean morning heart rate (250 mg vs. placebo) and 24-h mean diastolic blood pressure (150 mg and 250 mg combined vs. placebo) were reported (both p < 0.05).21
Patients with OSA were required to maintain regular use of CPAP therapy during the study, as armodafinil does not address the underlying pathology (i.e., airway obstruction). The average nightly duration of CPAP therapy reported in this study (6.3 h) falls within the range of values reported previously for long-term use of CPAP therapy (4.3–6.5 h)32–36 and exceeds the threshold associated with reduced cardiovascular risk.37 Thus, the duration of nightly CPAP therapy use remained high with armodafinil, and the reduction in use (24 min) was not considered clinically meaningful. The reported duration of CPAP use may not reflect usual trends over 12 months in clinical practice.
The improvements in subjectively determined wakefulness and clinical condition that were shown in this study are consistent with outcomes reported previously for shorter-term studies of armodafinil. In 12-week, double-blind, placebo-controlled studies conducted in patients with treated OSA22,23 and patients with narcolepsy,21 armodafinil 150 and 250 mg/day significantly reduced patient-reported sleepiness on the ESS (∼5-point decrease for armodafinil vs. ∼3-point decrease for placebo in the treated OSA studies; ∼4-point decrease for armodafinil vs. 2-point decrease for placebo in the narcolepsy study) (all p < 0.01). In the 12-week studies, armodafinil significantly improved overall clinical condition on the CGI-I (range, 69%–73% [armodafinil] vs. 33%–53% [placebo]) (all p < 0.01).21–23 In addition, these 12-week studies and another 12-week study that was conducted in patients with SWD20 reported significant improvements on objective measures of wakefulness (i.e., mean sleep latency) with armodafinil compared with placebo (all p < 0.05).
Several factors related to the design of the study place limits on the interpretation or the generalizability of the present findings. The study employed an open-label design that did not include a placebo comparator. The flexible nature of armodafinil dosing did not allow for comparisons of different doses or for correlations between doses and adverse events. No objective assessments of efficacy were used. However, as mentioned, shorter-term studies have reported improvements on objective measures (e.g., changes in mean sleep latency) with armodafinil.20–23 The general pattern of patient attrition across the 12-month study is typical of longer-term studies.
Armodafinil was generally well tolerated. Administration of armodafinil once daily or before night shifts for 12 months or more was associated with improvements in wakefulness in patients with excessive sleepiness associated with treated OSA, SWD, or narcolepsy. Armodafinil also improved the overall clinical condition of patients with treated OSA or narcolepsy. The tolerability and efficacy profiles are comparable to and confirm those reported in shorter-term studies of armodafinil in patients with excessive sleepiness associated with treated OSA, SWD, or narcolepsy.
DISCLOSURE STATEMENT
This study was sponsored by Cephalon, Inc. Dr. Schwartz has served as a consultant or speaker for AstraZeneca, Boehringer Ingelheim, Cephalon, GlaxoSmithKline, Pfizer, Medpointe, and Teva.
Dr. Khan has served as a principal investigator of over 280 trials sponsored by more than 55 pharmaceutical companies and more than 20 clinical research organizations. He discloses that he has done no consulting or speaking on their behalf and did not receive financial compensation for authorship of this paper. Dr. McCall was a local principal investigator and an author of this study. He reports no other potential conflicts of interest. Dr. Weintraub did not receive any fees or honoraria for this study. He reports no other potential conflicts of interest. Dr. Tiller is a Cephalon employee.
ACKNOWLEDGMENTS
The authors would like to acknowledge Ronghua Yang, PhD, and Sanjay Arora, PhD, for their statistical contribution to the completion of this manuscript. Funding for manuscript preparation was provided by Cephalon, Inc., to Deb DiMaggio.
The authors would also like to acknowledge all of the centers that participated in this study. In the USA: Aaronson, Robert, MD, Radiant Research Tucson, Tucson, Arizona; Anderson, Donald, MD, Anderson Clinical Research, Redlands, California; Bari, Mohammed, MD, Synergy Clinical Research Center, Chula Vista, California; Bastani, Bijan, MD, North Coast Clinical Trials, Inc., Beechwood, Ohio; Beckett, Louise, MD, IPS Research Company, Oklahoma City, Oklahoma; Bogan, Richard, MD, Sleep Disorders Center of South Carolina, Palmetto Baptist Medical Center, Columbia, South Carolina; Burns, Gerald, MD, APMC, Neurotrials Research of New Orleans, Metairie, Louisiana; Clevinger, Sidney, MD, Renstar Medical Research, Ocala, Florida; Clifford, Dennis, MD, Rocky Mountain Center for Clinical Research, Wheat Ridge, Colorado; Feldman, Neil, MD, St. Petersburg Sleep Disorder Center, St. Petersburg, Florida; Ferguson, James, MD, Radiant Research Salt Lake City, Salt Lake City, Utah; Geohas, Jeffrey, MD, Radiant Research Chicago, Chicago, Illinois; Grosz, Daniel, MD, Pharmacology Research Institute, Northridge, California; Hassman, Howard, DO, CNS Research Institute, Clementon, New Jersey; Herron, James, MD, Herron Medical Center, Chicago, Illinois; Hull, Steven, MD, Vince and Associates Clinical Research, Overland Park, Kansas; Jennings, William, MD, Radiant Research San Antonio (formerly Protocare Trials San Antonio), San Antonio, Texas; Kirby, Louis, MD, Pivotal Research, Peoria, Arizona; LaMarca, Anthony, MD, Therafirst Medical Center, Fort Lauderdale, Florida; Massie, Clifford, PhD, Radiant Research Alexian Brothers, Elk Grove Village, Illinois; Pellegrino, Richard, MD, PhD, Central Arkansas Research, Hot Springs, Arkansas; Pinto, John, MD, Clinical Research Center of Nevada, Las Vegas, Nevada; Pogue, Bryan, MD, Radiant Research Boise, Boise, Idaho; Raphaelson, Marc, MD, PA, Medical Neurology, P.A., Frederick, Maryland; Riffer, Ernie, MD, Central Phoenix Medical Clinic, Phoenix, Arizona; Johnson, Kyle, MD (formerly Sack, Robert, MD), Oregon Health Sciences University, Portland, Oregon; Stafford, Calvin, MD, Neurological Associates of Delaware Valley, Upland, Pennsylvania; Stroebel, Richard, MD, Lehigh Valley Hospital, Allentown, Pennsylvania; Toups, Kathleen, MD, Bay Area Research Institute, Lafayette, California; Trapp, John, MD, Somnos Laboratories, Lincoln, Nebraska; Singh, Baldev, MD (formerly Weiss, Laurence, MD), Precision Research, Hallandale, Florida; Wylie, Paul, MD, Arkansas Center for Sleep Medicine, Little Rock, Arkansas; Shubin, Richard, MD, Neurotherapeutics, Inc., Pasadena, California; Lahmeyer, Henry, MD, Henry Lahmeyer, MD and Associates, Northfield, Illinois. In The Russian Federation: Skoromets, Alexander, Saint-Petersburg State Medical University, Saint Petersburg; Babak, Sergey, Institute of Pulmonology, Moscow; Belov, Alexander, Presidential Medical Center of Russian Federation United Hospital and Out-Patient Clinic, Moscow; Kallistov, Dimitriy, Presidential Medical Center, Center of Rehabilitation, Moscow; Chizhova, Olga, St Petersburg Medical Clinic, Saint Petersburg; Merkulov, Vyacheslav, Cardioclinic, Center of Cardio-Respiratory Monitoring, Saint Petersburg; Lubshina, Olga, City Clinic Hospital 81, Moscow.
REFERENCES
- 1.Baldwin CM, Vishesh KK, Holberg CJ, et al. Associations between gender and measures of daytime somnolence in the Sleep Heart Health Study. Sleep. 2004;27:305–11. doi: 10.1093/sleep/27.2.305. [DOI] [PubMed] [Google Scholar]
- 2.Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the Multiple Sleep Latency Test in community adults. Brain. 2006;129:1609–23. doi: 10.1093/brain/awl079. [DOI] [PubMed] [Google Scholar]
- 3.Singh M, Drake CL, Roth T. The prevalence of multiple-sleep-onset REM periods in a population-based sample. Sleep. 2006;29:890–5. doi: 10.1093/sleep/29.7.890. [DOI] [PubMed] [Google Scholar]
- 4.Leger D. The cost of sleep-related accidents: a report for the national commission on sleep disorders research. Sleep. 1994;17:84–93. doi: 10.1093/sleep/17.1.84. [DOI] [PubMed] [Google Scholar]
- 5.Mitler M, Carskadon M, Czeisler C, et al. Catastrophes, sleep, and public policy: consensus report. Sleep. 1988;11:100–9. doi: 10.1093/sleep/11.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinges DF. An overview of sleepiness and accidents. J Sleep Res. 1995;4:4–14. doi: 10.1111/j.1365-2869.1995.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 7.Lindberg E, Carter N, Gislason T, et al. Role of snoring and daytime sleepiness in occupational accidents. Am J Respir Crit Care Med. 2001;164:2031–5. doi: 10.1164/ajrccm.164.11.2102028. [DOI] [PubMed] [Google Scholar]
- 8.Powell NB, Schectman KB, Riley RW, et al. The road to danger: the comparative risks of driving while sleepy. Laryngoscope. 2001;111:887–93. doi: 10.1097/00005537-200105000-00024. [DOI] [PubMed] [Google Scholar]
- 9.MacLean AW, Davies DRT, Thiele K. The hazards and prevention of driving while sleepy. Sleep Med Reviews. 2003;7:507–21. doi: 10.1016/s1087-0792(03)90004-9. [DOI] [PubMed] [Google Scholar]
- 10.Drake CL, Roehrs T, Richardson G, et al. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–62. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 11.Gander PH, Marshall NS, Harris RB, et al. Sleep, sleepiness and motor vehicle accidents: a national survey. Aust N Z J Public Health. 2005;29:16–21. doi: 10.1111/j.1467-842x.2005.tb00742.x. [DOI] [PubMed] [Google Scholar]
- 12.Powell NB, Schectman KB, Riley RW, et al. Sleepy driver near-misses may predict accident risks. Sleep. 2007;30:331–42. doi: 10.1093/sleep/30.3.331. [DOI] [PubMed] [Google Scholar]
- 13.American Academy of Sleep Medicine. 2nd edition. Westchester: American Academy of Sleep Medicine; 2005. International classification of sleep disorders: diagnostic and coding manual. [Google Scholar]
- 14.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–9. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong YN, King SP, Simcoe D, et al. Open-label, single-dose pharmacokinetic study of modafinil tablets: influence of age and gender in normal subjects. J Clin Pharmacol. 1999;39:281–8. [PubMed] [Google Scholar]
- 16.Wong YN, Simcoe D, Hartman LN, et al. A double-blind, placebo-controlled, ascending-dose evaluation of the pharmacokinetics and tolerability of modafinil tablets in healthy male volunteers. J Clin Pharmacol. 1999;39:30–40. doi: 10.1177/00912709922007534. [DOI] [PubMed] [Google Scholar]
- 17.Robertson P, Jr, Hellriegel ET. Clinical pharmacokinetic profile of modafinil. Clin Pharmacokinet. 2003;42:123–37. doi: 10.2165/00003088-200342020-00002. [DOI] [PubMed] [Google Scholar]
- 18.Darwish M, Kirby M, Hellriegel ET, Yang R, Robertson P., Jr. Pharmacokinetic profile of armodafinil in healthy subjects: pooled analysis of data from 3 randomized studies. Clin Drug Investig. 2009;29:87–100. doi: 10.2165/0044011-200929020-00003. [DOI] [PubMed] [Google Scholar]
- 19.NUVIGIL. Frazer, PA: Cephalon, Inc.; 2007. [package insert] [Google Scholar]
- 20.Drake C, Walsh J, Roth T. Armodafinil improves sleep latency in patients with shift work disorder. Sleep. 2006;29:A64. [Google Scholar]
- 21.Harsh JR, Hayduk R, Rosenberg R, et al. The efficacy and safety of armodafinil as treatment for adults with excessive sleepiness associated with narcolepsy. Curr Med Res Opin. 2006;22:761–74. doi: 10.1185/030079906X100050. [DOI] [PubMed] [Google Scholar]
- 22.Roth T, White D, Schmidt-Nowara W, et al. Effects of armodafinil in the treatment of residual excessive sleepiness associated with obstructive sleep apnea/hypopnea syndrome: a 12-week, multicenter, double-blind, randomized, placebo-controlled study in nCPAP-adherent adults. Clin Ther. 2006;28:689–706. doi: 10.1016/j.clinthera.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Hirshkowitz M, Black JE, Wesnes K, et al. Adjunct armodafinil improves wakefulness and memory in obstructive sleep apnea/hypopnea syndrome. Respir Med. 2007;101:616–27. doi: 10.1016/j.rmed.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 24.United States Department of Health and Human Services. Rockville, MD: United States Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research; 1996. Apr, Guidance for Industry. E6 Good Clinical Practice: Consolidated Guidance. Available at: http://www.fda.gov/cder/guidance/959fnl.pdf. [Google Scholar]
- 25.American Sleep Disorders Association. International classification of sleep disorders: diagnostic and coding manual. Rochester: American Sleep Disorders Association; 2000. [Google Scholar]
- 26.Guy W. ECDEU assessment manual for psychopharmacology. Rockville: National Institute of Mental Health; 1976. [Google Scholar]
- 27.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 28.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 29.Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 30.Nieto FJ, Young TB, Lind BK, et al. Association of sleep disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 31.Mignot E. Genetic and familial aspects of narcolepsy. Neurology. 1998;50:S16–22. doi: 10.1212/wnl.50.2_suppl_1.s16. [DOI] [PubMed] [Google Scholar]
- 32.Engleman HM, Martin SE, Douglas NJ. Compliance with CPAP therapy in patients with the sleep apnoea/hypopnoea syndrome. Thorax. 1994;49:263–6. doi: 10.1136/thx.49.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reeves-Hoche MK, Meck R, Zwillich CW. Nasal CPAP: an objective evaluation of patient compliance. Am J Respir Crit Care Med. 1994;149:149–54. doi: 10.1164/ajrccm.149.1.8111574. [DOI] [PubMed] [Google Scholar]
- 34.Krieger J, Kurtz D, Petiau C, et al. Long-term compliance with CPAP therapy in obstructive sleep apnea patients and in snorers. Sleep. 1996;19:S136–43. doi: 10.1093/sleep/19.suppl_9.s136. [DOI] [PubMed] [Google Scholar]
- 35.Pieters T, Collard P, Aubert G, et al. Acceptance and long-term compliance with nCPAP in patients with obstructive sleep apnoea syndrome. Eur Respir J. 1996;9:939–44. doi: 10.1183/09031936.96.09050939. [DOI] [PubMed] [Google Scholar]
- 36.Collard P, Pieters T, Aubert G, et al. Compliance with nasal CPAP in obstructive sleep apnea patients. Sleep Med Rev. 1997;1:33–44. doi: 10.1016/s1087-0792(97)90004-6. [DOI] [PubMed] [Google Scholar]
- 37.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]