Abstract
Rationale
Over the last decade, Asian ginseng (Panax ginseng) has been shown to improve aspects of human cognitive function. American ginseng (Panax quinquefolius) has a distinct ginsenoside profile from P. ginseng, promising cognitive enhancing properties in preclinical studies and benefits processes linked to human cognition.
Objectives
The availability of a highly standardised extract of P. quinquefolius (Cereboost™) led us to evaluate its neurocognitive properties in humans for the first time.
Methods
This randomised, double-blind, placebo-controlled, crossover trial (N = 32, healthy young adults) assessed the acute mood, neurocognitive and glycaemic effects of three doses (100, 200 400 mg) of Cereboost™ (P. quinquefolius standardised to 10.65% ginsenosides). Participants' mood, cognitive function and blood glucose were measured 1, 3 and 6 h following administration.
Results
There was a significant improvement of working memory (WM) performance associated with P. quinquefolius. Corsi block performance was improved by all doses at all testing times. There were differential effects of all doses on other WM tasks which were maintained across the testing day. Choice reaction time accuracy and ‘calmness’ were significantly improved by 100 mg. There were no changes in blood glucose levels.
Conclusions
This preliminary study has identified robust working memory enhancement following administration of American ginseng. These effects are distinct from those of Asian ginseng and suggest that psychopharmacological properties depend critically on ginsenoside profiles. These results have ramifications for the psychopharmacology of herbal extracts and merit further study using different dosing regimens and in populations where cognition is fragile.
Keywords: Cognitive, Working memory, Mood, Ginseng, Nootropic, Herbal extract
Introduction
‘Ginseng’ usually refers to extracts of plants from the Panax genus of the Araliaceae family. Extracts of ginseng have been used for millennia in Traditional Chinese Medicine for the prevention and treatment of a variety of diseases, and as general health elixirs and performance enhancers (including in the neurocognitive area). Empirical studies have attributed these effects to the action of a group of ginseng-specific saponins known as ginsenosides. There is a growing body of evidence to support Panax ginseng (Asian ginseng) as a cognitive enhancer; Panax quinquefolius (American ginseng) is another prominent species with a ginsenosides profile distinct to that of P. ginseng. Traditional use of American ginseng and, more importantly, preclinical efficacy studies coupled with the development of a highly standardised extract prompted us to assess its potential cognition-enhancing properties, which have not hitherto been investigated in humans.
Regarding the neurocognitive effects of ginseng, a series of previous studies has assessed the behavioural effects of administration of P. ginseng on cognition in young healthy individuals (Kennedy and Scholey 2003; Kennedy et al. 2001a, b, 2002; Scholey and Kennedy 2002) where P. ginseng differentially improved scores on a ‘secondary memory’ factor (a composite of four memory tasks). In the first study, doses of 200, 400 and 600 mg Ginseng (G115) were administered. Enhancement of ‘secondary memory’ was found following 400 mg at four post-dose testing sessions while the lower and higher dosage reduced performance on the ‘speed of attention’ factor (Kennedy et al. 2001b). Kennedy et al. (2002) replicated the finding that 400 mg dosage improved ‘secondary memory’. In a different study, assessing combinations of Ginseng and Ginkgo (ratio 100:60) at dosages of 320, 640, 960 mg, a similar pattern was observed (Kennedy et al. 2001a). There was improved performance of secondary memory following 960 mg, with reduced performance on speed of attention for the other dosages (320 and 640 mg). A later study assessed the effects of 200 and 400 mg Ginseng during sustained cognitive demand—repeated cycles of Serial Threes, Serial Sevens and the Bakan Rapid Visual Information Processing (RVIP) task. Serial Sevens performance was improved by the 200 mg dose (Reay et al. 2005); in a follow-up study, the same dose improved Serial Threes and RVIP performance (Reay et al. 2006). It appears that P. ginseng or its constituents are capable of producing tangible cognitive enhancing effects and that 200–400 mg appears to be the optimal dose range for young healthy adults when administered acutely prior to a cognitive test.
The constituents of the Panax genus which are thought to contribute to its bioactivity are the ginsenoside saponins. Ginsenosides can be classified into three groups on the basis of their chemical structure (Tachikawa et al. 1999); the Panaxadiol group (Rb1, Rb2, Rb3, Rc etc.), Panaxatriol group (Re, Rf, Rg1, Rg2, Rh1), and the oleanolic acid group (e.g. Ro). Many of these ginsenosides have been isolated and evaluated for pharmacological effects relevant to cognition. They have been reported to exert effects on the cholinergic system; isolated Rb1 was observed to both increase synaptosomal choline uptake, and stimulate acetylcholine release (Benishin 1992; Benishin et al. 1991). Furthermore, Salim et al. (1997) found that in Rb1 increased expression of rat brain choline acetyltransferase as well as nerve growth factor messenger RNA. Ginsenosides Rg1 and Rb1 elicit marked alterations in brain serotonin concentrations (Zhang et al. 1990). Other ginsenosides may affect specific physiological mechanisms, including corticosterone secretion by Rd (Hiai et al. 1983), inhibition of synaptosomal uptake of norepinephrine, dopamine, serotonin and GABA by Rd and Re (Tsang et al. 1985). These physiological effects are not straightforward, for example two types of in vivo modulation of long-term potentiation (LTP) in the rat hippocampal formation by Rb1 have been observed (Abe et al. 1994). Neutral Rb1 attenuated the LTP induced by a strong stimulus train only, whereas as slightly different (also naturally occurring) variant malonyl-Rb1 facilitated generation of LTP induced by a weaker stimulation only.
The most widely used standardised Ginseng extract (discussed above), both commercially and for research purposes, is G115, a concentrated aqueous extract, which is standardised to contain an invariable 4% ginsenosides (Soldati and Sticher 1980). However ginsenoside expression is substantially higher in P. quinquefolius (American Ginseng) than P. ginseng (Zhu et al. 2004). To date there has been surprisingly little research evaluating potential cognitive enhancement by whole extract P. quinquefolius. One study observed that scopolamine-associated spatial learning impairment in rats was partially reversed by P. quinquefolius, which also increased choline uptake in synaptosomal preparations (Sloley et al. 1999). However despite established associations between memory and cholinergic function and despite its high ginsenoside content, studies into the psychogenic benefits of P. quinquefolius in humans have not previously been conducted.
Although members of the Panax genus contain many saponins in common P. quinquefolius has its own characteristic profile, in this case with high expression of the ginsenoside Rb1. The extract used in the present study contains 11.65% ginsenosides—Rb1 (5.68%), Re (2.05%), Rc (1.86%), Rd (1.47%), Rb2 (0.29%), Rg1 (0.27%).
The above provides sufficient evidence to suggest that P. quinquefolius may improve cognitive function with similar or greater benefits than P. ginseng. Given that P. ginseng has been shown to be most efficacious as a cognitive enhancer at 200–400 mg in young healthy individuals, the present study investigated the effect of P. quinquefolius at similar ginsenoside levels.
The lack of standardised extracts has delayed the progress of herbal medicine research considerably, including in the psychopharmacology domain (Scholey et al. 2005). Indeed the consistent cognitive results obtained with P. ginseng are partly attributable to the use of a highly standardised extract (G115). The availability of a standardised American ginseng extract (Cereboost™) along with evidence of efficacy from the above studies has allowed us to start a systematic assessment of its neurocognitive effects. The first study in this series is a controlled dose-ranging study of cognitive and mood effects in healthy young adults. Another well-documented effect of P. quinquefolius is its action on blood glucose. P. quinquefolius appears to have significant hypoglycaemic action in rodents (Martinez and Staba 1984; Oshima et al. 1987). In humans, P. quinquefolius also reduced blood glucose levels following a 25-g glucose challenge in both diabetic patients who had ingested 300, 600 and 900 mg (Vuksan et al. 2000a,b) and non diabetics administered 100, 200 and 300 mg (Vuksan et al. 2000a, 2001). There is growing interest in the relationship between blood glucose and cognitive function. In particular the rate at which blood glucose levels fall during testing can correlate with better cognitive performance (Donohoe and Benton 1999a; Kennedy and Scholey 2000). The present study therefore also assessed the effect of P. quinquefolius extract on blood glucose in healthy young adults.
Method
Design
The study followed a randomised, double-blind, placebo-controlled crossover methodology. It used multi-dose, multiple-testing-times with a 7-day washout period between treatments.
Participants
Thirty two participants (16 female) were recruited via advertisements in local newspapers and university bulletin boards . Ages ranged from 18 to 40 years (M = 25.2, SD = 4.97). All participants reported that they were in good health, not taking any drugs or medications (excluding the contraceptive pill), had no known food allergies and were non smokers.
Volunteers completed an initial health screening questionnaire which excluded participants with a number of medical conditions (e.g. diabetes, hypoglycaemia, psychiatric disorders, epilepsy and gastrointestinal disorders) or who were taking prescribed medications, were pregnant or lactating. They were advised to refrain from taking any vitamins, other herbal supplements and over the counter medicines for the whole period of study. On the testing days, participants were advised to abstain from consuming alcohol, caffeine products and energy drinks. They were required to eat a light breakfast (toast or cereal) at least 2 h before the onset of the experiment and were provided with sandwich for lunch (with either chicken and salad, or cheese and salad), the same foods were consumed on each study day. The study, which was conducted according to the Declaration of Helsinki, was approved by the Swinburne University Human Research Ethics Committee and all participants gave written informed consent. Volunteers received AU $200 for their participation.
Treatments
A commercial extract of P. quinquefolius Cereboost™ contained a standardised 11.65% ginsenosides was prepared and provided by Naturex as detailed below. This was used to prepare opaque capsules containing 0 mg, 100 mg and 200 mg Cereboost™ with maltodextrose as excipient. Encapsulation was performed locally by Thompsons Amcal Pharmacy, Melbourne. Study day treatments took the form of two capsules corresponding to a dose of either 0 (placebo), 100, 200 or 400 mg of Cereboost™. In order to maintain the double-blind, each individual's treatments were prepared by a disinterested third party who took no further part in the study.
Extract preparation
The roots of American ginseng (P. quinquefolius) were collected from the region of Ontario, Canada. The roots were authenticated using macroscopic, microscopic, and high performance thin layer chromatography techniques (Reich and Schibli 2006). The American ginseng extract was obtained through an industrial process (Cereboost™, Naturex, USA, Reference: GA505000, Lot number E15/05/D8). Firstly, the ginseng roots were ground to be between 1/4 and 1/2 in., and then the ground roots were soaked three times over 5 h intervals in an ethanol/water (75/25, v/v) solution at 40°C. After filtration, the clarified solution was concentrated under vacuum at 45°C. The three pools were combined and concentrated again until the total solids on dry basis were around 60%. This is the Native Extract, which was then mixed with maltodextrine as a carrier and spray-dried to obtain a fine powder. The moisture content in the extract was less than 5%. After extraction, the sample was analyzed for its content of pesticides (USP 2008) and heavy metals (method 993.14, AOAC 2005) at Covance Laboratories (Madison, WI, USA) for compliance. The American ginseng extract was confirmed to be below the Maximum Residue Limits established for pesticides and heavy metals (Durgnat et al. 2005).
Analysis of ginsenosides
An HPLC method was developed for the quantification of ginsenosides in the American ginseng extract. To determine the concentration of ginsenosides, the standards (ginsenosides Rg1, Re, Rf, Rb1, Rc, Rb2 and Rd. Sigma-Aldrich, USA) and the American ginseng extract were dissolved in methanol. The HPLC system used was a Hewlett Packard 1100 equipped with an UV detector at 203 nm. The stationary phase used was a Waters Symmetry C18 column (5 μm, 4.6 mm ID × 250 mm, Waters, USA) thermostated at 25°C. The mobile phases were (a) acetonitrile (Sigma-Aldrich, USA) and (b) water (Milli-Q, USA) running at 1.5 mL/min. A solution of 20% a and 80% b was maintained for 20 min, and then changed to 22% a and 78% b, following a step gradient to 46% a and 54% b after 45 min, 55% a and 45% b after 50 min, reaching 100% a after 51 min (total time is 55 min). The system was then re-equilibrated to the initial composition for 10 min.
Blood glucose measurement
Blood glucose levels were measured using a MediSense Optium Xceed Blood Glucose Sensor and disposable MediSense Blood Glucose Electrodes (MediSense Britain Ltd., Birmingham, UK). The accuracy and consistency of MediSense blood glucose sensors has previously been established (Matthews et al. 1987). Blood samples were taken using Owen Mumford ‘Unistik 2’ single use capillary blood sampling devices (Owen Mumford Ltd., Oxford, UK). Alcohol-soaked Briemarpak skin cleansing swabs were used for pre-sampling sterilisation.
On each of the four active study days, blood glucose levels were measured via capillary finger prick at a baseline, 1-h post-treatment (prior to the commencement of the first post-dose battery), 3-h post-treatment (before the commencement of the second post-dose battery) and 6 h (before the commencement of the third post-dose cognitive assessment battery) and at the end of the day's visit.
Cognitive measures
The Computerised Mental Performance Assessment System (COMPASS) battery has been developed to include tests which have been shown to be sensitive to nutritional manipulations. The tasks in this study were designed to allow assessment across the major cognitive domains i.e. attention, working memory, secondary memory and executive function. Parallel versions of each of the following tasks allowed for multiple testing.
Word presentation
Words matched for linguistic familiarity, concreteness and frequency were drawn from http://www.math.yorku.ca/SCS/Online/paivio. Individual words were presented sequentially on the monitor. Stimulus duration was 1 s, as was the inter-stimulus interval.
Immediate word recall
The participant was allowed 60 s to write down as many of the words as possible. The task was scored for number of correct answers, errors and intrusions and the resulting score was converted into a percentage.
Picture presentation
Twenty line-drawings of everyday items were presented on the computer screen. Stimulus duration was 3 s, with a 1 s inter-stimulus duration.
Face presentation
Twelve photographic images of the faces downloaded from the Productive Aging Lab Face Database http://www.agingmind.cns.uiuc.edu/facedb/ were presented on the computer screen for the participants to remember. Stimulus duration was 3 s with inter-stimulus duration of 1 s.
Simple reaction time
A series of upwards pointing arrows appeared in the centre of the screen with a randomly varying inter-stimulus interval between 1 and 3 s. The participant pressed the space bar as quickly as possible to the on screen appearance of each stimulus and response times were recorded in milliseconds.
Choice reaction time
Arrows pointing to the left or right were presented in the centre of the screen with a randomly varying inter-stimulus interval of 1–3 s. The volunteer pressed the corresponding ‘left’ or ‘right’ cursor key on the computer keyboard as quickly as possible. The task was scored for accuracy (%) and reaction time (ms).
Four choice reaction time
A representation of the four directional arrow keys on the computer keyboard appeared at the centre of the screen. Sequentially, single arrows were illuminated on the screen with the inter-stimulus interval randomly varying between 1 and 3 s. The participants were required to make a response as quickly and as accurately as possible with the corresponding ‘left’, ‘right’, ‘up’ or ‘down’. The task was scored for accuracy (% correct) and reaction time (ms).
Stroop colour-word task
The Stroop task (MacLeod 1992; Stroop 1935) is a classic psychological test of interference, selective attention and response inhibition. In the current form, four colour blocks (blue, yellow, red and green) were displayed on the right hand side of the screen. At a given time interval, words describing one of the four colours (‘red’, ‘yellow’, ‘green’, blue’) were presented in different coloured fonts on the left side of the module area. The participants were instructed to click on colour panels on the right in order to identify the font colour (e.g. if the word ‘green’ is presented in a blue font, the correct response would be to click on the blue panel). The task was scored for accuracy (%) and response times (msec).
Numeric working memory
A series of five digits was presented on the computer screen sequentially for the participants to hold in their memory. This was followed by a series of 30 probe digits. The participants indicated whether or not the digit was from the original series by pressing corresponding keys labelled ‘yes’ and ‘no’. This was repeated three further times with different stimulus sets. Reaction times (ms) and accuracy (% correct) were measured.
Alphabetic working memory
This was similar to the numeric working memory but using letters. A series of five letters appeared on the screen for participants to remember. After 4 s the letters disappeared and were followed by a series of 30 probe letters. Participants were instructed to indicate whether the target letter had appeared in the original list of five letters by pressing corresponding ‘yes’ or ‘no’ key as quickly as possible. The measures were the percentage of the correctly identified stimuli and the average reaction time (ms).
Corsi blocks
The Corsi block-tapping task is a span task and a visuospatial analogue of the digit span of verbal working memory (Lezak et al. 1995; Lezak and Loring 2004). A computerised version of the Corsi blocks task was employed in the study. A series of squares appeared on the screen. A number of these illuminated sequentially in quasi-random order. The volunteer then attempted to repeat the pattern by clicking the boxes in the same order using the mouse and cursor. The task becomes progressively more difficult as the number of boxes increases from four upwards. The task gives a measure of spatial span as well as speed of responding.
N-back
Single letters were presented on the computer screen (total number of presented letters was 45 with 15 targets to be identified). The volunteers were instructed to make key presses labelled ‘yes’ or ‘no’ to indicate whether the digit is the same as the one N previously (e.g. for 1, back the previous digit; for 3, back the digit three previously). The task is scored for speed of reaction (ms) and accuracy (% correct).
Delayed word recall
Approximately 20 min after the word presentation, the participant was allowed 60 s to write down as many of the items from word presentation as possible. The task was scored as for immediate word recall.
Delayed word recognition
Word recognition was tested by representation of the words from the original list randomly interspersed with an equal number of distracter words. Participants responded either ‘yes’ or ‘no’ by pressing corresponding key to indicate whether the word had previously been presented or not. The task was scored for accuracy (%), and reaction time (ms).
Delayed picture recognition
Picture recognition was tested by the presentation of the original drawings and an equal number of distracters in random order. Participants responded either ‘yes’ or ‘no’ by key press in order to indicate whether the picture had been presented previously. The task was scored for accuracy (%) and reaction time (ms).
Delayed face recognition
The originally presented faces were presented along with an equal number of distracters in random sequence Participants were responded via key presses to indicate if they recognised the face from the initial sequence. Accuracy (% errors) and reaction time (ms) were measured.
Serial sevens subtraction task
Computerised versions of serial subtraction task were implemented (Scholey et al. 2001), here using tests of 2 min duration. For the serial sevens task participant was required to count backwards in sevens from a random starting number between 800 and 999, presented on the computer screen, as quickly and accurately as possible, using the numeric key pad to enter each response. Each three-digit response was entered via the numeric key pad with each digit being represented on the screen by an asterisk. The task was scored for total number of subtractions and number of errors. In case of incorrect responses, subsequent responses were scored as positive if they were correct in relation to the new number.
Serial threes subtraction task
The serial threes task was identical to serial sevens, except that it involved serial subtraction of threes.
Rapid visual information processing or Bakan task
This task has been widely used to study the cognitive effects of psychotropic interventions and is sensitive to blood glucose levels (Donohoe and Benton 1999b) and ginseng (Reay et al. 2006) as well as cholinergic modulation (Wesnes and Warburton 1984). A series of digits were presented on the screen, one at a time, at the rate of 100/min and in quick succession. The participant monitors the continuous series for targets of three consecutive odd or three consecutive even digits. The participant responds to the detection of a target string by pressing a space bar as quickly as possible. The task lasted for 3 min, with eight correct target strings being presented in each minute. The task was scored for percentage of target strings correctly detected, the average reaction time (ms) for correct detections, and the number of false alarms.
Mood measures
Visual analogue scales
Mood was assessed during each testing session via visual analogue scales (VAS) incorporated into the cognitive battery. These included the 16 Bond–Lader VAS (Bond and Lader 1974). These scales have high reliability and validity (Ahern 1997) and were originally designed for assessing the mood effects of anxiolytics and have been subsequently utilised in numerous pharmacological, psychopharmacological, medical trials and research programmes assessing dietary manipulations. Participants marked their current subjective state by using the computer mouse to place a mark on sixteen 100 mm VAS linking pairs of antonyms. From the resultant scores, three measures are derived: ‘alertness’ (from individual VAS of alert-drowsy, attentive-dreamy, lethargic-energetic, muzzy-clearheaded, well-coordinated-clumsy, mentally slow-quick witted, strong-feeble, interested-bored, incompetent-proficient); ‘calmness’ (calm-excited, tense-relaxed); and ‘contentedness’ (contented–discontented, trouble–tranquil, happy–sad, antagonistic–friendly, withdrawn–sociable).
Two other visual analogue scales assessed stress and mental fatigue. Using a computer mouse, participants were required to place a cross along the line of the visual analogue scale with end points anchored by ‘not at all’ and ‘extremely’ in response to the questions “How (stressed/mentally fatigued) do you feel right now?” Scores ranged from 1 to 100 with higher scores reflecting higher subjective feelings of stress/mental fatigue.
Pencil-and-paper measures
Depression anxiety and stress scale (DASS, Lovibond and Lovibond 1995)
The shortened 21-item version of the DASS was used to assess three negative affective states of depression, anxiety and stress on seven-item scales. The Depression subscale (DASS-D) measures symptoms relating to dysphoric mood (e.g. sadness), for example ‘I could not seem to experience any positive feeling at all’. The Anxiety subscale (DASS-A) assesses symptoms associated with physiological hyperarousal, for example ‘I felt I was close to panic’. The Stress subscale (DASS-S) assesses symptoms associated with nervous arousal, for example ‘I tended to over-react to situations’. Participants were required to indicate on a 4-point scale whether each statement applied to them not at all, to some degree, a considerable degree, or most of the time. Subscales were calculated by summing the scores of the appropriate items. Good internal consistency and validity for the DASS have been found with samples of clinical patients and non-clinical volunteers (Antony et al. 1998).
State-trait anxiety inventory
The State-Trait Anxiety Inventory (STAI) (Spielberger et al. 1983) comprises of two scales. The ‘State’ (STAI-S) subscale is a widely used instrument for measuring fluctuating levels of anxiety. The subscale contains 20 statements (e.g. ‘I am calm’). Participants rate how much they feel like each statement at the time of making the response by marking a 4-point scale ranging from ‘not at all’ to ‘very much so’. The ‘Trait’ (STAI-T) subscale comprises 20 different statements (e.g. ‘Some unimportant thought runs through my mind and bothers me’). Participants were asked to indicate how they generally feel on a scale ranging from ‘almost never’ to ‘almost always’. Scores on both sections of the STAI range from 20 to 80, with higher scores indicating more anxiety.
Symptom checklist
The symptom checklist was developed at the Brain Sciences Institute specifically for use with natural medicines and consists of 28 physiological/psychological problems people might have, e.g. ‘I feel dizzy’, ‘I have a dry mouth’, ‘I feel anxious more than usual’ (the items can be found in Stough et al. 2001). Participants indicated how much the problem had bothered them in the last 7 days including today using a 5-point scale from ‘not at all’ to ‘very much so’.
Procedure
On the arrival at the laboratory participants underwent a health screen, provided morphometric and demographic data and signed their informed consent.
Each participant was required to attend a total of five occasions (one practice session and four study days). Testing days were 7 days apart to ensure a sufficient washout between conditions.
Testing took place in a suite of dedicated laboratories with participants visually isolated from each other. They also wore headphones to further minimise distraction. On the first visit participants signed their informed consent followed by a health screen, were familiarised with the protocol of the study, including the COMPASS computerised test battery, questionnaires and procedures. Data from the first day were not included in any analysis.
On arrival for their first testing participants were randomly allocated to a treatment regime using a Latin square design that counterbalanced the order of treatments across the four active days. Each study day comprised four identical sessions using parallel versions of the COMPASS battery. The first was a pre-dose testing session that established baseline performance for that day and was immediately followed by the day's treatment (0, 100, 200 or 400 mg Cereboost™). Further testing sessions began at 1, 3 and 6 h following the consumption of the treatment. Each assessment involved blood glucose measurements, pencil-and-paper mood scales and the completion of the COMPASS cognitive battery. A light lunch was provided at the end of 1 h post-treatment session (the same for each participant on each study day). The symptom checklist was completed at the end of the final testing session of the day.
Statistical analysis
Data analyses were similar to those used in a series of similar previous acute, dose-ranging studies (Haskell et al. 2007; Kennedy et al. 2001a, b, 2002; Kennedy et al. 2003; Reay et al. 2005, 2006; Scholey et al. 2008; Tildesley et al. 2005) and followed the recommendations of Keppel (1991).
Change-from-baseline scores on study days were computed for each cognitive measure, at each time-point for every dose. These data were subjected to a General Linear Model ANOVA with terms fitted to the model for dose (placebo, 100, 200 and 400 mg), session (1, 3 and 6 h), dose × session and participant. The initial omnibus ANOVA was employed only to determine the MSError for the appropriate planned comparisons which were performed using t tests incorporating MSError from the ANOVA (Keppel 1991).
The primary analysis therefore involved planned comparisons of the change-from-baseline scores comparing each of the active treatments and placebo at each time point utilising t tests calculated with the mean squares error from the initial ANOVA as an error term. Given the exploratory nature of the study no specific adjustment was made for multiple comparisons, however we reduced the potential for type I error by implementing a number of safeguards. Firstly, we utilised an a priori statistical plan which restricted pre-planned comparisons to measures where there was a significant main effect of treatment, or a treatment × time interaction only. Secondly all these analyses were two-tailed, restricted to planned comparisons and constrained by the number of conditions minus one (i.e. three) at each time point. Finally, compared with single time-points differences, there is an exponentially decreasing probability for significant differences at two and three time-points for the same dose, so here we avoid any interpretation of significant differences isolated to one time-point (though they are reported).
Results
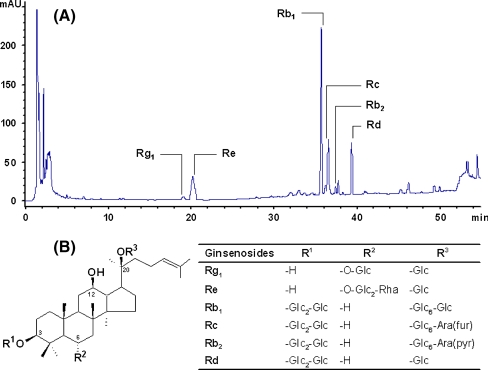
The American ginseng extract used in this clinical trial contained 0.28% of Rg1, 2.06% of Re, 5.69% of Rb1, 1.87% of Rc, 0.29% of Rb2 and 1.48% of Rd. The total ginsenosides, calculated as the sum of above individual ginsenosides, represented 11.65% in the American ginseng extract. As expected, the ginsenoside Rf was not found (Harkey et al. 2001). The ginsenoside Rf is not present in American ginseng but it is present in Asian ginseng (P. ginseng), and is used as a marker to determine adulterations in American ginseng. The chromatogram of the American ginseng extract and the structures of its ginsenosides are presented in Fig. 1.
Fig. 1.
a Chromatogram of the American ginseng (Panax quinquefolius) extract. b Structures of ginsenosides
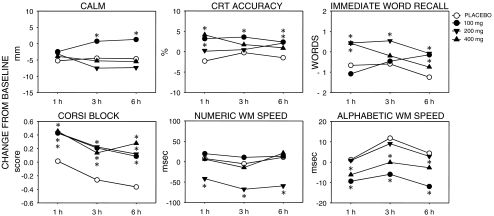
Initial one-way analyses on each cognitive and mood measure revealed no significant baseline differences between conditions confirming that post-treatment effects were not attributable to differences in baseline performance. Significant results are presented graphically in Fig. 2.
Fig. 2.
Significant effects of Panax quinquefolius on cognitive function and mood. Graphs depict mean change-from-baseline scores following a placebo and 100, 200 and 400 mg of a standardised extract. Significant differences from placebo at each time point are indicated (*p < 0.05, see text for details)
For Immediate Word Recall there was a significant main effect of Treatment [F(3,78) = 4.41, p = 0.006] and a Treatment x Time interaction [F(6,156) = 3.183, p = 0.006]. Comparisons of each dose at each time point revealed significant improvements associated with the 200 mg dose at all three time-points [t = 3.206, p = 0.003; t = 3.313, p = 0.002; t = 3.407, p = 0.002 at 1 h, 3 h and 6 h respectively]. There were also significant improvements for 400 mg at 1 h [t = 3.15, p = 0.004] and 100 mg at 6 h only [t = 3.212, p = 0.003].
For Choice Reaction Time accuracy there was a significant main effect of treatment [F(3,54) = 3.223, p = 0.030]. Comparisons of each dose at each time point revealed significant improvements associated with the 100 mg dose at all three time-points [t = 3.03, p = 0.006; t = 2.109, p = 0.047; t = 2.117, p = 0.046 at 1 h, 3 h and 6 h respectively]. There were also significant improvements for 400 mg at 1 h [t = 4.60, p < 0.001] and both 200 and 100 mg at 6 h [t = 2.066, p = 0.048; t = 2.533, p = 0.017, respectively].
There was a significant main effect of Treatment for Numeric Working Memory speed [F(3,63) = 4.39, p = 0.007]. Comparisons of each dose at each time point revealed significant improvements associated with the 200 mg dose at all three time-points [t = 2.155, p = 0.039; t = 2.73, p = 0.011; t = 3.07, p = 0.005 at 1 h, 3 h and 6 h, respectively]. There was a significant main effect of Treatment for speed of Alphabetic Working Memory [F(3,48) = 3.22, p = 0.04]. Comparisons of each dose at each time point revealed significant improvements at all three time-points associated with the 100 mg [t = 2.13, p = 0.041; t = 2.28; p = 0.03; t = 2.09, p = 0.045 at 1 h, 3 h and 6 h, respectively] and 400 mg doses [t = 2.24, p = 0.033; t = 2.17, p = 0.038; t = 2.20, p = 0.036 at 1 h, 3 h and 6 h, respectively]. There was a significant main effects of Treatment on mean Corsi block score [F(3,114) = 2.925, p = 0.041]. Comparisons of each dose at each time point revealed significant improvements at all time-points associated with 100 mg [t = 2.18, p = 0.037; t = 2.50, p = 0.019; t = 2.40, p = 0.023 at 1 h, 3 h and 6 h, respectively], 200 mg [t = 2.21, p = 0.035; t = 2.57, p = 0.015; t = 2.57, p = 0.015 at 1 h, 3 h and 6 h, respectively] and 400 mg [t = 2.33, p < 0.027; t = 2.11, p = 0.042; t = 3.41, p = 0.002 at 1 h, 3 h and 6 h, respectively].
There was a single effect of treatment on mood. The Treatment x Time interaction on self-rated calmness was significant [F6,150 = 2.345, p = 0.034]. Pre-planned comparisons of each dose at each time point revealed significant improvements associated with the 100 mg dose at 3 h and 6 h only [t = 3.34, p = 0.002; t = 3.80, p = 0.001, respectively].
There were no significant effects on any other measure.
Discussion
This is first study to evaluate the acute mood and cognitive effects of P. quinquefolius (American ginseng) extract in humans. We found treatment-related improvements in cognitive performance and increased calmness in healthy young adults. Compared to a placebo, all doses of Cereboost™ were found to improve some aspect of cognition. The present study did not observe any effect of on blood glucose levels thereby ruling out any interpretation of the cognitive facilitation effects as being due to impact on glucose or insulin-mediated mechanisms.
All three active doses improved Corsi blocks performance compared with placebo at all post-dose time-points with the most beneficial effects being observed for the lower two doses. Other than this the most robust effects were observed for the 200 mg dose, (although all doses influenced cognition to some degree). Immediate Word Recall accuracy and numeric working memory speed were differentially improved by the 200 mg dose at all three post-dose time-points. Conversely alphabetic working memory speed was improved at all three time-points following the 100 and 400 mg doses. Choice Reaction Time accuracy was significantly improved following both 100 and 400 mg at 1 h post-administration. At 3 h post-administration 100 mg continued to improve performance, and at 6 h post-administration choice reaction time accuracy was significantly improved by all treatment doses compared with placebo. The differential dose- and time- effects may indicate differential sensitivity of biological systems to specific neural substrates affected by ginseng and its constituent ginsenosides.
Regarding mood effects, compared to placebo 100 mg Cereboost™ improved self-rated calmness at 3 h and 6 h following administration. As this is the first study to assess the effects of this extract on mood, few comparisons can be drawn with the existing research. However a number of studies have assessed the effect of P. ginseng on mood using the same Bond Lader mood scale as the present study (Kennedy et al. 2001a, b, 2002). Both 200 and 400 mg reduced alertness at 6 h (Kennedy et al. 2001a) and calmness at 2.5 and 4 h (Reay et al. 2010). Other studies have also reported reduced mental fatigue during sustained mental effort following P. ginseng in healthy young individuals (Reay et al. 2005, 2006). In the present study 100 mg Cereboost™ was associated with increased calmness ratings at later time-points. Previous research in rodents has shown that Ginseng saponins and Ginsenoside Rb1 inhibit the stress-induced increases in plasma corticosterone (Kim et al. 1998a,b; Luo et al. 1993) later found that this inhibitory action of Ginseng was blocked by a co-administered inhibitor of nitric oxide (NO) synthase, suggesting that ginsenosides may modulate the stress-induced hypothalamus–pituitary–adrenal response by inducing NO production in the brain. Clearly at present this interpretation is purely speculative (indeed we found no effect of ginseng on self-ratings of stress), but may merit further investigation.
The mechanism(s) by which extracts of Ginseng or individual components derived from Ginseng exert their effects on cognition are not known. A number of potentially complementary effects may be involved. For example neuroprotective effects of ginsenosides have been demonstrated in vitro (Rudakewich et al. 2001) and in vivo. Such effects include protection of hippocampal CA1 neurons, reduction of infarct area (Zhang and Liu 1996), reduced lipid peroxidation, scavenging of oxygen free radicals (Chen et al. 1987) and preservation of local cerebral glucose utilisation (Choi et al. 1996) following ischaemia in rodents. Increased NO synthesis has been proposed to underlie these neuroprotective effects. The enzyme NO synthase has been shown to be present throughout the brain with a particular prevalence in the cerebellum and is reported to be involved in hippocampal LTP (Salemme et al. 1996) and general memory processes (Prast and Philippu 2001). Kennedy and Scholey (2003) note that it may be significant that following ginsenoside administration, the release of NO from endothelial cells has been shown to be specific to the Panaxatriol rather than the Panaxadiol ginsenosides (Kang et al. 1995). Jin et al. (1999) suggest that memory enhancing effects in rodents are restricted to extracts with a high ratio of Panaxatriol to Panaxadiol ginsenoside content. Indeed the present study has observed substantial memory enhancement using the extract Cereboost™, which has a high Panaxatriol to Panaxadiol ginsenoside content (see Fig. 1).
While Ginseng and Ginseng saponins boast an array of neuroprotective effects it seems unlikely that these attributes would greatly benefit cognition acutely. It seems more likely that any neuroprotective effects might be more pronounced in chronic trauma and/or deficit populations. However the effects of Ginsengosides are not limited to neuroprotective effects. As well as increased NO-mediated blood flow (Kim et al. 1998b), ginsenosides can increase choline uptake (Zhang et al. 1990), acetylcholine release (Benishin et al. 1991) and monoamine metabolism (Petkov 1990) all of which may contribute to acute positive neurocognitive effects.
The data presented here demonstrate enhancement effects of P. quinquefolius predominantly on working memory processes (Corsi blocks, and both numeric and alphabetic working memory). There is also some evidence of positive effects on short-term verbal declarative memory (immediate word recall) and attention (choice reaction time). Such findings tempt speculation about the neuroanatomical loci of these effects. As well as its well-documented role in long-term episodic memory (Eichenbaum and Cohen 2001), there is increasing evidence that the hippocampus may be involved in working memory (Axmacher et al. 2009) particularly, as in the tests used here, when multiple items are processed. However, if the effects here are largely driven by hippocampal activation we might expect a more pronounced effect on secondary memory above the enhancement of immediate word recall. Working and short-term memory systems are thought be localized to parietal, hippocampal and pre-frontal cerebral circuitry, with the pre-frontal cortex dealing with higher order working memory/executive functions including manipulating working memory (Gabrieli et al. 1998; Goldman-Rakic et al. 1996). Currently, there is a paucity of data regarding brain areas involved in the cognition-enhancing of effects of Ginseng, we hope that neuroimaging studies will soon reveal which areas are activated by Ginseng during cognitive processing.
It has been well documented that the cholinergic pathways projecting to the cerebral cortex and hippocampus play a key role in learning and memory. It has also been argued that the cholinergic system is a specific target for cognitively enhancing agents (Giovannini et al. 1995). A number of studies have identified cholinergic properties associated with isolated ginsenosides. A direct interaction between Rg2 and nicotinic receptor subtypes has been observed (Sala et al. 2002). Moreover Benshin (1992) demonstrated modulation by Rb1 of acetylcholine release and reuptake, along with a number of choline uptake sites in the hippocampus, and to a lesser extent, the cortex. Both ginsenosides Rg1 (Zhang et al. 1990) and Rb1 (Salim et al. 1997; Zhang et al. 1990) have also been shown to increase choline acetyltransferase levels in the rat brain. Scopolamine-induced deficits are attenuated by P. quinquefolius in rodents (Sloley et al. 1999). Protection against scopolamine-induced amnesia by P. quinquefolius was most evident in trials where animals were required to remember the task learned the previous day. In the same study, it was observed that Ginseng increased choline uptake into synaptosomes prepared from rat brain. In the human brain crude extracts of P. ginseng exhibited an affinity for both nicotinic and muscarinic receptors in cerebral cortex membrane (Lewis et al. 1999). As discussed previously, the P. quinquefolius extract profile has 2–3 times the ginsenoside content than the more commonly researched P. ginseng, with the highest expression of Rb1 and Re. Thus the cholinergic system is one potential central mechanism of action on the enhancement of memory by Cereboost™. On the other hand, the RVIP task used here may been regarded as a prototypical cholinergic task, and we found no effect of the ginseng extract on this measure.
A second aim of the study was to assess the acute effects of P. quinquefolius on glucoregulation on young healthy adults. The results of the present study show that, at least at the dosages used here American ginseng has no effect on blood glucose levels. Vuksan et al. (2000a) previously observed that 300 mg P. quinquefolius lowered blood glucose levels during a glucose challenge in both healthy and diabetic subjects. In that study, however, Ginseng administration lowered blood glucose levels only when taken 40 min prior to the glucose challenge in healthy individuals. Conversely, diabetic subjects experienced a fall in blood glucose either whether Ginseng was administered 40 min prior to, or concurrently with, the glucose challenge, suggesting that this effect is somewhat more robust in diabetic subjects (or at least that the temporal aspects are less important in that population). It is also noteworthy that in the Vuksan study there was a substantial age difference between the ‘healthy’ individuals (34 ± 7 year) and the diabetic individuals (62 ± 7 year) raising the possibility that the effect of P. quinquefolius on blood glucoregulation is more robust in older individuals. In the present study the ‘healthy’ individuals were in their mid-20s, somewhat younger than those in the study by Vuksan's group. Additionally the largest body of research assessing the effects of Ginseng on peripheral circulating blood glucose in humans has tended to investigate chronic administration (Vuksan et al. 2008; Vuksan et al. 2000b). It would be of great interest to evaluate glucoregulatory properties would emerge with chronic dosing using cereboost™, and any relationship with neurocognitive effects of such a regimen.
It is worth noting that these findings should be treated with a degree of caution. Firstly, this is the first investigation into the neurocognitive effects of American ginseng. Clearly the study needs at least partial replication possibly with more focus on specific working memory processes. Secondly, given the exploratory nature of the study, no adjustment was made for multiple comparisons (although we did implement a number of safeguards against conflated Type 1 error). This follows the recommendations of Keppel (1991) and is consistent with analyses utilised in a series of similar, acute dose-ranging studies (Haskell et al. 2007; Kennedy et al. 2001a, b, 2002; Kennedy et al. 2003; Reay et al. 2005, 2006; Scholey et al. 2008; Tildesley et al. 2005). We are aware that adjusting the alpha level to allow for multiple comparisons would have yielded fewer significant findings. On the other hand this should be balanced against the observation that for the majority of outcomes where there were significant differences, these were at all three time-points (and for Alphabetic Working Memory and Corsi blocks for two and three doses, respectively) suggesting that they are unlikely to have arisen from Type 1 errors.
Overall the findings of the present study were the first to demonstrate cognitive and mood enhancement following Cereboost™ administration. Furthermore cognition-enhancing effects of the extract were observed across a range of cognitive modalities at a range of dosages. The lack of glycaemic effects suggest that these effects can occur independently of changes in blood glucose, at least in healthy younger populations. Further research is required assessing the neurocognitive effects of P. quinquefolius in other populations (e.g. older individuals and those with cognitive problems) as well as evaluating the neurocognitive effects of chronic administration.
Acknowledgement
This work was supported by a grant from Naturex Inc.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Abe K, Cho S, Kitagawa I, Nishiyama N, Saito H. Differential effects of ginsenoside Rb1 and malonylginsenoside Rb1 on long-term potentiation in the dentate gyrus of rats. Brain Res. 1994;649:7–11. doi: 10.1016/0006-8993(94)91042-1. [DOI] [PubMed] [Google Scholar]
- Ahern EP. The use of visual analogue scales in mood disorders: a critical review. J Psychiatr Res. 1997;31:569–579. doi: 10.1016/S0022-3956(97)00029-0. [DOI] [PubMed] [Google Scholar]
- Antony M, Bieling P, Cox B, Enns M, Swinson R. Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychol Assess. 1998;10:176–181. doi: 10.1037/1040-3590.10.2.176. [DOI] [Google Scholar]
- Axmacher N, Lenz S, Haupt S, Elger C, Fell J. Electrophysiological signature of working and long-term memory interaction in the human hippocampus. Eur J Neurosci. 2009;31:177–188. doi: 10.1111/j.1460-9568.2009.07041.x. [DOI] [PubMed] [Google Scholar]
- Benishin C. Actions of ginsenoside Rb1 on choline uptake in central cholinergic nerve endings. Neurochem Int. 1992;21:1–5. doi: 10.1016/0197-0186(92)90061-U. [DOI] [PubMed] [Google Scholar]
- Benishin CG, Lee R, Wang LCH, Liu HJ. Effects of ginsenoside Rb 1 on central cholinergic metabolism. Pharmacology. 1991;42:223–229. doi: 10.1159/000138801. [DOI] [PubMed] [Google Scholar]
- Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47:211–218. [Google Scholar]
- Chen X, Li Y, Deng H, Yang B, Li D, Shen N. Protective effects of ginsenosides on anoxia/reoxygenation of cultured rat myocytes and on reperfusion injuries against lipid peroxidation. Biomed Biochim Acta. 1987;46:S646. [PubMed] [Google Scholar]
- Choi S, Saji H, Iida Y, Magata Y, Yokoyama A. Ginseng pretreatment protects against transient global cerebral ischemia in the rat: measurement of local cerebral glucose utilization by [14C] deoxyglucose autoradiography. Biol Pharm Bull. 1996;19:644. doi: 10.1248/bpb.19.644. [DOI] [PubMed] [Google Scholar]
- Donohoe R, Benton D. Cognitive functioning is susceptible to the level of blood glucose. Psychopharmacology. 1999;145:378–385. doi: 10.1007/s002130051071. [DOI] [PubMed] [Google Scholar]
- Donohoe R, Benton D. Declining blood glucose levels after a cognitively demanding task predict subsequent memory. Nutr Neurosci. 1999;2:413–424. doi: 10.1080/1028415X.1999.11747295. [DOI] [PubMed] [Google Scholar]
- Durgnat J, Heuser J, Andrey D, Perrin C. Quality and safety assessment of ginseng extracts by determination of the contents of pesticides and metals. Food Addit Contam: Part A. 2005;22:1224–1230. doi: 10.1080/02652030500199439. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: memory systems of the brain. London, Uk: Oxford University Press; 2001. [Google Scholar]
- Gabrieli J, Poldrack R, Desmond J. The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci. 1998;95:906. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannini MG, Camilli F, Mundula A, Bianchi L, Colivicchi MA, Pepeu G. Differential regulation by N-methyl–aspartate and non-N-methyl–aspartate receptors of acetylcholine release from the rat striatum in vivo. Neuroscience. 1995;65:409–415. doi: 10.1016/0306-4522(94)00503-W. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P, Cools A, Srivastava K. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive [and Discussion] Philos Trans: Biol Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Harkey M, Henderson G, Gershwin M, Stern J, Hackman R. Variability in commercial ginseng products: an analysis of 25 preparations. Am J Clin Nutr. 2001;73:1101. doi: 10.1093/ajcn/73.6.1101. [DOI] [PubMed] [Google Scholar]
- Haskell C, Kennedy D, Wesnes K, Milne A, Scholey A. A double-blind, placebo-controlled, multi-dose evaluation of the acute behavioural effects of guaraná in humans. J Psychopharmacol Oxf Engl. 2007;21:65. doi: 10.1177/0269881106063815. [DOI] [PubMed] [Google Scholar]
- Hiai S, Yokoyama H, Oura H, Kawashima Y. Evaluation of corticosterone secretion-inducing activities of ginsenosides and their prosapogenins and sapogenins. Chem Pharm Bull. 1983;31:168. doi: 10.1248/cpb.31.168. [DOI] [PubMed] [Google Scholar]
- Jin S, Park J, Nam K, Park S, Jung N. Korean red ginseng saponins with low ratios of protopanaxadiol and protopanaxatriol saponin improve scopolamine-induced learning disability and spatial working memory in mice. J Ethnopharmacol. 1999;66:123–129. doi: 10.1016/S0378-8741(98)00190-1. [DOI] [PubMed] [Google Scholar]
- Kang S, Schini-Kerth V, Kim N. Ginsenosides of the protopanaxatriol group cause endothelium-dependent relaxation in the rat aorta. Life Sci. 1995;56:1577–1586. doi: 10.1016/0024-3205(95)00124-O. [DOI] [PubMed] [Google Scholar]
- Kennedy D, Scholey A. Glucose administration, heart rate and cognitive performance: effects of increasing mental effort. Psychopharmacology. 2000;149:63–71. doi: 10.1007/s002139900335. [DOI] [PubMed] [Google Scholar]
- Kennedy D, Scholey A. Ginseng: potential for the enhancement of cognitive performance and mood. Pharmacol Biochem Behav. 2003;75:687–700. doi: 10.1016/S0091-3057(03)00126-6. [DOI] [PubMed] [Google Scholar]
- Kennedy D, Scholey A, Wesnes K. Differential, dose dependent changes in cognitive performance following acute administration of a Ginkgo biloba/Panax ginseng combination to healthy young volunteers. Nutr Neurosci. 2001;4:399–412. doi: 10.1080/1028415x.2001.11747376. [DOI] [PubMed] [Google Scholar]
- Kennedy D, Scholey A, Wesnes K. Dose dependent changes in cognitive performance and mood following acute administration of ginseng to healthy young volunteers. Nutr Neurosci. 2001;4:295–310. doi: 10.1080/1028415x.2001.11747370. [DOI] [PubMed] [Google Scholar]
- Kennedy D, Scholey A, Wesnes K. Modulation of cognition and mood following administration of single doses of Ginkgo biloba, ginseng, and a ginkgo/ginseng combination to healthy young adults. Physiol Behav. 2002;75:739–752. doi: 10.1016/S0031-9384(02)00665-0. [DOI] [PubMed] [Google Scholar]
- Kennedy D, Wake G, Savelev S, Tildesley N, Perry E, Wesnes K, Scholey A. Modulation of mood and cognitive performance following acute administration of single doses of Melissa officinalis (Lemon balm) with human CNS nicotinic and muscarinic receptor-binding properties. Neuropsychopharmacology. 2003;28:1871–1881. doi: 10.1038/sj.npp.1300230. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and analysis. A researcher’s handbook. 3. Englewood Cliffs, NJ: Prentice-Hall; 1991. [Google Scholar]
- Kim D, Jung J, Suh H, Huh S, Min S, Son B, Park J, Kim N, Kim Y, Song D. Inhibition of stress-induced plasma corticosterone levels by ginsenosides in mice: involvement of nitric oxide. NeuroReport. 1998;9:2261. doi: 10.1097/00001756-199807130-00021. [DOI] [PubMed] [Google Scholar]
- Kim H, Hong Y, Oh K, Seong Y, Rheu H, Cho D, Oh S, Park W, Jang C. Inhibition by ginsenosides Rb1 and Rg1 of methamphetamine-induced hyperactivity, conditioned place preference and postsynaptic dopamine receptor supersensitivity in mice. Gen Pharmacol. 1998;30:783–789. doi: 10.1016/S0306-3623(97)00330-3. [DOI] [PubMed] [Google Scholar]
- Lewis R, Wake G, Court G, Court JA, Pickering AT, Kim YC, Perry EK. Non-ginsenoside nicotinic activity in ginseng species. Phytother Res. 1999;13:59–64. doi: 10.1002/(SICI)1099-1573(199902)13:1<59::AID-PTR423>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Lezak M, Loring D. Neuropsychological assessment. New York: Oxford University Press; 2004. [Google Scholar]
- Lezak M, Howieson D, Loring D. Neuropsychological assessment. New York: Oxford University Press; 1995. [Google Scholar]
- Lovibond P, Lovibond S. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the beck depression and anxiety inventories. Behav Res Ther. 1995;33:335–343. doi: 10.1016/0005-7967(94)00075-U. [DOI] [PubMed] [Google Scholar]
- Luo Y, Cheng X, Yuan W. Effects of ginseng root saponins and ginsenoside Rb1 on immunity in cold water swim stress mice and rats. Zhongguo Yao Li Xue Bao. 1993;14:401–404. [PubMed] [Google Scholar]
- MacLeod C. The Stroop task: the“gold standard” of attentional measures. J Exp Psychol Gen. 1992;121:12–14. doi: 10.1037/0096-3445.121.1.12. [DOI] [Google Scholar]
- Martinez B, Staba E. The physiological effects of Aralia, Panax and Eleutherococcus on exercised rats. Jpn J Pharmacol. 1984;35:79. doi: 10.1254/jjp.35.79. [DOI] [PubMed] [Google Scholar]
- Matthews D, Holman R, Bown E, Steemson J, Watson A, Hughes S, Scott D. Pen-sized digital 30-second blood glucose meter. Lancet. 1987;1:778. doi: 10.1016/S0140-6736(87)92802-9. [DOI] [PubMed] [Google Scholar]
- Oshima Y, Sato K, Hikino H. Isolation and hypoglycemic activity of quinquefolans A, B, and C, glycans of Panax quinquefolium roots. J Nat Prod. 1987;50:188–190. doi: 10.1021/np50050a010. [DOI] [PubMed] [Google Scholar]
- Petkov V. Ginseng as a remedy regulating aging process in brain (experiments of rats) In: Shibata YOaHS S, editor. Recent advances in ginseng study. Tokyo: Hirokawa; 1990. pp. 83–98. [Google Scholar]
- Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog Neurobiol. 2001;64:51–68. doi: 10.1016/S0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- Reay J, Kennedy D, Scholey A. Single doses of Panax ginseng (G115) reduce blood glucose levels and improve cognitive performance during sustained mental activity. J Psychopharmacol. 2005;19:357. doi: 10.1177/0269881105053286. [DOI] [PubMed] [Google Scholar]
- Reay J, Kennedy D, Scholey A. Effects of Panax ginseng, consumed with and without glucose, on blood glucose levels and cognitive performance during sustained’mentally demanding’tasks. J Psychopharmacol. 2006;20:771. doi: 10.1177/0269881106061516. [DOI] [PubMed] [Google Scholar]
- Reay J, Scholey AB, Kennedy DO (2010) Panax ginseng (G115) improves aspects of working memory performance and subjective ratings of calmness in healthy young adults. Hum Psychopharmacol: Clin Exp in press doi:10.1002/hup.1138 [DOI] [PubMed]
- Reich E, Schibli A. High-performance thin-layer chromatography for the analysis of medicinal plants. New York: Thieme Medical Pub; 2006. [Google Scholar]
- Rudakewich M, Ba F, Benishin C. Neurotrophic and neuroprotective actions of ginsenosides Rb 1 and Rg 1. Planta Med. 2001;67:533–537. doi: 10.1055/s-2001-16488. [DOI] [PubMed] [Google Scholar]
- Sala F, Mulet J, Choi S, Jung S, Nah S, Rhim H, Valor L, Criado M, Sala S. Effects of ginsenoside Rg2 on human neuronal nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2002;301:1052. doi: 10.1124/jpet.301.3.1052. [DOI] [PubMed] [Google Scholar]
- Salemme E, Diano S, Maharajan P, Maharajan V. Nitric oxide, a neuronal messenger. Riv Biol. 1996;89(1):87–107. [PubMed] [Google Scholar]
- Salim K, McEwen B, Chao H. Ginsenoside Rb1 regulates ChAT, NGF and trkA mRNA expression in the rat brain. Mol Brain Res. 1997;47:177–182. doi: 10.1016/S0169-328X(97)00042-9. [DOI] [PubMed] [Google Scholar]
- Scholey A, Kennedy D. Acute, dose-dependent cognitive effects of Ginkgo biloba, Panax ginseng and their combination in healthy young volunteers: differential interactions with cognitive demand. Hum Psychopharmacol: Clin Exp. 2002;17:35–44. doi: 10.1002/hup.352. [DOI] [PubMed] [Google Scholar]
- Scholey A, Harper S, Kennedy D. Cognitive demand and blood glucose. Physiol Behav. 2001;73:585–592. doi: 10.1016/S0031-9384(01)00476-0. [DOI] [PubMed] [Google Scholar]
- Scholey A, Kennedy D, Wesnes K. The psychopharmacology of herbal extracts: issues and challenges. Psychopharmacology. 2005;179:705–707. doi: 10.1007/s00213-004-2062-9. [DOI] [PubMed] [Google Scholar]
- Scholey A, Tildesley N, Ballard C, Wesnes K, Tasker A, Perry E, Kennedy D. An extract of Salvia (sage) with anticholinesterase properties improves memory and attention in healthy older volunteers. Psychopharmacology. 2008;198:127–139. doi: 10.1007/s00213-008-1101-3. [DOI] [PubMed] [Google Scholar]
- Sloley B, Pang P, Huang B, Ba F, Li F, Benishin C, Greenshaw A, Shan J. American ginseng extract reduces scopolamine-induced amnesia in a spatial learning task. J Psychiatry Neurosci. 1999;24:442. [PMC free article] [PubMed] [Google Scholar]
- Soldati F, Sticher O. HPLC separation and quantitative determination of ginsenosides from Panax ginseng, Panax quinquefolium and from ginseng drug preparations. Planta Med. 1980;39:348–357. doi: 10.1055/s-2008-1074929. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Jacobs G, Russell S, Crane R. Assessment of anger: the state-trait anger scale. Adv Pers Assess. 1983;2:159–187. [Google Scholar]
- Stough C, Lloyd J, Clarke J, Downey L, Hutchison C, Rodgers T, Nathan P, Ltd S. The chronic effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy human subjects. Psychopharmacology (Berl) 2001;156:481–484. doi: 10.1007/s002130100815. [DOI] [PubMed] [Google Scholar]
- Stroop J. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. doi: 10.1037/h0054651. [DOI] [Google Scholar]
- Tachikawa E, Kudo K, Harada K, Kashimoto T, Miyate Y, Kakizaki A, Takahashi E. Effects of Ginseng saponins on responses induced by varoius receptor stimuli. Eur J Pharmacol. 1999;369:23–32. doi: 10.1016/S0014-2999(99)00043-6. [DOI] [PubMed] [Google Scholar]
- Tildesley N, Kennedy D, Perry E, Ballard C, Wesnes K, Scholey A. Positive modulation of mood and cognitive performance following administration of acute doses of Salvia lavandulaefolia essential oil to healthy young volunteers. Physiol Behav. 2005;83:699–709. doi: 10.1016/j.physbeh.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Tsang D, Yeung H, Tso W, Peck H. Ginseng saponins: influence on neurotransmitter uptake in rat brain synaptosomes. Planta Med. 1985;3:221–224. doi: 10.1055/s-2007-969463. [DOI] [PubMed] [Google Scholar]
- Vuksan V, Sievenpiper J, Koo V, Francis T, Beljan-Zdravkovic U, Xu Z, Vidgen E. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Arch Intern Med. 2000;160:1009. doi: 10.1001/archinte.160.7.1009. [DOI] [PubMed] [Google Scholar]
- Vuksan V, Stavro MP, Sievenpiper JL, et al. Similar postprandial glycemic reductions with escalation of dose and administration time of American ginseng in type 2 diabetes. Diab Care. 2000;23:1221–1226. doi: 10.2337/diacare.23.9.1221. [DOI] [PubMed] [Google Scholar]
- Vuksan V, Sievenpiper J, Wong J, Xu Z, Beljan-Zdravkovic U, Arnason J, Assinewe V, Stavro M, Jenkins A, Leiter L. American ginseng (Panax quinquefolius L.) attenuates postprandial glycemia in a time-dependent but not dose-dependent manner in healthy individuals. Am J Clin Nutr. 2001;73:753. doi: 10.1093/ajcn/73.4.753. [DOI] [PubMed] [Google Scholar]
- Vuksan V, Mi-Kyung S, John LS, Stavro PM, Alexandra LJ, Di Marco B, Kwang-Seung L, Lawrence AL, Ki Yeul N, John TA, Melody C, Asima N. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutrition, Metabolism, and Cardiovascular Diseases: NMCD. 2008;18:46–56. doi: 10.1016/j.numecd.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Warburton D. Effects of scopolamine and nicotine on human rapid information processing performance. Psychopharmacology. 1984;82:147–150. doi: 10.1007/BF00427761. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu T. Influences of ginsenosides Rb1 and Rg1 on reversible focal brain ischemia in rats. Zhongguo Yao Li Xue Bao. 1996;17:44–48. [PubMed] [Google Scholar]
- Zhang J, Qu Z, Liu Y, Deng H. Preliminary study on antiamnesic mechanism of ginsenosides Rg1 and Rb1. China Med J. 1990;103:932–938. [PubMed] [Google Scholar]
- Zhu S, Zou K, Fushimi H, Cai S, Komatsu K. Comparative study on triterpene saponins of Ginseng drugs. Planta Med. 2004;70:666–677. doi: 10.1055/s-2004-827192. [DOI] [PubMed] [Google Scholar]