Abstract
Study Objectives:
The aims of this study were to: (a) assess the prevalence of diagnosed OSA and symptoms of undiagnosed OSA in a cohort of ambulatory surgical patients, and (b) characterize the frequency of postoperative complications in outpatients with a diagnosis of or a propensity to OSA.
Methods:
Patients presenting for ambulatory surgery completed a self-administered questionnaire. Using a previously validated prediction model, the probability for OSA was determined. Patients with ≥ 70% propensities were considered to be at high risk of having the disorder. Relevant perioperative data and complications were tracked and recorded, and differences in median estimated propensities for OSA were considered by these data.
Results:
Three-thousand five hundred fifty-three patients completed the preoperative survey. A total of 2139 patients had perioperative data and estimated propensity scores. Ninety-four of the 2139 (4.4%) patients gave a self-reported prior diagnosis of OSA. One hundred three (4.8%) patients were found to be at high risk of OSA based on the survey and prediction model. Seventy-five percent of the patients with > 70% propensity for OSA had not yet been diagnosed. There was no association between OSA propensity scores and unplanned hospital admission, however there was an association of increased propensity with difficult intubation, intraoperative use of pressors, and postoperative oxygen saturation in the PACU.
Conclusion:
The results of this study suggest that undiagnosed OSA may be relatively common in an ambulatory surgical population. There was no relationship between unplanned hospital admission and diagnosis of or increased risk of OSA. However, there was an association of increased perioperative events requiring additional anesthetic management in patients with a diagnosis of, or with a higher propensity to OSA.
Citation:
Stierer TL; Wright C; George A; Thompson RE; Wu CL; Collop N. Risk assessment of obstructive sleep apnea in a population of patients undergoing ambulatory surgery. J Clin Sleep Med 2010;6(5):467-472.
Keywords: Outpatient, sleep apnea, anesthesia, outcome, complications
Obstructive sleep apnea (OSA) has been recognized as a potential independent risk factor for adverse perioperative outcome.1 Although the prevalence of OSA in the general population has been estimated to be between 2% to 4%, with a higher incidence in certain subpopulations such as males and obese subjects, many people with OSA have not been diagnosed.2,3 The actual prevalence of OSA in the general population may be higher with as many as 80% to 90% of patients with the disease being undiagnosed.2 While findings on physical examination such as cricomental space ≤ 1.5 cm, pharyngeal grade > II, and presence of overbite may be predictive of patients with OSA, otorhinolaryngological evaluation of anatomic parameters may not be sufficient to distinguish OSA patients from snoring non-OSA patients.4,5 The gold standard for the diagnosis of OSA, polysomnography (PSG), is impractical as a routine preoperative assessment tool for OSA as the test is expensive and labor intensive.
Approximately 35 million patients undergo ambulatory surgical procedures annually in the United States (US).6 Determining the prevalence of OSA in an ambulatory surgical population could be particularly important as these patients are scheduled to be discharged to an unmonitored setting (i.e., home) following the procedure. The physiologic perturbations resulting from anesthesia and postoperative analgesics are believed to place OSA patients undergoing outpatient surgery at risk for perioperative morbidity and mortality. Compared to non-OSA patients, OSA patients undergoing surgical procedures are vulnerable to postoperative airway obstruction, myocardial ischemia, congestive heart failure, stroke, and oxygen desaturation.1,7 Although the American Society of Anesthesiologists (ASA) has created guidelines for perioperative care of OSA patients, the consultants were equivocal regarding whether even superficial procedures could be performed on an outpatient basis in patients who were either diagnosed with OSA or at risk for OSA.8 Moreover, they concurred that the literature is insufficient to be able to support evidence based guidelines that delineate which surgical patients at risk for OSA can be safely managed on an outpatient basis. Because of the lack of data on the prevalence of OSA in patients undergoing ambulatory surgery, we designed a prospective observational study to define the prevalence of OSA symptoms in this population using a previously validated self-report instrument.9 Additionally, outcome data was recorded in order to correlate the frequency of adverse events in patients at risk for OSA.
BRIEF SUMMARY
Current Knowledge/Study Rationale: The prevalence of patients at risk for OSA presenting for ambulatory surgical procedures at an academic medical center has not been previously well defined. This study sought to identify these patients as well as assess potential associations of propensity for OSA with adverse outcomes.
Study Impact: This study suggests that patients with previously unrecognized risk for OSA frequently present to our center for a wide variety of ambulatory surgical procedures. While current guidelines are equivocal regarding the safety of discharge of these patients to a unmonitored setting, study subjects at risk for OSA were safely managed as outpatients at our center.
METHODS
This study was approved by the Johns Hopkins University Institutional Research Review board, and written informed consent was obtained from all patients enrolled. Patients undergoing outpatient surgery were enrolled from May 2004 to May 2006. The Johns Hopkins Outpatient Surgery Center is an integrated separate facility connected to the main inpatient hospital in downtown Baltimore, Maryland. The catchment area is the surrounding urban and suburban populations. Consecutive patients 18 years and older completed a self-administered questionnaire to assess demographics (age, gender, race, body mass index [BMI]) and sleep disturbance symptoms. History of angina, myocardial infarction, stroke, heart failure, and coronary artery revascularization was recorded based on self-report. Frequency of sleep related symptoms (e.g., snoring, witnessed apneas) was recorded on a 6-point Likert Scale (never, rarely, sometimes, often, usually, and always). Procedures included were otolaryngological, gynecological, orthopedic, plastic, general, neurological, and urological surgeries. Patients were excluded if they were pregnant, required supplemental home oxygen, or had a tracheostomy. Patients were considered at high risk for OSA if they had a ≥ 70% calculated probability based on demographic and questionnaire data.
Perioperative Management
Anesthetic care was provided by faculty anesthesiologists often supervising residents or nurse anesthetists. Each anesthesiologist conducted the usual preoperative assessment and physical exam, and was unaware of the study survey results. The anesthetic plan was determined by the attending anesthesiologist independent of the patient's participation in the investigation. Routine ASA monitoring was employed. The following intraoperative data was collected by a trained research assistant not involved in the patient's perioperative care using a predesigned data collection sheet: choice of anesthetic technique, changes in anesthetic plan (i.e., need to alter airway management from the original plan because of the inability to adequately ventilate the patient's lungs), difficulty of endotracheal intubation, including use of fiberoptic endoscopy, and vasoactive medications administered. Data recorded included the use of an oral airway or nasal trumpet during conscious sedation, as well as progression to unplanned placement of laryngeal mask airway or endotracheal intubation. Difficulty with tracheal intubation was assessed as multiple attempts at laryngoscopy, or inability to intubate secondary to poor visualization of anatomic structures.
Postoperatively, patients were transferred from the operating suite to the post-anesthesia care unit with supplemental oxygen delivered by face mask. Patients were weaned to room air immediately after transfer if oxygen saturation > 95% was maintained. Appropriateness of discharge was determined per usual criteria using a modified Aldrete Scoring System.10 The following data were documented during the postoperative course: (1) supplemental oxygen requirement > 0.40 FiO2 to maintain oxygen saturation > 95%; (2) occurrence of cardiac arrhythmias including ≥ 3 consecutive premature ventricular contractions (PVC) or 6 PVCs per minute, bradycardia (heart rate < 50/min), or tachycardia (heart rate > 100 beats/min); (3) administration of naloxone; (4) need for assisted lung ventilation; (5) re-intubation of the trachea; and (6) unplanned hospital admission. Postoperative phone calls were made to each patient's home 24 hours postoperatively to ascertain: (7) re-admission within 24 hours of discharge; and (8) perioperative cardiac symptoms, stroke, or death within 24 hours.
Data analysis was performed using STATA version 9.0 (StataCorp. 2005. College Station, TX). Summary statistics were calculated for the demographic variables, and group differences were assessed using the Student t-test for continuous measures and the nonparametric Kruskal-Wallis for continuous measures that were non-normal, and the χ2 goodness-of-fit test for categorical data. The probability of OSA was calculated from questions related to sleep disturbance using the multivariable logistic regression model validated by Maislin in patients who underwent polysomnography (Appendix 1). This index calculates the probability of a patient having OSA based on the patient's BMI, age, gender, and average index score of sleep disturbance.9 Propensity for OSA was determined for each patient with complete BMI, age, gender, and sleep disturbance data.
RESULTS
Preoperative screens were completed in 3553 consecutive subjects; perioperative data and propensity data were available for 2139 of these patients. Subsequent analyses are based on the latter number. A summary of patient demographic characteristics and medical history are given in Table 1 for the overall sample as well as by risk of OSA, as based on the propensity model. Patients ranged in age from 19 to 94 years. Ethnic breakdown was as follows: Caucasian (70.80%), African American (21.7%), Asian (4.1%), Hispanic (1.7%), American Indian (0.40%), and other (1.50%). Females (68.2%) who were enrolled were younger than males (mean of 46.8 y vs. 51.0 y, p < 0.001). From Table 1, we see that those with an OSA propensity ≥ 70% tended to be older (p < 0.001), have a higher BMI (p < 0.001), and were more likely to have had a CABG (p < 0.001). There was also a marginally significant association between race and risk of OSA (p = 0.020), with more African American (35.9% v. 20.9%) and fewer Caucasians (62.1% v. 71.2%) in the high OSA risk group. Ninety-four patients reported having been told that they have or had sleep apnea. Using the logistic regression model, 9.8% of men and 2.5 % of women were found to have > 70% probability of OSA. Among these at-risk patients, only 31.3% (21/67) of the males and 22.2% (8/36) of the females gave a previous self-reported diagnosis of OSA. However, of the 4 patients with an OSA risk probability ≥ 0.90, all had a self-reported diagnosis of sleep apnea. In contrast, 729 patients had a deduced OSA probability ≤ 0.10. Of these low-risk patients, only 3 (0.4%) had a self-reported diagnosis of OSA.
Table 1.
Demographic variables by OSA propensity and overall cohort
| Demographic Variable | < 70% Propensity (N = 2036) | ≥ 70% Propensity (N = 103) | Overall Cohort (N = 2139) |
|---|---|---|---|
| Age y [mean (SD)]1 | 47.6 (15.5) | 58.7 (14.5)* | 48.1 (15.7) |
| BMI [mean (SD)]1 | 26.8 (5.5) | 39.8 (8.1)* | 27.4 (6.3) |
| Race (% Caucasian)2 | 71.20% | 62.10%* | 70.80% |
| MI (% yes)3 | 1.5% | 6.9%* | 1.74% |
| Stroke (% yes)3 | 1.9% | 4.00%* | 2.00% |
| CHF (% yes)3 | 0.8% | 2.00%* | 0.90% |
| Angina (% yes)3 | 1.68% | 4.00% | 1.79% |
| CABG (% yes)3 | 0.99% | 2.00%* | 1.03% |
| PTCA (% yes)3 | 1.28% | 4.00% | 1.41% |
p < 0.05 when compared to < 70 % propensity; SD, standard deviation; BMI, body mass index; MI, myocardial infarction; CHF, congestive heart failure; CABG, coronary artery bypass graft; PTCA, percutaneous transluminal coronary angioplasty
There were no unplanned admissions for the patients who had a self-reported diagnosis of OSA. Furthermore, there was no association between OSA propensity scores obtained using the Maislin predictive model with the 11 patients who required unplanned hospital admission. Reasons for unplanned hospital admissions included need for continuous bladder irrigation (3), surgery more extensive than planned (1), observation of flap (2), pain management (1), dizziness (2), chest pain (1), and postoperative bleeding (1).
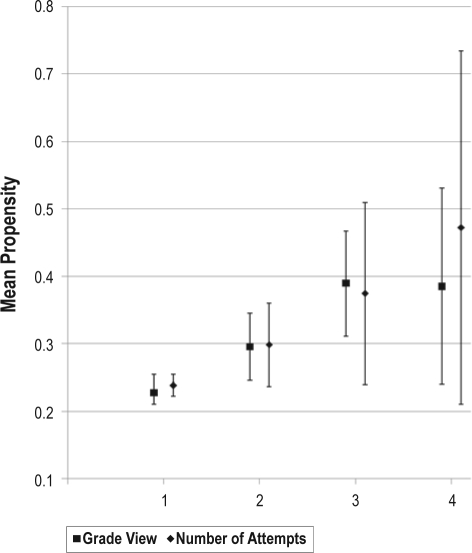
Table 2 shows the association of median propensities for OSA and variables related to airway assessment and management. Mallampati scores, decreased neck range of motion, numbers of attempts required for tracheal intubation and poor view of vocal cords with direct laryngoscopy were associated with increasing OSA propensity scores. Figure 1 shows number of intubation attempts and grade view based on mean propensity scores. Fiberoptic intubation was also associated with higher median propensity scores, while there was no correlation between median propensity and intraoperative change in anesthetic management (See Table 2).
Table 2.
Airway assessment and intraoperative airway management by median propensity to OSA
| Variable | Median Propensity | p value | n1 |
|---|---|---|---|
| Mallampati | |||
| I | 0.129 | < 0.001 | |
| II | 0.213 | ||
| III | 0.313 | ||
| IV | 0.515 | ||
| Neck Extension | |||
| Normal | 0.167 | < 0.001 | |
| Moderately decreased | 0.337 | ||
| Severely decreased | 0.309 | 4 | |
| Neck Flexion | |||
| Normal | 0.170 | 0.015 | |
| Moderately decreased | 0.328 | ||
| Severely decreased | 0.101 | ||
| Number of attempts at laryngoscopy | |||
| 1 | 0.154 | 0.001 | |
| 2 | 0.250 | ||
| 3 | 0.344 | ||
| 4 | 0.504 | 5 | |
| Laryngoscopic Grade View | |||
| 1 | 0.150 | < 0.001 | |
| 2 | 0.224 | ||
| 3 | 0.383 | ||
| 4 | 0.400 | ||
| Fiberoptic Bronchoscopy | |||
| No | 0.160 | 0.010 | |
| Yes | 0.494 | 10 | |
| Stylette Needed | |||
| No | 0.163 | 0.100 | |
| Yes | 0.230 | ||
| Planned LMA | |||
| No | 0.170 | 0.601 | |
| Yes | 0.175 | ||
| Change in A.M. | |||
| No | 0.171 | 0.101 | |
| Yes | 0.307 | 8 | |
| Intubation due to change in A.M. | |||
| No | 0.171 | ||
| Yes | 0.227 | 0.420 | 4 |
Number of patients ≤ 10 for this variable result.
LMA, laryngeal mask airway; A.M., anesthetic management
Figure 1.
Associations of mean number of attempts at endotracheal intubation, laryngoscopic grade view,and propensity for OSA
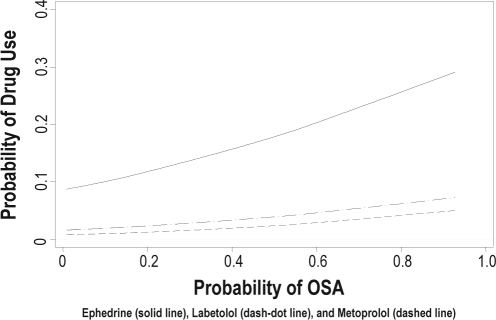
Table 3 illustrates median propensity scores associated with the administration of intraoperative medications. Higher propensity scores were associated with administration of intraoperative ephedrine (p < 0.001), labetalol (p < 0.001), metoprolol (p = 0.003), and succinylcholine (p = 0.001). Figure 2 shows probability of medication use (ephedrine, metoprolol, and labetalol) as a function of propensity for OSA. Choice of regional anesthesia over GA was associated with increased propensity score (p = 0.001). Table 4 demonstrates the association of propensity for OSA and postoperative variables. Prolonged supplemental oxygen and lower post operative oxygen saturations were associated with higher OSA propensity scores. The only additional variable associated with increased propensity was postoperative tachycardia. Two patients experienced supraventricular tachycardia; their median propensity score was 0.551; however, this was not found to be statistically significant. No correlation was found between increased propensity to OSA and administration of naloxone, need for postoperative ventilatory assistance, re-intubation, or postoperative cardiac symptoms. When contacted by phone 24 hours postoperatively there were no patients or family caretakers who reported patient MI, CVA, or death.
Table 3.
Choice of anesthesia and medications administered by median propensity for OSA
| Variable | Median Propensity | p-value |
|---|---|---|
| Type of Anesthesia | ||
| General | 0.238 | < 0.001 |
| Regional | 0.500 | |
| MAC | 0.268 | |
| Propofol | ||
| No | 0.248 | <0.001 |
| Yes | 0.169 | |
| Midazolam | ||
| No | 0.217 | <0.001 |
| Yes | 0.166 | |
| Ephedrine | ||
| No | 0.162 | <0.001 |
| Yes | 0.246 | |
| Labetalol | ||
| No | 0.169 | <0.001 |
| Yes | 0.311 | |
| Succinylcholine | ||
| No | 0.167 | 0.001 |
| Yes | 0.201 | |
| Metoprolol | ||
| No | 0.170 | 0.003 |
| Yes | 0.313 | |
| Neostigmine | ||
| No | 0.175 | 0.014 |
| Yes | 0.145 | |
| Vecuronium | ||
| No | 0.175 | 0.068 |
| Yes | 0.154 | |
| Fentanyl | ||
| No | 0.170 | 0.69 |
| Yes | 0.172 |
MAC, monitored anesthesia care
Figure 2.
Association of administration of medications to correct hemodynamic abnormalities and propensity for OSA
Table 4.
Postoperative results by median propensity of OSA
| Variable | Median Propensity | p value | n1 |
|---|---|---|---|
| Increased O2 requirement | |||
| No | 0.091 | 0.016 | |
| Yes | 0.174 | ||
| PVCs > 6/min or 3 consecutive beats | |||
| No | 0.172 | 0.116 | |
| Yes | 0.344 | ||
| SVT | |||
| No | 0.178 | 0.084 | |
| Yes | 0.551 | 2 | |
| Bradycardia | |||
| No | 0.152 | 0.139 | |
| Yes | 0.198 | ||
| Tachycardia | |||
| No | 0.243 | 0.026 | |
| Yes | 0.134 | ||
| Naloxone | |||
| No | 0.172 | 0.285 | |
| Yes | 0.077 | 6 | |
| Need for Ventilatory Assistance | |||
| No | 0.171 | 0.848 | |
| Yes | 0.221 | 3 | |
| Reintubated | |||
| No | 0.172 | 0.524 | |
| Yes | 0.146 | 2 | |
| Unplanned Admission | |||
| No | 0.171 | 0.769 | |
| Yes | 0.244 | ||
| Discharged Home | |||
| No | 0.186 | 0.277 | |
| Yes | 0.170 |
Number of patients ≤ 10 for this variable result.
PVC, premature ventricular complex; SVT, supraventricular tachycardia
DISCUSSION
The results of our study suggest that the presence of OSA may be relatively common in an ambulatory surgical population and that a majority of these patients remain undiagnosed. Based on the previously validated prediction model, 4.8% of patients had a greater than 70% propensity for OSA. Although we did not find a relationship between unplanned admission, life-threatening events (e.g., re-intubation, cardiac arrhythmia), or death in patients with either a diagnosis of OSA or higher propensity for OSA, there was an increased risk of serious perioperative events (increased difficulty of intubation, increased need for supplemental oxygen, and use of medications to correct hemodynamic derangements) in patients with a high propensity for OSA.
Our findings are similar to other prior cohort studies that suggest that the majority of patients with OSA have not undergone polysomnography and therefore, carry no formal diagnosis. Furthermore, women with OSA are less likely to be evaluated and diagnosed.11 This is important as the cohort in this study were predominately female. It is possible, based on current national surgical volume, that more than a million patients with undiagnosed obstructive sleep apnea undergo ambulatory surgery each year.
It is uncertain if patients with OSA can be managed safely as outpatients. Performing surgery in OSA patients on an ambulatory basis poses several dilemmas for clinicians as there are few prospective trials available to guide management decisions.12 The ASA practice parameters for the anesthetic care of patients with OSA do not support discharge to home after most ambulatory procedures. In fact, the consultants agreed only that patients who had lithotripsy and minor peripheral procedures performed with local anesthetic or regional anesthesia could be safely discharged to an unmonitored setting. The length of postoperative observation and rules for discharge to home after outpatient surgery for OSA patients are unclear; however, recently published practice guidelines suggested a return to baseline room air oxygen saturation without evidence of clinical airway obstruction.8 These guidelines also recommend that monitoring be continued for a median of 3 hours longer than non-OSA patients and 7 hours after the last episode of airway obstruction prior to discharge home.
Not surprisingly, in this investigation, OSA patients had a significantly greater likelihood of intraoperative issues. Patients with diagnosed OSA are presumed to be at increased risk for difficult airways. As noted by Siyam et al., OSA patients have a higher likelihood of airway-related problems with approximately 21.9% of OSA (vs. 2.6% in non-OSA) patients having a difficult intubation.13 However, the authors are unaware of any large studies directly linking the two. In our cohort, higher Mallampati score, increased number of attempts of direct vision laryngoscopy, and poorer grade view were all associated with increased propensity to OSA (Table 3). Additionally, choice of fiberoptic intubation to secure the airway was similarly associated with increased propensity scores. Increased propensity to OSA was associated with presence of intraoperative tachycardia, and use of intravenous ephedrine, metoprolol, and labetalol, although none of these patients required postoperative admission. Again, these findings may not be unexpected, as OSA (vs. non-OSA) subjects have a higher frequency of baseline cardiac dysrhythmias, ST-segment depression, and increased sympathetic tone.14–16 Of note, patients with an increased propensity to OSA were more likely to have been prescribed preoperative antihypertensive medications.
While there was a positive association of decreased oxygen saturation upon arrival to the PACU with increased OSA propensity in our study, there was not an association with need to assist ventilation or re-intubation. This is consistent with prior data by Sabers and Plevak.17 Although OSA patients may be more likely to have postoperative and post-discharge complications, a retrospective analysis of 234 ambulatory surgical patients with polysomnography-confirmed OSA did not have a higher incidence of unanticipated hospital admission or other adverse events than non-OSA case controls. Prior studies reporting outcome in ambulatory surgical patients have studied subjects with either a prior diagnosis of OSA or with a known propensity for obstructive sleep apnea.
There are several limitations to our study. The questionnaires used in this investigation are not 100% predictive, and may fail to identify patients with OSA, particularly in those who are young, thin, or female. These limitations exist with all predictive questionnaires used to identify patients at risk for OSA.19 Chung et al. examined outcome in an elective surgical population who were assessed for risk of OSA using the Berlin Questionnaire and the ASA checklist.20 These tools were reported to be moderately sensitive, with lower specificity than diagnosis by polysomnography. In that study, the anesthesiologists were notified if the patient's AHI exceeded 30 episodes per hour and required that these patients be admitted to the ICU for overnight observation. While validated using PSG, the STOP-BANG predictive model also suffered from a significant self-selection bias of participants who agreed to overnight sleep study, and was found to be most useful in identifying patients with severe OSA. This is in part because of the difficulty in arranging formal polysomnography for patients who are dealing with the stress of having to undergo a surgical procedure, and is therefore potentially unavoidable. Additionally, the questionnaires used in our study were designed to determine the propensity of a patient to have OSA, but did not address severity. Although the anesthesia team was blinded to the survey results in our study, it appears that perhaps information obtained in the routine history and physical exam somehow influenced the anesthesiologist to choose regional anesthesia over general anesthesia in patients with increased propensity to OSA. Intraoperatively, choice of regional anesthesia over general anesthesia or monitored sedation was associated with increased propensity to OSA. Although our study is one of the largest cohorts to specifically examine the issue of perioperative morbidity and mortality in patients at risk for OSA undergoing outpatient surgery, the incidence of death after outpatient surgery even in patients with significant comorbidities such as OSA is relatively low, and a larger database may be better able to elucidate other factors that may predict morbidity and mortality in this population.21
In summary, our study suggests that there may be many patients with unrecognized OSA that present to an ambulatory surgical center. Although additional large scale studies are needed, our study supports the concept that patients with obstructive sleep apnea may be able to undergo ambulatory surgical procedures without increased risk of major adverse outcome. OSA patients who undergo outpatient surgical procedures may require additional perioperative interventions; however, they can be safely treated in an ambulatory care center with subsequent discharge to home.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was funded in part by the International Anesthesia Research Society
The authors wish to acknowledge Naresh Punjabi, M.D., Ph.D. for his assistance with this project.
Appendix 1. Maislin Index Score
The index score used in the Maislin et al. (1995) formula is based on 3 questions concerning the frequency of snorting or gasping; loud snoring; and cessation of breathing, choking, or struggling for air. This frequency was recorded on a 5-point Likert scale that ranged from never to 5-7 times/week. In this study, the highest 2 values in the 6-point Likert scale used here (usually and always) were combined prior to regression analyses to conform to the highest value of the 5-point Likert scale (5-7 times/week) used by Maislin et al. A missing response to a question used to calculate the average index score was imputed from the mean of the other 4 questions if only one question per patient was missing. Patients with ≥ 2 missing responses were excluded from the logistic regression analyses. Of the 3557 patients in the data, 36 were excluded from the logistic analyses due to lack of complete data. Missing responses for the 8-question ESS were imputed from the mean responses of the other questions if no more than 2 responses per patient were missing. Twenty-six patients had insufficient data to calculate an ESS. Similarly, 17 patients had missing data on the question of habitual snoring.
Probability = ex / (1 + ex ),
where: x = −8.160 + 1.22*Index 1 + 0.163*BMI – 0.028*Index 1*BMI + 0.032* Age + 1.278* Male,
and Male = 1 if male and 0 if female.
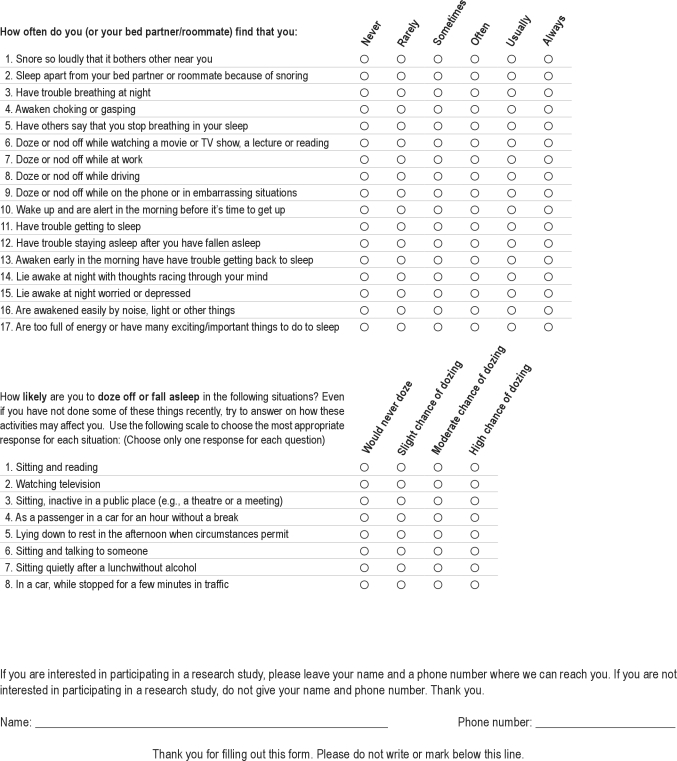
Supplemental Material
Screening Sleep Survey
We are asking you to complete this survey today as a part of a research study on sleep apnea. Your decision on whether or not to complete this survey is voluntary, and will have no impact on your clinic visit today. There are no anticipated risks or benefits to you for completing the survey. If you would like us to contact you regarding further participation in this research study, please provide your name and telephone number at the end. Your survey responses will otherwise remain anonymous and confidential, and will not be a part of your medical record for this clinic visit.
REFERENCES
- 1.Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc. 2001;76:897–905. doi: 10.4065/76.9.897. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Stierer T, Punjabi NM. Demographics and diagnosis of obstructive sleep apnea. Anesthesiol Clin North America. 2005;23:405–20. doi: 10.1016/j.atc.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Tsai WH, Remmers JE, Brant R, Flemons WW, Davies J, Macarthur C. A decision rule for diagnostic testing in obstructive sleep apnea. Am J Respir Crit Care Med. 2003;167:1427–32. doi: 10.1164/rccm.200112-110OC. [DOI] [PubMed] [Google Scholar]
- 5.Dreher A, de la Chaux R, Klemens C, et al. Correlation between otorhinolaryngologic evaluation and severity of obstructive sleep apnea syndrome in snorers. Arch Otolaryngol Head Neck Surg. 2005;131:95–8. doi: 10.1001/archotol.131.2.95. [DOI] [PubMed] [Google Scholar]
- 6.Cullen KA, Hall MJ, Golosinskiy A. Hyattsville, MD: National Center for Health Statistics; 2009. Ambulatory surgery in the United States, 2006. National health statistics reports; no 11. Revised. [PubMed] [Google Scholar]
- 7.Cullen DJ. Obstructive sleep apnea and postoperative analgesia--a potentially dangerous combination. J Clin Anesth. 2001;13:83–5. doi: 10.1016/s0952-8180(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 8.American Society of Anesthesiologists Task Force: Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists task force on perioperative management of patients with obstructive sleep apnea. Anesthesiology. 2006;104:1081–93. doi: 10.1097/00000542-200605000-00026. [DOI] [PubMed] [Google Scholar]
- 9.Maislin G, Pack A, Kribb N, et al. A survey screen for prediction of apnea. Sleep. 1995;18:158–66. doi: 10.1093/sleep/18.3.158. [DOI] [PubMed] [Google Scholar]
- 10.Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth. 1995;7:89–91. doi: 10.1016/0952-8180(94)00001-k. [DOI] [PubMed] [Google Scholar]
- 11.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 12.Bryson GL, Chung F, Finegan BA, et al. Patient selection in ambulatory anesthesia - an evidence-based review: part I. Can J Anaesth. 2004;51:768–81. doi: 10.1007/BF03018449. [DOI] [PubMed] [Google Scholar]
- 13.Siyam MA, Benhamou D. Difficult endotracheal intubation in patients with sleep apnea syndrome. Anesth Analg. 2002;95:1098–102. doi: 10.1097/00000539-200210000-00058. [DOI] [PubMed] [Google Scholar]
- 14.Knill RL, Moote CA, Skinner MI, Rose EA. Anesthesia with abdominal surgery leads to intense REM sleep during the first postoperative week. Anesthesiology. 1990;73:52–61. doi: 10.1097/00000542-199007000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Alonso-Fernandez A, Garcia-Rio F, Racionero MA, et al. Cardiac rhythm disturbances and ST-segment depression episodes in patients with obstructive sleep apnea-hypopnea syndrome and its mechanisms. Chest. 2005;127:15–22. doi: 10.1378/chest.127.1.15. [DOI] [PubMed] [Google Scholar]
- 16.Adlakha A, Shepard JW., Jr. Cardiac arrhythmias during normal sleep and in obstructive sleep apnea syndrome. Sleep Med Rev. 1998;2:45–60. doi: 10.1016/s1087-0792(98)90053-3. [DOI] [PubMed] [Google Scholar]
- 17.Sabers C, Plevak DJ, Schroeder DR, Warner DO. The diagnosis of obstructive sleep apnea as a risk factor for unanticipated admissions in outpatient surgery. Anesth Analg. 2003;96:1328–35. doi: 10.1213/01.ANE.0000061585.09157.66. [DOI] [PubMed] [Google Scholar]
- 18.Stierer T, Fleisher LA. Challenging patients in an ambulatory setting. Anesthesiol Clin North America. 2003;21:2243–61. doi: 10.1016/s0889-8537(03)00002-6. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandran SK, Josephs LA. A meta-analysis of clinical screening tests for obstructive sleep apnea. Anesth. 2009;110:928–39. doi: 10.1097/ALN.0b013e31819c47b6. [DOI] [PubMed] [Google Scholar]
- 20.Chung F, Yegneswaran B, Liao P, et al. Validation of the Berlin Questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesth. 2008;108:822–30. doi: 10.1097/ALN.0b013e31816d91b5. [DOI] [PubMed] [Google Scholar]
- 21.Warner MA, Shields SE, Chute CG. Major morbidity and mortality within 1 month of ambulatory surgery and anesthesia. JAMA. 1993;270:1437–41. doi: 10.1001/jama.270.12.1437. [DOI] [PubMed] [Google Scholar]