Abstract
Study Objectives:
Armodafinil is a wakefulness-promoting medication. Its efficacy and tolerability have been established in 12-week studies of patients with excessive sleepiness (ES) associated with treated obstructive sleep apnea (OSA), shift work disorder (SWD), or narcolepsy. This study evaluated the tolerability and efficacy of armodafinil for ≥ 12 months.
Methods:
Patients with ES associated with treated OSA, SWD, or narcolepsy who completed one of four 12-week, double-blind studies were eligible for this multicenter, open-label study of ≥ 12 months' duration of treatment with armodafinil (50 to 250 mg/day). Adverse events and other criteria of tolerability were monitored throughout the study. Efficacy assessments included the Clinical Global Impression of Change (CGI-C), Brief Fatigue Inventory (BFI), and Epworth Sleepiness Scale (ESS).
Results:
Of 743 enrolled patients (474 with treated OSA, 113 with SWD, and 156 with narcolepsy), 57% of patients (420/743) completed 12 months or more of treatment. Discontinuations due to adverse events occurred in 13% of patients (95/743) during the initial 12-month period. Throughout the ≥ 12-month study, adverse events were generally of mild-to-moderate intensity; headache (25% [180/731]), nasopharyngitis (17% [123/731]), and insomnia (14% [99/731]) were the most common. Modest increases were observed in vital sign measurements (blood pressure [3.6/2.3 mm Hg], heart rate [6.7 beats per minute]) across all patient groups; most of the changes occurred by month 3. Improvements from baseline in efficacy assessments started at month 1 and were maintained throughout the study.
Conclusions:
Armodafinil remained effective and was generally well tolerated. Increased monitoring of blood pressure may be appropriate in patients on armodafinil. Armodafinil represents an option for long-term treatment of patients with ES associated with treated OSA, SWD, or narcolepsy.
Citation:
Black JE; Hull SG; Tiller J; Yang R; Harsh JR. The long-term tolerability and efficacy of armodafinil in patients with excessive sleepiness associated with treated obstructive sleep apnea, shift work disorder, or narcolepsy: an open-label extension study. J Clin Sleep Med 2010;6(5):458-466.
Keywords: Armodafinil, wakefulness, narcolepsy, obstructive sleep apnea, shift work disorder, clinical study, long-term treatment
Excessive sleepiness (ES) is the inability to consistently sustain wakefulness and alertness, which interferes with the tasks of daily living.1 It is one of the major complaints of patients presenting with obstructive sleep apnea (OSA) and shift work disorder (SWD), and is the primary manifestation of narcolepsy.2 The prevalence of OSA with ES has been estimated at 4% and 2% in men and women, respectively, in the middle-aged work force of the United States.3 The standard treatment for OSA in the United States is continuous positive airway pressure (CPAP), which has been shown to decrease ES4; however, an important subset of patients using CPAP experience residual ES, as measured using the multiple sleep latency test (MSLT).5 The prevalence of SWD is unknown; up to 45% of shift workers report having ES and/or insomnia and are at risk for the disorder.6,7 Narcolepsy is a rare disorder: its prevalence is 0.03% to 0.05%.8 However, all patients with narcolepsy experience ES, and it is a debilitating symptom for most of them.9 ES can lead to sudden and irresistible sleep episodes, which may occur during daily activities such as walking and driving.2
BRIEF SUMMARY
Current Knowledge/Study Rationale: It has been reported that ES doubles the risk for occupational accidents, decreases work productivity, and increases the risk of motor vehicle accidents. Narcolepsy and OSA are chronic disorders, and SWD may be chronic if the work schedule is unremitting. Therefore, treatment for ES associated with these disorders requires sustained efficacy and tolerability. This long-term, open-label study evaluated the efficacy and tolerability of armodafinil for 12 months or more.
Study Impact: Armodafinil remained effective during this study. All 3 diagnostic groups reported high levels of ES and fatigue at baseline that had improved by month 1 and remained improved during the study. The intermittent use of armodafinil in patients with SWD did not diminish its efficacy. Patients with SWD reported baseline levels of fatigue on the BFI similar to those reported by patients with treated OSA, and demonstrated similar reductions in fatigue throughout the study. Armodafinil was also generally well tolerated whether used daily (by patients with treated OSA or narcolepsy) or intermittently (by those with SWD) for up to 2 years; however, increased monitoring of blood pressure may be appropriate in patients on armodafinil.
ES associated with any of these disorders—treated OSA, SWD, or narcolepsy—has serious consequences. It has been reported that ES doubles the risk for occupational accidents,10 decreases work productivity,11,12 and increases the risk of motor vehicle accidents.11 Patients with ES report impairment in attention, daily functioning, and psychological well-being,11,13 as well as limitations in work performance.12 Despite the substantial risks related to ES, OSA, SWD, and narcolepsy remain underdiagnosed and undertreated.3,7,9
Narcolepsy and OSA are chronic disorders that may persist for many years, and SWD may be chronic if the work schedule is unremitting. Treatment for ES associated with these disorders requires sustained efficacy and tolerability. Armodafinil, the longer-lasting isomer of modafinil, is a wakefulness-promoting medication. When armodafinil is compared with modafinil on a milligram-to-milligram basis, higher plasma concentrations are observed with armodafinil later in the waking day.14 In four 12-week, randomized, double-blind clinical studies, armodafinil significantly improved wakefulness throughout the day in patients with ES associated with treated OSA, SWD, or narcolepsy compared with placebo.15–18 This long-term, open-label study evaluated the efficacy and tolerability of armodafinil for 12 months or more in patients who completed a double-blind, 12-week study of ES associated with treated OSA, SWD, or narcolepsy.15–18
METHODS
Study Design
This was a multicenter, flexible-dose, open-label extension study of the four 12-week, double-blind studies of armodafinil. The baseline visit during the double-blind study was used as the baseline visit of the open-label study. The study was conducted at sites in the United States, Canada, France, Germany, Russia, and Australia. The study lasted 12 months at all centers, except in the United States and Canada, where patients were permitted to continue in the study for an additional 12 months. Patient follow-up visits were scheduled at months 1, 3, 6, 9, and 12 of the initial 12-month period, and then every 3 months thereafter for those patients continuing in the study beyond 12 months. Patients were contacted by telephone every month between clinic visits. Patients were considered “study completers” if they had a month 12 visit and/or ≥ 365 days of treatment with armodafinil.
The protocol was approved by an independent ethics committee or institutional review board, according to national or local regulations; and the study was conducted in full accordance with the Good Clinical Practice: Consolidated Guideline approved by the International Conference on Harmonisation and any applicable national and local laws and regulations. All patients provided informed written consent.
Patients
Patients who had completed a double-blind, placebo-controlled study of armodafinil for ES associated with CPAP-treated OSA,17,18 SWD,15 or narcolepsy16 were eligible for this study. Participants who met the key entry criteria for the double-blind studies were male or female outpatients aged 18 to 65 years with a diagnosis of OSA, SWD, or narcolepsy according to International Classification of Sleep Disorders criteria.2 Patients with OSA had to have been compliant with CPAP therapy during the 2-week screening period of their double-blind study, i.e., used their CPAP machine for ≥ 4 h/night on ≥ 70% of nights. Those patients with SWD worked ≥ 5 nights a month: night shifts included ≥ 6 h between 22:00 and 08:00 and lasted ≤ 12 h. Evidence of clinically relevant ES was required, i.e., an Epworth Sleepiness Scale (ESS) score ≥ 10 for treated OSA patients and an MSLT ≤ 6 min for patients with SWD or narcolepsy. Women had to be surgically sterile, 2 years postmenopausal, or, if they were capable of childbearing, using a medically accepted method of birth control (steroidal contraceptives had to be used in conjunction with a barrier method).
Patients were excluded for clinically relevant, uncontrolled medical conditions; a probable diagnosis of a current sleep disorder other than OSA, SWD, or narcolepsy; consuming > 600 mg/day of caffeine; taking prescription drugs disallowed by the protocol; or clinically relevant treatment with over-the-counter drugs within 7 days of the first visit. A history of alcohol, narcotic, or any other drug abuse or a positive urine drug screening was also grounds for exclusion.
Study Drug
Armodafinil was titrated to a dose based on the efficacy and tolerability observed for each individual patient. Patients with treated OSA or narcolepsy started treatment at 100 mg daily. The dose could be increased in increments of 50 mg/day on days 4, 8, and 10 to a maximum dose of 250 mg daily. These patients were instructed to take their daily dose of armodafinil in the morning at approximately 08:00 or, if arising after 08:00, immediately upon waking. Patients with SWD took armodafinil only on nights worked. The initial dose was 50 mg/night and could be titrated to 100 mg/night for doses 2 and 3, to 150 mg/night for doses 4 and 5, to 200 mg/night for doses 6 and 7, and to 250 mg/night for all subsequent doses. Patients were instructed to take their dose 30 min to 1 h before beginning each night shift, but no later than 23:00.
Evaluations
Tolerability
Adverse events, concomitant medication usage, and compliance with CPAP by patients with OSA were assessed during all clinic or telephone contacts with patients through 24 months. Concomitant medications included all medications taken while being treated with armodafinil. Adverse event severity and the relationship to study treatment were determined by the investigator. Vital sign measurements, clinical laboratory testing (serum chemistry, hematology, and urinalysis), and electrocardiograms (ECGs) were performed at all clinic visits. Physical exams were performed at the first visit, at yearly intervals thereafter, and at the final visit.
Efficacy
Efficacy measures were assessed at all clinic visits during the first 12 months and at the final visit for those patients who were enrolled in the study longer than 12 months. The clinician-rated Clinical Global Impression of Change (CGI-C)19 was used to assess change (using 7 categories from “very much worse” to “very much improved”) in the overall clinical condition of patients with treated OSA or narcolepsy and to assess change in ES during the night shift, including the commute to and from work, in patients with SWD. Patients with treated OSA or narcolepsy assessed their propensity to fall asleep in various situations using the ESS.20 Scores on the ESS range from 0 to 24, a score ≥ 10 being commonly associated with clinically relevant ES. All patients completed the Brief Fatigue Inventory (BFI)21 to assess fatigue and its impact on daily functioning. The “global” BFI score was the average of the ratings for all items on the BFI, while the “worst” fatigue score was the rating for the individual item of the worst level of fatigue over the previous 24 h. Scores on BFI range from 0 to 10: a higher score indicates more severe fatigue.
Data Analysis
The safety analysis set included all enrolled patients who took ≥ 1 dose of armodafinil in this open-label study; the efficacy analysis set included patients in the safety analysis set who had ≥ 1 efficacy assessment post baseline in this study. Descriptive statistics were computed for all variables. Efficacy data were summarized for each visit through month 12 and at final visit. Final visit efficacy analyses include the last observation carried forward for all patients enrolled in the study regardless of when the last visit occurred. Final visit could have occurred in either the 12-month period or at a point beyond 12 months of treatment.
RESULTS
Patients
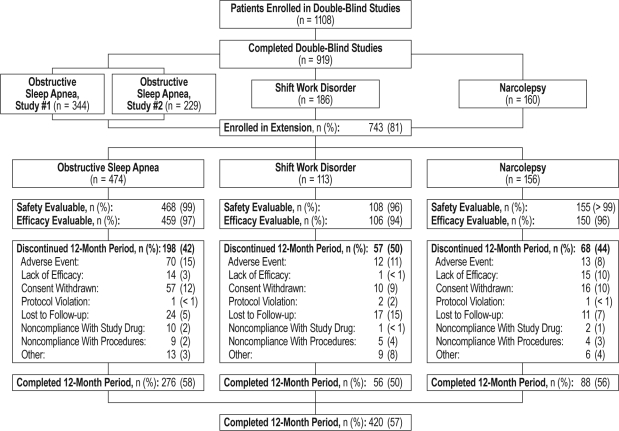
The study enrolled 743 patients: 474 with CPAP-treated OSA, 113 with SWD, and 156 with narcolepsy. Patients with treated OSA tended to be older, were more likely to be male, and had a greater body mass index (BMI) than those with SWD or narcolepsy (Table 1). Overall, 731 patients received ≥ 1 dose of study drug and were evaluable for safety; 715 were evaluable for efficacy; and 420 patients completed at least 12 months of treatment (Figure 1). During the 12-month period, 323 patients discontinued: the reasons for discontinuation included adverse event (13% [95/743]), withdrawal of consent (11% [83/743]), lost to follow-up (7% [52/743]), noncompliance with study drug or procedures (4% [31/743]), lack of efficacy (4% [30/743]), and protocol violation (< 1% [4/743]). There were no relevant differences in baseline demographic or clinical characteristics between those patients who discontinued during the 12-month period and those patients who were study completers.
Table 1.
Baseline patient demographics and clinical characteristics for safety analysis set
| Characteristic | OSA (n = 468) | SWD (n = 108) | Narcolepsy (n = 155) | Total (N = 731) |
|---|---|---|---|---|
| Age, year | ||||
| Mean (SD) | 50.2 (8.80) | 42.7 (9.89) | 38.9 (12.55) | 46.7 (10.97) |
| Rangea | 25–69 | 19–63 | 18–67 | 18–69 |
| Sex, n (%) | ||||
| Men | 343 (73) | 61 (56) | 70 (45) | 474 (65) |
| Women | 125 (27) | 47 (44) | 85 (55) | 257 (35) |
| Race, n (%) | ||||
| White | 404 (86) | 80 (74) | 115 (74) | 599 (82) |
| Black | 31 (7) | 21 (19) | 22 (14) | 74 (10) |
| Asian | 4 (< 1) | 1 (< 1) | 2 (1) | 7 (< 1) |
| Other | 28 (6) | 6 (6) | 10 (6) | 44 (6) |
| Missing | 1 (< 1) | 0 | 6 (4) | 7 (< 1) |
| BMI, kg/m2, mean (SD) | 36.9 (8.00) | 29.3 (6.97) | 29.1 (6.47) | 34.1 (8.40) |
| Use of concomitant therapy, n (%) | 456 (97) | 90 (83) | 135 (87) | 681 (93) |
| CPAP use, h/d, mean (SD) | 6.9 (1.05) | — | — | — |
| CGI-S, n (%) | ||||
| Moderately ill | 252 (54) | 55 (51) | 51 (33) | 358 (49) |
| Markedly ill | 144 (31) | 40 (37) | 75 (48) | 259 (35) |
| Severely ill | 64 (14) | 11 (10) | 27 (17) | 102 (14) |
| Extremely ill | 8 (2) | 2 (2) | 2 (1) | 12 (2) |
| ESS, mean (SD)b | 15.8 (3.52) | — | 16.9 (4.12) | — |
| BFI, mean (SD)c | ||||
| Global | 4.9 (1.85) | 5.1 (1.71) | 5.7 (1.98) | — |
| Worst | 7.2 (2.02) | 7.8 (2.01) | 7.8 (2.25) | — |
BFI refers to Brief Fatigue Inventory; BMI, body mass index; CGI-S, Clinical Global Impression of Severity of Illness; CPAP, continuous positive airway pressure; ESS, Epworth Sleepiness Scale; OSA, obstructive sleep apnea; SWD, shift work disorder.
Five patients over the age of 65 were granted a protocol exception and were enrolled in the study.
Baseline ESS scores were reported for 454 patients with treated OSA and 147 patients with narcolepsy.
Baseline BFI scores were reported for 459 patients with treated OSA, 106 patients with SWD, and 150 patients with narcolepsy.
Figure 1.
Patient disposition by diagnosis group for patients who completed the 12-month period
The mean number of days of exposure to armodafinil was 372.3 ± 241.5 (median: 369.0; range: 1 to 766 days). The most common dose used overall was 250 mg (in 67% of 731 patients), followed by 150 mg (14%), 200 mg (10%), 100 mg (8%), and 50 mg (< 1%).
At baseline, abnormal systolic blood pressure (SBP; ≥ 140 mm Hg) was recorded in 22% (103/468) of patients with treated OSA, 11% (12/108) with SWD, and 10% (15/155) with narcolepsy. Abnormal baseline diastolic blood pressure (DBP; ≥ 90 mm Hg) was recorded in 11% (52/468) of patients with treated OSA, 8% (9/108) with SWD, and 8% (12/155) with narcolepsy.
Tolerability
Adverse Events
Among all patients treated with at least 1 dose of study drug, the most commonly reported adverse events were headache (25% [180/731]), nasopharyngitis (17% [123/731]), insomnia (14% [99/731]), and upper respiratory tract infection (URTI; 10% [76/731]; Table 2). Most events were mild or moderate in intensity. Fifteen percent of enrolled patients discontinued during the entire study because of adverse events (18% [85/474] of those with treated OSA, 11% [12/113] of those with SWD, and 10% [16/156] of those with narcolepsy). The adverse events that led to discontinuation by at least 1 patient in each diagnostic group were headache (2% [18/731]) and anxiety (1% [10/731]). One death (associated with atherosclerotic heart disease) occurred in a patient with treated OSA and was considered unlikely to be related to the study drug by the investigator.
Table 2.
Most commonly reported adverse eventsa for safety analysis set
| Adverse Event, n (%) | OSA (n = 468) | SWD (n = 108) | Narcolepsy (n = 155) | Total (N = 731) |
|---|---|---|---|---|
| Headache | 114 (24) | 20 (19) | 46 (30) | 180 (25) |
| Nasopharyngitis | 71 (15) | 19 (18) | 33 (21) | 123 (17) |
| Insomnia | 76 (16) | 10 (9) | 13 (8) | 99 (14) |
| URTI | 55 (12) | 13 (12) | 8 (5) | 76 (10) |
| Nausea | 38 (8) | 7 (6) | 23 (15) | 68 (9) |
| Sinusitis | 52 (11) | 4 (4) | 8 (5) | 64 (9) |
| Arthralgia | 47 (10) | 4 (4) | 8 (5) | 59 (8) |
| Anxiety | 39 (8) | 9 (8) | 9 (6) | 57 (8) |
| Influenza | 39 (8) | 9 (8) | 7 (5) | 55 (8) |
| Back pain | 41 (9) | 5 (5) | 7 (5) | 53 (7) |
| Dry mouth | 30 (6) | 7 (6) | 12 (8) | 49 (7) |
| Dizziness | 31 (7) | 2 (2) | 14 (9) | 47 (6) |
| Cough | 23 (5) | 6 (6) | 13 (8) | 42 (6) |
| Hypertension | 31 (7) | 3 (3) | 8 (5) | 42 (6) |
OSA refers to obstructive sleep apnea; SWD, shift work disorder; URTI, upper respiratory tract infection.
Events reported by > 5% of the safety analysis set.
Serious adverse events were reported by 8% of the safety population: 10% (45/468) of those with treated OSA, 4% (4/108) of those with SWD, and 5% (7/155) of those with narcolepsy. Serious adverse events reported by more than 1 patient included chest pain (6 patients with treated OSA), myocardial infarction (4 patients with treated OSA), nephrolithiasis (4 patients with treated OSA), coronary artery disease (1 patient with treated OSA, 1 with narcolepsy), hemorrhoidal hemorrhage (1 patient with treated OSA, 1 with narcolepsy), cellulitis (2 patients with treated OSA), prostate cancer (2 patients with treated OSA), and hypertension (1 patient with treated OSA, 1 with narcolepsy). Ninety percent of serious adverse events (83/92) were considered by the investigator not or unlikely to be related to treatment.
Cardiovascular Adverse Events
Cardiac and vascular adverse events were reported in 10% (46/468) and 9% (41/468) of patients with treated OSA, 5% (5/108) and 6% (6/108) with SWD, and 7% (11/155) and 9% (14/155) with narcolepsy, respectively. Overall, 15 patients discontinued the study due to cardiac adverse events and 5 due to vascular adverse events. The most common cardiac adverse event was palpitations (3% [24/731]), and the most common vascular adverse event was hypertension (6% [42/731]): all other cardiac and vascular adverse events were reported in < 1% of patients over the entire study. While none of the palpitations cases were considered serious, 3 patients with treated OSA did discontinue the study due to this adverse event. Hypertension was considered a serious adverse event in 2 patients and led to the discontinuation of 3 patients (1 patient with narcolepsy, 2 patients with OSA).
Overall, mean values for SBP, DBP, and heart rate increased at final visit from baseline by 3.6 mm Hg for SBP, 2.3 mm Hg for DBP, and 6.7 beats per minute (bpm) for heart rate (Table 3). Increases were observed at month 1, and thereafter, from month 3 through month 18, the mean values remained within ± 2 mm Hg for SBP, ± 1 mm Hg for DBP, and ± 1 bpm for heart rate compared to month 3 values. Clinically relevant changes in BP, defined as an increase of ≥ 10% from baseline and a value above the predefined WHO threshold for hypertension, were found in all diagnostic groups. Clinically relevant increases in SBP were found in 34% (158/468) of patients with treated OSA, 18% (19/108) with SWD, and 20% (31/155) with narcolepsy. Twenty-four percent (113/468) of patients with treated OSA, 22% (24/108) with SWD, and 24% (37/155) with narcolepsy had clinically relevant increases in DBP. The mean heart rate increased by 6.8 bpm for patients with treated OSA, 4.3 bpm with SWD, and 8.1 bpm with narcolepsy. No patient had a change in heart rate that was reported to be clinically relevant (i.e., ≥ 120 bpm and increase ≥ 15 bpm relative to baseline).
Table 3.
Vital sign means at baseline and changes at final visit for safety analysis set
| Variable, Mean (SD) | OSA (n = 457) | SWD (n = 106) | Narcolepsy (n = 152) | Total (N = 715) |
|---|---|---|---|---|
| Heart rate, beats/min | ||||
| Baseline | 67.2 (9.49) | 67.7 (9.58) | 68.0 (11.32) | 67.4 (9.91) |
| Final visit | 74.0 (10.66) | 72.1 (9.67) | 76.2 (10.99) | 74.2 (10.65) |
| Change | 6.8 (11.14) | 4.3 (12.08) | 8.1 (12.25) | 6.7 (11.57) |
| SBP, mm Hg | ||||
| Baseline | 127.8 (14.24) | 121.7 (16.27) | 119.6 (14.13) | 125.2 (14.94) |
| Final visit | 131.3 (13.60) | 123.3 (14.79) | 124.4 (15.61) | 128.6 (14.64) |
| Change | 3.6 (16.31) | 1.4 (13.68) | 5.0 (15.40) | 3.6 (15.77) |
| DBP, mm Hg | ||||
| Baseline | 78.0 (9.01) | 76.2 (9.62) | 74.4 (9.99) | 76.9 (9.41) |
| Final visit | 79.9 (9.30) | 78.6 (9.63) | 77.9 (9.39) | 79.3 (9.39) |
| Change | 2.0 (10.35) | 2.3 (10.03) | 3.5 (9.77) | 2.3 (10.19) |
DBP refers to diastolic blood pressure; OSA, obstructive sleep apnea; SBP, systolic blood pressure; SWD, shift work disorder.
Twelve patients had ECG abnormalities reported as adverse events, and 2 patients withdrew because of these events; none were considered serious by the study investigator.
Other Tolerability Evaluations
There were no clinically meaningful changes in mean clinical laboratory results or physical examination. Five patients had clinically relevant abnormal chemistry values reported as adverse events (3 of whom withdrew because of elevated liver function tests), and 3 patients had abnormal hematology values as adverse events. None of these values were considered serious by the study investigator.
For patients with OSA, in general, CPAP therapy use remained high during the study. CPAP use was 6.9 ± 1.05 h/night at baseline and 6.2 ± 1.28 h/night at final visit.
Efficacy
CGI-C
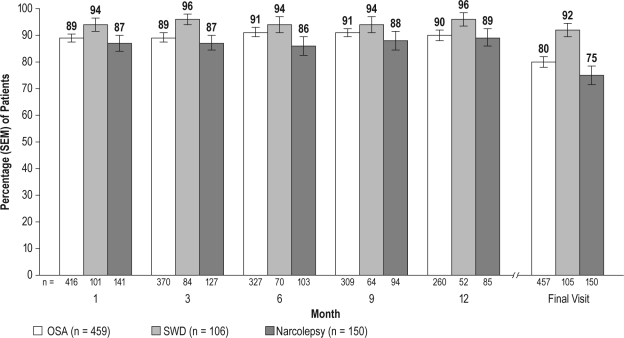
Minimal or greater improvement, compared with baseline, on the CGI-C was reported by most patients in the 3 diagnostic groups (75% to 92%) at final visit; patients in the SWD group reported the greatest improvement. Improvements in clinical condition for the treated OSA and narcolepsy groups and in ES during the night shift, including the commute to and from work, in the SWD group were reported early (at month 1) and were sustained through month 12 and at the final visit (Figure 2). Substantial improvement (“much” or “very much improved”) was reported at the final visit by 65% (295/457) of patients with treated OSA (95% CI: 60.2, 68.9), 88% (92/105) with SWD (95% CI: 81.3, 93.9), and 62% (93/150) with narcolepsy (95% CI: 54.2, 69.8).
Figure 2.
Percentage of patients with at least minimal improvement on the Clinical Global Impression of Change (CGI-C) across visits
OSA refers to obstructive sleep apnea; SWD, shift work disorder.
ESS
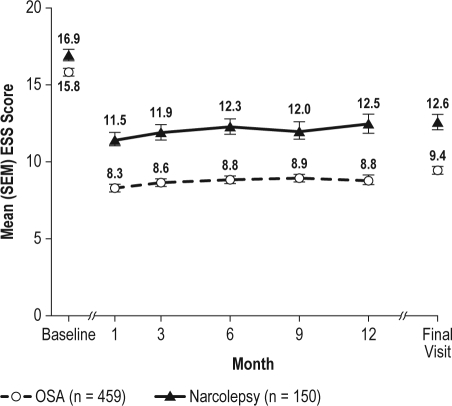
Armodafinil improved wakefulness, as measured by the ESS in the treated OSA and narcolepsy groups at all follow-up visits compared with baseline (Figure 3). At baseline, the proportion of patients with a normal ESS score (i.e., < 10) was 0.4% (2/454) in the treated OSA group and 3.4% (5/147) in the narcolepsy group. At the final visit, mean ESS score was reduced by 6.4 (95% CI: −6.90, −5.94) in the treated OSA group and by 4.3 (95% CI: −5.20, −3.49) in the narcolepsy group. The proportion of patients with an ESS score < 10 at final visit was 54.8% (249/454) for treated OSA and 31.3% (46/147) for narcolepsy.
Figure 3.
Epworth Sleepiness Scale (ESS) total scores (mean ± SEM) across visits
OSA refers to obstructive sleep apnea; SEM, standard error of mean.
BFI
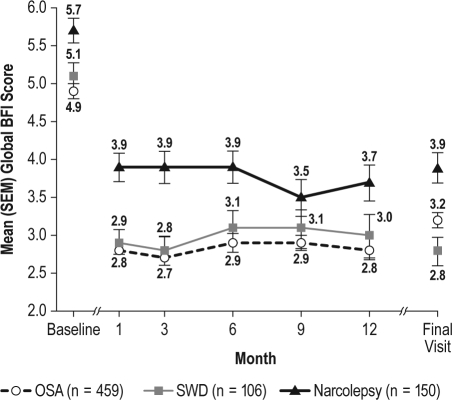
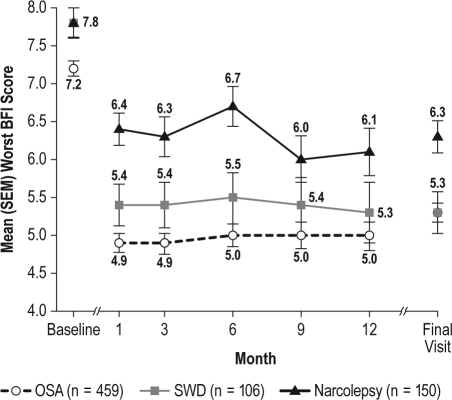
The level of fatigue and its impact on daily activities was consistently reduced from baseline, at all visits, in each of the diagnostic groups, as measured by global and worst BFI scores (Figures 4 and 5). At final visit, mean global scores were reduced by 1.7 (95% CI: −1.88, −1.43) in the treated OSA group, 2.3 (95% CI: −2.75, −1.87) in the SWD group, and 1.7 (95% CI: −2.13, −1.35) in the narcolepsy group; mean worst fatigue scores were reduced by 1.8 (95% CI: −2.13, −1.57) in the treated OSA group, 2.4 (95% CI: −3.06, −1.83) in the SWD group, and 1.5 (95% CI: −2.00, −1.07) in the narcolepsy group.
Figure 4.
Global Brief Fatigue Inventory (BFI) scores (mean ± SEM) across visits
OSA refers to obstructive sleep apnea; SEM, standard error of mean; SWD, shift work disorder.
Figure 5.
Worst Brief Fatigue Inventory (BFI) scores (mean ± SEM) across visits
OSA refers to obstructive sleep apnea; SEM, standard error of mean; SWD, shift work disorder.
Among observed cases of study completers, numerical values for all efficacy outcomes remained stable across individual visits (data not shown).
DISCUSSION
Treatment of ES associated with CPAP-treated OSA, SWD, or narcolepsy typically requires long-term management. This study is the first to report on the efficacy and tolerability of armodafinil in patients with ES associated with these conditions treated for up to 2 years.
Armodafinil was generally well tolerated in this long-term, open-label study. Most adverse events were mild to moderate in intensity; the most commonly reported events included headache, nasopharyngitis, insomnia, and URTI. Incidences of adverse events were similar across diagnostic groups. CPAP use among patients with OSA remained high, but there was a slight decrease over the period of the study, which reinforces the need for routine assessment of CPAP adherence of all patients with OSA. This constitutes best clinical practice.21 The incidence of withdrawals associated with adverse events was 8% to 15% (13% overall) during the 12-month period, compared with 4% to 9% in the 12-week, double-blind studies.16–18
Modest, yet consistent, increases in mean vital sign values were noted in all diagnostic groups, and clinically relevant blood pressure changes were more common in patients with treated OSA compared with patients with narcolepsy or SWD. Increases in mean vital sign values in patients receiving armodafinil compared with placebo were reported previously in a double-blind study of patients with ES associated with treated OSA.18 In the double-blind studies, patients with ES associated with treated OSA who received placebo had clinically relevant increases of 34% and 26% in SBP and DBP, respectively. Among patients who received placebo in the double-blind narcolepsy or SWD studies, 14% of both populations had a clinically relevant increase in SBP, and 16% of the narcolepsy group and 20% of the SWD group had a clinically relevant increase in DBP.
The mechanism underlying these changes is unknown, although preexisting abnormalities in blood pressure (noted in approximately 10% to 20% of patients at baseline) may have played a role. The changes in vital signs are consistent with the known safety profile of armodafinil, and increased monitoring of blood pressure may be warranted following armodafinil treatment in patients at risk for cardiovascular disorders—in particular those patients with treated OSA.
The higher rate of adverse event-related withdrawals, serious events, and changes in blood pressure among patients with treated OSA may reflect the cardiovascular risk factors noted at baseline, including advanced age and high BMI, as well as the well-recognized comorbidities associated with this condition. Cardiac and vascular events, although noted in all groups, were the most common serious adverse events (10/45) reported in the treated OSA group, as might be expected given the well-established link between OSA and cardiovascular disease.22,23 Hypertension, hypercholesterolemia, type 2 diabetes, and ischemic heart conditions were the most common comorbid illnesses noted in a database composed of > 60,000 inpatients with OSA.24Atherosclerosis has been associated with OSA, independent of other risk factors, including obesity.25
Armodafinil remained effective during this study. All 3 diagnostic groups reported high levels of ES and fatigue at baseline that had improved by month 1 and remained improved during the study. In patients with CPAP-treated OSA, the average baseline level of ES, as measured by the ESS score (mean = 15.8), was high and decreased to a level that was within normal range at month 1 (8.3) and at all subsequent visits (8.6 to 8.9).26 Improvement in overall clinical condition and reduction in fatigue were also substantial in the treated OSA group.
The intermittent use of armodafinil in patients with SWD did not appear to diminish its efficacy. Patients with SWD reported baseline levels of fatigue on the BFI similar to those reported by patients with treated OSA, and demonstrated similar reductions in fatigue throughout the study. In the SWD group, 88% of patients were rated as “very much” or “much improved” at final visit on the CGI-C. The CGI-C was used to measure change in ES only (during the night shift) in the SWD group, whereas in the other groups it was used to measure improvement in general clinical condition.
Patients with narcolepsy reported the most severe ES and fatigue at baseline, as reflected in the ESS and BFI measures. The mean ESS score, which was high at baseline (16.9), improved considerably over the course of the study but did not achieve a value at follow-up visits that is considered normal. At baseline, only 3.4% had an ESS score < 10, while 31.3% had a score in this range at final visit. Mean worst fatigue score was also severe at baseline (7.8; ≥ 7 is severe),21 but was reduced to a moderate level at month 1 (6.4), with improvements sustained throughout the study. Mean changes in efficacy measures were generally somewhat less in the narcolepsy group than those seen in the treated OSA or SWD groups. This reflects the more severe levels of ES observed in patients with narcolepsy.20 Despite this, most patients were “much” or “very much improved” at final visit.
The results of this long-term, open-label study are consistent with those of the double-blind studies that preceded it.15–18 The proportions of patients with improvements on the CGI-C and the mean changes on the ESS and BFI suggest that efficacy was generally well maintained in most patients who enrolled in the study. This is supported by the finding that only 4% of patients withdrew due to lack of efficacy during the 12-month period, while < 1% withdrew during the period of treatment beyond 12 months. Efficacy results at final visit (i.e., all postbaseline data included) are consistent with those of patients who had a 12-month visit, suggesting maintenance of effectiveness.
The limitations of this study include its open-label design and use of subjective measures only, both of which could have biased results in favor of overrated efficacy. Additionally, because only patients who completed the double-blind studies were enrolled, those patients who had a tolerable response and meaningful improvement on the study medication were more likely to be recruited. Only a double-blind, placebo-controlled study that does not restrict enrollment to completers of a previous study and that incorporates both objective and subjective measures can truly discern long-term efficacy and tolerability. Unfortunately, these types of studies are not often feasible because of practical and ethical concerns related to long-term administration of placebo or difficulties in patient recruiting.
In conclusion, throughout this long-term study, armodafinil consistently improved subjective wakefulness and reduced fatigue of patients with ES associated with treated OSA, SWD, or narcolepsy. Armodafinil was also generally well tolerated whether used daily (by patients with treated OSA or narcolepsy) or intermittently (by those with SWD) for up to 2 years. Monitoring for changes in blood pressure may be appropriate in patients, especially those with treated OSA, being treated with armodafinil. Armodafinil represents an effective treatment for the chronic management of ES for many patients with these sleep disorders.
DISCLOSURE STATEMENT
This study was supported by Cephalon, Inc. Dr. Black has received investigator-initiated research support from Cephalon, Inc., Jazz Pharmaceuticals, GlaxoSmithKline, and Sanofi-Aventis. He is currently employed by Actelion Pharmaceuticals and is a consulting professor at Stanford University. Dr. Hull has disclosed that he has received grants for clinical research from Pfizer, Neurocrine, Somaxon, Cephalon, Takeda, Sanofi-Aventis, Merck, and Sepracor. Dr. Hull has also disclosed that he has served as an advisor or consultant to Pfizer, Neurocrine, Somaxon, Cephalon, Takeda, Sanofi-Aventis, Merck, and Sepracor, and has served on the speakers' bureau for Sepracor and Cephalon. Both Drs. Tiller and Yang are Cephalon employees. Dr. Harsh reports no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was sponsored by Cephalon, Inc., Frazer, PA. Funding for editorial support was provided by Cephalon, Inc., to the Curry Rockefeller Group, LLC, Tarrytown, NY. Editorial support was provided by Debbie Due, Ph.D., and the Curry Rockefeller Group.
The authors would also like to acknowledge all of the centers that participated in this study. In the USA: Aaron, Joshua, M.D., Elkton, Maryland; Ahmed, Mansoor, M.D., F.C.C.P., Middleburg Heights, Ohio; Asnis, Gregory, M.D., Bronx, New York; Bastani, Bijan, M.D., Beechwood, Ohio; Biber, Michael, M.D., Newton, Massachusetts; Black, Jed, M.D., Stanford, California; Bogan, Richard, M.D., Columbia, South Carolina; Bonnet, Michael, Ph.D., Dayton, Ohio; Burns, Gerald, M.D., A.P.M.C., Metairie, Louisiana; Candal, Francisco, M.D., Slidell, Louisiana; Cook, James, M.D., Danville, Indiana; Corser, Bruce, M.D., Cincinnati, Ohio; Dorsey, Cynthia, Ph.D., Belmont, Massachusetts; Duntley, Stephen, M.D., St. Louis, Missouri; Dyken, Mark, M.D., Iowa City, Iowa; Emsellem, Helene, M.D., Chevy Chase, Maryland; Erman, Milton, M.D., San Diego, California; Fakih, Faisal, M.D., Winter Park, Florida; Feldman, Neil, M.D., St. Petersburg, Florida; Ferguson, James, M.D., Salt Lake City, Utah; Flescher, Jonathan, M.D., Raleigh, North Carolina; Foldvary, Nancy, D.O., Cleveland, Ohio; Fried, David, M.D., Warwick, Rhode Island; Furman, Yury, M.D., Los Angeles, California; Haberman, Paul, M.D., Santa Monica, California; Hansbrough, James, M.D., Ph.D., Bowling Green, Kentucky; Harris, Barbara, Ph.D., Phoenix, Arizona; Harsh, John, Ph.D., Hattiesburg, Mississippi; Haynes, J. Brevard, M.D., Nashville, Tennessee; Hill, Dennis, M.D., Salisbury, North Carolina; Hirshkowitz, Max, Ph.D., D.A.B.S.M., Houston, Texas; Hudson, John, M.D., Austin, Texas; Hull, Steven, M.D., Overland Park, Kansas; Jiménez, Lissette, M.D., San Juan, Puerto Rico; Kaelin, Thomas Jr., D.O., Charlestown, South Carolina; Kriengkairut, Siriwan, M.D., Bismarck, North Dakota; Krystal, Andrew, M.D., Durham, North Carolina; Lahmeyer, Henry, M.D., Northfield, Illinois; Laman, David, M.D., Pittsburgh, Pennsylvania; Lankford, D. Alan, Ph.D., Atlanta, Georgia; Leeds, William, D.O., Topeka, Kansas; Leo, Gary, D.O., Wauwatosa, Wisconsin; Levin, Stuart, M.D., Raleigh, North Carolina; Levine, Bernard, M.D., Phoenix, Arizona; Loewy, Derek, Ph.D., Tucson, Arizona; Lorch, Daniel, M.D., Brandon, Florida; Marcus, Richard, M.D., Hickory, North Carolina; Mark, Burton, D.O., West Chester, Pennsylvania; Mayleben, David, Ph.D., Crestview, Kentucky; Medoff, Jeffrey, M.D., Greensboro, North Carolina; Menn, Stuart, M.D., Palm Springs, California; Murphy, John, M.D., Beverly Hills, California; Neeb, Michael, Ph.D., Toledo, Ohio; Pascualy, Ralph, M.D., Seattle, Washington; Pegram, Vernon Jr., Ph.D., Birmingham, Alabama; Pellegrino, Richard, M.D., Ph.D., Hot Springs, Arkansas; Pinto, John, M.D., Las Vegas, Nevada; Richter, Ralph, M.D., F.A.C.P., Tulsa, Oklahoma; Riff, Dennis, M.D., F.A.C.G., Anaheim, California; Rosenberg, Russell, Ph.D., Atlanta, Georgia; Rosenthal, Murray, D.O., San Diego, California; Scharf, Martin, Ph.D., Cincinnati, Ohio; Schmidt, Markus, M.D., Ph.D., Dublin, Ohio; Schmidt-Nowara, Wolfgang, M.D., Plano, Texas; Simmons, Jerald, M.D., The Woodlands, Texas; Simon, Stuart, M.D., Marietta, Georgia; Smith, J. Baldwin III, M.D., Winston-Salem, North Carolina; Sweer, Leon, M.D., F.C.C.P., Carlisle, Pennsylvania; Swick, Todd, M.D., Houston, Texas; Thein, Stephen, Ph.D., San Diego, California; Wells, Charles Jr., M.D., Macon, Georgia; Whitten, Patrick, M.D., Peoria, Illinois; Winslow, David, M.D., Louisville, Kentucky; Zammit, Gary, Ph.D., New York, New York.
In Canada: Alexander, Michael, M.D., Niagara Falls, Ontario; Botros, Wagdy, F.R.C.P., Kitchener, Ontario; Kayumov, Leonid, Ph.D., Scarborough, Ontario; Leech, Judith, M.D., Ottawa, Ontario; Mamelak, Mortimer, M.D., Toronto, Ontario; Moscovitch, Adam, M.D., Calgary, Alberta; Reinish, Lawrence, M.D., Parry Sound, Ontario; Shapiro, Colin, Ph.D., M.R.C.P., F.R.C.P., Toronto, Ontario.
In France: Dauvilliers, Yves, M.D., Montpellier; Escourrou, Pierre, M.D., Clamart; d'Ortho, Marie-Pia, M.D., Ph.D., Creteil; Krieger, Jean, M.D., Strasbourg.
In Australia: Cistulli, Peter, M.B.B.S., F.R.A.C.P., Ph.D., F.C.C.P., MBA, Kogarah, New South Wales; Desai, Anup, M.B.B.S., Camperdown, New South Wales; Ho, Michael, M.B.B.S., Clayton, Victoria; Swieca, John, M.B.B.S., F.R.A.C.P., East Melbourne, Victoria; Wheatley, John R., Ph.D., M.B.B.S., F.R.A.C.P., Westmead, New South Wales.
In Germany: Becker, Heinrich, LPK, Marburg; Geisler, Peter, Ph.D., Regensburg; Mayer, Geert, M.D., Schwalmstadt; Voderholzer, Ulrich, M.D., Freiburg.
In The Russian Federation: Kalinkin, Alexander, M.D., Ph.D., Moscow; Levin, Yakov, M.D., Ph.D., Moscow; Oganesyan, Genrikh, M.D., Ph.D., Saint Petersburg; Zakharov, Alexander, Samara.
REFERENCES
- 1.Lee-Chiong T. Manifestations and classifications of sleep disorders. In: Lee-Chiong T, Sateia M, Carskadon M, editors. Sleep medicine. Philadelphia: Hanley & Belfus, Inc.; 2002. pp. 125–41. [Google Scholar]
- 2.American Academy of Sleep Medicine. 2nd edition. Westchester: American Academy of Sleep Medicine; 2005. International classification of sleep disorders: diagnostic and coding manual. [Google Scholar]
- 3.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 4.Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163:565–71. doi: 10.1001/archinte.163.5.565. [DOI] [PubMed] [Google Scholar]
- 5.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–9. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–62. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz JR, Roth T. Shift work sleep disorder: burden of illness and approaches to management. Drugs. 2006;66:2357–70. doi: 10.2165/00003495-200666180-00007. [DOI] [PubMed] [Google Scholar]
- 8.Hublin C, Partinen M, Kaprio J, Koskenvuo M, Guilleminault C. Epidemiology of narcolepsy. Sleep. 1994;17:S7–12. doi: 10.1093/sleep/17.suppl_8.s7. [DOI] [PubMed] [Google Scholar]
- 9.Green PM, Stillman MJ. Narcolepsy. Signs, symptoms, differential diagnosis, and management. Arch Fam Med. 1998;7:472–8. doi: 10.1001/archfami.7.5.472. [DOI] [PubMed] [Google Scholar]
- 10.Fransen M, Wilsmore B, Winstanley J, et al. Shift work and work injury in the New Zealand Blood Donors' Health Study. Occup Environ Med. 2006;63:352–8. doi: 10.1136/oem.2005.024398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hillman DR, Murphy AS, Antic R, Pezzullo L. The economic cost of sleep disorders. Sleep. 2006;29:299–305. doi: 10.1093/sleep/29.3.299. [DOI] [PubMed] [Google Scholar]
- 12.Omachi TA, Claman DM, Blanc PD, Eisner MD. Obstructive sleep apnea: a risk factor for work disability. Sleep. 2009;32:791–8. doi: 10.1093/sleep/32.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 14.Darwish M, Kirby M, Hellriegel ET, Robertson P. Armodafinil and modafinil have substantially different pharmacokinetic profiles despite having the same terminal half-lives. Clin Drug Investig. 2009;29:613–23. doi: 10.2165/11315280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Czeisler CA, Walsh JK, Wesnes KA, Arora S, Roth T. Armodafinil for treatment of excessive sleepiness associated with shift work disorder: a randomized controlled study. Mayo Clin Proc. 2009;84:958–72. doi: 10.1016/S0025-6196(11)60666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harsh JR, Hayduk R, Rosenberg R, et al. The efficacy and safety of armodafinil as treatment for adults with excessive sleepiness associated with narcolepsy. Curr Med Res Opin. 2006;22:761–74. doi: 10.1185/030079906X100050. [DOI] [PubMed] [Google Scholar]
- 17.Hirshkowitz M, Black JE, Wesnes K, Niebler G, Arora S, Roth T. Adjunct armodafinil improves wakefulness and memory in obstructive sleep apnea/hypopnea syndrome. Respir Med. 2007;101:616–27. doi: 10.1016/j.rmed.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Roth T, White D, Schmidt-Nowara W, et al. Effects of armodafinil in the treatment of residual excessive sleepiness associated with obstructive sleep apnea/hypopnea syndrome: a 12-week, multicenter, double-blind, randomized, placebo-controlled study in nCPAP-adherent adults. Clin Ther. 2006;28:689–706. doi: 10.1016/j.clinthera.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Guy W, editor. Clinical global impressions. Rockville: National Institute of Mental Health; 1976. ECDEU assessment manual for psychopharmacology; pp. 217–22. [Google Scholar]
- 20.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 21.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–96. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 22.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 23.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 24.Huang QR, Qin Z, Zhang S, Chow CM. Clinical patterns of obstructive sleep apnea and its comorbid conditions: a data mining approach. J Clin Sleep Med. 2008;4:543–50. [PMC free article] [PubMed] [Google Scholar]
- 25.Minoguchi K, Yokoe T, Tazaki T, et al. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:625–30. doi: 10.1164/rccm.200412-1652OC. [DOI] [PubMed] [Google Scholar]
- 26.Johns M, Hocking B. Daytime sleepiness and sleep habits of Australian workers. Sleep. 1997;20:844–9. doi: 10.1093/sleep/20.10.844. [DOI] [PubMed] [Google Scholar]