Abstract
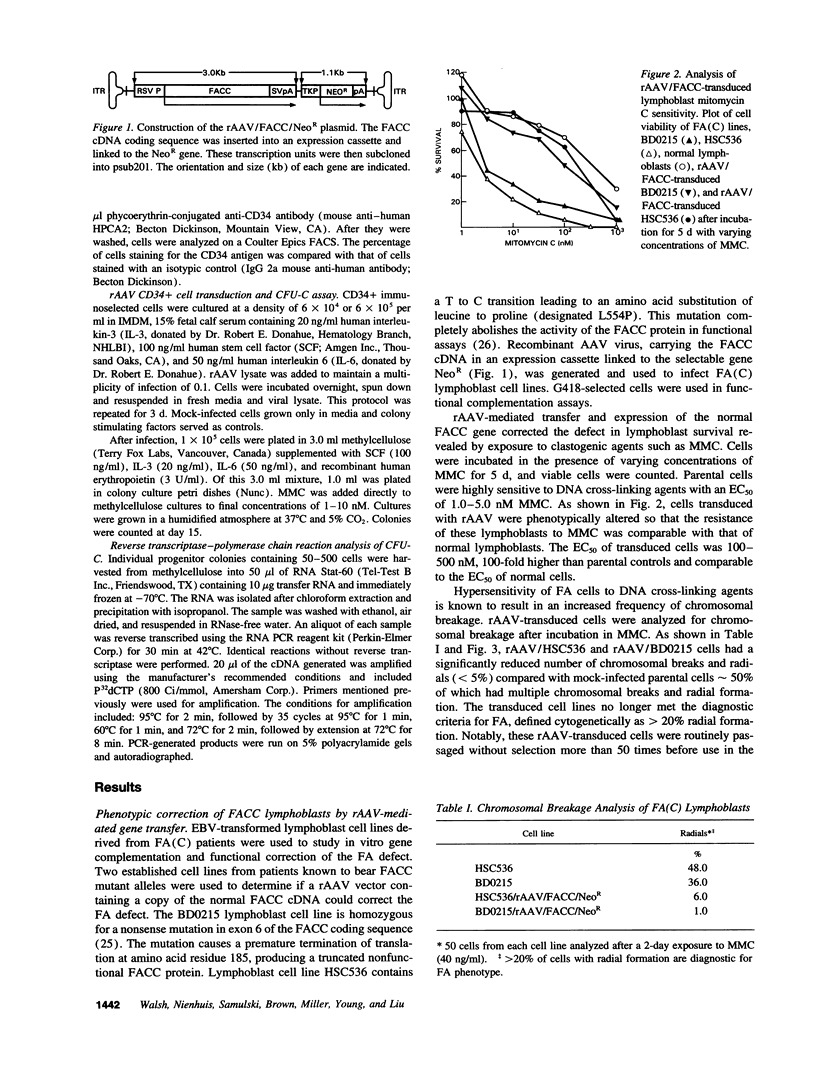
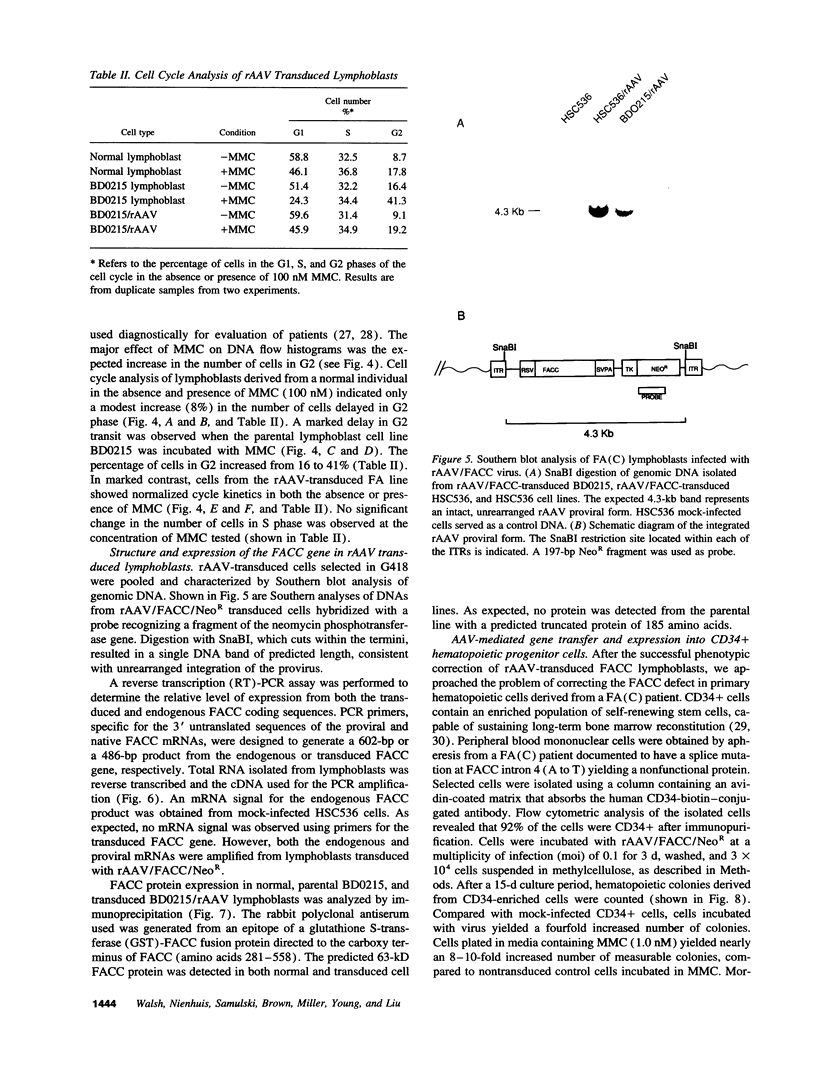
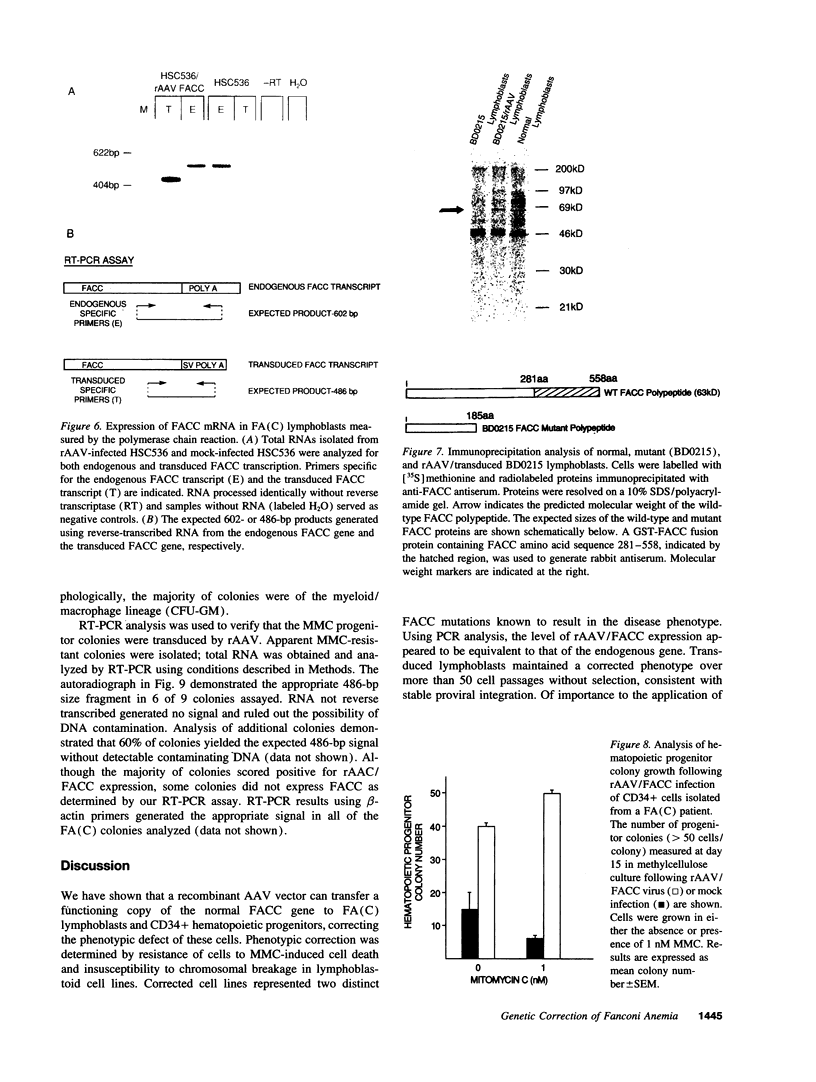
Fanconi anemia (FA) is a recessive inherited disease characterized by defective DNA repair. FA cells are hypersensitive to DNA cross-linking agents that cause chromosomal instability and cell death. FA is manifested clinically by progressive pancytopenia, variable physical anomalies, and predisposition to malignancy. Four complementation groups have been identified, termed A, B, C, and D. The gene for the FA complementation group C, FACC, has been cloned. Expression of the FACC cDNA corrects the phenotypic defect of FA(C) cells, resulting in normalized cell growth in the presence of DNA cross-linking agents such as mitomycin C (MMC). Gene transfer of the FACC gene should provide a survival advantage to transduced hematopoietic cells, suggesting that FA might be an ideal candidate for gene therapy. We demonstrated efficient transduction, expression, and phenotypic correction in lymphoblastoid cell lines derived from FA (C) patients using a recombinant adeno-associated virus (rAAV) vector containing the FACC gene. Molecular characterization of the transduced FACC gene showed an intact unrearranged proviral genome with expression sufficient to normalize cell growth, cell cycle kinetics and chromosomal breakage in the presence of MMC. These observations were extended by testing rAAV transduction in hematopoietic progenitor cells. Peripheral blood CD34+ cells isolated from a FA (C) patient and transduced with rAAV/FACC virus yielded 5-10-fold more progenitor colonies than mock-infected cells, consistent with genetic "rescue" of corrected cells. This is the first demonstration of rAAV gene correction in primary human hematopoietic progenitor cells and has important implications for gene therapy of hematopoietic disorders, specifically FA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter B. P., Knobloch M. E., He L., Gillio A. P., O'Reilly R. J., Reilly L. K., Weinberg R. S. Effect of stem cell factor on in vitro erythropoiesis in patients with bone marrow failure syndromes. Blood. 1992 Dec 15;80(12):3000–3008. [PubMed] [Google Scholar]
- Andrews R. G., Singer J. W., Bernstein I. D. Human hematopoietic precursors in long-term culture: single CD34+ cells that lack detectable T cell, B cell, and myeloid cell antigens produce multiple colony-forming cells when cultured with marrow stromal cells. J Exp Med. 1990 Jul 1;172(1):355–358. doi: 10.1084/jem.172.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A. D., Rogatko A., Schroeder-Kurth T. M. International Fanconi Anemia Registry: relation of clinical symptoms to diepoxybutane sensitivity. Blood. 1989 Feb;73(2):391–396. [PubMed] [Google Scholar]
- Bagby G. C., Jr, Segal G. M., Auerbach A. D., Onega T., Keeble W., Heinrich M. C. Constitutive and induced expression of hematopoietic growth factor genes by fibroblasts from children with Fanconi anemia. Exp Hematol. 1993 Oct;21(11):1419–1426. [PubMed] [Google Scholar]
- Berger R., Le Coniat M., Gendron M. C. Fanconi anemia. Chromosome breakage and cell cycle studies. Cancer Genet Cytogenet. 1993 Aug;69(1):13–16. doi: 10.1016/0165-4608(93)90104-t. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Johnson P. R., Wong K. K., Jr Dual-target inhibition of HIV-1 in vitro by means of an adeno-associated virus antisense vector. Science. 1992 Nov 27;258(5087):1485–1488. doi: 10.1126/science.1359646. [DOI] [PubMed] [Google Scholar]
- Dutrillaux B., Aurias A., Dutrillaux A. M., Buriot D., Prieur M. The cell cycle of lymphocytes in Fanconi anemia. Hum Genet. 1982;62(4):327–332. doi: 10.1007/BF00304549. [DOI] [PubMed] [Google Scholar]
- Frazelle J. H., Harris J. S., Swift M. Responses of Fanconi anemia fibroblasts to adenine and purine analogues. Mutat Res. 1981 Feb;80(2):373–380. doi: 10.1016/0027-5107(81)90109-3. [DOI] [PubMed] [Google Scholar]
- Gavish H., dos Santos C. C., Buchwald M. A Leu554-to-Pro substitution completely abolishes the functional complementing activity of the Fanconi anemia (FACC) protein. Hum Mol Genet. 1993 Feb;2(2):123–126. doi: 10.1093/hmg/2.2.123. [DOI] [PubMed] [Google Scholar]
- Gibson R. A., Hajianpour A., Murer-Orlando M., Buchwald M., Mathew C. G. A nonsense mutation and exon skipping in the Fanconi anaemia group C gene. Hum Mol Genet. 1993 Jun;2(6):797–799. doi: 10.1093/hmg/2.6.797. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Weinert T. A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989 Nov 3;246(4930):629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Ishida R., Buchwald M. Susceptibility of Fanconi's anemia lymphoblasts to DNA-cross-linking and alkylating agents. Cancer Res. 1982 Oct;42(10):4000–4006. [PubMed] [Google Scholar]
- Kaiser T. N., Lojewski A., Dougherty C., Juergens L., Sahar E., Latt S. A. Flow cytometric characterization of the response of Fanconi's anemia cells to mitomycin C treatment. Cytometry. 1982 Mar;2(5):291–297. doi: 10.1002/cyto.990020505. [DOI] [PubMed] [Google Scholar]
- Kotin R. M., Siniscalco M., Samulski R. J., Zhu X. D., Hunter L., Laughlin C. A., McLaughlin S., Muzyczka N., Rocchi M., Berns K. I. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubbies M., Schindler D., Hoehn H., Schinzel A., Rabinovitch P. S. Endogenous blockage and delay of the chromosome cycle despite normal recruitment and growth phase explain poor proliferation and frequent edomitosis in Fanconi anemia cells. Am J Hum Genet. 1985 Sep;37(5):1022–1030. [PMC free article] [PubMed] [Google Scholar]
- LaFace D., Hermonat P., Wakeland E., Peck A. Gene transfer into hematopoietic progenitor cells mediated by an adeno-associated virus vector. Virology. 1988 Feb;162(2):483–486. doi: 10.1016/0042-6822(88)90491-6. [DOI] [PubMed] [Google Scholar]
- Lau C. C., Pardee A. B. Mechanism by which caffeine potentiates lethality of nitrogen mustard. Proc Natl Acad Sci U S A. 1982 May;79(9):2942–2946. doi: 10.1073/pnas.79.9.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. L., Walsh C. E., Ney P. A., Samulski R. J., Nienhuis A. W. Single-copy transduction and expression of human gamma-globin in K562 erythroleukemia cells using recombinant adeno-associated virus vectors: the effect of mutations in NF-E2 and GATA-1 binding motifs within the hypersensitivity site 2 enhancer. Blood. 1993 Sep 15;82(6):1900–1906. [PubMed] [Google Scholar]
- Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- Pincheira J., Bravo M., López-Sáez J. F. Fanconi's anemia lymphocytes: effect of caffeine, adenosine and niacinamide during G2 prophase. Mutat Res. 1988 May;199(1):159–165. doi: 10.1016/0027-5107(88)90241-2. [DOI] [PubMed] [Google Scholar]
- Rigaud G., Grange T., Pictet R. The use of NaOH as transfer solution of DNA onto nylon membrane decreases the hybridization efficiency. Nucleic Acids Res. 1987 Jan 26;15(2):857–857. doi: 10.1093/nar/15.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier L., Dutrillaux B. Effect of caffeine in Fanconi anemia. I. Restoration of a normal duration of G2 phase. Hum Genet. 1988 Jul;79(3):242–244. doi: 10.1007/BF00366244. [DOI] [PubMed] [Google Scholar]
- Samulski R. J. Adeno-associated virus: integration at a specific chromosomal locus. Curr Opin Genet Dev. 1993 Feb;3(1):74–80. doi: 10.1016/s0959-437x(05)80344-2. [DOI] [PubMed] [Google Scholar]
- Samulski R. J., Chang L. S., Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989 Sep;63(9):3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski R. J., Zhu X., Xiao X., Brook J. D., Housman D. E., Epstein N., Hunter L. A. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 1991 Dec;10(12):3941–3950. doi: 10.1002/j.1460-2075.1991.tb04964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M. S., Tonomura A. A high susceptibility of Fanconi's anemia to chromosome breakage by DNA cross-linking agents. Cancer Res. 1973 Aug;33(8):1829–1836. [PubMed] [Google Scholar]
- Saunders E. F., Freedman M. H. Constitutional aplastic anaemia: defective haematopoietic stem cell growth in vitro. Br J Haematol. 1978 Oct;40(2):277–287. doi: 10.1111/j.1365-2141.1978.tb03664.x. [DOI] [PubMed] [Google Scholar]
- Schroeder T. M., Anschütz F., Knopp A. Spontane Chromosomenaberrationen bei familiärer Panmyelopathie. Humangenetik. 1964;1(2):194–196. doi: 10.1007/BF00389636. [DOI] [PubMed] [Google Scholar]
- Stark R., Thierry D., Richard P., Gluckman E. Long-term bone marrow culture in Fanconi's anaemia. Br J Haematol. 1993 Apr;83(4):554–559. doi: 10.1111/j.1365-2141.1993.tb04690.x. [DOI] [PubMed] [Google Scholar]
- Strathdee C. A., Duncan A. M., Buchwald M. Evidence for at least four Fanconi anaemia genes including FACC on chromosome 9. Nat Genet. 1992 Jun;1(3):196–198. doi: 10.1038/ng0692-196. [DOI] [PubMed] [Google Scholar]
- Strathdee C. A., Gavish H., Shannon W. R., Buchwald M. Cloning of cDNAs for Fanconi's anaemia by functional complementation. Nature. 1992 Apr 30;356(6372):763–767. doi: 10.1038/356763a0. [DOI] [PubMed] [Google Scholar]
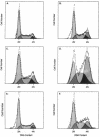
- Verfaillie C., Blakolmer K., McGlave P. Purified primitive human hematopoietic progenitor cells with long-term in vitro repopulating capacity adhere selectively to irradiated bone marrow stroma. J Exp Med. 1990 Aug 1;172(2):509–502. doi: 10.1084/jem.172.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C. E., Liu J. M., Miller J. L., Nienhuis A. W., Samulski R. J. Gene therapy for human hemoglobinopathies. Proc Soc Exp Biol Med. 1993 Dec;204(3):289–300. doi: 10.3181/00379727-204-43665. [DOI] [PubMed] [Google Scholar]
- Walsh C. E., Liu J. M., Xiao X., Young N. S., Nienhuis A. W., Samulski R. J. Regulated high level expression of a human gamma-globin gene introduced into erythroid cells by an adeno-associated virus vector. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7257–7261. doi: 10.1073/pnas.89.15.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert T. A., Hartwell L. H. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science. 1988 Jul 15;241(4863):317–322. doi: 10.1126/science.3291120. [DOI] [PubMed] [Google Scholar]
- Wevrick R., Clarke C. A., Buchwald M. Cloning and analysis of the murine Fanconi anemia group C cDNA. Hum Mol Genet. 1993 Jun;2(6):655–662. doi: 10.1093/hmg/2.6.655. [DOI] [PubMed] [Google Scholar]
- Whitney M. A., Saito H., Jakobs P. M., Gibson R. A., Moses R. E., Grompe M. A common mutation in the FACC gene causes Fanconi anaemia in Ashkenazi Jews. Nat Genet. 1993 Jun;4(2):202–205. doi: 10.1038/ng0693-202. [DOI] [PubMed] [Google Scholar]
- Zhou S. Z., Broxmeyer H. E., Cooper S., Harrington M. A., Srivastava A. Adeno-associated virus 2-mediated gene transfer in murine hematopoietic progenitor cells. Exp Hematol. 1993 Jul;21(7):928–933. [PubMed] [Google Scholar]