Abstract
Previous studies of interference competition have shown an asymmetric effect on intake rate of foragers on clumped resources, with only subordinate individuals suffering. However, the food distributions in these studies were uniform or highly clumped, whereas in many field situations, food aggregation is intermediate. Here we investigated whether food distribution (i.e., uniform, slightly clumped, and highly clumped) affects the behavioral response of mallards foraging alone or competing with another. Although the amount of food was the same in all distributions, the mallards reached higher intake rates, visited fewer patches, and showed longer average feeding times in the highly clumped distribution. Competing mallards had lower intake rates on the slightly clumped than on the uniform or highly clumped food distributions. Subordinates generally visited more patches and had shorter feeding times per patch, but their intake rates were not significantly lower than those of dominants. Therefore, we propose that subordinates do not necessarily suffer from interference competition in terms of intake rate, but do suffer higher search costs. In addition, although dominants had significantly higher average feeding times on the best quality patches of the highly clumped food distribution, such an effect was not found in the slightly clumped distribution. These findings indicate that in environments where food is aggregated to a lesser extent, monopolization is not the best strategy for dominants. Our results suggest that interference experiments should use food distributions that resemble the natural situation animals are faced with in the field.
Keywords: Anas platyrhynchos, Feeding time, Intake rate, Intraspecific competition, Search cost, Spatial autocorrelation of food density
Introduction
Animals have to continuously make decisions on what, where, and how much to eat. Their optimal decision is likely to be affected by the presence of other individuals with similar preferences, leading to competition for limited food resources. When a number of individuals utilize (and deplete) common resources, exploitative competition occurs (Krebs 2001). However, individuals might also hinder each other's intake rate, regardless of the resource depletion, through interference competition. This latter form of competition was defined as the decline in intake rate due to the mere presence of competitors (Goss-Custard 1980).
Interference competition can be active, arising from overt aggression and displacement. Competitors may also avoid one another to reduce the number of aggressive encounters (Cresswell 1997), and thus spend less time foraging. Eventually, when interference becomes too intense, some individuals may be forced into suboptimal habitats (Goss-Custard 1980). Another form of active interference competition is kleptoparasitism, where one individual steals food or takes over good foraging spots from another (e.g., Ens and Goss-Custard 1984; Monaghan and Metcalfe 1985; Triplet et al. 1999). Interference competition can also be passive, when foragers monitor their competitors and consequently get distracted from food searching or face a reduced foraging time (Ens and Goss-Custard 1984; Goss-Custard et al. 1982; Stillman et al. 1997). An important aspect of interference competition is that it is also “immediately reversible” (Goss-Custard 1980): when the density of competitors decreases, the negative effects of interference will often immediately be eliminated.
It has been a common practice to evaluate interference competition by varying forager densities (e.g., Ruxton and Moody 1997; Sutherland 1983; Vahl et al. 2005b; van Gils and Piersma 2004). In addition, numerous authors have shown that individuals differing in their dominance status are differentially affected by interference competition (Alonso et al. 1997; Hupp et al. 1996; Klaassen et al. 2006a; Lendvai et al. 2006; Rowcliffe et al. 2004; Stahl et al. 2001; Stillman et al. 2002). In most studies, interference competition had little, if any, effect on dominants, and could even lead to increased intake rate. The observed increased intake rates of dominants could be a result of the higher detection rates in groups of foraging animals of the most profitable patches compared to an individual forager (Stahl et al. 2001). Subsequently, dominants can easily monopolize these patches (Bautista et al. 1995; Monaghan and Metcalfe 1985; Taillon and Cote 2007). In these cases, subordinates are either displaced or simply avoid the dominants. Therefore, they have to cope with longer searching times (Alonso et al. 1997; Klaassen et al. 2006a; Rowcliffe et al. 2004; Stahl et al. 2001) and a reduced intake rate due to foraging in lower quality patches (Dolman 1995).
When interference is due to active displacement from high-quality feeding areas, food distribution can also have a detrimental effect on the level of interference competition. Vahl et al. (2005a) emphasized that the effects of interference competition are largely determined by the spatial distribution of prey. They highlighted that models of interference competition commonly ignore that in most natural systems food items occur in aggregations (Benhamou 1992; Fryxell et al. 2005; Kraan et al. 2009; Li and Reynolds 1995; Sparrow 1999; Spencer et al. 1994; Turchin and Kareiva 1989), and instead, models assume simple homogenous food distributions. However, in terms of food intake, there is little variation in interference competition in environments where food is homogenously distributed: one area is just as profitable as any other (Theimer 1987; Vahl et al. 2005a).
In contrast, if food is clumped, interference competition can strongly affect individual intake rate because dominant individuals are likely to monopolize the food patch (e.g., Monaghan and Metcalfe 1985; Theimer 1987; Vahl et al. 2005a). Studies on the effect of spatial clumping on interference competition have typically used clumped or concentrated food distributions (basically a single clump (Theimer 1987; Vahl et al. 2005a)). However, many naturally occurring food items are neither completely spread nor fully concentrated. Typically, their distribution takes an intermediate form, characterized by positive spatial autocorrelation: high-density patches will tend to be next to other high-density patches and low-density patches next to low-density patches (Klaassen and Nolet 2008; Klaassen et al. 2006b; Kolasa and Rollo 1991; Kotliar and Wiens 1990; Kraan et al. 2009; Li and Reynolds 1995; Nolet and Mooij 2002).
We aimed to experimentally compare the effects of interference competition in a design with an intermediate food aggregation (henceforth, “slightly clumped” (SC)) with designs where food is distributed homogeneously (“uniform” (UN)) or concentrated (“highly clumped” (HC)). We measured food intake, mean feeding time per patch, and the number of visited patches of female mallards (Anas platyrhynchos) in trials where they were foraging alone or with a competitor of either a relatively lower or higher dominance status.
We expected the effect of interference competition to vary among the tested food distributions, from no effect in the homogeneous distribution to the strongest effect in the highly clumped food distribution, with the effect in the slightly clumped distribution being somewhere intermediate. In addition, we hypothesized that the dominant bird would take control over the best quality patches (Bautista et al. 1995; Monaghan and Metcalfe 1985) and consequently reach a higher intake rate than the subordinate (Dolman 1995). However, we expected that subordinates would suffer to a lesser degree in the slightly clumped distribution, because in that environment monopolization of the best foraging spots by dominants would only result in a small advantage.
Material and methods
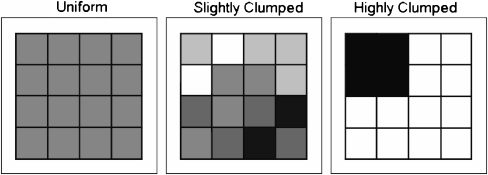
The trials were carried out in the waterfowl experimental facility of the NIOO-KNAW in Nieuwersluis. Sixteen trays (measuring 30 × 30 × 30 cm), forming a continuous foraging area of 1.44 m2, were placed at the bottom of a basin of 2 × 2 m, with water depth set at 30 cm above the sediment. Wheat grains were used as food items, covered with a layer of sand to avoid visual detection. By manipulating food densities in the individual trays, three different food distributions were created. All food distributions contained the same total amount of seeds (1,200 seeds; 61.7 ± 3.5 g). For the uniform food distribution (Fig. 1), a homogeneous environment was created by filling all 16 trays with an intermediate amount of seeds (75 seeds per tray). The SC food distribution (Fig. 1) consisted of five different food densities ranging from 25 seeds per tray (n = 2), through 50 (n = 4), 75 (n = 4) and 100 (n = 4) seeds per tray to 125 seeds per tray (n = 2) with a positive spatial autocorrelation between food densities. For the degree of clumping, the spatial distribution of fennel pondweed (Potamogeton pectinatus) tubers was used as a template (see Fig. 5b in Klaassen and Nolet 2008). The highly clumped distribution (Fig. 1) contained a clump of four trays with a very high food density (225 seeds per tray) and 12 with a low food density (25 seeds per tray). Special care was taken that birds could not learn the position of the best patches in the clumped distributions. For each trial in the SC distribution, a different spatial configuration was taken from the field measurements (Nolet et al. 2006). The position of the food clump in the HC distribution was rotated daily in a random order of 90°, 180°, or 270°.
Fig. 1.
The uniform (left 75 seeds per tray) food distribution and configurations for the slightly clumped (middle 25, 50, 75, 100, and 125 seeds per tray) and highly clumped (right 25 or 225 seeds per tray) food distributions. Color shades represent different food densities
We used mallards (>1-year old) for the experiment. Because the focus was on the effects of interference competition and dominance on foraging decisions, we used only females to eliminate potential mate competition. Nine birds were purchased from a breeder (Kooij and Sons Waterfowl Breeding Farm, 't Zand, The Netherlands), but they were all kept at our waterfowl facility for at least a year prior to the experiment. The animals were housed in an aviary measuring 10 × 6 m with access to a large pond and were only moved to the experimental room immediately prior to the trials.
In order to familiarize birds with the experimental facilities and procedure, they were trained for 4 weeks prior to the experiment and for 1 week during the experiment before switching to a new food distribution (see below). Three of the birds regularly displayed stereotype behavior of stress during the training trials (i.e., continuously swimming back and forth along the edge of the basin), and hence were not included in the experiment. The six remaining birds were fitted with different color leg bands to allow individual recognition. During the training and the experiment, the birds had access to food only during the trials in the morning hours and for 1 h in the afternoon in the aviary.
Prior to the experiment, dominance relations were determined while observing priority of access to the feeding tray after at least 8 h of starvation (cf. Syme 1974; Wagner and Gauthreaux 1990), verifying a linear dominance hierarchy in mallards (Poisbleau et al. 2006). The scoring of dominance was repeated four times throughout the experimental period, confirming that the rank order of the birds remained stable during the experiment.
Experimental trials were carried out in February and March 2006. Trials were done in one-bird designs (single) with each mallard being used once and in two-bird designs (pair), where each combination of individuals was tested. In order to minimize potential effects of food depletion, the foraging trials lasted only 120 s from when (one of) the mallard(s) started foraging. All the trials took place in the morning, between 0800 and 1300 hours. The trial sequence and the selection of individual ducks for the trials were randomized, with the restriction that individuals were used in only one trial per day. The experiment consisted of 21 trials (i.e., six single trials and 15 combinations of pairs) for each food distribution (i.e., 63 trials in total). However, mallards in two single trials and in five-paired trials did not eat, reducing the sample size to 56.
Mallards are highly capable of learning the food distribution they are foraging in (Klaassen et al. 2007). As we aimed to study the effects of interference competition while foraging in a certain food distribution and not whilst learning a food distribution, we applied the three distributions sequentially, and not randomly. First the UN, then the HC, and finally the SC distribution was tested, with 1 week of training trials in between to allow for the learning of new food distributions. The trials were filmed with a video camera (Panasonic NV-GS15) positioned above the basin. Videos were analyzed using Observer 5.0 (Noldus Information Technology bv.).
After a trial, all trays were removed from the basin and the sediment was sieved to collect the remaining wheat grains. Wheat grains were counted, and the number of seeds consumed per patch (per individual) was divided by 120 s to calculate intake rates per second over the whole trial. Functional responses measured in individual training trials on the slightly clumped distribution revealed that the instantaneous intake rates (i.e., amount of food eaten in a certain foraging time) per food density did not significantly differ among individuals (F 17,18 = 0.59, p = 0.86; Table 1). Therefore, when both individuals ate from the same tray (29% of the cases), the total number of seeds eaten from a tray was divided in proportion to the feeding time of each individual on that tray. The final intake rate (seeds s−1) was subjected to a square root transformation to obtain normality. The number of patches visited and the mean feeding time (i.e., time with head under water; s) per patch were analyzed from the videos. These results were subjected to ln-transformations to reach normality.
Table 1.
Mean (SD) instantaneous intake rate of the six individuals (rows indicated by the codes of the individuals) at different food densities (columns)
| 25 | 50 | 75 | 100 | 125 | |
|---|---|---|---|---|---|
| BM | 0.00 | 0.39 (0.18) | 0.83 (0.15) | 1.62 (0.45) | 2.03 (0.71) |
| LG | 0.00 | 0.76 (0.05) | 1.40 (0.48) | 2.02 (1.93) | 2.99 (0.81) |
| LR | 0.00 | 0.32 (0.03) | 0.41 (0.13) | 1.24 (0.38) | 1.70 (0.34) |
| RB | 0.00 | 0.61 (0.01) | 1.14 (0.39) | 1.25 (0.31) | 1.29 (0.49) |
| RM | 0.20 (0.04) | 0.57 (0.33) | 1.43 (0.43) | 1.03 (0.47) | 1.06 (0.93) |
| RR | 0.00 | 0.60 (0.12) | 1.10 (0.52) | 1.12 (0.03) | 1.44 (0.51) |
Two full-factorial Generalized Linear Models (GLM) were used to test the effect of the interaction of food distribution (i.e., UN, SC, and HC) with number of foragers (i.e., single or pair trials; GLM1) and with dominance status (i.e., single, subordinate, dominant; GLM2) on intake rate, mean feeding time per patch and number of visited patches. Type III sums of squares of the software Statistica 8.0 (Statsoft, Inc. 1984–2008) were applied, which compares least square means, correcting for unequal sample sizes. Post hoc tests were conducted using HSD for unequal sample sizes.
Results
Intake rate
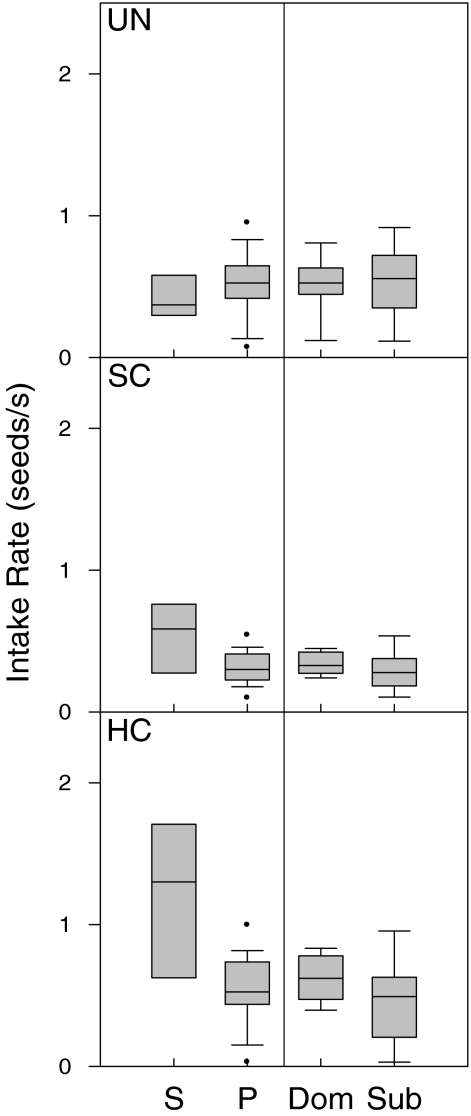
The effect of the number of foragers on intake rate varied with food distribution (GLM1 interaction term: F 2,96 = 8.4, p = 0.0004). The post hoc test revealed that this was primarily because birds in single-trials feeding in the highly clumped distribution reached higher intake rates than in any other trial types (all p = 0.001; Fig. 2). In addition, pairs in the slightly clumped distribution reached lower intake rates than in the other two distributions (both p < 0.05; Fig. 2).
Fig. 2.
Intake rate (seeds s−1) for all food distributions (uniform (UN), slightly clumped (SC), and highly clumped (HC)). Mallards were foraging alone ((S) single trials) or together with a competitor ((P) paired trials). These latter results are also subdivided into dominants (Dom) and subordinates (Sub). Box plot shows median (line in box), interquartile range (box), 10th and 90th percentile (bars) and outliers (dots; data points outside the 10th and 90th percentiles)
Dominance differences between the paired birds did not affect food intake in any of the distributions, but single birds in the highly clumped food distribution reached higher intake rates than in any other case (GLM2: F 4, 93 = 4.9, p = 0.001; post hoc test: all p < 0.05; Fig. 2).
Number of visited patches
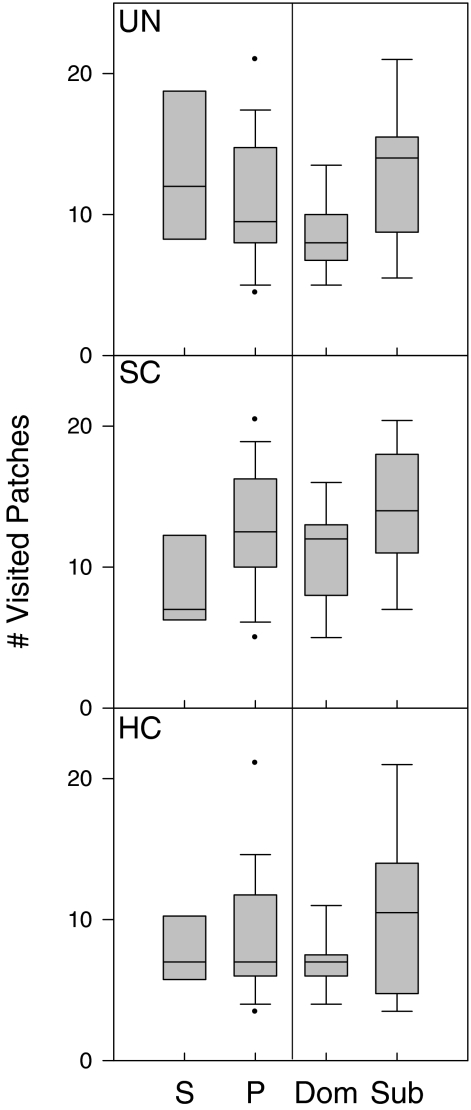
Although the number of foragers did not affect the number of visited patches differently in the three food distributions (GLM1 interaction term: F 2,96 = 1.97, p = 0.1; Fig. 3), there was a main difference between the food distributions (GLM1: F 2,96 = 3.33, p = 0.04): birds in the highly clumped distribution visited significantly fewer patches than birds in the other food distributions (post hoc test: both p < 0.05; Fig. 3).
Fig. 3.
Number of visited patches for all food distributions (uniform (UN), slightly clumped (SC) and highly clumped (HC)). Mallards were foraging alone ((S) single trials) or together with a competitor ((P) paired trials). These latter results are also subdivided into dominants (Dom) and subordinates (Sub). Box plot shows median (line in box), interquartile range (box), 10th and 90th percentile (bars) and outliers (dots; data points outside the 10th and 90th percentiles)
Similarly, the interaction of food distribution with dominance was not significant (GLM2: F 4,93 = 1.14, p = 0.3; Fig. 3). However, the main effect of dominance was significant (GLM2: F 2,93 = 5.95, p = 0.004): subordinates generally visited more patches than dominants (post hoc test: p = 0.003; Fig. 3).
Feeding time
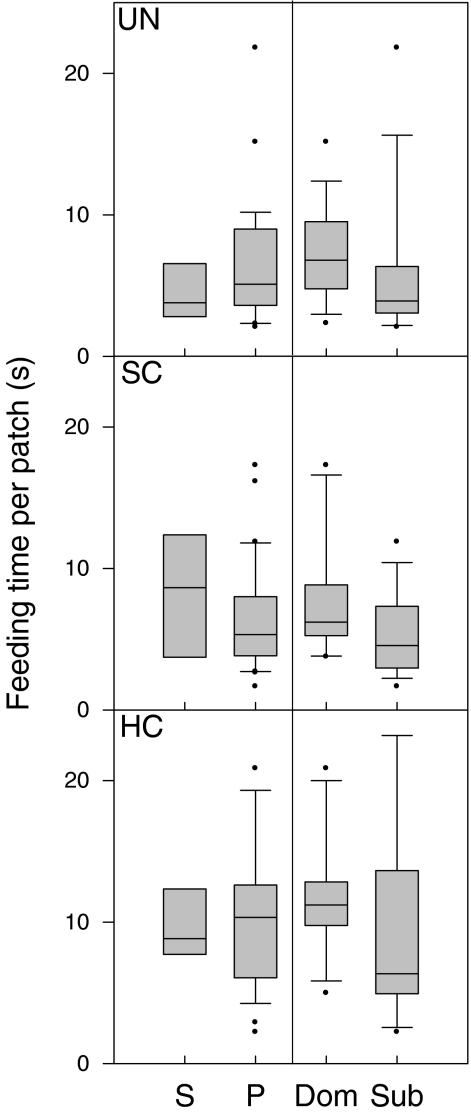
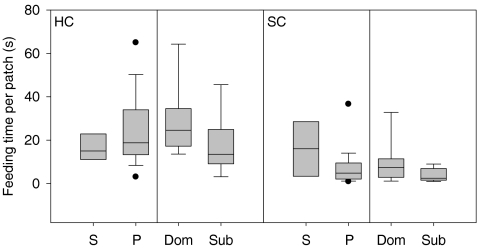
The number of competitors did not affect the average feeding time per patch differently in the three food distributions (GLM1 interaction term: F 2,96 = 1.15, p = 0.3), but there was a main difference between the food distributions (GLM1: F 2,96 = 6.04, p = 0.003): mallards showed longer average feeding times per patch in the highly clumped distribution (Fig. 4).
Fig. 4.
Average feeding times (s) per patch for all food distributions (uniform (UN), slightly clumped (SC) and highly clumped (HC)). Mallards were foraging alone ((S) single trials) or together with a competitor ((P) paired trials). These latter results are also subdivided into dominants (Dom) and subordinates (Sub). Box plot shows median (line in box), interquartile range (box), 10th and 90th percentile (bars) and outliers (dots; data points outside the 10th and 90th percentiles)
The interaction of food distribution with dominance level was not significant (GLM2: F 4,93 = 0.64, p = 0.6; Fig. 4). However, there was a main effect of dominance (GLM2: F 2,93 = 6.09, p = 0.003; Fig. 4), which was due to subordinates showing generally shorter average feeding times per patch compared to dominants (post hoc tests, both p < 0.01; Fig. 4).
An additional analysis revealed that in the highly clumped distribution, dominant individuals spent more time on the high-quality patches than subordinates (one-way ANOVA: F 2,31 = 3.53, post hoc test: p = 0.04; Fig. 5). However, the same effect was not found in the slightly clumped food distribution (one-way ANOVA: F 2,23 = 2.4, p = 0.1; Fig. 5).
Fig. 5.
Average feeding times (s) on the best quality patches for the highly clumped (HC) and slightly clumped (SC) food distributions. Mallards were foraging alone (S) or in pairs, subdivided into dominants (Dom) and subordinates (Sub). Box plot shows median (line in box), interquartile range (box), 10th and 90th percentile (bars) and outliers (dots; data points outside the 10th and 90th percentiles)
Discussion
Although total food resources were the same in all three tested food distributions, mallards reached higher intake rates, visited fewer patches, and showed longer average feeding times in the highly clumped distribution than in the other two distributions. Pairs of birds had lower intake rates in the slightly clumped distribution than in the other two distributions. Although we did not observe the expected higher intake rates for dominants compared with subordinates, subordinates did visit more patches and had shorter feeding times. Therefore, the disadvantage for subordinates of foraging with dominants may not necessarily be evident in intake rates, but they may suffer higher costs. In addition, dominants had significantly higher average feeding times on the best quality patches of the highly clumped food distribution, which was not evident in the slightly clumped distribution. This indicates that subordinates are more easily excluded from better foraging circumstances when food aggregation is higher.
Our results for the uniform food distribution, where all patches were equal in quality, match the finding from previous studies (Theimer 1987). In this distribution, no effect of interference competition was detected. In such environments, spending time competing with other individuals for food patches incurs only costs, since the achievable intake rates are the same throughout the environment. Therefore, dominants cannot profit from their higher status in the hierarchy through kleptoparasitism of patches (Theimer 1987; Vahl et al. 2005a).
The similar individual intake rates measured in single and paired trials on homogeneously distributed food provide support for the absence of a food depletion effect in our 2-min long trials. Otherwise, if food exploitation through exploitative competition would have played a role, the longer cumulative foraging time in the paired trials (i.e., 240 s due to two birds foraging) would have led to lower individual intake rates compared to the 120 s in the single trials, even in the absence of interference competition.
The foraging scale (i.e., the scale above which foragers respond to spatial heterogeneity, also called “grain”) was defined for mallards as 2 × 2 cm (Klaassen et al. 2006c). Therefore, we are confident that the mallards could distinguish between the three food distributions in our experiment. Consequently, we expected dominant birds to dominate the higher quality patches in the two clumped food distributions, and therefore spend relatively more time in these patches than subordinate foragers (e.g., Hupp et al. 1996; Klaassen et al. 2006a; Lendvai et al. 2006). However, dominant individuals did not have higher intake rates than subordinates in either of the clumped distributions (Fig. 2). This might simply be a consequence of the limited number of animals competing with each other. Alternatively, this effect may be because subordinates generally visited more patches (Fig. 3) and had shorter feeding times (Fig. 4) than dominants. This might have been the result of subordinates either passively avoiding dominants or being actively displaced (Smith et al. 2001; Stahl et al. 2001). Subsequently, this may result in longer searching times for a suitable patch, leaving less time for feeding (Belanger and Bedard 1992; Klaassen et al. 2006a; Vahl et al. 2005b). In this experiment, we focused on the effects of interference competition; therefore, we used very short trials to avoid food depletion. Using longer trials and assuming that individuals were foraging at their maximum instantaneous intake rate after food deprivation, the constantly shorter feeding times of subordinates would likely restrict them to reach comparable long-term intake rates to dominants.
This is especially likely for the highly clumped food distribution, where even in our short trials, dominants spent more feeding time on the high-quality patches than subordinates (Fig. 5). Animals can learn the distribution of the patches that they are foraging on (Benhamou 1992; Kotliar and Wiens 1990), including mallards (Klaassen et al. 2006c). Hence, in a highly clumped food distribution, individuals are expected to rapidly and accurately assess the quality of the patches: a good one and a bad one. Subsequently, in highly clumped distributions, once a high-quality patch is located, individuals do not need to continue searching or to fight for the other patches, as they are logically of lower quality (Vahl et al. 2005a). This results in animals showing a nearly omniscient behavior with shorter search times (i.e., lower number of visited patches, Fig. 3), longer average feeding times (Fig. 4) and highly increased intake rates (Fig. 2) compared to other food distributions.
In contrast, in food distributions where food items are less aggregated, dominants do not occupy the best quality patches longer than subordinates (Fig. 5). In such a situation, quality differences between patches are less pronounced (Li and Reynolds 1995; Nachman 2006; Nolet et al. 2006). Hence, even if subordinates are displaced from the best quality patches, they are not excluded from food altogether, which could be the case in a highly clumped food distribution. If a dominant is confident that it is occupying the best patch in the environment, it should not leave until it has depleted it to its final quitting intake rate (Charnov 1976; Nolet et al. 2006). However, in a slightly clumped food distribution, it becomes more difficult for individuals to instantaneously assess relative patch quality. This can have a large impact on the decision of dominants about whether to stay and monopolize the current patch, or to continue searching or to steal a patch from a subordinate. This uncertainty could result in lower intake rates in a slightly clumped food distribution compared to highly clumped or homogeneous food distributions for both the subordinate and dominant individuals (Fig. 2).
In experimental designs, the complexity of natural environments cannot always be fully incorporated. However, although our non-significant results have to be handled with caution, owing to the limited sample size, the three food distributions used in the experiment provide a good representation of the food aggregations possibly encountered by animals in the wild. Testing interference competition in a slightly clumped food distribution by using two animals is obviously a first step, and future research should aim to test these findings with a larger group.
The necessity of studying interference competition in different food distributions has been highlighted in previous studies (e.g., Monaghan and Metcalfe 1985; Theimer 1987; Vahl et al. 2007), but most studies commonly used uniform (homogeneous) and/or highly clumped food distributions (for review, see Vahl et al. 2005a). Many natural environments, however, contain intermediate distributions (Benhamou 1992; Gross et al. 1995; Kraan et al. 2009; Li and Reynolds 1995; Sparrow 1999). The strength of our experiment is that we also use a clumped distribution with a lower degree of food aggregation, based on the belowground distribution of fennel pondweed tubers (Nolet et al. 2006). This is a common food source of many herbivorous waterfowl species, not just in mallards (Anderson and Low 1976), but, for example, also in Bewick's swans (Cygnus bewickii) (Nolet and Drent 1998), whistling swans (Cygnus columbianus) and canvasbacks (Aythya valisineria) (Anderson and Low 1976). Occassionally, coots (Fulica atra), tufted ducks (Aythya fuligula), pochards (Aythya ferina) and goldeneyes (Bucephala clangula) may also rely on pondweed tubers (Hilt 2006). All these species are gregarious, at least partly during the year, and hence interference competition for food most likely takes place. In addition, similar slightly clumped spatial distribution has been observed in other belowground macrophytes, such as Vallisneria winter buds (Lovvorn and Gillingham 1996), in macrobenthic invertebrates (Kraan et al. 2009), the food source of numerous shorebird and crab species, and in aphids, the food source of several beetle species (Turchin and Kareiva 1989).
Our results show that the food distribution used in interference experiments is important. In less aggregated environments, uncertainty about the location of the best patches may restrict the dominant's monopolization of the food, and the intake of both dominants and subordinates. Therefore, together with findings from other interference studies, our results highlight the importance of using food distributions that mimic the natural situation that animals are faced with in the field, and not a hypothetical distribution that is easy to create and analyze in an experiment.
Acknowledgements
We thank Peter de Vries, Thijs de Boer, and Koos Swart for assistance during the experiment and Bart van Lith for taking care of the ducks before and after the experiment. Special thanks to Sarah Pryke for improving the readability of the paper. We greatly appreciate that Bruno Ens, Marcel Klaassen, Theunis Piersma, Charles Brown, Tatiana Czeschlik and two anonymous referees took time to comment on the manuscript. The experiment received approval by the KNAW animal experimentation committee (DEC license CL0504). The project was financed by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO grant 814.01.008). This is publication 4791 of the Netherlands Institute of Ecology (NIOO-KNAW).
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Alonso JC, Bautista LM, Alonso JA. Dominance and the dynamics of phenotype-limited distribution in common cranes. Behav Ecol Sociobiol. 1997;40:401–408. doi: 10.1007/s002650050356. [DOI] [Google Scholar]
- Anderson MG, Low JB. Use of sago pondweed by waterfowl on the delta marsh, Manitoba. J Wildl Manage. 1976;40:233–242. doi: 10.2307/3800420. [DOI] [Google Scholar]
- Bautista LM, Alonso JC, Alonso JA. A field test of ideal free distribution in flock-feeding common cranes. J Anim Ecol. 1995;64:747–757. doi: 10.2307/5853. [DOI] [Google Scholar]
- Belanger L, Bedard J. Flock composition and foraging behavior of greater snow geese (Chen caerulescens atlantica) Can J Zool-Rev Can Zool. 1992;70:2410–2415. doi: 10.1139/z92-323. [DOI] [Google Scholar]
- Benhamou S. Efficiency of area-concentrated searching behaviour in a continuous patchy environment. J Theor Biol. 1992;159:67–81. doi: 10.1016/S0022-5193(05)80768-4. [DOI] [Google Scholar]
- Charnov EL. Optimal foraging, the marginal value theorem. Theor Popul Biol. 1976;9:129–136. doi: 10.1016/0040-5809(76)90040-X. [DOI] [PubMed] [Google Scholar]
- Cresswell W. Interference competition at low competitor densities in blackbirds Turdus merula. J Anim Ecol. 1997;66:461–471. doi: 10.2307/5941. [DOI] [Google Scholar]
- Dolman PM. The intensity of interference varies with resource density: evidence from a field study with snow buntings, Plectrophenax nivalis. Oecologia. 1995;102:511–514. doi: 10.1007/BF00341364. [DOI] [PubMed] [Google Scholar]
- Ens BJ, Goss-Custard JD. Interference among oystercatchers Haematopus ostralegus, feeding on mussels, Mytilus edulis, on the Exe estuary. J Anim Ecol. 1984;53:217–231. doi: 10.2307/4353. [DOI] [Google Scholar]
- Fryxell JM, Wilmshurst JF, Sinclair ARE, Haydon DT, Holt RD, Abrams PA. Landscape scale, heterogeneity, and the viability of Serengeti grazers. Ecol Lett. 2005;8:328–335. doi: 10.1111/j.1461-0248.2005.00727.x. [DOI] [Google Scholar]
- Goss-Custard JD. Competition for food and interference among waders. Ardea. 1980;68:31–52. [Google Scholar]
- Goss-Custard JD, Durell SEAL, Ens BJ. Individual differences in aggressiveness and food stealing among wintering Oystercatchers, Haematopus ostralegus L. Anim Behav. 1982;30:917–928. doi: 10.1016/S0003-3472(82)80166-8. [DOI] [Google Scholar]
- Gross JE, Colleen Z, Hobbs NT, Spalinger DE. Movement rules for herbivores in spatially heterogeneous environments: responses to small scale pattern. Landsc Ecol. 1995;10:209–217. doi: 10.1007/BF00129255. [DOI] [Google Scholar]
- Hilt S. Recovery of Potamogeton pectinatus L. stands in a shallow eutrophic lake under extreme grazing pressure. Hydrobiologia. 2006;570:95–99. doi: 10.1007/s10750-006-0167-3. [DOI] [Google Scholar]
- Hupp JW, White RG, Sedinger JS, Robertson DG. Forage digestibility and intake by lesser snow geese: effects of dominance and resource heterogeneity. Oecologia. 1996;108:232–240. doi: 10.1007/BF00334646. [DOI] [PubMed] [Google Scholar]
- Klaassen RHG, Nolet BA. Persistence of spatial variance and spatial pattern in the abundance of a submerged plant. Ecology. 2008;89:2973–2979. doi: 10.1890/07-974.1. [DOI] [PubMed] [Google Scholar]
- Klaassen RHG, Nolet BA, Bankert D. The influence of social interactions on the foraging path of Bewick's Swans Cygnus columbianus bewickii. Ardea. 2006;94:477–484. [Google Scholar]
- Klaassen RHG, Nolet BA, Bankert D. Movement of foraging tundra swans explained by spatial pattern in cryptic food densities. Ecology. 2006;87:2244–2254. doi: 10.1890/0012-9658(2006)87[2244:MOFTSE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Klaassen RHG, Nolet BA, de Fouw J. Intake rate at differently scaled heterogeneous food distributions explained by the ability of tactile-foraging mallard to concentrate foraging effort within profitable areas. Oikos. 2006;112:322–331. doi: 10.1111/j.0030-1299.2006.13461.x. [DOI] [Google Scholar]
- Klaassen RHG, Nolet BA, van Leeuwen CHA. Prior knowledge about spatial pattern affects patch assessment rather than movement between patches in tactile-feeding mallard. J Anim Ecol. 2007;76:20–29. doi: 10.1111/j.1365-2656.2006.01184.x. [DOI] [PubMed] [Google Scholar]
- Kolasa J, Rollo CD. The heterogeneity of heterogeneity: a glossary. In: Kolasa J, Pickett STA, editors. Ecological Heterogeneity. New York: Springer-Verlag; 1991. pp. 1–23. [Google Scholar]
- Kotliar NB, Wiens JA. Mutiple scales of patchiness and patch structure: a hierarchical framework for the study of heterogeneity. Oikos. 1990;59:253–260. doi: 10.2307/3545542. [DOI] [Google Scholar]
- Kraan C, van der Meer J, Dekinga A, Piersma T. Patchiness of macrobenthic invertebrates in homogenized intertidal habitats: hidden spatial structure at a landscape scale. Mar Ecol Prog Ser. 2009;383:211–224. doi: 10.3354/meps07994. [DOI] [Google Scholar]
- Krebs CJ. Ecology: the experimental analysis of distribution and abundance. New York: Benjamin Cummings Pub Co; 2001. [Google Scholar]
- Lendvai AZ, Liker A, Barta Z. The effects of energy reserves and dominance on the use of social-foraging strategies in the house sparrow. Anim Behav. 2006;72:747–752. doi: 10.1016/j.anbehav.2005.10.032. [DOI] [Google Scholar]
- Li H, Reynolds JF. On definition and quantification of heterogeneity. Oikos. 1995;73:280–284. doi: 10.2307/3545921. [DOI] [Google Scholar]
- Lovvorn JR, Gillingham MP. Food dispersion and foraging energetics: a mechanistic synthesis for field studies of avian benthivores. Ecology. 1996;77:435–451. doi: 10.2307/2265620. [DOI] [Google Scholar]
- Monaghan P, Metcalfe NB. Group foraging in wild brown hares—effects of resource distribution and social-status. Anim Behav. 1985;33:993–999. doi: 10.1016/S0003-3472(85)80033-6. [DOI] [Google Scholar]
- Nachman G. A functional response model of a predator population foraging in a patchy habitat. J Anim Ecol. 2006;75:948–958. doi: 10.1111/j.1365-2656.2006.01114.x. [DOI] [PubMed] [Google Scholar]
- Nolet BA, Drent RH. Bewick's Swans refuelling on pondweed tubers in the Dvina Bay (White Sea) during their spring migration: first come, first served. J Avian Biol. 1998;29:574–581. doi: 10.2307/3677178. [DOI] [Google Scholar]
- Nolet BA, Klaassen RHG, Mooij WM. The use of a flexible patch leaving rule under exploitative competition: a field test with swans. Oikos. 2006;112:342–352. doi: 10.1111/j.0030-1299.2006.13460.x. [DOI] [Google Scholar]
- Nolet BA, Mooij WM. Search paths of swans foraging on spatially autocorrelated tubers. J Anim Ecol. 2002;71:451–462. doi: 10.1046/j.1365-2656.2002.00610.x. [DOI] [Google Scholar]
- Poisbleau M, Jenouvrier S, Fritz H. Assessing the reliability of dominance scores for assigning individual ranks in a hierarchy. Anim Behav. 2006;72:835–842. doi: 10.1016/j.anbehav.2006.01.024. [DOI] [Google Scholar]
- Rowcliffe JM, Pettifor RA, Carbone C. Foraging inequalities in large groups: quantifying depletion experienced by individuals in goose flocks. J Anim Ecol. 2004;73:97–108. doi: 10.1111/j.1365-2656.2004.00783.x. [DOI] [Google Scholar]
- Ruxton GD, Moody AL. The ideal free distribution with kleptoparasitism. J Theor Biol. 1997;186:449. doi: 10.1006/jtbi.1997.0405. [DOI] [Google Scholar]
- Smith RD, Ruxton GD, Cresswell W. Dominance and feeding interference in small groups of blackbirds. Behav Ecol. 2001;12:475–481. doi: 10.1093/beheco/12.4.475. [DOI] [Google Scholar]
- Sparrow AD. A heterogeneity of heterogeneities. Trends Ecol Evol. 1999;14:422–423. doi: 10.1016/S0169-5347(99)01735-8. [DOI] [PubMed] [Google Scholar]
- Spencer DF, Ksander GG, Whitehand LC. Estimating the abundance of subterranean propagules of submersed aquatic plants. Freshw Biol. 1994;31:191–200. doi: 10.1111/j.1365-2427.1994.tb00853.x. [DOI] [Google Scholar]
- Stahl J, Tolsma PH, Loonen M, Drent RH. Subordinates explore but dominants profit: resource competition in high Arctic barnacle goose flocks. Anim Behav. 2001;61:257–264. doi: 10.1006/anbe.2000.1564. [DOI] [PubMed] [Google Scholar]
- Stillman RA, Bautista LM, Alonso JC, Alonso JA. Modelling state-dependent interference in common cranes. J Anim Ecol. 2002;71:874–882. doi: 10.1046/j.1365-2656.2002.00652.x. [DOI] [Google Scholar]
- Stillman RA, GossCustard JD, Caldow RWG. Modelling interference from basic foraging behaviour. J Anim Ecol. 1997;66:692–703. doi: 10.2307/5922. [DOI] [Google Scholar]
- Sutherland WJ. Aggregation and the ideal free distribution. J Anim Ecol. 1983;52:821–828. doi: 10.2307/4456. [DOI] [Google Scholar]
- Syme GJ. Competitive orders as measures of social dominance. Anim Behav. 1974;22:931–940. doi: 10.1016/0003-3472(74)90016-5. [DOI] [Google Scholar]
- Taillon J, Cote SD. Social rank and winter forage quality affect aggressiveness in white-tailed deer fawns. Anim Behav. 2007;74:265–275. doi: 10.1016/j.anbehav.2006.11.018. [DOI] [Google Scholar]
- Theimer TC. The effect of seed dispersion on the foraging success of dominant and subordinate Dark-Eyed Juncos, Junco hyemalis. Anim Behav. 1987;35:1883–1890. doi: 10.1016/S0003-3472(87)80081-7. [DOI] [Google Scholar]
- Triplet P, Stillman RA, Goss-Custard JD. Prey abundance and the strength of interference in a foraging shorebird. J Anim Ecol. 1999;68:254–265. doi: 10.1046/j.1365-2656.1999.00280.x. [DOI] [Google Scholar]
- Turchin P, Kareiva P. Aggregation in Aphis varians—an effective strategy for reducing predation risk. Ecology. 1989;70:1008–1016. doi: 10.2307/1941369. [DOI] [Google Scholar]
- Vahl WK, Lok T, van der Meer J, Piersma T, Weissing FJ. Spatial clumping of food and social dominance affect interference competition among ruddy turnstones. Behav Ecol. 2005;16:834–844. doi: 10.1093/beheco/ari067. [DOI] [Google Scholar]
- Vahl WK, van der Meer J, Weissing FJ, van Dullemen D, Piersma T. The mechanisms of interference competition: two experiments on foraging waders. Behav Ecol. 2005;16:845–855. doi: 10.1093/beheco/ari073. [DOI] [Google Scholar]
- Vahl WK, Van Der Meer J, Meijer K, Piersma T, Weissing FJ. Interference competition, the spatial distribution of food and free-living foragers. Anim Behav. 2007;74:1493–1503. doi: 10.1016/j.anbehav.2007.03.006. [DOI] [Google Scholar]
- van Gils JA, Piersma T. Digestively constrained predators evade the cost of interference competition. J Anim Ecol. 2004;73:386–398. doi: 10.1111/j.0021-8790.2004.00812.x. [DOI] [Google Scholar]
- Wagner SJ, Gauthreaux SA. Correlates of dominance in intraspecific and interspecific interactions of song sparrows and white-throated sparrows. Anim Behav. 1990;39:522–527. doi: 10.1016/S0003-3472(05)80417-8. [DOI] [Google Scholar]