Abstract
Summary:
Noninvasive positive pressure ventilation (NPPV) devices are used during sleep to treat patients with diurnal chronic alveolar hypoventilation (CAH). Bilevel positive airway pressure (BPAP) using a mask interface is the most commonly used method to provide ventilatory support in these patients. BPAP devices deliver separately adjustable inspiratory positive airway pressure (IPAP) and expiratory positive airway pressure (EPAP). The IPAP and EPAP levels are adjusted to maintain upper airway patency, and the pressure support (PS = IPAP-EPAP) augments ventilation. NPPV devices can be used in the spontaneous mode (the patient cycles the device from EPAP to IPAP), the spontaneous timed (ST) mode (a backup rate is available to deliver IPAP for the set inspiratory time if the patient does not trigger an IPAP/EPAP cycle within a set time window), and the timed (T) mode (inspiratory time and respiratory rate are fixed). During NPPV titration with polysomnography (PSG), the pressure settings, backup rate, and inspiratory time (if applicable) are adjusted to maintain upper airway patency and support ventilation. However, there are no widely available guidelines for the titration of NPPV in the sleep center. A NPPV Titration Task Force of the American Academy of Sleep Medicine reviewed the available literature and developed recommendations based on consensus and published evidence when available. The major recommendations derived by this consensus process are as follows:
General Recommendations:
The indications, goals of treatment, and side effects of NPPV treatment should be discussed in detail with the patient prior to the NPPV titration study.
Careful mask fitting and a period of acclimatization to low pressure prior to the titration should be included as part of the NPPV protocol.
NPPV titration with PSG is the recommended method to determine an effective level of nocturnal ventilatory support in patients with CAH. In circumstances in which NPPV treatment is initiated and adjusted empirically in the outpatient setting based on clinical judgment, a PSG should be utilized if possible to confirm that the final NPPV settings are effective or to make adjustments as necessary.
NPPV treatment goals should be individualized but typically include prevention of worsening of hypoventilation during sleep, improvement in sleep quality, relief of nocturnal dyspnea, and providing respiratory muscle rest.
When OSA coexists with CAH, pressure settings for treatment of OSA may be determined during attended NPPV titration PSG following AASM Clinical Guidelines for the Manual Titration of Positive Airway Pressure in Patients with Obstructive Sleep Apnea.
Attended NPPV titration with PSG is the recommended method to identify optimal treatment pressure settings for patients with the obesity hypoventilation syndrome (OHS), CAH due to restrictive chest wall disease (RTCD), and acquired or central CAH syndromes in whom NPPV treatment is indicated.
Attended NPPV titration with PSG allows definitive identification of an adequate level of ventilatory support for patients with neuromuscular disease (NMD) in whom NPPV treatment is planned.
Recommendations for NPPV Titration Equipment:
The NPPV device used for titration should have the capability of operating in the spontaneous, spontaneous timed, and timed mode.
The airflow, tidal volume, leak, and delivered pressure signals from the NPPV device should be monitored and recorded if possible. The airflow signal should be used to detect apnea and hypopnea, while the tidal volume signal and respiratory rate are used to assess ventilation.
Transcutaneous or end-tidal PCO2 may be used to adjust NPPV settings if adequately calibrated and ideally validated with arterial blood gas testing.
An adequate assortment of masks (nasal, oral, and oronasal) in both adult and pediatric sizes (if children are being titrated), a source of supplemental oxygen, and heated humidification should be available.
Recommendations for Limits of IPAP, EPAP, and PS Settings:
The recommended minimum starting IPAP and EPAP should be 8 cm H2O and 4 cm H2O, respectively.
The recommended maximum IPAP should be 30 cm H2O for patients ≥ 12 years and 20 cm H2O for patients < 12 years.
The recommended minimum and maximum levels of PS are 4 cm H2O and 20 cm H2O, respectively.
The minimum and maximum incremental changes in PS should be 1 and 2 cm H2O, respectively.
Recommendations for Adjustment of IPAP, EPAP, and PS:
IPAP and/or EPAP should be increased as described in AASM Clinical Guidelines for the Manual Titration of Positive Airway Pressure in Patients with Obstructive Sleep Apnea until the following obstructive respiratory events are eliminated (no specific order): apneas, hypopneas, respiratory effort-related arousals, and snoring.
The pressure support (PS) should be increased every 5 minutes if the tidal volume is low (< 6 to 8 mL/kg)
The PS should be increased if the arterial PCO2 remains 10 mm Hg or more above the PCO2 goal at the current settings for 10 minutes or more. An acceptable goal for PCO2 is a value less than or equal to the awake PCO2.
The PS may be increased if respiratory muscle rest has not been achieved by NPPV treatment at the current settings for 10 minutes of more.
The PS may be increased if the SpO2 remains below 90% for 5 minutes or more and tidal volume is low (< 6 to 8 mL/kg).
Recommendations for Use and Adjustment of the Backup Rate/Respiratory Rate:
A backup rate (i.e., ST mode) should be used in all patients with central hypoventilation, those with a significant number of central apneas or an inappropriately low respiratory rate, and those who unreliably trigger IPAP/EPAP cycles due to muscle weakness.
The ST mode may be used if adequate ventilation or adequate respiratory muscle rest is not achieved with the maximum (or maximum tolerated) PS in the spontaneous mode.
The starting backup rate should be equal to or slightly less than the spontaneous sleeping respiratory rate (minimum of 10 bpm).
The backup rate should be increased in 1 to 2 bpm increments every 10 minutes if the desired goal of the backup rate has not been attained.
The IPAP time (inspiratory time) should be set based on the respiratory rate to provide an inspiratory time (IPAP time) between 30% and 40% of the cycle time (60/respiratory rate in breaths per minute).
If the spontaneous timed mode is not successful at meeting titration goals then the timed mode can be tried.
Recommendations Concerning Supplemental Oxygen:
Supplemental oxygen may be added in patients with an awake SpO2 < 88% or when the PS and respiratory rate have been optimized but the SpO2 remains < 90% for 5 minutes or more.
The minimum starting supplemental oxygen rate should be 1 L/minute and increased in increments of 1 L/minute about every 5 minutes until an adequate SpO2 is attained (> 90%).
Recommendations to Improve Patient Comfort and Patient-NPPV Device Synchrony:
If the patient awakens and complains that the IPAP and/or EPAP is too high, pressure should be lowered to a level comfortable enough to allow return to sleep.
NPPV device parameters (when available) such as pressure relief, rise time, maximum and minimum IPAP durations should be adjusted for patient comfort and to optimize synchrony between the patient and the NPPV device.
During the NPPV titration mask refit, adjustment, or change in mask type should be performed whenever any significant unintentional leak is observed or the patient complains of mask discomfort. If mouth leak is present and is causing significant symptoms (e.g., arousals) use of an oronasal mask or chin strap may be tried. Heated humidification should be added if the patient complains of dryness or significant nasal congestion.
Recommendations for Follow-Up:
Close follow-up after initiation of NPPV by appropriately trained health care providers is indicated to establish effective utilization patterns, remediate side effects, and assess measures of ventilation and oxygenation to determine if adjustment to NPPV is indicated.
Citation:
Berry RB; Chediak A; Brown LK; Finder J; Gozal D; Iber C; Kushida CA; Morgenthaler T; Rowley JA; Davidson-Ward SL. Best clinical practices for the sleep center adjustment of noninvasive positive pressure ventilation (NPPV) in stable chronic alveolar hypoventilation syndromes. J Clin Sleep Med 2010;6(5):497-509.
Keywords: NPPV, NPPV titration, bilevel positive airway pressure, chronic hypoventilation, BPAP, sleep related breathing disorder, sleep disordered breathing
1.0. INTRODUCTION
Compelling evidence exists to support the use of noninvasive positive pressure ventilation (NPPV) via mask during sleep in the management of selected chronic alveolar hypoventilation (CAH) syndromes in adults and children.1–46 Improved sleep, nocturnal arterial oxygen saturation, diurnal and nocturnal arterial PCO2, and quality of life indicators have been ascribed to nocturnal respiratory assistance in a divergent spectrum of disorders. NPPV has been used in CAH syndromes secondary to central respiratory control disturbances (CRCD), restrictive thoracic cage disorders (RTCD), neuromuscular diseases (NMD), and the obesity hypoventilation syndrome (OHS). Application of NPPV via a mask interface avoids the morbidity involved with tracheostomy. The most common approach is delivery of bilevel positive airway pressure47 (BPAP) in which separately adjustable inspiratory positive airway pressure (IPAP) and expiratory positive airway pressure (EPAP) are delivered. The pressure support mode devices are inherently leak tolerant and therefore ideal for use with mask ventilation.
Clinical Guidelines for the manual titration of positive airway pressure titration in patients with obstructive sleep apnea have recently been published regarding adjustment of CPAP and BPAP.48 However, a standard approach to NPPV titration has not yet appeared in the published literature. Many sleep centers have limited experience in dealing with patients with CAH syndromes other than the obesity hypoventilation syndrome. Individual sleep centers that care for a large number of patients with CAH syndromes do have protocols. However, these are not widely available and may reflect a particular mix of patients (e.g., predominantly neuromuscular disease). Protocols for NPPV titration are available from individual NPPV device manufacturers but often contain device-specific recommendations. Recognizing the need for development of guidelines for NPPV titration using polysomnography (PSG), the AASM Board of Directors appointed the NPPV Titration Task Force to develop recommendations for NPPV titration during PSG in sleep centers. The goal of the NPPV Task Force was the development of evidence- and consensus-based standardized NPPV titration guidelines, with the underlying concept that a successful titration is one in which there is an optimized trade-off between increasing pressure to yield efficacy in supporting ventilation and decreasing pressure to minimize emergence of pressure-related side effects.
2.0. METHODS
The AASM Board of Directors approved the development of NPPV titration recommendations in October of 2007 and approved the appointments of Task Force members in February 2008. The mandate for the scope of the titration recommendations included CAH syndromes due to defects in CRCD, RTCD, NMD, and OHS. The use of NPPV in patients with chronic obstructive pulmonary disease (COPD) was not included in the Task Force's mandate, which also did not include establishing indications for NPPV treatment. A literature search was conducted using the key words: NPPV titration, mask ventilation, nasal ventilation, non-invasive positive pressure ventilation, kyphoscoliosis, NPPV treatment, bilevel positive airway pressure titration, bi-level pressure titration, BPAP titration, obesity hypoventilation syndrome, central hypoventilation, congenital central hypoventilation, neuromuscular disease (including ALS and muscular dystrophy), and BPAP adjustment. All literature searches were computer-based using PubMed. The objective was to identify all studies that described NPPV titration protocols and NPPV treatment of the disorders of interest. Additional publications were obtained by review of the bibliographies of the initial set of publications. The Task Force also reviewed NPPV titration protocols developed by industry for background information. However, these protocols were not used to support the final recommendations. All relevant publications were assigned an evidence level based on the classification49 shown in Table 1.
Table 1.
AASM classification of evidence (Adapted from Oxford Centre for Evidence-based Medicine49)
| Evidence Levels | Study Design |
|---|---|
| 1 | High quality randomized clinical trials with narrow confidence intervals |
| 2 | Low quality randomized clinical trials or high quality cohort studies |
| 3 | Case-control studies |
| 4 | Case series or poor case control studies or poor cohort studies or case reports |
Summaries of studies relevant to the titration of NPPV are located in the Appendix. (The Appendix is available online only at www.aasmnnet.org/jcsm.) The evidence grades listed indicate the level of evidence available to support the findings of the study. In most instances, studies were not designed to validate the NPPV titration protocol.
As high level evidence was lacking in most areas concerning NPPV titration, a consensus process was felt to be necessary in order to guide the process. The Rand/UCLA Appropriateness Method50 was selected for this purpose given its use by the AASM Standards of Practice Committee (SPC) and the Clinical Guidelines for the titration of CPAP and BPAP Task Force.48 The first conference call of the NPPV Task Force was held on October 8, 2008 to discuss the Rand consensus process and to assign literature search topics to the committee members. A set of potential recommendations was developed to reflect the available evidence and NPPV titration protocols supplied by device manufacturers and several sleep centers with experience in NPPV titration. The proposed ballot was discussed during a second conference call on December 8, 2008, and the task force members made recommendations for revisions. After compiling these revisions the first ballot was sent to task force members who then independently voted on the recommendations. For balloting, the possible recommendations were rated on a 9-point scale. The “classic” definition of agreement was assessed using definitions from the RAND manual: Agreement for or against: No more than 2 Task Force members rate the indication outside the 3-point region (1-3, 4-6, 7-9) containing the median. Disagreement: At least 3 Task Force members rate the indication in the 1-3 region, and at least 3 Task Force members rate it in the 7-9 region. Indeterminate: Criteria are not met for agreement or disagreement. A third conference call took place on Feb 27, 2009, to review the tabulated balloting and discuss areas in which no consensus was reached. A second ballot for items that did not reach consensus was created and distributed to task force members in March 2008. A third conference call on May 28, 2009, was convened to review the 2nd ballot results and reach consensus on the remaining items.
The recommendations in section 4.0 were developed based on the voting results. The nomenclature for the recommendations and levels of recommendation are listed in the Table 2. The recommendations were reviewed by the Task Force members and two outside reviewers, the Chair of the AASM Standards of Practice Committee, and the AASM Board of Directors. The AASM Board of Directors approved these recommendations in August 2010.
Table 2.
Levels of recommendation
| Term | Level | Evidence Levels | Explanation |
|---|---|---|---|
| Recommended/Not Recommended | A | 1 or 2 | Assessment supported by a substantial amount of high quality (Level 1 or 2) evidence and/or based on a consensus of clinical judgement |
| Suggested/Not suggested | B | 1 or 2 - few studies 3 or 4 - many studies and expert consensus | Assessment supported by sparse high grade (Level 1 or 2) data or a substantial amount of low-grade (Level 3 or 4 data and/or clinical consensus by the task force |
| May be considered/Probably should not be considered | C | 3 or 4 | Assessment supported by low grade data without the volume to recommend more highly and likely subject to revision with further studies |
All members of the Task Force and the Board of Directors completed detailed conflict-of-interest statements; none had Level 1 conflicts relative to the scope of their roles. All participants in the development of this report are directors or active staff members of sleep disorders centers, and all have substantial experience with PAP and NPPV titration. These recommendations should not be considered inclusive of all proper methods of care or exclusive of other methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding the propriety of any specific aspect of care must be made by the clinician in light of the individual circumstances presented by the patient and the availability of diagnostic and treatment options and resources.
The definitions, protocols, procedures, and indications for the diagnosis and management of OSA as specified in the AASM practice parameters for polysomnography51,52 and PAP treatment,53 the AASM Manual for the Scoring of Sleep and Associated Events (i.e., respiratory rules),54 and the clinical guidelines for titration of positive airway pressure for obstructive sleep apnea should be followed where applicable.48
3.0. BACKGROUND
Treatment with NPPV via mask has been used effectively in a broad spectrum of disorders with CAH. Many of the initial studies of NPPV used volume ventilators with a mask interface. The volume ventilators were set at relatively high tidal volumes (10-15 mL/kg) to compensate for leak. Today, the most common approach is to use a bilevel positive airway pressure (BPAP) device which delivers separately adjustable inspiratory positive airway pressure (IPAP) and expiratory positive airway pressure (EPAP).47 The IPAP and EPAP are adjusted to maintain upper airway patency and the IPAP-EPAP difference provides pressure support (PS) to augment tidal volume. The delivered tidal volume may vary depending on respiratory system impedance and the patient's ventilatory effort. However, BPAP devices are relatively leak tolerant and are thus preferred for use with mask interfaces. Comparisons of volume and pressure preset NPPV have found similar effects on gas exchange and sleep quality.27,55 One study found more gastrointestinal side effects from volume preset NPPV.55 Volume preset NPPV devices may have advantages for individual patients. This document addresses only the use of BPAP devices for delivery of NPPV as these devices are available in sleep centers.
NPPV using BPAP may be delivered in the spontaneous mode (S) in which the patient determines the time spent in IPAP (within the device IPAPtime limits) as well as the respiratory rate. NPPV in the spontaneous-timed (ST) mode provides a backup rate to ensure a minimum respiratory rate (Figure 1). If the patient fails to initiate an IPAP/EPAP cycle within a time window based on the backup rate, the device will deliver a machine-triggered IPAP cycle for the inspiratory time (IPAP time) set by the prescribing physician. For example, if the back-up rate is 10 bpm, the time window following the previous breath is 6 seconds. If a spontaneous breath does not occur, the device provides a machine triggered breath. In the timed mode (T) the NPPV device delivers IPAP/EPAP cycles at a set respiratory rate with a set inspiratory time. Recently, volume targeted BPAP (VT-BPAP) has been developed in which the IPAP-EPAP difference is automatically adjusted to deliver a target tidal volume.8–11
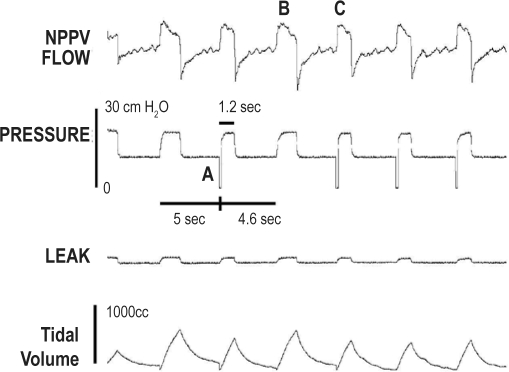
Figure 1.
Tracing of NPPV flow, pressure, leak, and tidal volume in a patient receiving BPAP in the ST mode
The backup rate is 12, and as the patient did not trigger a breath for 5 seconds, a machine triggered breath was provided (A). Note that spontaneous and machine triggered breaths have similar peak flows (B, C) but different durations and different tidal volumes. The negative pressure spike (A) is an artifact generated by the NPPV device to denote a machine triggered breath.
NPPV is often initiated after an attended NPPV titration with PSG. This approach allows determination of an appropriate interface, NPPV mode, IPAP, EPAP, as well as backup rate and inspiratory time (IPAP time), if applicable. IPAP and EPAP can also be adjusted to eliminate obstructive apnea, hypopnea, respiratory effort related arousals (RERAs), and snoring. Some sleep centers use transcutaneous PCO2 monitoring, end-tidal PCO2, or arterial blood gas sampling to guide adjustments to the NPPV device. Monitoring respiratory muscle EMG is performed in some sleep centers to help assess if adequate respiratory muscle rest has been attained during the titration (Figure 2). A NPPV delivery system consists of three main components: a NPPV device; a mask interface (nasal mask, nasal pillows mask, oronasal mask, or oral interface) held snug to the face by headgear; and a flexible hose that connects the device to the interface. NPPV devices used for titration with PSG also typically provide analog or digital outputs of flow, tidal volume, delivered pressure, and leak (total or unintentional depending on the manufacturer) that may be recorded along with other standard PSG information.
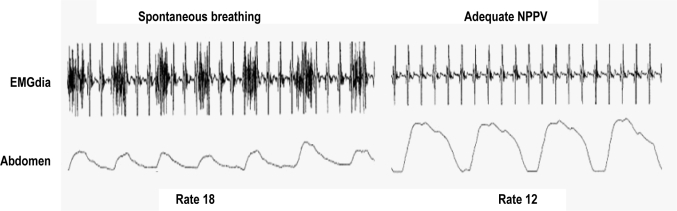
Figure 2.
When adequate NPPV was provided to this patient the diaphragmatic EMG (EMGdia) decreased as did the respiratory rate. Note the EKG artifact superimposed on the diaphragmatic EMG (figure courtesy of Conrad Iber).
The optimal NPPV device settings may vary widely between patients with various disease processes. NPPV settings may result in variable minute ventilation in the same patient in response to changes in respiratory system impedance, progression of muscle weakness, or alterations in central control due to medications or the underlying disease process. Therefore, adequate follow-up of the patient during NPPV therapy by a physician knowledgeable in the delivery of NPPV treatment is essential. The recommendations in this report pertain only to nighttime NPPV titration studies. However, some recommendations in this document may be applicable to alternative methods of NPPV titration when under the direction of a physician knowledgeable in NPPV treatment.
4.0. RECOMMENDATIONS
The following are recommendations of the NPPV Titration Task Force and the AASM Board of Directors. The scope of these NPPV titration recommendations is restricted to adult and pediatric patients with stable CAH due to NMD, RTCD, CRCD (congenital and acquired), and OHS. Patients with chronic obstructive pulmonary disease, Cheyne-Stokes respiration, and primary central sleep apnea (formerly known as idiopathic central sleep apnea) are excluded. The scope of these NPPV titration recommendations is restricted to pressure preset BPAP ventilation by mask as this is the type of ventilatory support available in most sleep centers. The scope of these NPPV titration recommendations does not address the indications for NPPV treatment. The goal of these NPPV titration guidelines is to outline a practical approach for the titration of NPPV in the sleep center that could be widely available to provide effective NPPV treatment. The titration guidelines do not assert that other careful approaches under knowledgeable physician direction would not constitute acceptable medical care.
The optimal setting for the nocturnal titration of NPPV is in an AASM-accredited sleep center or laboratory with personnel experienced with both NPPV titration and managing patients with hypoventilation such as a registered polysomnography technologist or sleep-trained respiratory therapist. The NPPV titration should be reviewed and treatment pressures selected by a physician board certified in sleep medicine.
It is understood that the recommendations for minimum and maximum IPAP and EPAP may be constrained by the specific BPAP device used during the titration protocol. Lastly, the expectation of the Task Force is that these recommendations should not be followed in a “cookbook” manner; instead, sleep technologists and clinicians should combine their experience and judgment with the application of these recommendations to attain the best possible titration for any given patient
The AASM expects these recommendations to have a positive impact upon the practice of sleep medicine, patient treatment outcomes, and health care costs. These recommendations reflect the state of knowledge at publication and will be reviewed, updated, and revised as new information becomes available. It is important to note that most recommendations published in this report are not practice parameters, since the majority of these recommendations do not achieve the evidence level of typical practice parameters. Instead, most recommendations were developed using the consensus process, and the evidence grading was used only to indicate the level of evidence available to support the recommendations. AASM levels of recommendations (Table 2) are indicated in parentheses after each Task Force recommendation. Recommendations extracted from active AASM practice parameters are labeled “(Standard)” while those not based on published AASM practice parameters are labeled “(Consensus).”
4.1. General Recommendations for NPPV Titration PSG Studies in the Sleep Center for Pediatric or Adult Patients with CAH.
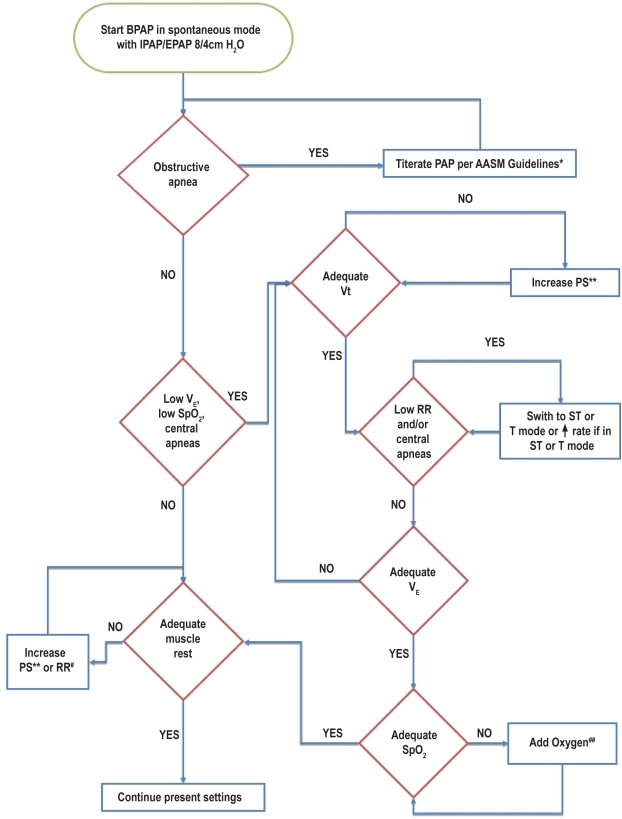
Unless otherwise stated it is understood that the recommendations apply to both pediatric and adult patients undergoing NPPV titration with PSG. A flow diagram summarizing the recommendations for NPPV titration is shown in Figure 3.
Figure 3.
Schematic of NPPV titration protocol
RR refers to respiratory rate; Vt, tidal volume; VE, minute ventilation; SpO2, oxyhemoglobin saturation by pulse oximetry; PAP, positive airway pressure; PS, pressure difference between EPAP and IPAP; ST, spontaneous timed mode; T, timed mode. *refer to section 1.4 and reference 48; **Increase PS 1-2 cm H2O every 5 minutes (section 4.4); #Add NPPV initiated breaths at 1-2 breaths per minute below the NPPV naïve rate or increase RR 1-2 every 10 minutes (section 4.5); ##Add oxygen at 1 L/min or increase by 1 L/min every 5 minutes (section 4.7).
4.1.1. The indications, goals of treatment, rationale for use, and side effects of NPPV treatment should be discussed in detail with the patient prior to the NPPV titration study. Careful mask fitting and a period of acclimatization to low pressure before the titration should be included as part of the NPPV protocol. (Standard A)
This recommendation is based on Standard level recommendation 4.3.4 (“The addition of a systematic educational program is indicated to improve PAP utilization”) in the 2006 practice parameters for the use of PAP devices53 and consensus agreement by the NPPV Titration Task Force. Orientation to the procedure can improve sleep quality and mask acceptance.56 The patient should be carefully fitted with an appropriate mask with the goals of minimizing leak, maximizing comfort, and compensating for significant nasal obstruction. The patient should be acclimated to the NPPV equipment (i.e., wearing the interface with the pressure on) prior to “lights off.”48 For pediatric patients behavioral modification techniques may be implemented to increase the tolerability and potential adherence to PAP therapy,57–59 since children frequently have problems adjusting to PAP.
4.1.2. NPPV titration with polysomnography is the recommended method to determine an effective level of nocturnal ventilatory support in patients with known daytime or nocturnal hypoventilation. In circumstances (based on clinical judgment) when NPPV treatment is initiated and adjusted empirically in the outpatient setting, a PSG should be utilized (if and when possible) to confirm that the final NPPV settings are effective or to make further adjustments as necessary. (Level A - Consensus)
This recommendation is based on consensus of the NPPV titration task force and is consistent with the recommendations for initiating PAP treatment in patients with OSA. Several studies documenting the effectiveness of NPPV in CAH syndromes used titration with PSG to determine the NPPV settings. These include studies in patients with NMD40,42,44 and OHS.13,14,21,22 In most of the studies, the titration protocols were not presented in detail. However, the stated goals of titration usually were to eliminate obstructive events and improve ventilation such that the SpO2 was > 90% and the transcutaneous PCO2 was less than a set goal (such as 45 to 50 mm Hg), if sufficient pressure to achieve these goals was tolerated.
NPPV treatment can be initiated without PSG.3,4 In some situations, based on clinical judgment, treatment can be empirically initiated at low pressure settings. Treatment pressures are then increased as tolerated over days to weeks based on daytime PCO2 measurements, nocturnal oximetry, subjective relief of symptoms, and in some cases nocturnal transcutaneous PCO2 monitoring. Such an approach may be suitable when the patient has difficulty tolerating NPPV or the major treatment goal is palliation. Several of the studies documenting the efficacy of NPPV titration in CAH syndromes did not use PSG for NPPV titration. NPPV treatment was initiated in RTCD,24–28,30 NMD,3,26,39 and OHS12 patients without PSG for titration. Many of these studies actually admitted patients to the hospital for NPPV treatment initiation (Appendix). In most settings today, hospital admission for NPPV titration alone would not be financially feasible or considered as medically necessary. A randomized trial of inpatient versus outpatient initiation of home mechanical ventilation in patients with nocturnal hypoventilation found equivalent outcomes.60 However, the patients did not have diurnal hypoventilation at baseline and PSG was not used to establish effectiveness of treatment. Outpatient initiation of NPPV without a PSG titration requires an experienced team of providers (physicians and respiratory therapists) as well as systematic approach to intervene for initial problems and for careful follow-up.
Attended NPPV titration with PSG has a number of advantages.61 These include documentation of the effects of NPPV on sleep quality, obstructive apnea and hypopnea, ventilation, and nocturnal gas exchange. The NPPV device settings (EPAP, IPAP, backup rate, inspiratory time) can be manually adjusted to deliver adequate nocturnal ventilation and improve or normalize nocturnal PCO2. If necessary, supplemental oxygen can also be added. Mask interfaces can be changed or adjusted to maximize comfort and minimize leak. The PSG can document the effectiveness of NPPV settings in various body positions and sleep stages. It is common for NMD, RTCD, and OHS patients to have the most severe degree of hypoventilation during REM sleep.62 Therefore, it is important to document that NPPV settings selected for chronic treatment are effective in that situation.
Studies in patients with OHS21 and NMD63 have documented disturbances in sleep quality due to patient-NPPV device asynchrony. Other studies noted that leak (particularly mouth leak)64–66 also resulted in sleep disturbance and reduced the effectiveness of NPPV. Such sleep disturbance and problems with leak would usually not be recognized without PSG. Fanfulla and coworkers63 found that empiric NPPV settings that were effective and well tolerated during the day were associated with a substantial frequency of ineffective respiratory efforts and worsened sleep quality at night. Guo et al.21 found that PSG detected periods of patient-NPPV device desynchronization (uncoupling of the onset of the patient's respiratory effort as detected by thoraco-abdominal movement and the onset of an IPAP/EPAP pressure cycle), periodic breathing, or auto-triggering in a group of OHS patients chronically treated with NPPV. Desynchronization was present in 55% of the patients studied and was associated with arousals. These episodes were often not associated with changes in either transcutaneous PCO2 or oximetry. NPPV titration with PSG or PSG during treatment on the empirically selected NPPV settings would therefore be necessary to identify desynchronization or arousals from leaks.
4.1.3. The goals of NPPV titration and treatment should be individualized. Different levels of NPPV support may be needed depending on the specific goals in an individual patient. Attended NPPV titration with polysomnography is the standard method to determine an effective level of NPPV support when the treatment goal (s) is (are) to (1) reduce sleep fragmentation and improve sleep quality, (2) decrease the work of breathing and provide respiratory muscle rest, (3) normalize or improve gas exchange, and (4) relieve or improve nocturnal symptoms in patients with nocturnal hypoventilation. (Level A - Consensus)
This recommendation is based on consensus of the NPPV titration task force and acknowledges that titration goals may vary based on the individual case characteristics and by CAH etiology. Two published studies documented an improvement in sleep quality with NPPV treatment.8,23 If palliation is the main goal, finding a tolerable level of NPPV that improved symptoms (nocturnal dyspnea, morning headache, frequent awakenings) would be the primary goal of NPPV titration and treatment.
Optimal titration of NPPV to decrease the work of breathing during sleep requires monitoring of tidal volume, respiratory rate, and possibly respiratory muscle EMG to document the impact of NPPV. Patients with respiratory muscle weakness often exhibit considerable EMG activity of the accessory muscles of respiration (sternocleidomastoid, scalene, and others) during sleep.62,68–70 In addition, patients with respiratory muscle weakness or increased work of breathing often have a pattern of rapid shallow breathing (low tidal volume, high respiratory rate). Evidence that NPPV has reduced work of breathing includes an increase in tidal volume, a reduction in respiratory rate,71–73 and absence or reduction of inspiratory EMG activity of the muscle of respiration compared to baseline levels or those on lower amounts of NPPV support68 (Figure 2). Evidence of reduced work of breathing as just described, or by other validated methods, in the course of NPPV titration serves as the metric of successful NPPV titration where respiratory muscle rest during sleep is desirable.
During attended NPPV titration gas exchange can be monitored by pulse oximetry and the arterial PCO2 may be measured intermittently by arterial blood gas testing or continuously estimated by transcutaneous PCO2 or end-tidal PCO2 monitoring to allow precise documentation of an adequate level of NPPV support.
4.1.4. In instances where OSA coexists with CAH, NPPV titration with polysomnography is the standard method to determine effective pressure settings for the treatment of OSA. (Level A - Standard) The titration of positive airway pressure to eliminate obstructive events should follow the AASM Clinical Guidelines for PAP Titration in Patients with Obstructive Sleep Apnea. (Level A - Consensus)
This recommendation is based on the Standard recommendation in the practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders and the consensus based AASM Clinical Guidelines for PAP titration in patients with Obstructive Sleep Apnea.48 The majority of patients with the obesity hypoventilation syndrome12 have discrete apneas and hypopneas. In addition, several studies of patients with chronic hypoventilation due to chest wall disorders and neuromuscular disorders also were found to have obstructive respiratory events during PSG. For example, in one study by Gonzalez and coworkers24 of patients with kyphoscoliosis the AHI averaged 13.9 events/hour. While concurrent OSA may be suspected in candidates for NPPV who report snoring and witnessed apnea, absence of these symptoms does not rule out significant obstructive events.
4.1.5. Attended NPPV titration with polysomnography is the standard method to identify optimal treatment pressure settings for patients with the OHS in whom NPPV treatment is indicated (Level A - Consensus).
This recommendation is based on the AASM Practice Parameters for PSG and the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders.53 The majority of patients with OHS have obstructive sleep apnea.9,10,12–21 However, a minority simply have daytime hypoventilation that worsens during sleep in the absence of discrete obstructive events.18 This NPPV titration recommendation does not imply that CPAP may not be effective in preventing apnea, hypopnea, and snoring in individual OHS patients. Chronic CPAP treatment in OHS patients can also result in a reduction in daytime PCO2 in some patients.13 However, many OHS patients require high levels of CPAP and may manifest residual arterial oxygen desaturation or continue to hypoventilate after upper airway patency is restored.13,17 In such patients BPAP (NPPV) has been proven effective and by augmenting ventilation may prevent the need for mechanical ventilation or supplemental oxygen.12–21 A recent randomized trial22 compared CPAP and BPAP for treatment of patients with OHS. Patients with significant residual desaturation (SpO2 < 80% for > 10 min) on a level of CPAP that eliminated obstructive events, an acute rise in PCO2 > 10 mm Hg during REM sleep, or an increase in PCO2 > 10 mm Hg in the morning compared to the afternoon were excluded. An equivalent reduction in daytime PCO2 was noted at 3 months in patient randomized to CPAP or BPAP. Adherence to the treatment modalities was also not significantly different. In the BPAP group the mean IPAP and EPAP levels used were 16 and 10 cm H2O, respectively, and the spontaneous mode of BPAP was employed. A few patients in both groups required supplemental oxygen in addition to PAP. Of note, the most severe OHS patients were excluded from this study and were treated with NPPV outside of the study protocol. OHS patients may require high levels of EPAP to prevent obstructive apnea and this tends to limit the available range of pressure support unless very high IPAP levels are used. In a study of the effect of NPPV in OHS patients by Berger and coworkers,13 EPAP values up to 14 cm H2O and IPAP values up to 25 cm H2O were needed. The mean IPAP and EPAP values were 18 and 8 cm H2O, respectively.
4.1.6. Attended NPPV titration with polysomnography is the recommended method to identify an effective level of ventilatory support for patients with RTCD in whom NPPV treatment is indicated. (Level A - Consensus)
This recommendation is based on consensus of the NPPV titration task force. Several studies have documented an improvement in quality of life and gas exchange in patients with RTCD. To date, most published studies concerning NPPV treatment in RTCD patients23–25 have not used an attended NPPV titration study to select levels of pressure. However, in one study24 patients were admitted to the hospital for initiation of NPPV. Treatment NPPV settings were based on daytime arterial blood gas testing, nocturnal oximetry, or nocturnal transcutaneous PCO2 monitoring. One study also used nocturnal monitoring of arterial blood gases.25 In this study, the mean IPAP and EPAP values were 21.1 and 3.1 cm H2O, respectively, with a backup rate of 20 bpm. In this study improvement in daytime PCO2 was correlated with the amount of pressure support. A study by Gonzalez and coworkers24 used a mean backup rate of 15 bpm.
4.1.7. Attended NPPV titration with polysomnography should be performed before NPPV treatment in patients with acquired or congenital CRCD when NPPV treatment is deemed indicated. (Level A - Consensus)
This recommendation is based on consensus of the NPPV titration task force. Some patients with CRCD such as those with congenital central hypoventilation may have life-threatening events if not adequately ventilated during sleep. In these patients, documentation of the adequacy of NPPV treatment settings is essential before chronic NPPV treatment can be safely initiated. Patients with congenital central hypoventilation often require tracheostomy and volume ventilation from birth at least during sleep. Those patients who do not require daytime ventilation may be transitioned to NPPV if they are motivated, found to be reliably adherent, and a sleep study documents efficacy of NPPV.32–34 Often the transition to NPPV occurs when the patient attains an age sufficient to accept mask ventilation. However, selected patients have used NPPV from a very early age. A potential complication of early use of mask ventilation is development of central facial hypoplasia,74,75 possibly due to chronic mask pressure. This is most likely to occur in patients needing mask ventilation during the day as well as during sleep.
4.1.8. Attended NPPV titration with polysomnography allows definitive identification of an adequate level of ventilatory support for patients with NMD in whom NPPV treatment is planned. (Level A - Consensus)
This statement is based on consensus of the NPPV task Force. Numerous studies have demonstrated benefits in patients with NMD although the benefits may vary depending on the specific disorder.3,35–46 Attended NPPV titration with PSG in NMD patients can provide rapid determination of an adequate level of support, intervention for obstructive sleep apnea if present, and documentation of the effectiveness of the chosen NPPV settings in various sleep stages and body positions. Interventions for mask and leak problems can be made quickly. A number of studies in NMD patients did employ NPPV titration with PSG.40,42,44
It is acknowledged that some centers with considerable expertise in treating patients with NMD (e.g., ALS) have a structured program for initiating NPPV on an outpatient basis during the daytime. NMD patients are often started on BPAP at low pressures (IPAP = 8, EPAP = 4) after a period of daytime adaptation under direct supervision. If patients tolerate nocturnal NPPV with low pressures, the settings are increased over weeks to months based on symptoms and/or daytime arterial PCO2 measurement (or estimates of arterial PCO2 such as end-tidal PCO2). In one study of this approach in ALS patients,36 symptom relief was provided in 4 of 18 patients with the low initial settings, while most other patients required either one or two increases in pressure. Only 6/19 required a pressure support over 10 cm H2O. In patients with rapidly progressive NMD, the main goal of treatment is often palliation of symptoms and improvement in quality of life rather than normalization of nocturnal arterial PCO2.
In a randomized controlled study of the effect of BPAP in the ST mode on survival and quality of life in ALS,3 the average IPAP and EPAP settings were 15 and 6 cm H2O, respectively. In this study only the subgroup of ALS patients without moderate to severe bulbar dysfunction had improved survival. However, all patients treated with NPPV had improvement in the quality of life. Patients with bulbar involvement may have more difficulty tolerating NPPV and mask ventilation. These patients may also have a greater risk of aspiration than those without upper airway dysfunction.38
4.2. Recommendations Concerning the NPPV Device, Monitoring, Interfaces, and Other Equipment for NPPV Titration
4.2.1. Recommendations for the NPPV device used for titration
4.2.1.1. THE NPPV DEVICE USED FOR NPPV TITRATION SHOULD HAVE THE ABILITY TO FUNCTION IN THE SPONTANEOUS [SUPPORT] (S), SPONTANEOUS/TIMED [SUPPORT/TIMED] (ST), AND TIMED (T) MODES. IDEALLY THE DEVICE SHOULD PROVIDE ANALOG OR DIGITAL OUTPUTS OF FLOW, PRESSURE, LEAK, AND TIDAL VOLUME THAT MAY BE RECORDED DURING POLYSOMNOGRAPHY. (LEVEL A - CONSENSUS)
This recommendation is based on consensus of the NPPV titration task force. Patients with OHS may not require a backup rate. However, patients with severely defective central control of ventilatory drive often require a backup rate to prevent central apneas and/or hypoventilation. In patients with respiratory muscle weakness or decreased respiratory system compliance, feeble inspiratory efforts may fail to reliably trigger an IPAP/EPAP cycle; such patients often benefit from a backup rate. The timed mode is infrequently used in sleep centers. However, there are individual patients who are more adequately treated with the timed mode than the ST mode. The timed mode employing a set respiratory rate that is typical higher than the native rate may result in a more stable pattern of ventilation in some patients. Parreira and coworkers76 compared the spontaneous and timed modes in normal subjects awake and asleep. Using the timed mode, they were able to deliver higher minute ventilation at lower IPAP than with the spontaneous mode and the patient's sleeping breathing rate. Of note, a relatively high respiratory rate of 20 was used in the timed mode, with an inspiratory/expiratory ratio of 1 to 2. These findings may not generalize to patients with hypoventilation but illustrate the point that the timed mode or the ST mode with a relatively high rate is an option that could be tried in patients who do not tolerate high IPAP or in whom the maximally tolerated pressure support at the spontaneous respiratory rate does not deliver adequate minute ventilation. This may be a particularly effective strategy in patients with RTCD and reduced respiratory system compliance, in whom a high PS may be required to augment tidal volume.
4.2.2. Recommendations for parameters that should be recorded during the NPPV titration
4.2.2.1. RECORDING THE AIRFLOW SIGNAL DIRECTLY OBTAINED FROM THE NPPV DEVICE (DERIVED FROM THE ACCURATE INTERNAL FLOW SENSOR) IS THE RECOMMENDED METHOD FOR MONITORING AIRFLOW AND DETECTING APNEAS AND HYPOPNEAS DURING NPPV TITRATION. (LEVEL A - CONSENSUS)
4.2.2.2. MEASUREMENT OF AIRFLOW USING A THERMAL DEVICE OR A NASAL PRESSURE CANNULA UNDER THE MASK IS NOT RECOMMENDED. THERMAL DEVICE SIGNALS ARE NOT PROPORTIONAL TO FLOW, AND A NASAL PRESSURE CANNULA MAY CAUSE PROBLEMS WITH THE MASK SEAL AND RESULT IN UNDESIRED LEAK. (LEVEL A - CONSENSUS)
4.2.2.3. SAWTOOTH PATTERNS IN THE UNFILTERED INSPIRATORY AIRFLOW OR MASK PRESSURE TRACINGS AND/OR DETECTION OF VIBRATION BY PIEZOELECTRIC TRANSDUCERS OR MICROPHONES APPLIED TO THE NECK ARE ACCEPTABLE METHODS FOR DETECTING SNORING DURING NPPV TITRATION (LEVEL A - CONSENSUS).
Recommendations 4.2.2.1 to 4.2.2.3 are based on consensus of the NPPV titration task force and are consistent with the recommendations contained in the AASM clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea.48
NPPV devices designed for titration during polysomnography generate a digital or analog flow signal based on accurate flow sensors within the device. The majority also provide signals reflecting an estimate of leak and tidal volume as well as the delivered pressure. During NPPV titrations, the use of a standard nasal pressure sensor placed under the nares is problematic due to the difficulty in obtaining a good PAP mask seal, since the tubing must pass underneath the mask to reach the nares. The use of thermal devices under the mask is also not recommended, as the signal is not proportional to airflow. The flow output from most NPPV devices while accurate for assessing airflow and flow limitation is often too filtered or under-sampled to display snoring.
4.2.2.4. THE NPPV DEVICE ESTIMATE OF TIDAL VOLUME DERIVED FROM AN ACCURATE INTERNAL FLOW SENSOR IS AN ACCEPTABLE METHOD TO ESTIMATE TIDAL VOLUME. THE TIDAL VOLUME SIGNAL OBTAINED FROM THE NPPV DEVICE SHOULD BE RECORDED IF POSSIBLE. (LEVEL A - CONSENSUS)
This recommendation is based on consensus of the NPPV titration task force. While the flow signal readily allows detection of apnea and hypopnea, it may be more difficult to visually estimate a tidal volume that depends on both the inspiratory time was well as flow amplitude. Devices used for NPPV titration in sleep centers generally provide an analog or digital output of the estimated tidal volume derived from integration of the flow signal produced by an accurate flow sensor in the device. A breath-by-breath estimate of tidal volume is almost always available for display in the software or on the peripheral device remotely controlling the NPPV device. However, recording the tidal volume signal (Figure 1) clearly show trends and variation during acquisition and may benefit the physician reviewing the study. The product of the estimated tidal volume and respiratory rate provides an estimate of the minute ventilation.
4.2.2.5. THE NPPV DEVICE MEASUREMENT OF DELIVERED PRESSURE USING AN ACCURATE INTERNAL PRESSURE SENSOR IS AN ACCEPTABLE METHOD TO ESTIMATE DELIVERED PRESSURE. MEASUREMENT OF PRESSURE AT THE MASK OR DEVICE OUTLET USING AN ACCURATE PRESSURE TRANSDUCER IS ALSO AN ACCEPTABLE METHOD OF DETERMINING DELIVERED PRESSURE. A SIGNAL REFLECTING DELIVERED PRESSURE (AT THE MACHINE OR MASK) SHOULD BE RECORDED IF POSSIBLE. (LEVEL A - CONSENSUS)
Recording of delivered pressure is helpful to the technologist and physician reviewing the sleep recording. The pressure signal clearly shows the IPAP/EPAP cycle and is especially helpful in determining the pattern of response of the NPPV device in situations where the backup rate frequently intervenes. Some NPPV devices output a brief signal (intentional artifact) before each machine triggered breath (Figure 1). Another method to identify machine triggered breaths is to observe that they often have a different inspiratory time than spontaneous breaths.
4.2.2.6. THE NPPV DEVICE MEASUREMENT OF LEAK USING AN INTERNAL FLOW SENSOR IS AN ACCEPTABLE METHOD TO ESTIMATE LEAK. A SIGNAL REFLECTING LEAK SHOULD BE MONITORED AND RECORDED IF POSSIBLE. (LEVEL A - CONSENSUS).
Recording of estimates of leak is helpful to the technologist and the physician reviewing the sleep recording. The total flow is partitioned into a bias portion (leak) and a variable portion (patient flow). The leak is composed of the intentional leak + unintentional leak (mask or mouth leak). The intentional leak is due to flow through the non-rebreathing orifice. This leak depends on the interface type and delivered pressure. In some NPPV devices, the leak signal reflects the total leak; in others, a mask interface can be specified and the leak signal reflects unintentional leak. Several studies have documented that leak, especially mouth leak, can impair the effectiveness of NPPV and sleep quality.64–67 As delivered pressure increases during the titration, the total leak will increase. Therefore, what constitutes an inappropriately high leak will depend on interface and pressure (see also 4.8.6).
4.2.2.7. RECORDING RESPIRATORY MUSCLE EMG ACTIVITY USING BIPOLAR SURFACE ELECTRODES MAY BE USEFUL DURING NPPV TITRATION IN PATIENTS WITH NMD AND/OR AN INCREASED EFFORT OF BREATHING. ADEQUATE NPPV SUPPORT FOR MUSCLE REST IS ASSOCIATED WITH THE ABSENCE OR DECREASE IN INSPIRATORY EMG ACTIVITY OF THE RESPIRATORY MUSCLES DURING NREM SLEEP. (LEVEL A - CONSENSUS)
Some sleep centers with considerable experience in NPPV titration in patients with muscle weakness routinely record surface EMG from respiratory muscles using bipolar electrodes with techniques similar to those used for recording the anterior tibialis EMG. Surface diaphragm EMG recording utilizes two electrodes about 2 cm apart horizontally in the seventh and eighth intercostal spaces in the right anterior axillary line.68,70 The right side of the body is used to reduce EKG artifact. Other sites include the sternocleidomastoid (an accessory muscle of respiration) and the right parasternal intercostal muscle (2nd and 3rd intercostal spaces in the mid-clavicular line). In normal individuals, the accessory muscles are usually quiet during sleep except during periods following arousal. Inspiratory EMG activity is noted in the intercostal muscles and the diaphragm during NREM sleep. During REM sleep, the intercostal activity is inhibited but diaphragmatic activity persists (although frequently diminished during bursts of eye movements). In contrast, in patients with respiratory muscle weakness or increased work of breathing, the EMG of accessory muscles often shows inspiratory activity during NREM sleep. During the NPPV titration, a reduction in the EMG activity of respiratory muscles (accessory, intercostals, diaphragm) may be a useful indicator that sufficient pressure support is being administered to allow muscle rest (Figure 2).
4.2.2.8. RECOMMENDATIONS FOR THE MEASUREMENT OF PCO2 DURING NPPV TITRATION
4.2.2.8.1. ARTERIAL BLOOD GAS TESTING AT A GIVEN LEVEL OF NPPV SUPPORT (ONCE SETTINGS HAVE BEEN OPTIMIZED OR IN THE MORNING AFTER A NIGHT OF NPPV) IS THE MOST ACCURATE METHOD FOR DETERMINING THE EFFECTS OF NPPV ON THE ARTERIAL PCO2. CAPILLARY BLOOD GAS TESTING IS AN ACCEPTABLE ALTERNATIVE TO ARTERIAL BLOOD GAS TESTING. (LEVEL A - CONSENSUS)
Determination of the arterial PCO2 by arterial blood gas sampling is the gold standard but is invasive, and arterial blood gas sampling requires special expertise. In addition, rapid access to a laboratory where the sample can be processed may be problematic for sleep centers located outside of hospital. In pediatric patients, sampling of a capillary blood gas (arterialized blood) is often used as an alternative to arterial blood gas testing. Another limitation of arterial blood gas monitoring is that serial testing during sleep requires either an indwelling catheter or repeated arterial punctures, and obtaining a sample may well cause arousal and a change in the PCO2.
4.2.2.8.2. TRANSCUTANEOUS CO2 (PTCCO2) MONITORING MAYBE USEFUL FOR NPPV TITRATION WHEN THE DEVICE IS CALIBRATED AND THE READINGS ARE WITHIN 10 MM HG OF THE CONCURRENT PCO2 VALUE OBTAINED BY ARTERIAL BLOOD GAS (OR CAPILLARY BLOOD GAS) TESTING DURING STABLE BREATHING. DUE TO A SLOW RESPONSE TIME, CHANGES IN PTCCO2 GENERALLY ARE DELAYED FOLLOWING CHANGES IN THE ARTERIAL PCO2. (LEVEL A - CONSENSUS)
There have been relatively few studies of the performance of transcutaneous CO2 (PtcCO2) monitoring during NPPV titration and treatment.77–79 Storre and coworkers77 compared 250 paired samples of arterial blood gas and PtcCO2 measurements in a group of 8 subjects undergoing NPPV for acute or chronic hypoventilation due to chronic obstructive pulmonary disease. They found that the PtcCO2 provided a reasonable estimate of arterial blood gases, with the best results obtained by comparing a given arterial PCO2 value with a PtcCO2 result two minutes later. In an earlier study, Sanders et al.80 did not find transcutaneous PCO2 to be a valid indicator of the arterial PCO2during sleep. Pavia and coworkers80 performed transcutaneous PCO2 monitoring during NPPV and documented nocturnal hypoventilation (PtcCO2 > 50 mm Hg) in 21 patients of a group of 50 with normal daytime PCO2 (capillary blood gas) who had no nocturnal desaturation on oximetry. Certainly the information supplied by transcutaneous PCO2 may be very useful if the accuracy can be verified. The ideal situation is one in which the PtcCO2 device is calibrated before monitoring and the results are validated by ABG sampling at least at the start of or during the study. Caution is advised in making clinical decisions based on transcutaneous PCO2 monitoring alone especially if the results do not correlate with other findings.
4.2.2.8.3. END-TIDAL PCO2 (PETPCO2) MONITORING MAY BE USEFUL DURING NPPV TITRATION. IDEALLY, THE ACCURACY OF PETCO2 SHOULD BE VALIDATED BY CONCURRENT PCO2 MEASUREMENT USING ARTERIAL OR CAPILLARY BLOOD GAS TESTING DURING STABLE BREATHING. PETCO2 VALUES THAT DIFFER BY LESS THAN 10 MM HG FROM THE ARTERIAL (OR CAPILLARY) VALUE INDICATE A VALID READING. SAMPLING OF GAS AT THE NARES RATHER THAN AT THE MASK IS REQUIRED FOR OPTIMAL MEASUREMENT OF PETCO2 DURING NPPV TITRATION. IF THE PLATEAU IS LOST FROM THE PETCO2 WAVEFORM DURING THE TITRATION, THE MEASUREMENT CAN NO LONGER BE CONSIDERED ACCURATE. IF SIGNIFICANT LUNG DISEASE IS PRESENT, THE DIFFERENCE BETWEEN THE PETCO2 AND THE ARTERIAL PCO2 OFTEN EXCEEDS 10 MM HG. FOR THIS REASON, END-TIDAL PCO2 MEASUREMENT IS LESS FREQUENTLY USED IN PATIENTS WITH CAH WHO HAVE SIGNIFICANT CONCURRENT LUNG DISEASE. (LEVEL A - CONSENSUS)
This recommendation is based on consensus of the NPPV titration task force. We know of no published study that documents the accuracy of monitoring end-tidal PCO2 during NPPV titration. Of note, Sanders and coworkers80 found poor agreement between end-tidal PCO2 and arterial blood gas testing in adult patients during diagnostic polysomnography. However, gas was sampled from a mask covering the nose and mouth rather than the nares. In contrast Kirk and coworkers81 found reasonable agreement between end-tidal PCO2 and transcutaneous PCO2 during pediatric polysomnography. In the commonly used side-stream capnography method, exhaled gas is suctioned via a nasal cannula to an external CO2 sensor. The major challenge for end-tidal PCO2 monitoring during NPPV titration is dilution of the exhaled sample by flow from the NPPV device (especially if gas is sampled at the mask). One approach is to have the patient wear a sampling nasal cannula under the NPPV mask interface. Using this approach gas is sampled as it is exhaled from the nares rather than suctioned from the mask. A similar method was used by Parreira and coworkers76 during their study of bilevel PAP in normal subjects. A disadvantage of this approach is that the presence of the cannula passing under the nasal or oronasal mask seal could cause excessive leak. If an oral mask is used the sampling catheter would be directly connected to the mask.
4.2.2.9. NPPV MASK INTERFACES AND HUMIDIFICATION FOR NPPV TITRATION
4.2.2.9.1. THERE SHOULD BE SEVERAL DIFFERENT TYPES OF PAP INTERFACES (I.E., NASAL MASKS, NASAL PILLOWS, ORONASAL MASK) AND ACCESSORIES (CHINSTRAPS, HEATED HUMIDIFIER) AVAILABLE IF THE PATIENT ENCOUNTERS PROBLEMS (E.G., MOUTH LEAK, NASAL CONGESTION) DURING THE NIGHT. THE PATIENT SHOULD BE ACCLIMATED TO THE INTERFACE AND THE MASK FIT TESTED WITH THE PRESSURE ON PRIOR TO “LIGHTS OFF.” (LEVEL A - CONSENSUS)
4.2.2.9.2. PEDIATRIC (IN ADDITION TO SMALL ADULT) INTERFACES SHOULD BE AVAILABLE FOR TITRATION IN PATIENTS LESS THAN 12 YEARS OLD. PEDIATRIC PATIENTS BENEFIT FROM INTRODUCTION TO POTENTIAL INTERFACES AND ACCLIMATIZATION BEFORE THE NIGHT OF THE NPPV TITRATION. INTRODUCTION OF THE INTERFACES SHOULD BE DEVELOPMENTALLY APPROPRIATE FOR THE CHILD'S AGE AND MAY INCLUDE CHILD LIFE OR OTHER SUPPORT SERVICES PRIOR TO TITRATION. (LEVEL A - CONSENSUS)
Recommendations 4.2.2.9.1 and 4.2.2.9.2 are based on consensus of the NPPV titration task force and are consistent with the AASM clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea.48 As mentioned in an earlier section, pediatric patients require an introduction to masks prior to the NPPV titration. A Child Life Specialist is an example of a trained professional with expertise in helping children and their families overcome challenging life events in the medical setting. A Child Life Specialist or similar professional with expertise in child development can help children and their families adapt to masks and NPPV titration.
4.2.2.9.3. HEATED HUMIDIFICATION SHOULD BE AVAILABLE FOR PATIENTS UNDERGOING NPPV TITRATION. THE ADDITION OF HUMIDIFICATION IS INDICATED IF THE PATIENT COMPLAINS OF SIGNIFICANT ORAL OR NASAL DRYNESS (UNLESS HUMIDIFICATION IS NOT TOLERATED BY THE PATIENT). (LEVEL A - CONSENSUS)
This recommendation is based on consensus of the task force. It is consistent with clinical guidelines for the manual titration of CPAP and BPAP in patients with OSA. The AASM Standards of Practice for CPAP and BPAP treatment also states “the addition of heated humidification is indicated to improve PAP utilization.”53 Two controlled studies were not able to document an improvement in patient acceptance of CPAP treatment with the prophylactic use of heated humidity.82,83 However, the level of CPAP was relatively low (around 10 cm H2O) and the nasal symptoms were mild in one study. NPPV treatment frequently uses relatively high pressures and is associated with high intentional and unintentional leak. A bench study documented that NPPV devices deliver air at a reduced relative humidity compared to ambient air and that increasing IPAP lowered the delivered relative humidity.84 Nava and coworkers compared heated humidification with a heat and moisture exchanger using a crossover protocol and found similar adherence to NPPV.85 However, most patients preferred heated humidity. Tuggey and coworkers66 found that heated humidification reduced the impact of mouth leak on nasal resistance (increased with mouth leak) and delivered tidal volume in a group of normal subjects breathing on NPPV during the day. Initiation of humidification at the start of a NPPV titration PSG may be useful in selected patients with significant nasal congestion or a history of severe mucosal dryness during sleep, or in particularly dry climates such as the American southwest or during the winter in cold climates. At the minimum, heated humidity should be available for patients who develop severe nasal congestion or dryness during the night.
4.3. Recommendations for Initial and Maximum Pressures during NPPV Titration
4.3.1. The recommended minimum starting IPAP and EPAP should be 8 cm H2O and 4 cm H2O, respectively. (Level A - Consensus).
This recommendation is based on consensus agreement by the NPPV PAP Titration Task Force
An EPAP of 4 cm H2O is the lowest available on most NPPV devices. The recommendation of a starting IPAP is based on the minimum recommended pressure support. These recommendations are consistent with the AASM Clinical Guidelines for the manual titration of PAP in patients with OSA.48
4.3.2. The recommended minimum starting pressure support (difference between IPAP and EPAP) should be 4 cm H2O. (Level A - Consensus)
This recommendation is based on consensus agreement by the NPPV Titration Task Force.
While it is acknowledged that 4 cm H2O provides only a modest degree of pressure support, starting at this level will allow adaptation to positive airway pressure.
4.3.3. The recommended maximum pressure support (difference between IPAP and EPAP) should be 20 cm H2O. (Level A - Consensus)
This recommendation is based on consensus agreement by the NPPV titration task force.
In the AASM clinical guidelines for titration of CPAP and BPAP, the recommended minimum IPAP-EPAP difference was 4 cm H2O and the maximum was 10 cm H2O. However, in the case of OSA, the major objective is to maintain airway patency. Budweiser and coworkers25 used a mean pressure support of 18 cm H2O in a study of patients with RTCD. Patients with low respiratory system compliance may require high level of pressure support to augment ventilation during sleep.
4.3.4. The recommended maximum IPAP should be 20 cm H2O for patients < 12 years and 30 cm H2O for patients ≥ 12 years. (Level A - Consensus)
This recommendation is based on consensus agreement by the NPPV titration task force.
The maximum IPAP available on most NPPV devices is 25 or 30 cm H2O. A lower value for the maximum IPAP was recommended for patients less than 12 years of age in the clinical guidelines for manual titration of PAP in patients with OSA.48 It is acknowledged that individual patients may benefit from higher IPAP. High pressure levels are problematic for obtaining an adequate mask seal and often require significant mask tightening. There have been reports of changes in facial structure associated with NPPV in children.74,75 Although it is not known whether the interface itself or the pressure delivered is the issue, it seems prudent to avoid very high pressures if possible in children.
4.3.5. The minimum and maximum incremental changes in PS during NPPV titration should be 1 and 2 cm H2O, respectively. (Level A - Consensus).
This recommendation is based on consensus agreement by the NPPV titration task force.
Although smaller pressure changes than 1 cm H2O are possible, such changes are unlikely to be clinically meaningful. A maximum incremental change of 2 cm H2O is recommended to avoid over titration.
4.4. Recommendations for the Adjustment of Pressure Support During NPPV Titration (see Figure 3)
4.4.1. IPAP and EPAP should be adjusted to eliminate obstructive apneas, hypopneas, RERAs (respiratory effort related arousals), and snoring following the AASM Clinical Guidelines for the Manual Titration of PAP in Patients with OSA. (Level A - Consensus)
Recommendation 4.4.1 is based on a consensus of the NPPV titration task force and is consistent with the AASM clinical guidelines for PAP titration in patients with OSA.48 These guidelines provide a protocol for increasing in the IPAP and EPAP to eliminate obstructive apneas, hypopneas, RERAs, and snoring.
4.4.2. Recommendations for adjusting pressure support for low tidal volume or hypoventilation during sleep
4.4.2.1. THE PS SHOULD BE INCREASED EVERY 5 MINUTES IF THE TIDAL VOLUME IS BELOW THE ACCEPTABLE GOAL. AN ACCEPTABLE TIDAL VOLUME GOAL FOR MOST PATIENTS RANGES FROM 6 TO 8 ML/KG USING IDEAL BODY WEIGHT (FIGURE 3). (LEVEL A - CONSENSUS).
Recommendation 4.4.2.1 is based on consensus of the NPPV titration task force. An acceptable tidal volume may vary with the disorder being treated and the respiratory rate. Tidal volumes of 6 to 8 mL/kg at typical respiratory rates usually deliver normal minute ventilation. In patients with hypercapnia, a small increase or decrease in alveolar ventilation results in a relatively large decrease or increase in the PCO2 due to the hyperbolic relationship between the two variables. If lung disease is present, a higher minute ventilation is needed to deliver adequate alveolar ventilation due to an increase in physiological dead space. In normal individuals, the dead space is approximately equal to 2 mL/kg. The recommended tidal volume target for volume targeted BPAP is 8 mL/kg using ideal body weight.8–10 Slightly lower tidal volumes with higher respiratory rates may be better tolerated in individual patients (particularly in RTCD). It should also be noted that a reduction in leak by mask refit or change may improve the effectiveness of the current PS. Therefore, intervention for leak should be considered especially if prior increases in PS have been ineffective with respect to increasing tidal volume.65,66 The accuracy of NPPV device estimates of tidal volume is dependent on the accuracy of the flow signal. If the accuracy of the flow signal is degraded by mouth leak (nasal mask) or high mask leak, then the estimated tidal volume may not accurately reflect the tidal volume of the patient. Discrepancy between device estimates of tidal volume and the clinical scenario (including other related sensors) should prompt the technologist to check for excessive leak. As an example, increases in the pressure support (difference between IPAP and EPAP) that fail to raise tidal volume might herald excessive leak.
4.4.2.2. THE PS SHOULD BE INCREASED IF THE ARTERIAL PCO2 REMAINS 10 MM HG ABOVE GOAL AT THE CURRENT SETTINGS FOR 10 MINUTES OR MORE. AN ACCEPTABLE GOAL FOR PCO2 IS A VALUE LESS THAN OR EQUAL TO THE AWAKE PCO2(FIGURE 3). (LEVEL A - CONSENSUS).
4.4.2.3. THE PS SHOULD BE INCREASED IN 1 TO 2 CM H2O INCREMENTS IF THE TRANSCUTANEOUS PCO2 OR END-TIDAL PCO2REMAINS 10 MM HG OR MORE ABOVE GOAL FOR 10 MINUTES OR MORE. THIS ASSUMES THAT THESE MEASUREMENTS OF THE PCO2 HAVE BEEN DOCUMENTED TO ACCURATELY REFLECT THE ARTERIAL PCO2 IN A GIVEN PATIENT (FIGURE 3). (LEVEL A - CONSENSUS).
Recommendations 4.4.2.2 and 4.4.2.3 are based on consensus of the NPPV titration task force.
It is acknowledged that not all sleep centers have the ability to measure transcutaneous or end-tidal CO2or the expertise to use the information. This recommendation is included for sleep centers who have the capability to make these measurements and routinely use them during NPPV titrations. Several of the studies included in the Appendix did utilize measurements of CO2 to guide NPPV titration. It should be noted that in normal subjects there is a small increase in arterial PCO2 during sleep of about 5 to 10 mm Hg. However, as most patients being treated with NPPV have daytime hypoventilation, one ideal goal of treatment would consist of preventing a further increase in the arterial PCO2 during sleep. The daytime awake PCO2 may decrease with chronic nocturnal NPPV treatment in a substantial number of patients with CAH. Thus, the level of nocturnal PCO2 on NPPV may eventually be lower than the initial goal. On the other hand some patients may not initially tolerate a level of PS adequate to meet the chosen PCO2 goal. However, both daytime and nocturnal PCO2 may decrease over time with chronic NPPV treatment.
4.4.2.5. PRESSURE SUPPORT MAY BE INCREASED IF RESPIRATORY MUSCLE REST HAS NOT BEEN ACHIEVED BY NPPV TREATMENT AT THE CURRENT SETTINGS FOR 10 MINUTES OF MORE. ADEQUATE RESPIRATORY MUSCLE REST DURING NPPV IS ASSOCIATED WITH RESOLUTION OR IMPROVEMENT IN TACHYPNEA AND/OR EXCESSIVE INSPIRATORY EFFORT AS MEASURED BY PHASIC EMG ACTIVITY OF INSPIRATORY MUSCLES (FIGURE 3). (LEVEL A – CONSENSUS)
This recommendation is based on consensus of the NPPV titration task force. As noted in a previous section, the accessory muscles of respiration are not normally active during sleep. The absence or decrease in accessory muscle EMG and a decrease in intercostal and diaphragmatic EMG would suggest that a given level of NPPV was providing respiratory muscle rest.
4.4.2.6. PRESSURE SUPPORT MAY BE INCREASED IF THE SPO2 REMAINS BELOW 90% FOR 5 MINUTES OR MORE AND TIDAL VOLUME IS LOW (< 6 TO 8 ML/KG). IF DISCRETE OBSTRUCTIVE APNEAS OR HYPOPNEAS ARE PRESENT, THE CLINICAL GUIDELINES FOR PAP TITRATION IN OSA PATIENTS SHOULD BE FOLLOWED. (LEVEL A - CONSENSUS).
This recommendation is based on consensus of the NPPV titration task force. The rationale behind this recommendation is that an increase in pressure support may increase tidal volume and reduce or eliminate residual hypoventilation thereby improving oxygenation. If not successful the addition of supplemental oxygen may be needed. While criteria for chronic oxygen therapy often use a SpO2 of less than 88% as an indication for supplemental oxygen, the slightly higher goal of 90% was chosen to allow for a margin error as the tidal volume associated with a level of pressure support could vary somewhat with the clinical condition of the patient. In addition, in some circumstances the pulse oximetry values can overestimate the actual arterial oxyhemoglobin saturation.
4.5. Recommendations for the Use of ST and Timed Modes
4.5.1. The ST Mode (backup rate) should be used in all patients with central hypoventilation or significantly impaired respiratory drive. If the ST Mode is not successful the Timed Mode with a fixed respiratory rate may be tried (Level A - Consensus).
4.5.2. The ST Mode (backup rate) should be used if frequent and significant central apneas are present at baseline or during the NPPV titration, if the respiratory rate is inappropriately low, or if the patient fails to reliably trigger the NPPV device to transition from EPAP to IPAP due to muscle weakness (Figure 3). If the ST Mode is not successful, the Timed Mode with a fixed respiratory rate may be tried (Level A - Consensus).
Recommendations 4.5.1 and 4.5.2 are based on consensus of the task force and the fact that patients with central hypoventilation by definition may fail to trigger an IPAP/EPAP cycle for an inappropriately long period (central apnea) or may have an inappropriately low respiratory rate to provide adequate minute ventilation. In addition, many patients with disorders such as central congenital hypoventilation will not respond to worsening hypoxemia with an increase in respiratory effort. Other patients with CAH may unreliably trigger IPAP/EPAP cycle due to muscle weakness or develop central apnea during the NPPV titration. Given the fact that muscle strength may vary over time in patients with NMD, most clinicians would use a backup rate for chronic treatment in these patients even if not required during the NPPV titration. Several of the studies reviewed employed the ST mode in NMD,3,39,44 OHS,18 and RTCD.25,27,30
4.5.3. The ST Mode (backup rate) may be used if adequate ventilation or adequate respiratory muscle rest is not achieved with the maximum (or maximum tolerated) PS in the spontaneous mode. If the ST Mode is not successful, the Timed Mode with a fixed respiratory rate may be tried (Figure 3). (Level A - Consensus).
This recommendation is based on consensus of the task force. Using a higher respiratory rate can potentially deliver higher minute ventilation with the same tidal volume. However, at higher rates, the time for exhalation decreases. Choice of respiratory rate and inspiratory time are discussed below.
4.5.4. Recommendations for choosing the backup rate in the ST mode and the respiratory rate in the timed mode
4.5.4.1. THE STARTING BACKUP RATE FOR THE ST MODE SHOULD BE EQUAL TO OR SLIGHTLY LESS THAN THE PATIENT'S SPONTANEOUS SLEEPING RESPIRATORY RATE (MINIMUM OF 10 BPM). IF THE SLEEPING RESPIRATORY RATE IS NOT KNOWN, ONE MAY USE THE SPONTANEOUS AWAKE RESPIRATORY RATE. THE INITIAL SETTING FOR THE RESPIRATORY RATE IN THE TIMED MODE SHOULD BE EQUAL OR SLIGHTLY LESS THAN THE PATIENT'S SPONTANEOUS SLEEPING RESPIRATORY RATE OR THE CURRENT BACK-UP RATE IF SWITCHING FROM THE ST TO TIMED MODE. (LEVEL A – CONSENSUS)
4.5.4.2. THE BACKUP RATE (ST MODE) OR SPECIFIED RESPIRATORY RATE (TIMED MODE) SHOULD BE INCREASED IN INCREMENTS OF 1-2 BREATHS PER MINUTE, NO MORE FREQUENTLY THAN EVERY 10 MINUTES, IF THE DESIRED GOAL OF THE BACKUP RATE (OR FIXED RESPIRATORY RATE) IS NOT ATTAINED WITH LOWER RATES (FIGURE 3). (LEVEL A – CONSENSUS)
4.5.4.3. THE BACKUP RATE (ST MODE) OR RESPIRATORY RATE (TIMED MODE) SHOULD BE DECREASED IF THE PATIENT REPORTS DISCOMFORT THOUGHT TO BE RELATED TO A HIGH RESPIRATORY RATE OR IF NPPV DEVICE-TRIGGERED AND PATIENT-TRIGGERED BREATHS ARE FREQUENTLY SUPERIMPOSED (STACKING OF BREATHS). (LEVEL A – CONSENSUS)
These recommendations (4.5.4.1 to 4.5.4.3) are based on consensus of the NPPV titration task force and review of industry protocols. In a study of NMD patients Katz and coworkers44 used a back-up rate 10% below that patient's resting breathing rate. One industry protocol suggests starting at a minimum rate of 8 to 10 breaths per minute or 2 breaths per minute below the patient's resting rate.86 Gonzalez and coworkers24 used a backup rate of 15 in a group of patients with RTCD. Mellies et al.42 used backup rates ranging from 14 to 24 (mean 19.6) in a group of children with NMDs. Tuggey et al.27 used a mean backup rate of 15 in a study of NPPV and patients with RTCD, while another study25 used a backup rate of 20 breaths per minute in a similar population.
4.5.5. Recommendations for choice of the inspiratory time in the ST and timed modes
4.5.5.1. THE INITIAL INSPIRATORY TIME (IPAP TIME) FOR MACHINE-TRIGGERED BREATHS IN THE ST MODE OR ALL BREATHS IN THE TIMED MODE IS CHOSEN BASED ON THE RESPIRATORY RATE AND THE NEED TO PROVIDE BOTH AN ADEQUATE TIDAL VOLUME AND AN APPROPRIATE INSPIRATORY TIME TO EXPIRATORY TIME RATIO (I: E RATIO). ANOTHER METHOD OF EXPRESSING THE I: E RATIO IS THE %IPAP TIME WHICH IS INSPIRATORY TIME (IPAP TIME) AS A % OF THE CYCLE TIME (TABLE 3). THE RECOMMENDED %IPAP TIME IS USUALLY BETWEEN 30% AND 40%. THE DEFAULT INSPIRATORY TIME ON NPPV DEVICES IS COMMONLY 1.2 SECONDS. (LEVEL A - CONSENSUS)
Table 3.
| %IPAPtime (%) | RR | Cycle Time (sec) | Inspiratory Time = IPAPtime (sec) | EPAPtime (sec) | I:E |
|---|---|---|---|---|---|
| 30% | 10 | 6 | 1.8 | 4.2 | 1:2.3 |
| 12 | 5 | 1.5 | 3.5 | 1:2.3 | |
| 15 | 4 | 1.2 | 2.8 | 1:2.3 | |
| 20 | 3 | 0.9 | 2.1 | 1:2.3 | |
| 40% | 10 | 6 | 2.4 | 3.6 | 1:1.5 |
| 12 | 5 | 2.0 | 3.0 | 1:1.5 | |
| 15 | 4 | 1.6 | 2.4 | 1:1.5 | |
| 20 | 3 | 1.2 | 1.8 | 1:1.5 |
Inspiratory Time = IPAP time
%IPAP = IPAPtime X 100 / (IPAPtime+EPAPtime)
Cycle time = 60/respiratory rate in breaths per minute
Cycle time = (IPAPtime+EPAPtime)
I:E ratio = inspiratory time/expiratory time = IPAP time/EPAP time
4.5.5.2. A SHORTER INSPIRATORY TIME (%IPAPTIME OF APPROXIMATELY 30%) MAY BE USEFUL IN PATIENTS WITH CONCURRENT OBSTRUCTIVE AIRWAYS DISEASE (ESPECIALLY AT HIGHER RESPIRATORY RATES) TO ALLOW ADEQUATE TIME FOR EXHALATION. A LONGER INSPIRATORY TIME (%IPAPTIME OF APPROXIMATELY 40%) MAY BE USEFUL IN PATIENTS WITH RESTRICTIVE DISEASE SUCH AS RTCD (DECREASED RESPIRATORY SYSTEM COMPLIANCE). (LEVEL A - CONSENSUS)
4.5.5.3. THE INSPIRATORY TIME IN THE ST OR TIMED MODES SHOULD BE ADJUSTED TO MAXIMIZE VENTILATION, PATIENT/NPPV SYNCHRONY, AND PATIENT COMFORT (TABLE 3). (LEVEL A - CONSENSUS)
The recommendations 4.5.5.1 to 4.5.5.3 are based on consensus agreement by the NPPV titration task force. When NPPV devices are used in the ST mode both the respiratory rate and the duration of IPAP (inspiratory time or IPAP time) for machine triggered breaths must be specified (although the devices do have default values). Specifying the respiratory rate specifies the cycle time (60 sec/respiratory rate in breaths per minute). As the respiratory rate increases the maximum IPAP time decreases for a given inspiratory/expiratory ratio (I/E ratio). A convenient method is to specify the %IPAP time (IPAPtime X 100 / cycle time). In general the minimum recommended I:E ratio is 1:2, with longer exhalation recommended for patients with obstructive lung disease. The relationship of the respiratory rate, the %IPAPtime and the IPAP time are illustrated in Table 3. At a respiratory rate of 15 breaths per minute, the range of inspiratory time corresponding to 30% to 40% (%IPAP time) is 1.2 to 1.6 seconds.
The timed mode is rarely used in most sleep centers but is an option in patients unable to synchronize with the NPPV device or those with consistently feeble respiratory efforts during sleep. In this mode, one specifies the respiratory rate and the inspiratory time for all breaths.
A lower %IPAP time and I:E ratio is desirable in patients with obstructive airway disease to allow a sufficient expiratory time as expiratory airflow is reduced in these patients. A greater %IPAP time (greater I:E ratio) is preferred in patients with restrictive lung disease to allow for a longer inspiratory time. As the respiratory rate becomes faster, the inspiratory time must be decreased to maintain an adequate I:E ratio.
4.6. Recommendations for the Use of Volume Targeted BPAP
4.6.1. Volume targeted BPAP (VT-BPAP), a mode of NPPV that automatically adjusts the IPAP between limits (IPAPmin to IPAP max) to deliver a targeted tidal volume, is an acceptable method for providing NPPV treatment. Volume targeted BPAP may be used in the spontaneous, spontaneous-timed (ST), and timed modes. (Level A - Consensus)
4.6.2. The goals of NPPV titration using VT-BPAP are to select an effective EPAP (to eliminate obstructive events) and to document that the device delivers adequate pressure support. The EPAP may be adjusted using recommendations for adjustment of CPAP in the AASM Guidelines for the titration of PAP in patients with OSA. (Level A - Consensus)
4.6.3. Recommended initial settings for volume targeted pressure support include EPAP = 4 cm H2O, IPAP min = EPAP + 4 cm H2O, and IPAPmax = 25 to 30 cm H2O, with an initial target tidal volume setting of approximately 8 mL/kg ideal body weight. (Level A - Consensus)
4.6.4. If Volume targeted pressure support is used in the ST or timed modes, the backup rate and inspiratory time may be chosen based on recommendations in the above sections. (Level A - Consensus)
Recommendations 4.6.1 to 4.6.4 are based on consensus of the NPPV task force. Volume targeted BPAP has the potential advantage of automatically varying the pressure support to deliver a targeted tidal volume. For example, if respiratory muscle strength declined and the tidal volume decreased, the device would deliver higher pressure support to return the delivered tidal volume to the targeted level. Relatively few studies on volume targeted VT-BPAP devices have been published. To date, only one VT-BPAP device is available in the United States (Average Volume Assured Pressure Support or AVAPS, Philips-Respironics). Therefore, recommendations are confined to this device. The above recommendations are based on the manufacturer's guidelines. Storre and coworkers8 compared BPAP and AVAPS (both in the ST mode) using a randomized crossover trial in patients with the obesity hypoventilation syndrome. AVAPS resulted in a slightly higher ventilation and lower PCO2 without any better sleep quality or quality of life measures compared to BPAP ST. Using the same device, Ambrosio and coworkers9 studied an assortment of patients with CAH who were stable on BPAP support. A validation night preceded the study nights to ensure the BPAP settings were adequate and to provide guidance for the AVAPS settings. Patients then were studied on BPAP or AVAPS in random order. On AVAPS the minute ventilation was greater than BPAP, but sleep quality was comparable between the two NPPV modes. Using a different VT-BPAP device, Janssens et al.10 monitored patients with an obesity hypoventilation syndrome using either BPAP or VT-BPAP for one night each in random order. Sleep quality was worse but nocturnal PtcCO2 slightly lower on VT-BPAP. It is possible that patients might have exhibited better sleep quality after a longer period of adaptation to the VT-BPAP. Jaye and coworkers11 compared BPAP ST with an autotitrating bilevel ventilator (AutoVPAP) in patients with stable neuromuscular and chest wall disease and nocturnal hypoventilation and found AutoVPAP to produce comparable control of nocturnal oxygenation without compromising sleep quality. The mean nocturnal transcutaneous PCO2 was higher with AutoVPAP although the difference was unlikely to be clinically significant. AutoVPAP (ResMed, Poway, CA) is not currently available in the United States. These studies suggest VT-BPAP can be effective, but the role of VT-BPAP in the NPPV treatment of patients with CAH remains to be determined.
4.7. Recommendations for the Addition of Supplemental Oxygen
4.7.1. Supplemental O2 should be added during the NPPV titration when the patient's awake supine SpO2 while breathing room air is < 88% or when supplemental O2 is required to maintain an awake supine pre-NPPV titration SpO2 ≥ 88% during stable breathing. (Level A - Consensus)
4.7.2. Supplemental O2 should be added when PS and respiratory rate have been optimized but the SpO2 remains < 90% for 5 minutes or more. (Consensus)
4.7.3. The minimum starting O2 rate should be 1 L/minute in adult patients and pediatric patients. The O2 rate should be increased in increments of 1 L/minute about every 5 minutes, until SpO2 > 90%. (Level A - Consensus) (Figure 3)
4.7.4. Optimally, supplemental O2 should be connected to the PAP device outlet (using a T-connector). (Level A - Consensus)
4.7.5. “Weaning” down of O2 supplementation by employing a higher PS or respiratory rate (if the patient tolerates these increases) can be attempted. (Level A - Consensus)
Recommendations 4.7.1 to 4.7.5 are based on consensus and are similar to recommendations for supplemental oxygen in the AASM Clinical Guidelines for the Manual Titration of CPAP and BPAP in patients with Obstructive Sleep Apnea.48 Recommendation 4.7.2 is made with the understanding that pulse oximetry can overestimate the actual arterial oxyhemoglobin saturation in some circumstances and that the effective inspired oxygen concentration can fall if machine flow increases due to higher leak.87–90 A slightly higher goal than 88% (90%-94%) might be prudent in some circumstances. Of note, since the intentional leak increases with the magnitudes of either IPAP or EPAP, the effective fraction of oxygen in the inspired air (FiO2) for a given supplemental oxygen flow rate (bleed in) falls as these pressures increase.87–90 The FiO2 does not appear to vary with the amount of PS or the respiratory rate. Recommendation 4.7.4 is based on several studies of models that evaluated the optimal location at which supplemental oxygen should be connected to the NPPV circuit. A 3-orifice “T” shaped connector attached between the NPPV device outlet and the hose allows for the addition of supplemental oxygen into the circuit through the side arm of the T connector. The results of these studies varied somewhat possibly due to the position at which the FiO2 was sampled. One study found the best location to be at the NPPV outlet assuming the leak port was in the mask,89 while another found a connection midway between the machine and the exhalation valve87 was optimal. The latter attachment is not practical in most settings. In any case, an attachment position allowing adequate mixing and one providing a reservoir of enriched gas for inspiration appears to optimize the FiO2 for a given supplemental oxygen flow and level of BPAP.
4.8. Recommendations Concerning Patient Comfort and Leak
4.8.1. If the patient awakens and complains that the IPAP, EPAP, or both pressures is (are) too high, the appropriate pressure(s) should be decreased to a lower pressure(s), chosen so that the patient reports a degree of comfort adequate to allow return to sleep. Elevation of the head of the bed (if not contraindicated) may be used as a strategy to allow down-titration of EPAP if the required pressure is difficult for the patient to tolerate. (Consensus)
4.8.2. Use of pressure relief during EPAP (flexible PAP) may improve patient comfort to a given level of EPAP if the patient complains of difficulty exhaling. This option is available only in the spontaneous mode in selected devices. (Level A - Consensus)
Recommendations 4.8.1 and 4.8.2 are based on consensus agreement by the NPPV titration task Force. Recommendation 4.8.1 is consistent with the AASM guidelines for titration of CPAP and BPAP in sleep apnea patients.48 Regarding 4.8.2, there is currently no evidence to support the use of pressure relief BPAP. To date, there is conflicting evidence about the ability of flexible CPAP to improve adherence.91,92 However, some patients may find flexible BPAP more comfortable. Pressure relief BPAP is available only in the spontaneous mode.
4.8.3. The rise time (time duration for pressure change from EPAP to set IPAP) should be increased or decreased for patient comfort. Patients with obstructive airway disease often prefer shorter rise times (100 ms to 400 ms) and patients with restrictive disease (NMD, RTCD) patients often prefer longer rise times (300 ms to 600 ms). A rise time of approximately 200 ms is usually the default on NPPV devices. (Level A - Consensus)
This recommendation is based on consensus of the task force. Adjustment of the rise time may improve patient tolerance to NPPV in certain patients. Typical rise times vary from 100 to 600 milliseconds.
4.8.4. Minimum IPAP duration (if available) maybe increased if the device cycles from IPAP to EPAP prematurely (e.g., in restrictive chest wall disorders). The maximum IPAP duration (if available) may be decreased if the device cycles to EPAP too late for patient comfort/synchrony (e.g., when mask leak is excessive). (Level A - Consensus)
Recommendation 4.8.4 is based on consensus agreement by the NPPV titration task force. NPPV devices transition from IPAP to EPAP during a patient triggered breath when flow falls below a set value. In patients with a stiff chest wall (decreased compliance), flow rates may fall as the maximum inspiratory volume is approached. An early fall in the absolute flow rates may trigger the transition to EPAP prematurely. Certain devices provide a minimum IPAPtime to ensure that IPAP lasts long enough to allow delivery of an adequate tidal volume. In situations of high leak (continued flow) or muscle weakness, the IPAP to EPAP transition may be unduly delayed. A default maximum IPAP duration of 3 seconds exists on some devices, while in other devices a shorter maximal IPAP time may be chosen.
4.8.5. During the NPPV titration, mask refit, adjustment, or change in mask type should be performed whenever any significant unintentional leak is observed or the patient complains of mask discomfort. If mouth leak is present and is causing significant symptoms (arousals) use of an oronasal mask or chin strap can be tried. (Level A - Consensus)
4.8.6. In general, an unacceptable leak for NPPV at a given IPAP/EPAP is one that is significantly higher than the leak resulting from a given mask fitted securely in place at the current pressure settings. The expected value of leak will depend on the pressures, specific interface, and NPPV device (whether total or unintentional leak information is provided) as specified by the manufacturer. (Level A - Consensus)
The recommendations 4.8.5 and 4.8.6 are based on consensus agreement by the NPPV Titration Task Force and are consistent with the recommendations concerning leak in the Clinical Guidelines for Titration of PAP in patients with OSA.48 The total leak is equal to the sum of the intentional leak (required to prevent rebreathing) and unintentional leak (mask and/or mouth leak depending on whether a nasal or full face mask is being used). The intentional leak depends on the specific interface and the level of pressure. Intentional leak can be appreciable with most interfaces at high pressure. NPPV devices used in sleep centers provide an estimate of leak. Some devices allow specification of an interface type and adjust the reported leak values accordingly. The trend in leak is often more informative than the absolute leak value. For example, a sudden increase in leak with minor or no changes in treatment pressure is a hint that an increase in unintentional leak has occurred. Mask leak may be minimized by mask refit or readjustment and/or switch to another mask type. If mouth leak is suspected, use of a chin strap or switch to a full face mask may be beneficial. Mouth leak is a significant problem in patients on NPPV treatment and may cause arousals even if the NPPV device is able to maintain the desired IPAP and EPAP.64–66 Teschler and coworkers65 studied a group of patient being treated with nasal BPAP who complained of symptomatic mouth leak. Taping the mouth substantially reduced arousals and increased the amount of REM sleep. Substantial mouth leak is often associated with complaints of oral dryness that may persist despite the use of heated humidification.
Although BPAP devices are leak tolerant, high leak can reduce the ability of a given pressure support setting to augment tidal volume.65,66 If tidal volume is inadequate and leak relatively high, interventions to reduce leak may improve tidal volume without requiring an increase in pressure support.
4.9. Recommendations for Grading the Quality of an NPPV Titration, Indications for Repeating a Titration, and Recommendations for Follow-up
4.9.1. Recommendations for grading the quality of an NPPV titration
4.9.1.1. THE NPPV DEVICE SETTINGS USED FOR TREATMENT SHOULD IDEALLY REFLECT THE FOLLOWING TREATMENT GOALS: CONTROL OF AIRWAY OBSTRUCTION AS DEFINED BY A RESPIRATORY DISTURBANCE INDEX (RDI) < 5/HOUR, ABSENCE OF SNORING, A MINIMUM SPO2 > 90% AT SEA LEVEL, NORMALIZATION/IMPROVEMENT OF VENTILATION WITH A PCO2 (IF MEASURED) NO GREATER THAN 10 MM HG ABOVE THE TREATMENT GOAL, REDUCTION IN EXCESSIVE RESPIRATORY MUSCLE ACTIVITY, AND A MASK LEAK WITHIN ACCEPTABLE PARAMETERS FOR THE SELECTED PRESSURES AND MASK INTERFACE. IN THIS WORK RDI REFERS TO THE NUMBER OF APNEAS + HYPOPNEAS + RERAS AND THE HOURS OF SLEEP. (LEVEL A - CONSENSUS)
4.9.1.2. AN OPTIMAL TITRATION MEETS THE ABOVE TREATMENT GOALS AT THE SELECTED NPPV SETTINGS FOR AT LEAST A 15-MINUTE PERIOD THAT INCLUDES REM SLEEP IN THE SUPINE POSITION (UNLESS THIS POSITION IS CONTRAINDICATED) THAT IS NOT CONTINUALLY INTERRUPTED BY AROUSALS. (LEVEL A - CONSENSUS)
4.9.1.3. A GOOD TITRATION MEETS THE ABOVE TREATMENT GOALS AT THE SELECTED NPPV SETTINGS FOR AT LEAST A 15-MINUTE PERIOD THAT INCLUDES NREM SLEEP IN THE SUPINE POSITION (UNLESS THIS POSITION IS CONTRAINDICATED) AND REM SLEEP IN ANY POSITION AT THE SELECTED SETTINGS. (CONSENSUS B)
4.9.1.4. AN ADEQUATE TITRATION MEETS THE ABOVE TREATMENT GOALS, EXCEPT THAT THE RDI MUST BE LESS THAN 10/HOUR AT THE SELECTED NPPV SETTINGS FOR AT LEAST A 15-MINUTE PERIOD THAT INCLUDES NREM SLEEP IN THE SUPINE POSITION (UNLESS THIS POSITION IS CONTRAINDICATED) AND REM SLEEP IN ANY POSITION AT THE SELECTED SETTINGS. (LEVEL A - CONSENSUS)
4.9.1.5. AN UNACCEPTABLE TITRATION IS ONE THAT DOES NOT MEET ANY ONE OF THE ABOVE STANDARDS. (LEVEL A - CONSENSUS)
The recommendations 4.9.1.1 to 4.9.1.5 are based on consensus of the NPPV titration task force and are similar to the grading of an adequate PAP titration in the clinical guidelines for PAP titration in patients with OSA.48 This scheme was based on grading criteria proposed by Hirshkowitz and Sharafkhaneh.93 It is acknowledged that the above grading scheme may not be relevant for all patients and must be individualized. For example, if the major goal of NPPV treatment is palliation, an optimal titration may well consist of NPPV settings that significantly improve patient comfort. Finally, since the technologies used for assessment of ventilation and/or respiratory muscle activity varies considerably among sleep laboratories, the task force offers solely what it deemed reasonable treatment goals for said parameters. Adequacy in these parameters should be defined individually by each sleep laboratory.
4.9.2. Indications for repeating an NPPV titration
4.9.2.1. A REPEAT NPPV TITRATION STUDY SHOULD BE PERFORMED (IF CLINICALLY INDICATED) WHEN THE INITIAL TITRATION DOES NOT ACHIEVE A GRADE OF OPTIMAL, GOOD, OR ADEQUATE BASED ON THE ABOVE CRITERIA, OR IF LESS THAN 3 HOURS OF SLEEP WAS RECORDED DURING THE TITRATION. THE DECISION TO REPEAT THE TITRATION DEPENDS ON THE TOLERANCE OF THE PATIENT TO NPPV AND THE CLINICAL STATUS AND PROGNOSIS OF THE PATIENT. (LEVEL A - CONSENSUS)
4.9.2.2. A REPEAT NPPV TITRATION STUDY SHOULD BE CONSIDERED IF THE RESPIRATORY FUNCTION OR SLEEP QUALITY OF A PATIENT ON CHRONIC NPPV TREATMENT DETERIORATES. FACTORS TO CONSIDER INCLUDE PROGNOSIS OF THE UNDERLYING DISEASE PROCESS AND THE ABILITY OF THE PATIENT TO ADHERE TO NPPV TREATMENT. (LEVEL A - CONSENSUS)
Recommendations 4.9.2.1 and 4.9.2.2 are based on consensus. The clinician must consider the long-term prognosis and tolerance of the patient to both NPPV titration with PSG and NPPV treatment. A similar grading scheme was presented in the AASM clinical guidelines for the titration of CPAP and BPAP in sleep apnea patients.48
4.9.3. Recommendations for follow-up after NPPV titration
4.9.3.1. BEFORE TREATMENT IS STARTED, THE DURABLE MEDICAL EQUIPMENT COMPANY SHOULD EDUCATE THE PATIENT AND CAREGIVER ABOUT DEVICE PARTS AND ASSEMBLY, OPTIONAL EQUIPMENT, THE IMPORTANCE OF DAILY/NIGHTLY USE, ADHERENCE ISSUES, NECESSITY OF, AND TECHNIQUES FOR, CLEANING THE EQUIPMENT, AND IMPLICATIONS OF THE PURCHASE/RENTAL OF THE EQUIPMENT (WHEN APPLICABLE). (LEVEL A - CONSENSUS)
4.9.3.2. CLOSE FOLLOW-UP AFTER INITIATION OF NPPV BY APPROPRIATELY TRAINED HEALTH CARE PROVIDERS IS INDICATED TO ESTABLISH EFFECTIVE UTILIZATION PATTERNS (IDEALLY USING OBJECTIVE ADHERENCE DATA), REMEDIATE PROBLEMS INCLUDING NPPV SIDE EFFECTS AND INTERFACE ISSUES, AND ENSURE THAT THE EQUIPMENT IS MAINTAINED IN GOOD REPAIR AND DISPOSABLE EQUIPMENT IS CHANGED ON A REGULAR SCHEDULE AS CLINICALLY INDICATED. (LEVEL A - CONSENSUS)
Recommendations 4.9.3.1 and 4.9.3.2 are based on consensus of the task force and are consistent with recommendations made in the clinical guidelines for titration of CPAP and BPAP in patients with OSA.48 Minimal information is available concerning adherence to NPPV treatment except for patients with the OHS. As BPAP at fixed settings does not deliver a set tidal volume, pressure settings determined during a NPPV titration may not remain adequate if patient characteristics such as muscle strength change during chronic treatment. In addition, concurrent medical problems or medications might impact the effectiveness of treatment.
4.9.4.3. THE RESPIRATORY FUNCTION OF PATIENTS ON CHRONIC NPPV TREATMENT SHOULD BE ASSESSED WITH MEASURES OF OXYGENATION AND VENTILATION (ARTERIAL BLOOD GAS, END-TIDAL CO2, TRANSCUTANEOUS PCO2) ON A REGULAR FOLLOW-UP BASIS OR IF SIGNS OF CLINICAL DETERIORATION ARE PRESENT. (LEVEL A - CONSENSUS)
This recommendation is based on consensus of the task force and recognizes the fact that the clinical condition of patients may change (weight gain, reduction in muscle strength, progression of the underlying cause of CAH), with the result that current NPPV treatment may no longer be adequate. The assessment of oxygenation and ventilation in such cases is typically carried out during quiet breathing while awake and at rest.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This report could not have been completed without the administrative support of the American Academy of Sleep Medicine; specifically, Jerome A. Barrett, Jennifer Markkanen, and Jordana Money.
Appendix.
| First Author Reference Evidence Level | Disorder/Patient No. | Design | Titration | Equipment/Pressure | Outcome/Findings (abbreviations at end of table) |
|---|---|---|---|---|---|
| Ambrogio9 2009 Randomized Crossover Level I Average Volume Assured Pressure Support (AVAPS) vs BPAP |
Chronic hypercapnic respiratory failure using NPPV OHS (with/ without OSA) COPD NMD N = 28 |
3 PSGs 1. Prescription-Validation BPAP titration to validate chronic NPPV settings IPAP-T = chosen IPAP level from BPAP titration 2.AVAPS or BPAP treatment 3.Crossover to alternate treatment |
BPAP titration with PSG start BPAP = 8/4, titrate to eliminate obstructive events (apnea, hypop, FL) IPAP increased in 2 cm increments when suspected hypoventilation (SaO2 < 90% for 3 minutes) in absence of obstructive events |
AVAPS IPAPmax = 30 or IPAP chosen for IPAP-T + 10 IPAPmin = IPAP-T - 5 AVAPS tidal volume goal either 8 cc/kg or 110% of calm tidal breathing (patient preference) |
Equivalent sleep quality on AVAPS and BPAP Higher minute ventilation on AVAPS |
| Banerjee17 2007 Case Series Level IV |
Prospective study of patients with BMI ≥ 50kg/m2 undergoing PSG group 1: OSA AHI = 61.2/hr N =23 group 2: OSA+OHS OSA + PCO2 > 45 mmHg without lung disease N = 23 |
PSG #1 Diagnostic,if AHI ≥ 15/hr then CPAP PSG 2nd PSG with CPAP measurements during CPAP trial |
Manual PSG CPAP titration with auto-CPAP device CPAP titrated to normalize inspiratory flow pattern Once flow trace had normalized CPAP was increased by no more than 3 cm H2O in attempt to improve SaO2 |
CPAP values mean CPAP (cm H2O) OSA group 12.9 OHS group 13.9 |
AHI group 1: OSA AHI = 61.2/hr group 2: OSA+OHS AHI = 78/hr Baseline PCO2 group1: OSA PCO2 = 42.9 mmHg group1: OSA+OHS PCO2 = 54.3 mmHg In both groups CPAP improved AHI, REM duration, arousal indices, nocturnal desaturation Once obstructive events eliminated: 9% of OSA and 43% of OHS had >20% TST with SaO2 less than 90% |
| Berger13 2001 Retrospective Case Series Level IV |
OHS 49 retrospectively identified 23 came for follow-up |
PSG for Dx PSG for PAP titration --> ultimate Rx depends on response to CPAP during the titration group 1 treated with CPAP group 2 treated with BPAP |
PSG titration: 1. CPAP increased to eliminate obstructive events 2. If SaO2 < 90% + flow limitation, CPAP increased If effective --> group 1: CPAP Rx (noncompliant had trach) 3. If SaO2 < 90% when no Flow limitation: group 2 BPAP used (noncompliant had trach + volume vent) |
group1: 11 patients CPAP Rx CPAP 13 cm H2O group 2: 12 patients BPAP Rx IPAP 18 cm H2O range (12 to 25) cm H2O EPAP 8 range ( 3 to 14) |
Compliant patients had drop in daytime PCO2 to near normal values – in both groups group 1: [CPAP / trach] group 2: [BPAP / trach + volume ventilator] groups |
|
Bourke39 2003 Case series Level IV |
ALS patients N = 22 |
ALS group followed Quality of life measures every 2 months PSG every 4 months in group with ALS Those meeting criteria were offered NPPV Analysis: comparison of Pre-NPPV measures with post- NPPV measures |
NPPV started in Hospital BPAP adjusted on basis of nocturnal oximetry and daytime ABGs, + compliance 15 tried NPPV 10 continued NPPV |
nasal, oro-nasal, total face masks, mouthpieces BPAP in ST mode mean values: IPAP = 16 EPAP = 4 backup rate not specified Asynchrony due to leaks controlled by limiting IPAPmax |
NPPV associated with improved quality of life Survival correlated with compliance to NPPV orthopnea best indicator of benefit from NPPV and adherence (better than AHI, nocturnal symptoms, PCO2) |
| Bourke3 Lancet Neuro 2006 Randomized Controlled Trial Level I with respect to NPPV vs Standard care |
ALS patients NPPV N = 22 standard care N = 19 20 normal or mild impairment of bulbar function 21 severe bulbar involvement |
ALS patients randomized to standard care or NPPV baseline PSG at randomization AHI not specified “some obstr events during REM sleep” quality of life measures SALQI |
NPPV started in hospital Settings adjusted using daytime ABG, nocturnal oximetry, and NPPV use Goal = normal daytime arterial blood gas |
VPAP ST II ST mode (backup rate not specified nasal, oronasal, oral masks IPAP mean 15 EPAP mean 4 IPAP max =24 EPAP max = 5 |
Compared to standard care, NPPV improved survival and quality of life in the subgroup with better bulbar function Bulbar group – NPPV may improve nocturnal symptoms but not survival |
| Budweiser25 2006 Case Series Level IV |
RTCD N = 62 initial N = 44 patients available for analysis |
Testing during day after NPPV treatment Variable treatment duration but > 3.8 months |
NPPV started in hospital Adaptation phase NPPV gradually increased ABG measured twice at night 01:00 AM and 04:00 AM NPPV goals: target tidal volume= 10 cc/kg reduction in PCO2 10-15% |
nasal or full face mask Pressure NPPV ST mode IPAP = 21.1 ± 3.4 EPAP = 3.1 ± 2.3 PS = 18 ± 3.0 mean respiratory rate = 20.6 bpm Heated humidification if dryness |
Improvements in daytime PCO2, PO2, lung volumes, and muscle strength changes in daytime PCO2 was not correlated with duration of use. Improvements in daytime vital capacity (VC) correlated with IPAP Reduction in daytime PCO2 correlated with PS |
| Budweiser18 2007 Survival of OHS on NPPV Case Series Level IV |
OHS N =126 PCO2 ≥ 45 mm Hg |
PSG documents OHS 87 patients, (69%) had OSA NPPV treatment then---> 118 re-evaluated in hospital at 3 to 6 months with daytime and night time ABG |
NPPV started in hospital PSG? IPAP increased with goal of reaching a tidal volume of 10 cc/kg ideal body weight Oxygen added only after PS optimized to achieve SaO2 > 90% |
nasal or full face masks BPAP ST IPAP 22.5 ± 3.6 mbar EPAP 5.8 ± 3.1 mbar backup rate 19.2/min |
Daytime PCO2 decreased Adherence (mean) = 6.5 hrs PCO2 changed Daytime 55.5 ---> 42.1 mmHg Nighttime 59.0 ---> 44.7 mmHg 1, 2, 5 year survival 97%, 92%, 70% all cause-mortality |
| Chatwin60 2008 Randomized parallel group design inpatient versus out patient initiation of home mechanical ventilation Level II |
N = 28 RTCD and NMD Patients with nocturnal hypoventilation |
NPPV started in PSG after 2 months of treatment at home After 2 months in hospital overnight monitoring of SpO2 and transcutaneous PCO2, as well as daytime ABG Baseline daytime PCO2 in both groups 44.2 mmHg (inpatient) and 45.7 (outpatient) Baseline peak nocturnal transcutaneous PCO2: 75 mmHg in outpatient, 71.2 inpatient group |
Inpatient – stay sufficient for patient competence Outpatient – 3 visits Outpatient – patient reclined an adjustments made to achieve “good” tidal volume |
Pressure preset NPPV Starting settings: Peak insp pressure 16 cm H2O and backup rate 15-20 bpm Nasal masks used if possible, full face if mouth leak |
Improvements in nocturnal SpO2 similar in both groups Peak nocturnal transcutaneous PCO2 improved in both groups Daytime PCO2 decreased in outpatient group Conclusion: outpatient initiation of home ventilation is feasible |
| De Lucas-Ramos19 2004 Case Series Level IV |
OHS N = 13 non-consecutive patients |
type 3: Home Sleep Testing at baseline 12 mo of NPPV At baseline and after 12 mo NPPV treatment, overnight oximetry, respiratory muscle strength, and mouth occlusion pressure tested |
NPPV started in hospital daytime 2 hr adaptation period Initial pressure IPAP = 16, EPAP = 5 then IPAP adjusted during daytime based on arterial blood gas. Nocturnal treatment started IPAP, EPAP |
BPAP Nasal mask used end IPAP = 19 ± 2 EPAP of 5 cm H2O |
NPPV--> improved daytime PCO2, PO2, FVC, and the mouth occlusion pressure responses to hypercapnia 9/13 needed supplemental oxygen |
| Ellis23 1988 Case Series Level IV |
RTCD KS (N = 7) |
PSG before and after Rx using Transcutaneous PCO2 (snoring, ob apn found ) 3 months of treatment |
Titration method not specified | nasal mask 5 NPPV (volume cycled) 2 CPAP |
NPPV improved sleep quality with lower nocturnal PtcCO2 Daytime: NPPV increased respiratory muscle strength, improved ABGs, improved lung volumes |
| Gonzalez24 2003 Case Series Level IV |
RTCD N = 16 |
1. Baseline PSG 2. NPPV treatment for 36 months 3. At 6 mo PSG OFF NPPV Other outcomes monitored over 3 years |
NPPV started in hospital ( Respiratory Care Unit) Adaptation to NPPV during 2-3 hrs in the morning mask, mode settings based on comfort, SaO2, PtcCO2 then--> 2 - 3 nights with adjustment of settings based on SaO2, PtcCO2 Goal: PCO2 ≤ 45 mmHg SaO2 > 90 % either volume vent or BPAP FIO2 increased if goals not met |
Volume cycled NPPV in 7 Pressure cycled NPPV in 9 nasal mask 12, FFM 4 BPAP ST mode IPAP 15 to 22 cm H2O EPAP 4 mean IPAP = 15 ± 2.8 backup rate 15 bpm Volume vent: Tidal volume = 10-15 cc/kg BF 15-25 |
Before NPPV PSG AHI = 13.9 After NPPV treatment, PSG (off NPPV) AHI =12.9r SaO2 improved at night but not sleep efficiency Daytime PO2, FVC, Resp Muscle strength improved at 36 mo Less hospitalizations Supplemental O2 at 11pm in 6 patients |
| Gruis36 2006 retrospective Assessed Pressure changes needed in chronic NPPV treatment Level IV |
NMD 55 total 18 tolerated NPPV 19 intolerant followed classified as NPPV tolerant if > 4 hrs use (?objective) at follow-up visits |
Stared NPPV Patients followed 3 of 18 NPPV tolerant patients had symptoms of OSA 2 of 18 NPPV tolerant patients diagnosed with PSG |
NPPV started as out patient patient Initial: NPPV 8/3 cm H2O - changes based on symptoms - most pressure changes occurred in the first year IPAP increased in 2 cm H2O increments until symptoms improved |
Nasal prongs (Nasal Aire) interface Only 6/18 (33%) required PS > 10 cm H2O 3 ALS has symptoms of OSA PSG found 2 had OSA |
18 tolerated NPPV 4 used 8/3 cm H2O until death Max pressure 19/5 cm H2O patients with different numbers of pressure changes “for comfort” N = 4 no change 8 single change 4 two changes 1 three changes 1 five changes patients tolerating NPPV had longer survival |
| Guilleminault40 1998 Case Series Level IV |
NMD Muscular dystrophy and others N = 20 |
1. PSG for diagnosis + MSLT 2. PSG for BPAP titration 3. 4wks NPPV Rx 4. PSG on NPPV + MSLT |
PSG for BPAP titration Titration goal: eliminate apnea, hypopnea, hypoventilation |
BPAP S (N = 18) BPAP T (N =1) EPAP 5 cm H2O IPAP 11.5 (9 to 14) cm H2O |
Mean RDI 28.2/hr (8.9); 78% central apnea 19 /20 accepted NPPV Mean Sleep latency (MSLT) increased after NPPV treatment MSL increased from 8.2 minutes to 12 minutes 3 switched to volume ventilators |
|
Guo21 2007 Level IV |
OHS Using NPPV for at least 3 months N = 20 |
PSG during NPPV in patients on chronic NPPV treatment |
PSG on NPPV while monitoring machine pressure, mask pressure, airflow, transcutaneous PCO2 Note transcutaneous CO2 device calibrated with calibration gas before each measurement night |
nasal (17) or full face masks (3), 4 also on supplemental oxygen BPAP ST mode used mean IPAP = 18.5 ± 4.6 mean EPAP = 6.2 ± 1.4 mean backup rate = 13.7 ± 2.2 bpm |
55% had desynchronization 40% frequent periodic breathing auto-triggering uncommon but frequent in a single patient |
| Janssens10 2008 assess effect of volume targeted BPAP on sleep quality Randomized cross over Level II for VT-BPAP |
OHS stable on chronic NPPV N = 12 |
NPPV using current settings one night and VT-BPAP on the other night PSG with Ptc CO2 VT-BPAP adjusted during day to find tolerated settings |
no titration studied at current settings chronic settings (cmH2O): IPAP = 21.6 ± 4.7 EPAP 8.6 ± 2.7 backup rate 13.3 ± 2.0 range (10-17) bpm |
9 FFM, 3 nasal masks ST mode VT 7-8 cc/kg IPAPmax = 30 cm H2O IPAPmin = usual IPAP - 3 Backup rate same as chronic treatment |
mean tidal volume, ventilation, IPAP higher with VT-BPAP – all small differences Slight improvement in nocturnal PCO2 on VT-BPAP compared BPAP amounts of stage N2 and TST better with BPAP than VT-BPAP Better subjective sleep quality on BPAP WASO higher with VT-BPAP critique = lack of adaptation to VT-BPAP |
| Katz44 2002 Case Series Level IV |
children NMD Spinal muscular atrophy, DMD, myotonic dystrophy, myopathies N = 15 |
PSG for Diagnosis PSG baseline AHI = 7.3/hour PSG for BPAP titration then follow-up In 9 PSG repeated at follow-up |
NPPV titration with PSG PtcCO2 used NPPV Goal: a decrease of at least 10 mmHg if PtcCO2 was > 50 mmHg normalize respirations and SaO2 |
nasal mask nasal pillows IPAP 9 – 20 cm H2O EPAP 3-6 backup rate set at 10% below resting breathing rate |
Post NPPV initiation children spent 85% fewer days in hospital PSG repeated on NPPV Nocturnal PtcCO2 normalized AHI decreased |
| Leger29 1994 Case Series Level IV |
Mixed population with CAH KS post TB sequelae MD, COPD N= 276 |
NPPV started in hospital after exacerbation Long term f/u |
NPPV started in hospital after exacerbation Titration details not specified Humidity in 1/3 |
customized nasal masks volume ventilation 12=15cc/kg tidal volume assist-control or control mode breaths per minute 15 to 16 |
KS, post TB improved quality of life and daytime gas exchange, less hospital days MD – less continued NPPV, did reduce hospital days Side effects dryness, gastric distension, nasal congestion, eye irritation, nasal bridge soreness |
| Masa16 2001 compare NPPV treatment in OHS vs RTCD Cohort study Level IV |
OHS N =22 PCO2 > 47 RTCD (KS) N = 14 PCO2 > 47 |
PSG, if AHI < 20/hr admitted to hospital for protocol re-evaluation after 4 months of NPPV |
NPPV started in hospital Titration details not provided Adaptation to NPPV 3 to 7 days Goal: maximal reduction in PCO2 and maintenance of SaO2 > 90%. 11 OHS, 8 KS required oxygen supplement at start of study --> at end only 2 OHS using supplemental oxygen at night |
Volume cycled ventilator in most, BPAP in 5 OHS, 1 KS |
OHS group daytime PCO2 decreased from 58 to 45 mmHg RTCD group: daytime PCO2decreased from 59 to 45 mmHg after treatment, no change in muscle strength or PFTs Both OHS and RTCD had equivalent improvement in symptoms and daytime gas exchange with NPPV treatment Conclusion: NPPV effective in OHS |
| Masa31 1997 Oxygen vs NPPV Crossover trial not randomized order Level II |
RTCD 8 7KS+1 thoracoplasty 2 NMD 11 OHS No daytime hypercapnia. 27 nocturnal desaturators without OSA 21 completed the protocol |
baseline PSG to demonstrate desaturation without OSA RTCD patients had AHI 5.6/hour overall but 19 ± 17/hour during REM sleep. 2 wks of nocturnal oxygen then PSG on oxygen then 2 wks nocturnal NPPV then PSG on NPPV |
NPPV started in hospital Adaptation period 3 to 7 days. The protocol was not specified |
BPA P N = 7 Volume cycled ventilator N=20 |
NPPV not oxygen normalized nocturnal hypoventilation and improved symptoms oxygen treatment had greater nocturnal saturation Daytime PO2 improved only after NPPPV Conclusion: RTCD patients without daytime hypoventilation but with nocturnal hypoventilation and desaturation obtain more benefit from NPPV than nocturnal oxygen |
| Mellies42 2003 Case Series Level IV |
NMD children N = 30 |
1. PSG for dx 2. PSG for titration 3. Follow-up RDI 10.5 ± 13.1 REM RDI 20.5 ±21.1 |
NPPV titration using PSG BPAP ST mode goals: suppress SDB, SaO2 > 95%, PtcCO2 < 50 mmHg PetCO2 used, calibrated before each study |
Full face mask (10) nasal masks, others IPAP 13.9 range (8-19) EPAP 4.4 range (3-8) backup rate 19.6 (14-24) bpm |
Improvement in daytime PCO2 and PO2 Improvement in TST with PCO2 > 50 or TST with SAO2 < 90% |
| Perez de Llano12 2005 Retrospective Study Case Series Level IV |
OHS started on NPPV 20 elective 34 after exacerbation | NPPV treatment then follow-up PSG performed once stable and discharged from the hospital |
NPPV started in hospital PSG not used for titration daytime sessions for exacerbations night time session for all starting EPAP= 6 cmH2O, IPAP=10 titrated upward adjusted based on nocturnal SaO2 |
nasal mask At discharge: N=3 CPAP N=2 vol vent N= 49 BPAP mean IPAP=18 (12-30) mean EPAP = 9 (5-13) N=47 needed supplemental oxygen |
AHI > 5 in 87% 47% required supplemental oxygen ESS decreased from 16 to 6 mean decrease in daytime PCO2 was 17 mmHg PCO2 fell after discharge from hospital on treatment: not necessary to totally normalize PCO2 – continued improvement occurs over time |
| Perez de Llano14 2008 Level IV assess those who can be switched to CPAP after NPPV |
OHS N = 24 N=11 CPAP N=13 NPPV |
OHS stabilized on NPPV When clinically stable had PSG titration starting with CPAP, changed to BPAP and oxygen added if needed. |
NPPV titration by PSG CPAP used to eliminate obstructive events. If low SaO2 persisted BPAP used EPAP = CPAP and IPAP increased up to 20 to improve SaO2. If not effective supplemental oxygen was added |
CPAP group mean pressure 10.4 cm H2O NPPV group 11 BPAP, 2 volume vent. Pressures not presented |
CPAP group had higher AHI and worse SaO2 NPPV group had lower AHI and worse SaO2 |
| Piper and Sullivan26 1996 Does NPPV at night improve spontaneous ventilation during sleep (i.e., NPPV)? Case Series Level IV |
NMD 8 RTCD 6 N = 14 |
1. Baseline PSG before 2. 6 mo NPPV treatment Ventilator settings verified during PSG before discharge from hospital 3. Sleep study OFF NPPV after 6 mo or greater treatment. |
NPPV started in hospital NPPV protocol not clearly specified, NPPV adjusted based on patient tolerance, adjusted with nocturnal oximetry NPPV settings verified by PSG before discharge |
nasal mask 13 patients volume ventilator 1 patient pressure ventilator |
Daytime Chronic NPPV improved spontaneous daytime PO2 and PCO2 and muscle strength Night time: chronic NPPV improved nocturnal SaO2 and transcutaneous PCO2 during sleep (off treatment) |
| Piper22 2007 RCT Level I CPAP vs BPAP |
OHS stable awake PCO2 > 45, pH > 7.34 18 CPAP 18 BPAP |
Initial CPAP trial in all subjects -- excluded those with persistent nocturnal hypoxemia or CO2 retention despite CPAP those on BPAP had an additional PSG for BPAP titration compared long term rx with CPAP versus BPAP over 3 months |
NPPV titration with PSG starting EPAP = effective CPAP -2 (minimum 5) starting IPAP such that PS =4. EPAP increased in 1 cm H2O increments if inspiratory efforts did not trigger IPAP IPAP increased to eliminate hypopneas and improve SaO2 |
CPAP mean pressure was 14 cm H2O BPAP S mode mean IPAP = 16 cm H2O EPAP = 10 cm H2O 7 patients on supplemental oxygen |
Both groups decrease in daytime PCO2 – no difference between CPAP and BPAP Similar improvements in ESS, PVT, Adherence |
| Redolfi20 2006 Leptin in OHS prospective case controlled Level IV |
OHS N = 6 |
1. Type 3 monitor used excluded OHS patients with OSA (AHI > 5.hr) 2. Leptin before and after 10 months of NPPV 3. Results compared with 6 eucapnic obese subjects |
Location where NPPV started is unclear (hospital or clinic?). EPAP =4 IPAP adjusted on basis of overnight oximetry until SaO2 > 90%, if higher IPAP not tolerated supplemental oxygen was addded Type 3 device on NPPV to evaluate settings. |
BPAP Synchrony device mean pressures IPAP = 12 EPAP = 4 |
Daytime PO2 increased, PCO2 decreased leptin increased |
| Ramesh33 2008 Case Control Level IV |
Congenital Central Hypoventilation Syndrome N = 15 age range 9 months to 21 years |
9 patients started NPPV at later age 6 patients started NPPV at 5 to 26 weeks of age 3 with positive pressure ventilation via endotracheal tube, 1 on positive pressure ventilation via tracheosotomy |
NPPV protocol not presented | nasal and face masks Details of pressures used not presented |
NPPV started at later age took median of 3 years to wean from former mode of ventilation to mask ventilation |
| Simonds28 2006 Case Series Level IV |
NMD or RTCD N = 40 children 17 daytime resp failure 18 nocturnal hypoventilation 3 being weaned 2 frequent chest infections |
overnight SaO2, Ptc CO2 monitoring, some had full PSG monitoring repeated once NPPV settings finalized |
NPPV started in hospital NPPV settings determined by overnight SaO2, PtcCO2 monitoring, supplemental oxygen if needed |
BPAP in ST mode 20 ffm, 18 nasal masks, 2 nasal pillows mean pressures not given |
38 of 40 tolerated mask ventilation Nocturnal: peak PtcCO2 decreased, mean and minimum PO2 improved |
| Storre8 2006 AVAPS (Average Volume Assured Pressure Support) Randomized Crossover Level II AVAPS vs BPAP ST |
OHS N = 10 “Non-responders” to CPAP |
1. 6 weeks of AVAPS or BPAP-ST then PSG 2. 6 weeks of alternate mode then PSG |
NPPV settings according to daytime and nighttime tolerance and to maximally decrease PtcCO2 | AVAPS IPAPmax = 30 mbar target volume 7 to 10 cc/kg BPAP ST IPAP up to 20 mbar EPAP 4 to 8 mbar both modes: backup rate = 12 to 18 bpm I/E 1 : 2 |
Sleep quality and oxygen saturation, quality of life equivalent between AVAPS and BPAP ST Daytime PCO2 after 6 weeks slightly lower on AVAPS Nocturnal PtcCO2 lower on AVAPS mean difference 6.9 mm Hg |
| Tibballs32 2003 Case Series Level IV |
Congenital Central Hypoventilation N = 4 | 2 newborns treated with nasal mask and BPAP when parents refused tracheostomy 2 older children transitioned trach /volume ventilator to nasal mask BPAP |
Titration protocols not specified Case # 1 NPPV tried at age 5 and 6 unsuccessful mask not tolerated, age 9 tried not successful, tried again age 11 successful, tracheostomy removed on BPAP phrenic pacing during the day Case #2 BPAP 24/4 ST mode with 12 bpm backup |
Nasal masks Case # 3 developed mid-face hypoplasia uses negative pressure ventilator most nights, for travel BPAP used Case #4 BPAP started at age 9 months, developed mid-face hypoplasia, at age 3 years used combination of BPAP to fall asleep and negative pressure ventilator for most of night |
2 cases mid-face hypoplasia developed then switched to negative pressure ventilator for at least part of treatment maxilla may fail to grow in relation to mandible- lower jaw protrudes “pseudoprognathism” |
| Toussaint37 2008 Prospective Case Series Level IV |
NMD Duchenne muscular dystrophy group 1: no dyspnea, no support group 2: nocturnal hypercapnia group 3: nocturnal NPPV, no breathlessness group 4: nocturnal NPPV with breathlessness group 5 using 24 hr NPPV |
group 4 nightly NPPV but no breathlessness TT0.1 tension time index a measure of muscle load measured at 20:00 and at 8:00 after NPPV |
Not specified tidal volume 12 cc/kg then reduced slowly goal maintain normocapnia | NPPV Volume ventilator mean tidal volume 653 ml, mean RR 19.9 | Tension time index higher with greater weakness group 1 to 3 not improvement in tension time index after overnight NPPV group 4 and 5 showed significant reduction in tension time index after overnight NPPV NPPV improved AM muscle function |
| Tuggey and Elliot27 2005 Randomized Crossover pressure versus volume ventilation Level II for type of NPPV |
RTCD N = 13 |
Randomized crossover design after treatment for 4 weeks, PSG on NPPV with either pressure or volume mode Volume vs Pressure NPPV compared |
No EPAP provided daytime titration either pressure or volume ventilation adjusted to give equivalent minute ventilation Volume ventilatory = tidal volume increased in 100 cc increments IPAP increased in increments of 5 cm H2O between limits of 10 and 40 cm |
TV pressure/Volume to 548/.546 Pressure mode backup rate =15 IPAP =25 cm H2O RR =15 Volume mode: backup rate 15 TV 749 ml |
Night time: 2 modes provided equivalent sleep quality and SaO2, Daytime: 2 modes resulted in equivalent respiratory muscle strength, health status, ABGs, and daytime function Pressure NPPV showed more leak and lower ventilation Pressure and volume ventilation modes showed lower ventilation during sleep than wake titration |
| Tuggey72 2006 comparing titration goal of respiratory muscle rest and reverse nocturnal hypoventilation Level II for titration goal |
N = 24 12 COPD 12 RTCD |
daytime study Increase in pressure or tidal volume Pressure time product measured with esophageal balloon (PTPes) |
Pressures above 20-25 cm H2O or volumes above 6 cc/kg did not produce further drops in PTPes, ventilation did continue to increase although so did leak | Nasal mask Volume ventilator Pressure ventilator |
Little gain in increasing PS above 20 in RTCD Respiratory effort minimized below maximum ventilation |
| Vianello43 1994 Case controlled Level IV |
NMD Duchenne Muscular Dystrophy N = 5 on NPPV but 10 in total | compared 5 with NPPV with another 5 without NPPV but similar characteristics (case control) |
NPPV started in the Hospital 3 consecutive nights with overnight NPPV monitoring with transcutaneous PCO2 monitoring NPPV adjusted so PtcCO2 < 45 |
Volume Ventilator used Nasal mask | at 24 months 4/5 non NPPV had died 0/5 NPPV group had died at 6 months drop in FVC much lower in NPPV group |
| Ward30 2005 Randomized controlled trial standard care vs NPPV Manually adjusted overnight with SaO2, tcPCO2 ? EEG Level II |
NMD RTCD N = 26 daytime normocapnia and nocturnal hypercapnia most actually MD |
Baseline “overnight respiratory sleep study” including SaO2 and PtcCO2 Randomized patients to control or NPPV 12 randomized to control, 2 drop outs 13 randomized to NPPV but 3 elected not to start NPPV Studied every 6 months tcPCO2, SaO2 Control group followed, if met safety criteria started on NPPV |
NPPV adjusted by overnight “monitoring” separate from initial diagnostic with PSG. Uncertain if overnight monitoring means PSG Goal of overnight monitoring to adjust NPPV to normalize SaO2 and PCO2 |
BPAP ST mode pressures not presented nasal or full face masks |
Nocturnal PtcO2 decreased in NPPV group 9 /10 in control group deteriorated and treated with NPPV after 8 months |
| Windisch 55 2005 Cross-over trial Volume vs pressure preset ventilation Level II |
N = 15 9 COPD 6 Non-COPD OHS, achondroplasia, post-polio,post-TB, ALS, Duchenne MD Chronic hypercapnia 5 patients did not complete study |
Received either volume or pressure preset ventilation via mask for 6 weeks, then switched to alternate mode PSG used to assess sleep quality with transcutaneous PCO2 monitoring Volume NPPV (V-NPPV) and pressure NPPV (P-NPPV) compared |
Admitted to hospital for start of NPPV NPPV adjusted to NPPV adjusted to achieve maximal decrease in PCO2 Supplemental oxygen added to keep SpO2 > 90% Arterialized ear lobe blood tested |
ST/Assist control mode Nasal mask interface Passive humidification Mean values: In pressure mode set peak pressure = 26.6, delivered peak pressure = 22.9 (mbar) Respiratory rate = 20.5 Volume set = 677 ml |
Night time PCO2 decreased in both modes Baseline 54.6 mmHg P-NPPV 46.5 mm Hg V-NPPV 46.2 mmHg Similarly small decrease in daytime PCO2 in P-NPPV and V-NPPV similar Comparable improvements in sleep quality in both modes More gastric distension in volume mode |
RTCD refers to restrictive chest wall disease; NMD, neuromuscular disease; KS, kyphoscoliosis; OHS, obesity hypoventilation syndrome; FFM, full face mask (oronasal); VT-BPAP, volume targeted BPAP; CAH, chronic alveolar hypoventilation; vs, versus; PtcCO2, transcutaneous PCO2. IPAP and EPAP in cm H2O unless specified.
REFERENCES
- 1.Bach JR, Alba AS. Management of chronic alveolar hypoventilation by nasal ventilation. Chest. 1990;97:52–7. doi: 10.1378/chest.97.1.52. [DOI] [PubMed] [Google Scholar]
- 2.Alves RS, Resende MB, Skomro RP, Souza FJ, Reed UC. Sleep and neuromuscular disorders in children. Sleep Med Rev. 2009;13:133–48. doi: 10.1016/j.smrv.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomized controlled trial. Lancet Neurol. 2006;5:140–7. doi: 10.1016/S1474-4422(05)70326-4. [DOI] [PubMed] [Google Scholar]
- 4.Perrin C, Unterborn JN, Ambrosio CD, Hill NS. Pulmonary complications of chronic neuromuscular diseases and their management. Muscle Nerve. 2004;29:5–27. doi: 10.1002/mus.10487. [DOI] [PubMed] [Google Scholar]
- 5.Ozsancak A, D'Ambrosio C, Hill NS. Nocturnal noninvasive ventilation. Chest. 2008;133:1275–86. doi: 10.1378/chest.07-1527. [DOI] [PubMed] [Google Scholar]
- 6.Casey KR, Cantillo KO, Brown LK. Sleep-related hypoventilation/hypoxemic syndromes. Chest. 2007;1318:1936–48. doi: 10.1378/chest.06-2334. [DOI] [PubMed] [Google Scholar]
- 7.Robert D, Argaud L. Non-invasive positive ventilation in the treatment of sleep-related breathing disorders. Sleep Med. 2007;8:441–52. doi: 10.1016/j.sleep.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Storre JH, Seuthe B, Fiechter R, et al. Average volume-assured pressure support in obesity hypoventilation: a randomized crossover trial. Chest. 2006;130:815–21. doi: 10.1378/chest.130.3.815. [DOI] [PubMed] [Google Scholar]
- 9.Ambrogio C, Lowman X, Kuo M, Malo J, Prasad AR, Parthasarathy S. Sleep and non-invasive ventilation in patients with chronic respiratory failure. Intensive Care Med. 2009;35:306–13. doi: 10.1007/s00134-008-1276-4. [DOI] [PubMed] [Google Scholar]
- 10.Janssens JP, Metzger M, Sforza E. Impact of volume targeting on efficacy of bi-level non-invasive ventilation and sleep in obesity hypoventilation syndrome. Respir Med. 2009;103:165–72. doi: 10.1016/j.rmed.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Jaye J, Chatwin M, Dayer M, et al. Autotitration versus standard noninvasive ventilation: a randomized crossover trial. Eur Respir J. 2009;33:566–73. doi: 10.1183/09031936.00065008. [DOI] [PubMed] [Google Scholar]
- 12.Perez de Llano LA, Golpe R, Montserrat OP, Racamonde AV, et al. Short-term and long-term effects of nasal intermittent positive pressure ventilation in patients with obesity-hypoventilation syndrome. Chest. 2005;128:587–94. doi: 10.1378/chest.128.2.587. [DOI] [PubMed] [Google Scholar]
- 13.Berger KI, Ayappa I, Chatr-Amontri B, et al. Obesity hypoventilation syndrome as a spectrum of respiratory disturbances during sleep. Chest. 2001;120:1231–8. doi: 10.1378/chest.120.4.1231. [DOI] [PubMed] [Google Scholar]
- 14.Pérez de Llano LA, Golpe R, Piquer MO, et al. Clinical heterogeneity among patients with obesity hypoventilation syndrome: therapeutic implications. Respiration. 2008;75:34–9. doi: 10.1159/000105460. [DOI] [PubMed] [Google Scholar]
- 15.Rapoport DM, Sorkin B, Garay SM, Goldring RM. Reversal of the “Pickwickian syndrome” by long-term use of nocturnal nasal-airway pressure. N Engl J Med. 1982;307:931–3. doi: 10.1056/NEJM198210073071507. [DOI] [PubMed] [Google Scholar]
- 16.Masa JF, Bartolome RC, Riesco JA, et al. The obesity hypoventilation syndrome can be treated with noninvasive mechanical ventilation. Chest. 2001;119:1102–7. doi: 10.1378/chest.119.4.1102. [DOI] [PubMed] [Google Scholar]
- 17.Banerjee D, Yee BJ, Piper AJ, Zwillich CW, Grunstein RR. Obesity hypoventilation syndrome: hypoxemia during continuous positive airway pressure. Chest. 2007;131:1678–84. doi: 10.1378/chest.06-2447. [DOI] [PubMed] [Google Scholar]
- 18.Budweiser S, Riedl SG, Jörres RA, Heinemann F, Pfeifer M. Mortality and prognostic factors in patients with obesity-hypoventilation syndrome undergoing noninvasive ventilation. J Intern Med. 2007;261:375–83. doi: 10.1111/j.1365-2796.2007.01765.x. [DOI] [PubMed] [Google Scholar]
- 19.de Lucas-Ramos P, de Miguel-Diez J, Santacruz-Siminiani A, et al. Benefits at 1 year of nocturnal intermittent positive pressure ventilation in patients with obesity hypoventilation syndrome. Respir Med. 2004;98:961–7. doi: 10.1016/j.rmed.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Redolfi S, Corda L, La Piana G, Spandrio S, et al. Long-term non-invasive ventilation increases chemosensitivity and leptin in obesity-hypoventilation syndrome. Respir Med. 2007;101:1191–5. doi: 10.1016/j.rmed.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Guo YF, Sforza E, Janssens JP. Respiratory patterns during sleep in obesity hypoventilation patients treated with nocturnal pressure support: a preliminary report. Chest. 2007;131:1090–9. doi: 10.1378/chest.06-1705. [DOI] [PubMed] [Google Scholar]
- 22.Piper AJ, Wang D, Yee BJ, Barnes DJ, Grunstein RR. Randomized trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax. 2008;63:395–401. doi: 10.1136/thx.2007.081315. [DOI] [PubMed] [Google Scholar]
- 23.Ellis ER, Grunstein RR, Chan S, Bye PT, Sullivan CE. Noninvasive ventilatory support during sleep improves respiratory failure in kyphoscoliosis. Chest. 1988;94:811–5. doi: 10.1378/chest.94.4.811. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez C, Ferris G, Diaz J, Fontana I, Nuñez J, Marín J. Kyphoscoliotic ventilatory insufficiency: effects of long-term intermittent positive-pressure ventilation. Chest. 2003;124:857–62. doi: 10.1378/chest.124.3.857. [DOI] [PubMed] [Google Scholar]
- 25.Budweiser S, Heinemann F, Fischer W, Dobroschke J, Wild PJ, Pfeifer M. Impact of ventilation parameters and duration of ventilator use on non-invasive home ventilation in restrictive thoracic disorders. Respiration. 2006;73:488–94. doi: 10.1159/000088712. [DOI] [PubMed] [Google Scholar]
- 26.Piper AJ, Sullivan CE. Effects of long-term nocturnal ventilation on spontaneous breathing during sleep in neuromuscular and chest wall disorders. Eur Respir J. 1996;9:1515–22. doi: 10.1183/09031936.96.09071515. [DOI] [PubMed] [Google Scholar]
- 27.Tuggey JM, Elliott MW. Randomized crossover study of pressure and volume non-invasive ventilation in chest wall deformity. Thorax. 2005;60:859–64. doi: 10.1136/thx.2005.041327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simonds AK, Ward S, Heather S, et al. Outcome of pediatric domicilliary mask ventilation in neuromuscular and skeletal disease. Eur Respir J. 2000;16:476–81. doi: 10.1034/j.1399-3003.2000.016003476.x. [DOI] [PubMed] [Google Scholar]
- 29.Leger R, Bedicam JM, Cornetta A, et al. Nasal intermittent positive pressure ventilation: long term follow-up in patients with severe chronic respiratory insufficiency. Chest. 1994;105:100–5. doi: 10.1378/chest.105.1.100. [DOI] [PubMed] [Google Scholar]
- 30.Ward S, Chatwin M, Heather S, Simonds AK. Randomised controlled trial of non-invasive ventilation (NIV) for nocturnal hypoventilation in neuromuscular and chest wall disease patients with daytime normocapnia. Thorax. 2005;60:1019–24. doi: 10.1136/thx.2004.037424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masa JF, Celli BR, Riesco JA, et al. Noninvasive positive pressure ventilation and not oxygen may prevent overt ventilatory failure in patients with chest wall disease. Chest. 1997;112:207–13. doi: 10.1378/chest.112.1.207. [DOI] [PubMed] [Google Scholar]
- 32.Tibballs J, Henning RD. Noninvasive ventilatory strategies in the management of a newborn infant and three children with congenital central hypoventilation syndrome. Pediatr Pulmonol. 2003;36:544–8. doi: 10.1002/ppul.10392. [DOI] [PubMed] [Google Scholar]
- 33.Ramesh P, Boit P, Samuels Mask ventilation in the early management of congenital central hypoventilation syndrome. Arch Dis Child Fetal Neonatal Ed. 2008;93:F400–3. doi: 10.1136/adc.2008.139931. [DOI] [PubMed] [Google Scholar]
- 34.Nielson DW, Black PG. Mask ventilation in congenital central alveolar hypoventilation syndrome. Pediatr Pulmonol. 1990;9:44–5. doi: 10.1002/ppul.1950090110. [DOI] [PubMed] [Google Scholar]
- 35.Ellis ER, Bye PT, Bruderer JW, Sullivan CE. Treatment of respiratory failure during sleep in patients with neuromuscular disease. Positive-pressure ventilation through a nose mask. Am Rev Respir Dis. 1987;135:148–52. doi: 10.1164/arrd.1987.135.1.148. [DOI] [PubMed] [Google Scholar]
- 36.Gruis KL, Brown DL, Lisabeth LD, Zebarah VA, et al. Longitudinal assessment of noninvasive positive pressure ventilation adjustments in ALS patients. J Neurol Sci. 2006;247:59–63. doi: 10.1016/j.jns.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Toussaint M, Chatwin M, Soudon P. Mechanical ventilation in Duchenne patients with chronic respiratory insufficiency: clinical implications of 20 years published experience. Chron Respir Dis. 2007;4:167–77. doi: 10.1177/1479972307080697. [DOI] [PubMed] [Google Scholar]
- 38.Benditt JO. Respiratory complications of amyotrophic lateral sclerosis. Semin Respir Crit Care Med. 2002;23:239–47. doi: 10.1055/s-2002-33032. [DOI] [PubMed] [Google Scholar]
- 39.Bourke SC, Bullock RE, Williams TL, Shaw PJ, Gibson GJ. Noninvasive ventilation in ALS: indications and effect on quality of life. Neurology. 2003;61:171–7. doi: 10.1212/01.wnl.0000076182.13137.38. [DOI] [PubMed] [Google Scholar]
- 40.Guilleminault C, Philip P, Robinson A. Sleep and neuromuscular disease: bilevel positive airway pressure by nasal mask as a treatment for sleep disordered breathing in patients with neuromuscular disease. J Neurol Neurosurg Psychiatry. 1998;65:225–32. doi: 10.1136/jnnp.65.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lechtzin N, Weiner CM, Clawson MC, et al. Use of noninvasive ventilation in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5:9–15. doi: 10.1080/14660820310017335. [DOI] [PubMed] [Google Scholar]
- 42.Mellies U, Ragette R, Dohna Schwake C, Boehm H, Voit T, Teschler H. Long-term noninvasive ventilation in children and adolescents with neuromuscular disorders. Eur Respir J. 2003;22:631–6. doi: 10.1183/09031936.03.00044303a. [DOI] [PubMed] [Google Scholar]
- 43.Vianello A, Bevilacqua M, Salvador V, Cardaioli C, Vincenti E. Long-term nasal intermittent positive pressure ventilation in advanced Duchenne's muscular dystrophy. Chest. 1994;105:445–8. doi: 10.1378/chest.105.2.445. [DOI] [PubMed] [Google Scholar]
- 44.Katz S, Selvadurai H, Keilty K, Mitchell M, MacLusky I. Outcome of non-invasive positive pressure ventilation in paediatric neuromuscular disease. Arch Dis Child. 2004;89:121–4. doi: 10.1136/adc.2002.018655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson CE, Lovitt S, Gowda N, Anderson F, Miller RG. Factors correlated with NPPV use in ALS. Amyotroph Lateral Scler. 2006;7:80–5. doi: 10.1080/14660820500504587. [DOI] [PubMed] [Google Scholar]
- 46.Berlowitz DJ, Detering K, Schachter L. A retrospective analysis of sleep quality and survival with domiciliary ventilatory support in motor neuron disease. Amyotroph Lateral Scler. 2006;7:100–6. doi: 10.1080/14660820500504645. [DOI] [PubMed] [Google Scholar]
- 47.Sanders MH, Kern N. Obstructive sleep apnea treated by independently adjusted inspiratory and expiratory positive airway pressures via nasal mask. Physiologic and clinical implications. Chest. 1990;98:317–24. doi: 10.1378/chest.98.2.317. [DOI] [PubMed] [Google Scholar]
- 48.Kushida CA, Chediak A, Berry RB, et al. Positive Airway Pressure Titration Task Force; American Academy of Sleep Medicine. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4:157–71. [PMC free article] [PubMed] [Google Scholar]
- 49.Phillips B, Ball C, Sackett D, et al. Oxford Centre for Evidence-Based Medicine - Levels of Evidence. 2009. Mar, http://www.cebm.net/index.aspx?o=1025.
- 50.Fitch K, Bernstein S, Aguilar M, et al. The RAND/UCLA Appropriateness Method User's Manual. Santa Monica, CA: RAND Corporation; 2001. [Google Scholar]
- 51.Chesson AL, Jr, Ferber RA, Fry JM, et al. The indications for polysomnography and related procedures. Sleep. 1997;20:423–87. doi: 10.1093/sleep/20.6.423. [DOI] [PubMed] [Google Scholar]
- 52.Kushida CA, Littner M, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 53.Kushida CA, Littner MR, Hirshkowitz M, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29:375–80. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 54.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. American Academy of Sleep Medicine. [Google Scholar]
- 55.Windisch W, Storre JH, Sorichter S, Virchow JC., Jr Comparison of volume and pressure-limited NPPV at night: a prospective randomized cross-over trial. Respir Med. 2005;99:52–9. doi: 10.1016/j.rmed.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 56.Silva RS, Truksinas V, de Mello-Fujita L, et al. An orientation session improves objective sleep quality and mask acceptance during positive airway pressure titration. Sleep Breath. 2008;12:85–9. doi: 10.1007/s11325-007-0138-6. [DOI] [PubMed] [Google Scholar]
- 57.Rains JC. Treatment of obstructive sleep apnea in pediatric patients. Behavioral intervention for compliance with nasal continuous positive airway pressure. Clin Pediatr (Phila) 1995;34:535–41. doi: 10.1177/000992289503401005. [DOI] [PubMed] [Google Scholar]
- 58.Kirk VG, O'Donnell AR. Continuous positive airway pressure for children: a discussion on how to maximize compliance. Sleep Med Rev. 2006;10:119–27. doi: 10.1016/j.smrv.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Slifer KJ, Kruglak D, Benore E, et al. Behavioral training for increasing preschool children's adherence with positive airway pressure: a preliminary study. Behav Sleep Med. 2007;5:147–75. doi: 10.1080/15402000701190671. [DOI] [PubMed] [Google Scholar]
- 60.Chatwin M, Nickol AH, Morrell MJ, Polkey, Simonds AK. Randomized trial of inpatients versus outpatient initiation of home mechanical ventilation in patients with nocturnal hypoventilation. Respir Med. 2008;102:1528–35. doi: 10.1016/j.rmed.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 61.Lofaso F, Quera-Salva MA. Polysomnography for the management of progressive neuromuscular disorders. Eur Respir J. 2001;19:989–90. doi: 10.1183/09031936.02.00752002. [DOI] [PubMed] [Google Scholar]
- 62.Weinberg J, Klefbeck, Borg J, Svanborg E. Polysomnography in chronic neuromuscular disease. Respiration. 2003:349–54. doi: 10.1159/000072896. [DOI] [PubMed] [Google Scholar]
- 63.Fanfulla F, Taurino AE, Lupo ND, Trentin R, D'Ambrosio C, Nava S. Effect of sleep on patient/ventilator asynchrony in patients undergoing chronic non-invasive mechanical ventilation. Respir Med. 2007;101:1702–7. doi: 10.1016/j.rmed.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 64.Meyer TJ, Pressman MR, Benditt J, et al. Air leaking through the mouth during nocturnal nasal ventilation: effect on sleep quality. Sleep. 1997;20:561–9. doi: 10.1093/sleep/20.7.561. [DOI] [PubMed] [Google Scholar]
- 65.Teschler H, Stampa J, Ragette R, Konietzko N, Berthon-Jones M. Effect of mouth leak on effectiveness of nasal bilevel ventilatory assistance and sleep architecture. Eur Respir J. 1999;14:1251–7. doi: 10.1183/09031936.99.14612519. [DOI] [PubMed] [Google Scholar]
- 66.Tuggey JM, Delmastro M, Elliott MW. The effect of mouth leak and humidification during nasal non-invasive ventilation. Respir Med. 2007;101:1874–9. doi: 10.1016/j.rmed.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 67.Bach JR, Robert D, Leger P, Langevin B. Sleep fragmentation in kyphoscoliotic individuals with alveolar hypoventilation treated by NIPPV. Chest. 1995;107:1552–8. doi: 10.1378/chest.107.6.1552. [DOI] [PubMed] [Google Scholar]
- 68.Carrey Z, Gottfried SB, Levy RD. Ventilatory muscle support in respiratory failure with nasal positive pressure ventilation. Chest. 1990;97:150–8. doi: 10.1378/chest.97.1.150. [DOI] [PubMed] [Google Scholar]
- 69.Bennett JR, Dunroy HMA, Corfield DR, et al. Respiratory muscle activity during REM sleep in patients with diaphragm paralysis. Neurology. 2004;61:134–7. doi: 10.1212/01.wnl.0000101719.84675.e0. [DOI] [PubMed] [Google Scholar]
- 70.White JES, Drinnan MJ, Smithson AJ, Griffiths CJ, Gibson GJ. Respiratory muscle activity and oxygenation during sleep in patients with muscle weakness. Eur Respir J. 1995;8:808–14. [PubMed] [Google Scholar]
- 71.Fanfulla F, Delmastro M, Berardinell A, et al. Effects of different ventilator settings on sleep and inspiratory effort in patients with neuromuscular disease. Am J Respir Crit Care Med. 2005:619–24. doi: 10.1164/rccm.200406-694OC. [DOI] [PubMed] [Google Scholar]
- 72.Tuggey JM, Elliott MW. Titration of non-invasive positive ventilation in chronic respiratory failure. Respir Med. 2006;100:1262–9. doi: 10.1016/j.rmed.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 73.Pankow W, Hijjeh N, Schullter F, Penzel T, et al. Influence of noninvasive positive pressure ventilation on inspiratory muscle activity in obese subjects. Eur Respir J. 1997;10:2847–52. doi: 10.1183/09031936.97.10122847. [DOI] [PubMed] [Google Scholar]
- 74.Fauroux B, Lavis JF, Nicot F, et al. Facial side effects during noninvasive positive pressure ventilation in children. Intensive Care Med. 2005;31:965–9. doi: 10.1007/s00134-005-2669-2. [DOI] [PubMed] [Google Scholar]
- 75.Villa MP, Pagani J, Ambrosio R, Ronchetti R, Bernkopf E. Mid-face hypoplasia after long-term nasal ventilation. Am J Respir Crit Care Med. 2002;166:1142–3. doi: 10.1164/ajrccm.166.8.257c. [DOI] [PubMed] [Google Scholar]
- 76.Parreira VF, Delguste P, Jounieaux V, et al. Effectiveness of controlled and spontaneous modes in nasal two-level positive pressure ventilation in awake and asleep normal subjects. Chest. 1997;112:1267–77. doi: 10.1378/chest.112.5.1267. [DOI] [PubMed] [Google Scholar]
- 77.Storre JH, Steurer B, Kabitz HJ, Dreher M, Windisch W. Transcutaneous PCO2 monitoring during initiation of noninvasive ventilation. Chest. 2007;132:1810–6. doi: 10.1378/chest.07-1173. [DOI] [PubMed] [Google Scholar]
- 78.Janssens JP, Howarth-Frey C, Chevrolet JC, Abajo B, Rochat T. Transcutaneous PCO2 to monitor noninvasive mechanical ventilation in adults: assessment of a new transcutaneous PCO2 device. Chest. 1998;113:768–73. doi: 10.1378/chest.113.3.768. [DOI] [PubMed] [Google Scholar]
- 79.Paiva R, Krivec U, Aubertin G, Cohen E, Clement A, Faurox B. Carbon dioxide monitoring during noninvasive respiratory support in children. Intensive Care Med. 2009;35:1068–74. doi: 10.1007/s00134-009-1408-5. [DOI] [PubMed] [Google Scholar]
- 80.Sanders MH, Kern NB, Costantino JP, Stiller RA, et al. Accuracy of End-tidal and transcutaneous pco2 monitoring during sleep. Chest. 1994;106:472–83. doi: 10.1378/chest.106.2.472. [DOI] [PubMed] [Google Scholar]
- 81.Kirk VG, Batuyong ED, Bohn SG. Transcutaneous carbon dioxide monitoring and capnography during pediatric polysomnography. Sleep. 2006;29:1601–8. doi: 10.1093/sleep/29.12.1601. [DOI] [PubMed] [Google Scholar]
- 82.Wiest GH, Harsch IA, Fuchs FS, Kitzbickler S, et al. Initiation of CPAP Therapy for OSA: does prophylactic humidification during CPAP titration improve initial patient acceptance and comfort? Respiration. 2002;69:406–12. doi: 10.1159/000064016. [DOI] [PubMed] [Google Scholar]
- 83.Duong M, Jayram L, Camfferman D, et al. Use of heated humidification during nasal CPAP titration in obstructive sleep apnea syndrome. Eur Respir J. 2005;26:679–85. doi: 10.1183/09031936.05.00131504. [DOI] [PubMed] [Google Scholar]
- 84.Holland AE, Denehy L, Buchan CA, Wilson JW. Efficacy of a heated passover humidifier during noninvasive ventilation: a bench study. Respir Care. 2007;52:38–44. [PubMed] [Google Scholar]
- 85.Nava S, Cirio S, Fanfulla F, et al. Comparison of two humidification systems for long-term noninvasive mechanical ventilation. Eur Respir J. 2008;32:460–4. doi: 10.1183/09031936.00000208. [DOI] [PubMed] [Google Scholar]
- 86.Titration Protocol Philips-Respironics. 2007 [Google Scholar]
- 87.Thys F, Liistro G, Dozin O, Marion E, Rodenstein DO. Determinants of FiO2 with oxygen supplementation during noninvasive two-level positive pressure ventilation. Eur Respir J. 2002;19:653–7. doi: 10.1183/09031936.02.00263102. [DOI] [PubMed] [Google Scholar]
- 88.Yoder EA, Klann K, Strohl KP. Inspired oxygen concentrations during positive pressure therapy. Sleep Breath. 2004;8:1–5. doi: 10.1007/s11325-004-0001-y. [DOI] [PubMed] [Google Scholar]
- 89.Schwartz AR, Kacmarek RM, Hess DR. Factors affecting oxygen delivery with bilevel positive airway pressure. Respir Care. 2004;49:270–5. [PubMed] [Google Scholar]
- 90.Miyoshi E, Fujino Y, Uchiyama A, Mashimo T, Nishimura M. Effects of gas leak on triggering function, humidification, and inspiratory oxygen fraction during noninvasive positive airway pressure ventilation. Chest. 2005;128:3691–8. doi: 10.1378/chest.128.5.3691. [DOI] [PubMed] [Google Scholar]
- 91.Aloia MS, Stanchina M, Arnedt JT, Malhotra A, Millman RP. Treatment adherence and outcomes in flexible vs standard continuous positive airway pressure therapy. Chest. 2005;127:2085–93. doi: 10.1378/chest.127.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nilius G, Happel A, Domanski U, Ruhle KH. Pressure-relief continuous positive airway pressure vs constant continuous positive airway pressure: a comparison of efficacy and compliance. Chest. 2006;130:1018–24. doi: 10.1378/chest.130.4.1018. [DOI] [PubMed] [Google Scholar]
- 93.Hirshkowitz M, Sharafkhaneh A. Positive airway pressure therapy of OSA. Semin Respir Crit Care Med. 2005;26:68–79. doi: 10.1055/s-2005-864209. [DOI] [PubMed] [Google Scholar]