Abstract
Study Objectives:
To evaluate the effects of a 2-year weight reduction program on respiratory disturbances, arousal index, daytime sleepiness, metabolic status, and quality of life in obese patients with obstructive sleep apnea syndrome (OSAS).
Methods:
Prospective intervention study of 33 consecutive obese OSAS patients (24 men, 9 women); 19 subjects used continuous positive airway pressure and 4 used mandibular retaining device, except during nights with sleep recording. The program consisted of 8 weeks of low calorie diet followed by group meetings with behavioral change support.
Results:
Seventy percent of the patients completed the program; 67% completed the sleep recordings. The success rate for the apnea-hypopnea index (AHI) (< 20 and reduction ≥ 50%) was 15% in the intention to treat (ITT) analysis. The AHI showed a nonsignificant decrease in mean values, from 43 to 28. The oxygen desaturation index (ODI) decreased from 42 to 23 (p = 0.010), arousal index from 24 to 11 (p = 0.019), body mass index from 40 to 35 (p = 0.003) and the Epworth Sleepiness Scale (ESS) from 9 to 5 (p = 0.026), all ITT. Metabolic status, physical functioning, and vitality evaluations improved only in the per protocol analysis. Reduction in weight correlated significantly to reductions in ESS (p = 0.038) and insulin levels (p = 0.002), respectively. There were no differences based on gender or use/non-use of OSAS treatment device.
Conclusions:
Our weight reduction program showed a limited success in reducing AHI. However, there were significant improvements in weight, ODI, arousal index, and subjective symptoms. We recommend the program as an adjunct treatment for well-motivated obese OSAS patients.
Citation:
Nerfeldt P; Nilsson BY; Mayor L; Uddén J; Friberg D. A two-year weight reduction program in obese sleep apnea patients. J Clin Sleep Med 2010;6(5):479-486.
Keywords: Sleep apnea, obesity, weight reduction, diet, quality of life, sleepiness, behavioral therapy
Obstructive sleep apnea syndrome (OSAS) is a common disorder, in which recurrent episodes of upper airway obstruction during sleep cause repetitive episodes of transient oxygen desaturations. The obstructive apneas cause arousals and other impairments of sleep architecture and are typically associated with excessive snoring and daytime sleepiness.1 OSAS is associated with mood effects and neurocognitive impairments. Further, OSAS is implicated in the pathogenesis of cardiovascular diseases.2 The mechanisms by which OSAS effects the vascular system include intermittent hypoxia, increased sympathetic activation, and endothelial dysfunction.3,4 In addition, there is increasing evidence that obstructive sleep apnea (OSA) is an independent risk factor for insulin resistance, a key contributor to the pathogenesis of cardiometabolic syndrome.5
Obesity and OSAS often covary; 60% to 90 % of OSAS patients have a body mass index (BMI kg/m2) > 28.6 Fat disposition around the pharynx, in the thorax as well as abdomen is considered to be a major cause of OSAS,7,8 and significant sleep apnea is present in about 40% of the severely obese.9,10 Previous studies on weight reduction in obese OSAS patients have shown a reduction of apnea frequency in the short term,11–14 because of decreased upper airway collapsibility15 and increased size of the upper airway passage.16
Compared to surgical intervention, conservative weight reduction involves a smaller risk of morbidity and mortality,17 but is not considered equally successful. In an early non-randomized dietary study, Suratt et al. showed reduced respiratory disturbances after a low calorie diet (LDC) in 8 obese subjects.14 The effect of LCD has been investigated, and complications have been found to be mild and transient.18,19 For OSAS patients there is scant information on the LCD method with regard to long-term effects and results on sleep quality.11
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obesity and OSAS most often covary, but conservative weight reduction is difficult to maintain and seldom used. We evaluated the effect of a 2-year program with full-night polysomnography on respiratory disturbances, arousal index, daytime sleepiness, metabolic status and quality of life.
Study Impact: Our behavioral change dietary program showed limited success in reducing apnea-hypopnea index. However, the significant improvements in weight, oxygen desaturation index, arousal index and subjective symptoms encourage us suggest the program as an adjunct treatment for well-motivated obese subjects with OSAS.
We previously published the results of a randomized controlled pilot study of dietary intervention with LCD for 8 weeks, in which there was reduction of weight and nocturnal desaturations (ODI) in the treatment group compared to the control group.20 We then proceeded with the present intervention study, in this same population of obese OSAS patients. After an initial LCD period, we added behavioral change support for 2 years. A short-term follow-up study after 6 months showed reductions in weight, nocturnal respiratory disturbance, blood pressure, metabolic status, and daytime sleepiness.21 In addition, there were improvements in the arousal index, sleep efficiency, percentage deep sleep, and total wake time.
The aim of the present study was to evaluate the effects after 2 years of the intervention program with LCD and behavioral change support on obese OSAS patients. The effects on nocturnal respiration and sleep quality were measured, as well as metabolic status, daytime sleepiness, and quality of life. Our hypothesis was that conservative weight reduction in obese OSAS patients would reduce the apnea-hypopnea index (AHI). The primary outcome of interest was change in AHI. The secondary outcome was change is the arousal index (ArousInd).
METHODS
This nonrandomized, prospective intervention study took place from 2001-2003.
Subjects
Forty consecutive OSAS patients from the waiting list at the Obesity unit at the Karolinska University Hospital were asked to participate in the study; 33 patients (24 males, 9 females) accepted and formed 4 consecutive therapy groups.
Inclusion criteria included age 30-69 years, body mass index (BMI) ≥ 30, and apnea-hypopnea index (AHI) ≥ 10 and/or oxygen desaturation index 4% or greater (ODI4) ≥ 6, plus subjective symptoms of OSAS. Exclusion criteria were serious psychiatric disease, insufficient knowledge of the Swedish language (preventing taking part in group therapy), or low motivation for behavioral change
The 33 participating patients included 23 who used OSAS treatment devices (19 patients used continuous positive airway pressure [CPAP], and 4 used mandibular retaining devices [MRD]). All were well adapted to their devices ≥ 3 months prior to beginning the study. The patients continued the same treatment throughout the entire study, except during nights with sleep recording. Of the 10 patients without OSAS treatment devices, 5 had failed CPAP, 1 had failed MRD, and 4 were newly diagnosed with OSAS and had chosen weight reduction as a primary treatment. The patients were not allowed to start any additional OSAS treatment during the study period. The patients who changed their antihypertensive medication during the study were excluded from the blood pressure analysis. Anthropometric and respiratory data for the study population are listed in Table 1.
Table 1.
Patient characteristics at baseline
| Mean (range) | All | Males | Females | With device | Without device | Drop-outs |
|---|---|---|---|---|---|---|
| Number of subjects | 33 | 24 | 9 | 23 | 10 | 11 |
| Age (years) | 52(31-68) | 52(31-68) | 51(32-64) | 50(31-64) | 55(38-68) | 52(32-64) |
| Weight (kg) | 122(87-157) | 128(104-157) | 105(87-135) | 121(93-155) | 123(87-157) | 123(93-155) |
| BMI (kg/m2) | 40(33-50) | 40(33-48) | 40(35-50) | 40(33-50) | 40(36-47) | 42(35-50) |
| AHI | 43(6-93) | 43(6-93) | 44(12-84) | 41(6-84) | 48(12-93) | 51(12-93) |
| ODI4 | 42(6-104) | 41(6-86) | 45(8-104) | 38(6-75) | 52(8-104) | 46(8-86) |
| ESS | 9(2-17) | 8(2-17) | 10(2-16) | 8(2-17) | 10(4-17) | 9(2-15) |
| None/CPAP/MRD | 10/19/4 | 6/16/2 | 4/3/2 | 0/19/4 | 10/0/0 | 2/8/1 |
All included patients and subgroups of males, females, patients with device (CPAP or MRD), without device, and drop-outs (patients with missing AHI at follow-up). OSAS refers to obstructive sleep apnea syndrome; CPAP, continuous positive airway pressure; MRD, mandibular retaining device; BMI, body mass index; AHI, apnea hypopnea index; ODI4, oxygen desaturation index; EES, Epworth Sleepiness Scale.
Thirteen patients had hypertension, 9 patients suffered from other cardiovascular diseases, and 2 patients had asthma. Nine patients had type 2 diabetes mellitus, and 14 patients were prediabetic, of whom 9 had coexisting hypertension or other cardiovascular diseases. Twenty-seven patients suffered from metabolic syndrome, as defined by the International Diabetes Federation, April 2005 (www.idf.org). Thirty-one patients were Caucasian; 2 were Asian.
Patients who did not have a scored AHI at 2-year follow-up were considered to be dropouts.
The study was approved by The Regional Ethical Review Board in Stockholm.
Procedure
Prior to acceptance for treatment at the Obesity unit, a physician met each patient in a screening process, to evaluate sufficient motivation for behavioral change. An interviewing technique was used in accordance with Prochaska/DiClementeés “Stages of Change.”22 Simple questions (e.g., “Why do you want to join the weight-loss program?”) were used to assess how ready the patient was for behavioral changes and lifestyle improvements. Two subjects were dismissed having problems with compliance and previous behavioral change programs. After acceptance and study inclusion the patients met a physician and nurse at the Obesity unit for baseline screening with anthropometric data collection, 4-electrode whole-body bioelectrical impedance analysis (BIA4), and laboratory blood sampling. The patients answered questionnaires, including the Epworth Sleepiness Scale (ESS) and the Short-Form Health Survey 36 (SF-36), regarding their symptoms. Thereafter, a sleep investigation followed with ambulant whole night polysomnography (PSG), which also included a sleep apnea recording. Patients treated with CPAP or MRD slept without those devices during the night of the sleep recording.
The weight reduction intervention started with 8 weeks of LCD, consisting of a protein drink (Nutrilett®) with approximately 800 kcal (3400 kJ) per day. During the first 7 weeks, the patients were instructed to consume only the LCD. During the final 8th week, they gradually began to eat balanced low-calorie meals. Group meetings for support, led by a specialized nurse, were held approximately every week in addition to physical check-ups and urine analysis for ketonuria, a marker for dietary compliance.
After the LCD, there was a time gap for a seasonal vacation of approximately 2 months, during which the patients were offered orlistat (Xenical®) or sibutramin (Reductil®) to maintain weight loss. Five patients accepted orlistat and one sibutramin as pharmacological temporary treatments. Thereafter, a second period followed with behavioral change support. These group meetings were led by specialists from different areas (nurse, dietician, physiotherapist, and physician); their purpose was to increase each individual's ability and knowledge in making behavioral changes. Nutritional education, cooking sessions, and individualized physical activity programs were included to encourage long-term lifestyle behavioral changes. The program was similar to cognitive behavioral therapy, but was more focused on behavioral changes. During the first 3 months the group meetings were held once a week between 08:00 and 15:00. After the 6-month follow-up,21 the meetings were shorter and less frequent, i.e., 2 h once a month, until 2-year follow-up. Patients who did not attend 3 group sessions were excluded from the program.
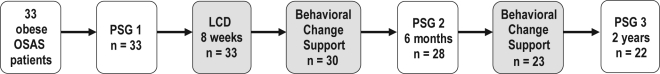
Weight controls were performed at each group meeting. Evaluations with ambulant polysomnography, questionnaires, laboratory samplings, and anthropometrics were performed at 6 months and 2 years (end of study). A quality of life questionnaire (SF-36) was added to the 2-year follow-up. This paper presents the results from the 2-year follow-up. Figure 1 presents a flowchart of the entire study.
Figure 1.
Flow-scheme
OSAS refers to Obstructive Sleep Apnea Syndrome; PSG, Polysomnography; LCD, Low Calorie Diet
Sleep Recordings
Full-night ambulatory polysomnography (PSG) was performed with portable equipment (Biosaca, HIC AB, Gothenburg, Sweden), comprising 6 EEG channels, 2 EOG channels, ECG, and submental EMG. The PSG data were transferred to the Nervus EEG System (NicoletOne nEEG, Viasys Healthcare Inc., Madison, WI, USA) before analysis. Simultaneous with PSG, patients underwent ambulatory sleep apnea recordings (Embletta, Medcare Flaga, Reykjavik, Iceland), comprising 7 channels (oronasal airflow recording with nasal cannula and pressure transducer, thoracic and abdominal respiratory efforts, pulse oximetry, pulse frequency, snoring, and body position). All sleep apnea recordings were analyzed with the Somnologica for Embletta scoring software program. Respiratory events were manually verified and interpreted by a qualified neurophysiologist. The PSG and sleep apnea recordings were time synchronized, and the sleep period and awakenings were derived from the PSG analysis. A qualified neurophysiologist interpreted the recordings. Apnea was defined by Embletta as > 80% reduction of airflow at the nose and mouth for ≥ 10 sec, and hypopnea as reduction ≥ 30% if > 3% desaturation was detected within 20 sec. For more detailed definitions of sleep parameters see Nerfeldt et al. 2008.21
Bioelectrical Impedance Analysis
A 4-electrode whole-body bioelectrical impedance analysis (BIA4) was performed (Tanita TBF-300, Tanita Corp., Tokyo, Japan) that measured the total body fat percentage (Body fat %) and the fat mass in kilograms (Body fat mass). Clothing was compensated at a reduction of 0.5 kg. The analysis required input on whether the patient was athletic or standard body type; all patients registered as standard.
Statistical Analyses
Results are presented as mean, standard deviation (SD), and range values. p-values < 0.1 are specified, and ≥ 0.1 abbreviated as n.s. (non-significant); but the limit for statistical significance was set to < 0.05 as standard. Nonparametric tests (Mann-Whitney-U [MWU] for unpaired groups, Wilcoxon signed rank test [WSR] for paired groups, and Spearman rank correlations [SRC]) were used, because the participants were limited in number and some parameters had skewed distribution. The small number of participants caused a high risk for type II errors and therefore, no adjustment for multiple comparisons was done. Two analysis methods were used; a per protocol analysis (PP) of all patients who fulfilled the program, and a stricter intention to treat analysis (ITT). In the ITT-analysis we assumed a worsening with one unit between baseline and follow-up for the dropouts. This conservative imputation method is used to not favor a positive treatment effect. A linear regression model was used to evaluate the impact of changes in weight compared to changes in AHI and ODI respectively, with adjustment for each other. Five parameters were chosen for this regression analysis, as they had reached a level of statistically significant reduction after 2 years. All statistical analyses were made in R 2.5 and Statistica.
RESULTS
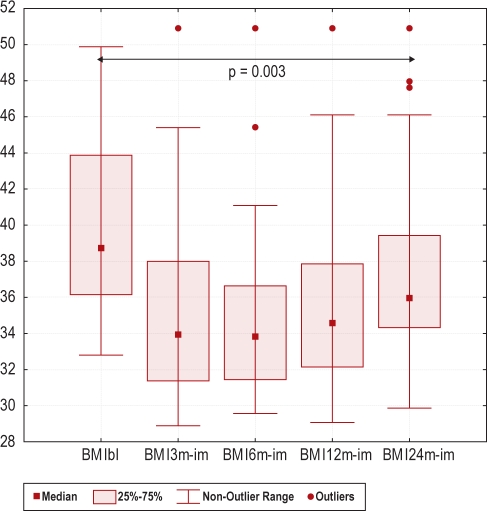
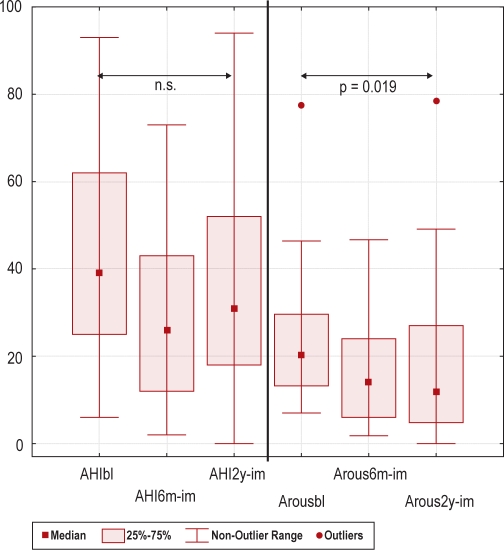
Of the 33 patients, 23 (70%) were still in the weight reduction program after 2 years. Twenty-two (67%) underwent all polysomnography recordings, with evaluation of the primary outcome parameter AHI. Dropout analyses on baseline data displayed only minor differences (Table 1). Table 2 presents the number of patients evaluated for each parameter and the results of the intervention. In the ITT-analysis AHI was unchanged, but reductions were found in ODI (p = 0.010), BMI (p = 0.003), and ESS (p = 0.026). Further, improvements were found in sleep quality (arousal index p = 0.019 and stage shift index p = 0.003). However, only one metabolic parameter was improved; HDL (p = 0.037). In the PP-analysis, a large part of the metabolic status was improved, including insulin levels (p = 0.014) and dyslipidemia (triglycerides p = 0.042, HDL p < 0.001, LDL p = 0.008). Further, the 2 domains “physical functioning” (p = 0.031) and “vitality” (p = 0.046) were improved in the SF-36 questionnaire, as shown in Table 2. Change in BMI during intervention is shown in Figure 2; and the primary and secondary outcomes (changes in AHI and arousal index) in ITT-analysis are shown in Figure 3. Compared to the results after 6 months, there was a slight increase in weight and AHI after 2 years. In contrast, the arousal index was slightly reduced after 2 years compared to 6 months.
Table 2.
Descriptive and statistical results for changes in weight, respiratory, sleepiness, sleep architecture, metabolic and quality of life parameters after the 2-year weight reduction program (per protocol analysis [PP] and intention to treat analysis [ITT])
| Numbers at 2 years | Baseline Mean (SD) | 2 years Mean (SD) | PP p-value WSR | ITT p-value WSR | |
|---|---|---|---|---|---|
| Weight (kg) | 23 | 122(19) | 110(15) | < 0.001 | 0.003 |
| BMI (kg/m2) | 23 | 40(5) | 35(3) | < 0.001 | 0.003 |
| Body fat % | 18 | 43(6) | 36(8) | < 0.001 | 0.040 |
| Body fat mass (kg) | 18 | 52(11) | 41(11) | < 0.001 | 0.007 |
| Waist circumference (cm) | 18 | 127(14) | 118(9) | 0.002 | n.s. |
| Waist Hip ratio | 18 | 1.01(0.1) | 1.02(0.1) | n.s. | n.s. |
| AHI | 22 | 43(24) | 28(19) | 0.054 | n.s. |
| ODI4 | 22 | 42(23) | 23(15) | < 0.001 | 0.010 |
| ESS | 22 | 9(4) | 5(3) | 0.003 | 0.026 |
| Arousal Index | 22 | 24(15) | 11(11) | < 0.001 | 0.019 |
| Deep sleep % | 22 | 16(11) | 19(10) | n.s. | n.s. |
| REM sleep % | 22 | 14(7) | 16(8) | n.s. | n.s. |
| Total sleep time | 22 | 357(67) | 338(83) | 0.097 | n.s. |
| Total wake time | 22 | 60(42) | 55(29) | n.s. | n.s. |
| Sleep efficiency | 22 | 78(10) | 79(12) | n.s. | n.s. |
| Stage shift index | 22 | 17(11) | 11(10) | < 0.001 | 0.003 |
| Awakening Index | 22 | 4(7) | 5(9) | n.s. | n.s. |
| Sleep latency | 22 | 31(19) | 24(17) | 0.076 | n.s. |
| fP-Glucose (mmol/L) | 21 | 7.2(2.7) | 7.0(3.3) | 0.082 | n.s. |
| B-HbA1C (%) | 21 | 5.7(1.3) | 5.6(1.7) | 0.059 | n.s. |
| fS-Insulin (pmol/L) | 21 | 147(78) | 90(52) | 0.014 | 0.078 |
| P-Cholesterol (mmol/L) | 21 | 5.3(1.1) | 4.9(1.2) | n.s. | n.s. |
| P-Triglycerides (mmol/L) | 21 | 1.8(0.8) | 1.6(0.7) | 0.042 | n.s. |
| P-HDL (mmol/L) | 21 | 1.2(0.3) | 1.4(0.5) | < 0.001 | 0.037 |
| P-LDL (mmol/L) | 21 | 3.4(1.0) | 2.7(1.1) | 0.008 | n.s. |
| P-ASAT (ukat/L) | 21 | 0.5(0.3) | 0.5(0.3) | 0.085 | n.s. |
| P-ALAT (ukat/L) | 21 | 0.7(0.4) | 0.6(0.5) | n.s. | n.s. |
| P-ALP (ukat/L) | 15 | 3.1(0.9) | 2.9(1.0) | n.s. | n.s. |
| P-GGT (ukat/L) | 21 | 0.9(0.8) | 0.7(0.7) | < 0.001 | n.s. |
| P-Urat (umol/L) | 21 | 400(69) | 418(86) | n.s. | n.s. |
| Syst BP (mm Hg) | 13 | 144(19) | 129(10) | n.s. | n.s. |
| Diast BP (mm Hg) | 13 | 89(14) | 81(6) | n.s. | n.s. |
| SF-36: PF | 18 | 62(27) | 77(26) | 0.031 | n.s. |
| SF-36: RP | 18 | 67(40) | 74(36) | n.s. | n.s. |
| SF-36: BPain | 18 | 62(29) | 67(29) | n.s. | n.s. |
| SF-36: GH | 18 | 57(24) | 61(27) | n.s. | n.s. |
| SF-36: VT | 18 | 49(24) | 62(27) | 0.046 | n.s. |
| SF-36: SF | 18 | 82(20) | 82(25) | n.s. | n.s. |
| SF-36: RE | 18 | 75(39) | 78(36) | n.s. | n.s. |
| SF-36: MH | 18 | 74(19) | 80(16) | n.s. | n.s. |
p-values < 0.1 are specified, and ≥ 0.1 abbreviated as non-significant (n.s.). BMI, body mass index; Body fat %, percentage body fat; AHI, apnea hypopnea index; ODI4, oxygen desaturation index; ESS, Epworth Sleepiness Scale; Deep sleep %, percentage slow wave sleep; REM sleep %, percentage REM sleep; fP-Glucos, fasting plasma glucose level; HbA1C, glycosylated hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; ASAT, aspartate aminotransferase; ALAT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transpeptidase; Syst BP, systolic blood pressure; Diast BP, diastolic blood pressure; SF-36, Short Form 36. PF, Physical Functioning; RP, Role Physical; BPain, Bodily Pain; GH, General Health; VT, Vitality; SF, Social Functioning; RE, Role Emotional; MH, Mental Health; WSR, Wilcoxon signed rank test; n.s., non significant
Figure 2.
Box-plots showing Body Mass Index (BMI) at baseline (bl) and after a 3, 6, 12 and 24 months (3m, 6m, 12m, and 24m) weight reduction program with an imputated (im) worsening with one unit between baseline and follow-up for the dropouts (intention to treat analysis, Wilcoxon Sign Rank test, n = 33).
Figure 3.
Box-plots showing Apnea-hypopnea index (AHI) and Arousal Index (Arous) at baseline (bl), after 6 months (6m) and 2 years (2y) weight reduction program with an imputated (im) worsening with one unit between baseline and follow-up for the dropouts (intention to treat analysis, Wilcoxon Sign Rank test, n.s.= non significant, n = 33).
The statistical analyses revealed no differences in results between patients treated with OSAS-device, CPAP or MRD (n = 23), compared to those without (n = 10), when examining changes in the following 7 parameters: percentage weight reduction, kg, BMI, AHI, ODI, arousal index, and ESS. The same 7 parameters were evaluated for gender differences; there were no significant differences between the 24 men and the 9 women.
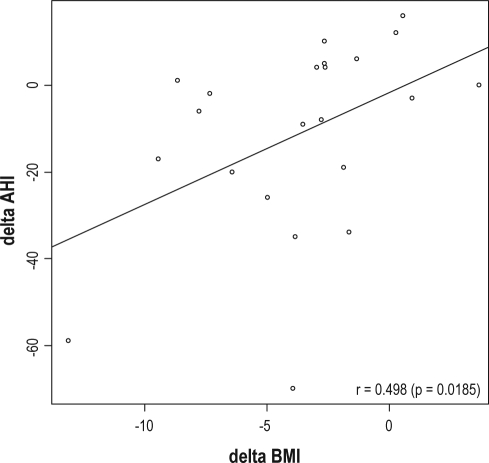
A positive correlation was seen between reduction in BMI and nocturnal respiratory disturbances (AHI), r = 0.498 (p < 0.05, SRC) (Figure 4). Further, the reduction in weight correlated to the reduction in ESS, r = 0.457 (p < 0.05), and insulin r = 0.586 (p < 0.001). There was no correlation between changes in daytime sleepiness (ESS) and percentage deep sleep r = −0.249 (p = 0.29).
Figure 4.
Scatter plot and correlation between improvement in AHI and BMI reduction after the 2-year weight reduction intervention (Spearman correlation (r) 0.498, p =
Delta AHI, changes in Apnea Hypopnea Index; Delta BMI, changes in Body Mass Index (kg/m2)
Five outcome parameters; insulin, SF-36 physical functioning, ESS, systolic and diastolic blood pressure were evaluated in a linear regression analysis, with delta kilogram and delta AHI or delta ODI, respectively. Change in weight was found to be a significant factor to improve ESS rating (p = 0.038) and insulin levels (p = 0.002), while AHI and ODI never were found to be significant predictors. In univariate analysis, change in weight showed significant correlation to ESS and insulin; AHI and ODI did not.
Three different treatment success levels (PP and ITT) are presented in Table 3. The LCD treatment was well tolerated by all participants, and no side effects were found. Of the 14 patients determined to be prediabetic at baseline, 5 had normalized their glucose levels after 6 months, and 4 after the 2-year treatment period.
Table 3.
Different criteria of success were used for the results after the 2-year weight reduction program, analysed with both per protocol and intention to treat analysis
| Success criteria | Per Protocol | Intention to treat |
|---|---|---|
| Reduction of weight > 10% | 7/23 (30%) | 7/33 (21%) |
| Reduction of AHI ≥ 50% and < 20 | 5/22 (23%) | 5/33 (15%) |
| Reduction of ODI4 ≥ 50% and < 20 | 8/22 (36%) | 8/33 (24%) |
AHI, apnea hypopnea index; ODI4, oxygen desaturation index
DISCUSSION
This study showed that our weight reduction program of obese sleep apnea patients was safe and tolerable. After 2 years there was a significant weight reduction and a small, although nonsignificant, decrease of the AHI index, and these reductions were correlated. In addition, ODI, ESS and arousal index were reduced in the stricter ITT-analysis. Furthermore, there was an improvement of metabolic status (blood insulin levels and dyslipidemia) as well as vitality and physical functioning rating, but only in the PP-analysis. There were no major differences in the baseline data for the subgroups, i.e., gender or kind of other OSAS treatment, and the subgroups responded similarly to the dietary intervention.
The analyses indicated that the reduction in weight, and not in respiratory disturbances (AHI or ODI), was the reason for improvement in cardiovascular risk factors, for example hyperinsulinemia and blood pressure. These results were somewhat unexpected since OSAS itself is considered an independent cardiovascular risk factor.23 We assume that the effects of weight reduction are stronger than those of improved nocturnal respiration, and that our study population was too small and diverse to demonstrate such possible effects.
The ITT analysis was used to evaluate the effectiveness of the program, and included the drop-outs who were presumed to have not achieved weight reduction. Weight reduction of 10% was used as an index for success24; with this criterion, 73% were successfully treated after 6 months, but only 21% after 2 years. Previous studies have shown success rates to vary between 5% and 42%.25–27 To measure nocturnal breathing, we used the same success criteria as in our 6-month follow-up; reduction of AHI ≥ 50 % and AHI < 20.21 The success rate (ITT) decreased from 27% at 6 months to 15% after 2 years. Another parameter for measuring nocturnal breathing, the ODI, showed a success rate of 24% at 2 years.
Seventy percent of the patients fulfilled our 2-year weight reduction program, and 67% underwent all polysomnography recordings. Thus, our compliance rate is acceptable compared to other weight reduction programs for OSAS patients,11,27 as well as compared to other OSAS treatments.28–31 The difference between the more successful study of Kajaste27 (88% compliance after 2 years) and our study may have several explanations, including a population with a more severe degree of obesity as well as OSAS, and the use of individual cognitive behavioral therapy vs. our group therapy. Between the 6-month follow-up and the 2-year follow-up, there was gain in weight. In addition, the 6-month improvements found for several other parameters were diminished. At the 2-year evaluation, there was no longer a correlation between changes in daytime sleepiness (ESS score) and percentage deep sleep, as seen in the 6-month follow-up. One reason for the decreasing effect of the program could be the well-known fact that it is hard to sustain a reduced weight for a long term. Another reason may be the differences between the two major parts of our program; first intensive six months with LCD diet and the behavioral change support program, and second less intense intervention focused mainly on maintenance. A review by Mustajoki and Pekkarinen stresses the importance of a concordant LCD and cognitive behavioral counseling.32
The short form health survey (SF-36) is frequently used as a method for quality of life evaluation. The use of SF-36 has revealed that OSA patients have lower quality of life compared to an age- and gender matched control group.33 The questionnaire has been used in several studies on CPAP intervention and the answers have shown that patients improved in some of the eight domains, most often the “role-physical” and the “vitality” domains.34 Also in the obese patients, the results from SF-36 have revealed impaired quality of life, with a correlation between increased degree of obesity and decreased quality of life.35 In addition, dietary weight loss methods have shown improvements in “vitality” and “physical functioning.”36,37 Our intervention in obese OSAS patients showed improvements in “physical functioning” and “vitality” in the per protocol analysis, results that correspond with previous findings.
There are several limitations in the present study. One obvious drawback is the design; a randomized control trial (RCT) has a higher degree of evidence than the chosen intervention. However, our experience from a previous RCT of obese OSAS patients recruited from the ENT-clinic showed a high drop-out rate, 9 of 20 (45%).20 We concluded that a majority of those patients were not motivated to undergo an 8-week LCD. In the present study, we tried to improve the compliance by recruiting more motivated patients from the waiting list at the Obesity unit. On the other hand, these patients are probably unwilling to act as untreated controls for 2 years. In a study design like this, when blinding of treatment is not possible, a placebo effect of the program on subjective parameters, i.e., sleepiness and quality of life, cannot be ruled out. On the other hand, our results revealed reductions of several objective parameters, i.e., ODI, arousal index, blood tests, etc., which are probably not equally affected by the placebo effect. The possibility to recruit a matched control population was considered, but rejected because of the medical and ethical risks associated with having severe obese OSAS patients with comorbidity untreated for two years. A second limitation is that patients were allowed to use their devices on nights other than the registration nights, since we considered being without treatment could be a health risk. Thus, a remaining effect from the device is likely. However, the same procedure was used both at baseline and evaluations, and an immediate return of the majority of the hypopneas and apneas already after one night's withdrawal of CPAP has been shown.38 A third limitation is the small and heterogeneous study population (i.e., patients with and without OSAS devices), factors which may cause variability in results. Nevertheless, the results of all measured parameters showed improvements; statistical significance status was reached by some. All parameters indicated a positive effect by the use of our program on the nocturnal respiration as well as the general health. Finally, we did not perform a cost-benefit analysis, which would be of interest since the program is rather resource demanding.
In conclusion, our 2-year weight reduction program with LCD and behavioral change support for obese OSAS patients did not improve the AHI; however, there were significant improvements in weight and a reduced amount of oxygen desaturations, arousals, as well as subjective symptoms. We recommend the program as an adjunct treatment for well-motivated patients who want to improve their sleep and overall health.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Financial support for this study was provided by the research fund of Acta Oto-laryngologica.
Statistical assistance for this study was provided by Professor Johan Bring, Statisticon AB; Nurses at the Obesity unit at the Endocrinology-department: Lena Mannström, Maria Klingvall, Gunilla Åkerlund and at the department of Oto-rhino-laryngology: Lisbeth Blidberg; Neurophysiology technicians at the Department of Clinical Neurophysiology: Magdalena Aguirre and Ulrika Arnersten; Secretary at the department of Oto-rhino-laryngology: Eva Lundholm Larsson.
REFERENCES
- 1.Guilleminault C, Van Den Hoed J, Mitler MM. Clinical overview of the sleep apnoea syndromes. In: Guilleminault C, Dement WC, editors. Sleep apnoea syndromes. New York: Alan R. Liss; 1987. pp. 1–12. [Google Scholar]
- 2.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 3.Jain V. Clinical perspective of obstructive sleep apnea-induced cardiovascular complications. Antioxid Redox Signal. 2007;9:701–10. doi: 10.1089/ars.2007.1558. [DOI] [PubMed] [Google Scholar]
- 4.Dyugovskaya L, Polyakov A, Lavie P, Lavie L. Delayed neutrophil apoptosis in patients with sleep apnea. Am J Respir Crit Care Med. 2008;177:544–54. doi: 10.1164/rccm.200705-675OC. [DOI] [PubMed] [Google Scholar]
- 5.Schuster SR, Tabba M, Sahota P. Relationship between the cardiometabolic syndrome and obstructive sleep apnea. J Cardiometab Syndr. 2006;1:204–8. doi: 10.1111/j.1559-4564.2006.05846.x. [DOI] [PubMed] [Google Scholar]
- 6.Bassiri A, Guilleminault C. Clinical features and evaluation of obstructive sleep apnea-hypopnea syndrome. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of Sleep Medicine. 3rd ed. Philadelphia: WB Saunders Company; 2000. pp. 869–78. [Google Scholar]
- 7.Shelton KE, Woodson H, Gay S, Suratt PM. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis. 1993;148:462–6. doi: 10.1164/ajrccm/148.2.462. [DOI] [PubMed] [Google Scholar]
- 8.Shepard JW, Gefter WB, Guilleminault C, et al. Evaluation of the upper airway in patients with obstructive sleep apnea. Sleep. 1991;14:361–71. doi: 10.1093/sleep/14.4.361. [DOI] [PubMed] [Google Scholar]
- 9.Vgontzas AN, Tan TL, Bixler EO, Martin LF, Shubert D, Kales A. Sleep apnea and sleep disruption in obese patients. Arch Intern Med. 1994;154:1705–11. [PubMed] [Google Scholar]
- 10.Richman RM, Elliott LM, Burns CM, Bearpark HM, Steinbeck KS, Caterson ID. The prevalence of obstructive sleep apnoea in an obese female population. Int J Obes Relat Metab Disord. 1994;18:173–7. [PubMed] [Google Scholar]
- 11.Noseda A, Kempenaers C, Kerkhofs M, Houben JJ, Linkowski P. Sleep apnea after 1 year domiciliary nasal-continuous positive airway pressure and attempted weight reduction. Potential for weaning from continuous positive airway pressure. Chest. 1996;109:138–43. doi: 10.1378/chest.109.1.138. [DOI] [PubMed] [Google Scholar]
- 12.Smith PL, Gold AR, Meyers DA, Haponik EF, Bleecker ER. Weight loss in mildly to moderately obese patients with obstructive sleep apnea. Ann Intern Med. 1985;103:850–5. doi: 10.7326/0003-4819-103-6-850. [DOI] [PubMed] [Google Scholar]
- 13.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 14.Suratt PM, McTier RF, Findley LJ, Pohl SL, Wilhoit SC. Effect of very-low-calorie diets with weight loss on obstructive sleep apnea. Am J Clin Nutr. 1992;56:182S–4S. doi: 10.1093/ajcn/56.1.182S. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz AR, Gold AR, Schubert N, et al. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1991;144:494–8. doi: 10.1164/ajrccm/144.3_Pt_1.494. [DOI] [PubMed] [Google Scholar]
- 16.Busetto L, Enzi G, Inelmen EM, et al. Obstructive sleep apnea syndrome in morbid obesity: effects of intragastric balloon. Chest. 2005;128:618–23. doi: 10.1378/chest.128.2.618. [DOI] [PubMed] [Google Scholar]
- 17.Flum DR, Salem L, Elrod JA, Dellinger EP, Cheadle A, Chan L. Early mortality among Medicare beneficiaries undergoing bariatric surgical procedures. JAMA. 2005;294:1903–8. doi: 10.1001/jama.294.15.1903. [DOI] [PubMed] [Google Scholar]
- 18.Gasteyger C, Larsen TM, Vercruysse F, Astrup A. Effect of a dietary-induced weight loss on liver enzymes in obese subjects. Am J Clin Nutr. 2008;87:1141–7. doi: 10.1093/ajcn/87.5.1141. [DOI] [PubMed] [Google Scholar]
- 19.Bravata DM, Sanders L, Huang J, et al. Efficacy and safety of low-carbohydrate diets: a systematic review. JAMA. 2003;289:1837–50. doi: 10.1001/jama.289.14.1837. [DOI] [PubMed] [Google Scholar]
- 20.Nerfeldt P, Nilsson BY, Uddén J, Rössner S, Friberg D. Weight reduction improves nocturnal respiration in obese sleep apnoea patients – a randomized controlled pilot study. Obes Res Clin Pract. 2008;2:119–24. doi: 10.1016/j.orcp.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Nerfeldt P, Nilsson BY, Mayor L, Uddén J, Rössner S, Friberg D. Weight reduction improves sleep, sleepiness and metabolic status in obese sleep apnoea patients. Obes Res Clin Pract. 2008;2:251–62. doi: 10.1016/j.orcp.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Prochaska JO, DiClemente CC. Stages of change in the modification of problem behaviors. Prog Behav Modif. 1992;28:183–218. [PubMed] [Google Scholar]
- 23.McNicholas WT, Bonsignore MR, Management Committee of EU COST ACTION B26 Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29:156–78. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415. [PubMed] [Google Scholar]
- 25.Pekkarinen T, Takala I, Mustajoki P. Two year maintenance of weight loss after a VLCD and behavioural therapy for obesity: correlation to the scores of questionnaires measuring eating behaviour. Int J Obes Relat Metab Disord. 1996;20:332–7. [PubMed] [Google Scholar]
- 26.Anderson JW, Vichitbandra S, Qian W, Kryscio RJ. Long-term weight maintenance after an intensive weight-loss program. J Am Coll Nutr. 1999;18:620–7. doi: 10.1080/07315724.1999.10718897. [DOI] [PubMed] [Google Scholar]
- 27.Kajaste S, Brander PE, Telakivi T, Partinen M, Mustajoki P. A cognitive-behavioral weight reduction program in the treatment of obstructive sleep apnea syndrome with or without initial nasal CPAP: a randomized study. Sleep Med. 2004;5:125–31. doi: 10.1016/j.sleep.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Marklund M, Franklin KA. Long-term effects of mandibular repositioning appliances on symptoms of sleep apnoea. J Sleep Res. 2007;16:414–20. doi: 10.1111/j.1365-2869.2007.00615.x. [DOI] [PubMed] [Google Scholar]
- 29.Marklund M, Sahlin C, Stenlund H, Persson M, Franklin KA. Mandibular advancement device in patients with obstructive sleep apnea: long-term effects on apnea and sleep. Chest. 2001;120:162–9. doi: 10.1378/chest.120.1.162. [DOI] [PubMed] [Google Scholar]
- 30.Grote L, Hedner J, Grunstein R, Kraiczi H. Therapy with nCPAP: incomplete elimination of sleep related breathing disorder. Eur Respir J. 2000;16:921–7. doi: 10.1183/09031936.00.16592100. [DOI] [PubMed] [Google Scholar]
- 31.Lindberg E, Berne C, Elmasry A, Hedner J, Janson C. CPAP treatment of a population-based sample--what are the benefits and the treatment compliance? Sleep Med. 2006;7:553–60. doi: 10.1016/j.sleep.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Mustajoki P, Pekkarinen T. Very low energy diets in the treatment of obesity. Obes Rev. 2001;2:61–72. doi: 10.1046/j.1467-789x.2001.00026.x. [DOI] [PubMed] [Google Scholar]
- 33.D'Ambrosio C, Bowman T, Mohsenin V. Quality of life in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure--a prospective study. Chest. 1999;115:123–9. doi: 10.1378/chest.115.1.123. [DOI] [PubMed] [Google Scholar]
- 34.Moyer CA, Sonnad SS, Garetz SL, Helman JI, Chervin RD. Quality of life in obstructive sleep apnea: a systematic review of the literature. Sleep Med. 2001;2:477–91. doi: 10.1016/s1389-9457(01)00072-7. [DOI] [PubMed] [Google Scholar]
- 35.Larsson U, Karlsson J, Sullivan M. Impact of overweight and obesity on health-related quality of life--a Swedish population study. Int J Obes Relat Metab Disord. 2002;26:417–24. doi: 10.1038/sj.ijo.0801919. [DOI] [PubMed] [Google Scholar]
- 36.Kolotkin RL, Meter K, Williams GR. Quality of life and obesity. Obes Rev. 2001;2:219–29. doi: 10.1046/j.1467-789x.2001.00040.x. [DOI] [PubMed] [Google Scholar]
- 37.Kaukua J, Pekkarinen T, Sane T, Mustajoki P. Health-related quality of life in obese outpatients losing weight with very-low-energy diet and behaviour modification--a 2-y follow-up study. Int J Obes Relat Metab Disord. 2003;27:1233–41. doi: 10.1038/sj.ijo.0802379. [DOI] [PubMed] [Google Scholar]
- 38.Phillips CL, Yang Q, Williams A, et al. The effect of short-term withdrawal from continuous positive airway pressure therapy on sympathetic activity and markers of vascular inflammation in subjects with obstructive sleep apnoea. J Sleep Res. 2007;16:217–25. doi: 10.1111/j.1365-2869.2007.00589.x. [DOI] [PubMed] [Google Scholar]