Abstract
Swimming may stimulate appetite and food intake but empirical data are lacking. This study examined appetite, food intake, and plasma acylated ghrelin responses to swimming. Fourteen healthy males completed a swimming trial and a control trial in a random order. Sixty min after breakfast participants swam for 60 min and then rested for six hours. Participants rested throughout the control trial. During trials appetite was measured at 30 min intervals and acylated ghrelin was assessed periodically (0, 1, 2, 3, 4, 6, and 7.5 h. N = 10). Appetite was suppressed during exercise before increasing in the hours after. Acylated ghrelin was suppressed during exercise. Swimming did not alter energy or macronutrient intake assessed at buffet meals (total trial energy intake: control 9161 kJ, swimming 9749 kJ). These findings suggest that swimming stimulates appetite but indicate that acylated ghrelin and food intake are resistant to change in the hours afterwards.
1. Introduction
Regular physical activity is important for the maintenance of body weight and its composition within a healthy range [1, 2]. All forms of physical activity can contribute to successful energy balance by increasing daily energy expenditure. Swimming is an attractive mode of physical activity due to the reduced musculoskeletal and thermoregulatory stresses (i.e., elevation in body temperature) imposed in comparison with other land-based activities such as running and cycling. Swimming may therefore offer an appealing form of physical activity for individuals seeking to prevent weight gain and/or to maintain a reduced body weight after successful weight loss.
Despite the attractiveness of swimming as a mode of physical activity, the ability of swimming to favourably influence body weight and body composition remains contentious. In obese individuals research has shown that swimming may not induce body weight and fat loss [3, 4] whereas walking and cycling interventions of similar intensity and duration do [3]. Considering the heightened energy output elicited by all forms of exertion the most logical explanation for these findings is that swimming stimulates a compensatory increase in energy intake [5]. This notion is consistent with anecdotal reports of swimming stimulating appetite. Specifically, it has been stated that individuals often feel like “eating a horse” after an acute bout of swimming [6]. This suggestion is consistent with empirical research which has described elevations in energy intake after cycling-based exercise performed on a modified ergometer in cold water [5, 7]. Despite these findings, there remains a paucity of data about the precise effects of swimming on appetite and food intake.
The mechanisms by which exercise influences appetite have recently begun to receive significant interest with specific attention being given to peptides implicated in the neuroendocrine regulation of feeding [8, 9]. Ghrelin is an acylated peptide secreted primarily from the stomach and remains unique as the only circulating gut peptide that stimulates appetite [10]. Defined roles of ghrelin in both short- and long-term feeding regulation have been uncovered [11], and more recently investigators have sought to determine how exercise influences circulating levels of ghrelin [12–14]. These studies suggest that intense exercise induces a transient suppression in circulating acylated ghrelin concentrations. Concomitant suppressions in hunger have been reported by Broom and colleagues [12, 14] raising the possibility that acylated ghrelin may be important in determining changes in appetite resulting from exercise.
The primary aim of this investigation was to examine the influence of an acute bout of swimming on appetite and energy intake in an effort to determine whether a stimulatory increase in these variables may explain data suggesting a relative inefficacy of swimming for the purposes of weight control. A subsidiary aim of this investigation was to explore the potential role of acylated ghrelin as a mediator of appetite and food intake, during and after exercise.
2. Methods
2.1. Participants
Following university ethical advisory committee approval 14 healthy male volunteers (age 22.0 ± 0.5 y, BMI 23.2 ± 0.6 kg·m2, body fat 17.2 ± 1.2%, mean ± SEM) gave their written informed consent to participate. Participants were nonsmokers, had no known history of cardiovascular/metabolic disease, were not dieting, did not have any atypical dietary habits (assessed by the three-factor eating questionnaire), were not taking medication, and were not obese (BMI ≤ 29.9 kg·m2) or hypertensive (resting blood pressure < 140/90 mmHg). Participants were habitually active but were not trained athletes, with most individuals typically participating in games activities such as soccer, hockey, and rugby on a regular basis at a recreational level. The nature of the study demanded that participants were competent at swimming; however, it was ensured that individuals taking part in swimming at a competitive level were not recruited for the study.
2.2. Procedure
Prior to main trials participants visited the laboratory to undergo screening and preliminary testing. On arrival at the laboratory participants were provided with an information sheet detailing the demands of the study. The information sheet stated that the aims of the study were to examine the effects of swimming on appetite, energy intake, and acylated ghrelin but did not provide any indication of the hypothesised direction of responses. After confirming that participants understood the study demands written informed consent was obtained. Thereafter, questionnaires were completed to assess health status, physical activity habits, and food preferences. Height was determined to the nearest 0.1 cm using a stadiometer (Seca 214, Seca Ltd, Germany), and body weight was measured to the nearest 0.1 kg using a digital scale (Seca 770, Seca Ltd, Germany). Body density was estimated via subcutaneous fat measurements [15] made using skinfold callipers (Baty International, West Sussex, UK), and body fat percentage was then ascertained [16].
Participants were then taken to the university swimming pool to confirm swimming competence and to be familiarised with procedures in anticipation of main trials. For this, participants were asked to complete a 60 min intermittent swimming set which was to be performed during the exercise trial. In this familiarisation session participants were accustomed to wearing heart rate monitors in the pool and taking recordings periodically. They were also familiarised with the ratings of perceived exertion scale [17].
After an interval of at least one week participants then completed two eight-hour trials (swimming and control) in a randomized-crossover fashion. Each trial was separated by at least one week. On the morning of main trials participants arrived at the laboratory having fasted overnight and not eaten breakfast. Main trials commenced at 09:00 with the consumption of a standard breakfast snack. This was consumed within 5 min. On the exercise trial participants rested within the laboratory for the first 40 min, after which they were escorted to the university swimming pool via motorised transport, in time for commencing swimming at the beginning of the second trial hour. At this time, participants began a 60 min intermittent swimming set. The set was composed of six 10 min blocks. In each block participants swam continuously for seven min using their preferred stroke and then rested for three min. The speed of swimming was ultimately determined by the participant although they were instructed to swim at a moderate intensity, defined as a rating of perceived exertion between 12 and 14. During exercise the distance completed was recorded. Heart rate was also assessed using short range telemetry. Upon completion of each swimming block participants rested on the pool side with their legs immersed in the water. Ratings of perceived exertion were then assessed. After completing the swimming protocol participants were escorted back to the research laboratory where they rested for a further six hours. Identical procedures were completed in the control trial except that no exercise was performed. Instead, during the equivalent time period resting metabolic rate was assessed via indirect calorimetry in order to permit the calculation of net energy expenditure (gross energy expenditure minus resting energy expenditure) during exercise.
2.3. Physical Activity and Dietary Standardization
Participants completed a weighed food record of all items consumed within the 24 h preceding their first main trial. Alcohol and caffeine were not permitted during this period. This feeding pattern was replicated prior to the second main trial. Participants refrained from strenuous physical activity during this time.
2.4. Appetite and Environmental Conditions
At baseline, 0.5-hour, 1-hour, and 30 min intervals thereafter appetite perceptions (hunger, satisfaction, fullness, and prospective food consumption) were assessed using 100 mm visual analogue scales [18]. Environmental temperature and humidity were also measured at these times using a handheld hygrometer (Omega RH85, Manchester, UK). The temperature of the swimming pool was monitored using a glass thermometer (Fisher Scientific, UK).
2.5. Breakfast and Ad Libitum Buffet Meals
During each main trial all food was consumed within the research laboratory and was quantified by the investigators. Main trials commenced with breakfast consumption (~09:00). The breakfast provided was standardised to body weight and consisted of a commercial cereal bar (Kellogg's Nutri-grain). Participants received 1.06 g per kilogram of body weight measured on the first trial visit. Identical amounts were consumed across trials. For a 70 kg individual this provided 1092 kJ of energy, 6 g of fat, 4 g of protein, and 48 g of carbohydrate.
At 3 h (~12:00) and 7.5 h (~16:30) into trials participants were given access to a buffet meal for a period of 30 min from which they could consume food ad libitum. The buffet meal provided diversity in protein, fat, and carbohydrate content in order to facilitate the detection of macronutrient preferences (Table 3). Food was presented in excess of expected consumption. Participants were told to eat until satisfied and that additional food was available if desired. Participants consumed meals in isolation so that social influence did not constrain food selection. Food consumption was ascertained by examining the weighted difference in each food item remaining compared with the weight of that initially presented. The energy and macronutrient content of the items consumed was ascertained using manufacturer values.
Table 3.
Items presented at buffet meals.
| Coco-pops—Cereal |
| Cornflakes—Cereal |
| Rice Krispies—Cereal |
| Frosties—Cereal |
| Milk |
| Cereal Bar |
| White Bread |
| Brown Bread |
| Tuna |
| Cheese |
| Ham |
| Butter |
| Mayonnaise |
| Salted Crisps |
| Apple |
| Orange |
| Banana |
| Chocolate rolls |
| Chocolate muffins |
| Plain muffins |
| Cookies |
| Chocolate bar (Mars fun size) |
2.6. Acylated Ghrelin
To explore the effects of swimming on circulating concentrations of acylated ghrelin, blood samples were collected from 10 of the 14 participants at baseline, 1 h (pre-exercise), 2 h (post-exercise), 3 h, 4 h, 6 h, and 7.5 h. (We did not measure acylated ghrelin in four participants for logistical reasons, i.e., the room we used for blood sampling at the swimming pool was not always available). In both the swimming and control trials baseline samples and the equivalent pre- and postexercise blood samples were taken via venepuncture of an antecubital vein. Thereafter, the remaining samples were collected via a cannula (Venflon, Becton Dickinson, Helsinborg, Sweden) positioned in an antecubital vein. Details of sample preparation, collection, and analysis have been described in depth previously [12, 14]. The within batch coefficient of variation for the acylated ghrelin ELISA assay was 6.4%.
2.7. Energy Expenditure Estimation
Energy expenditure during swimming was estimated using equations based on multiples of resting metabolism (METs) [19]. Specifically, energy expenditure was estimated by multiplying each participant's estimated resting energy expenditure (kJ·min−1) in the control trial by an appropriate MET value for the stroke used during each seven- minute block of swimming: general breast stroke (10 METs), general backstroke (7 METs), slow crawl (≤0.95 m·s−1—8.0 METs), and fast crawl (>0.95 m·s−1—11 METs).
2.8. Statistical Analysis
All data was analyzed using the Statistical Package for the Social Sciences (SPSS) software version 16.0 for Windows (SPSS Inc, Chicago, IL, US.). Area under the concentration versus time curve calculations were performed using the trapezoidal method. Student's t-tests for correlated data were used to assess differences between fasting and area under the curve values for appetite perceptions, acylated ghrelin, temperature, and humidity between the control and swimming trials. Repeated measures, two-factor ANOVA was used to examine differences between the swimming and control trials over time for appetite perceptions, energy and macronutrient intake, and acylated ghrelin. The Pearson product moment correlation coefficient was used to examine relationships between variables. Correction of acylated ghrelin values for changes in plasma volume did not alter the statistical significance of findings therefore for simplicity the unadjusted values are presented. Statistical significance was accepted at the 5% level. Results are presented as mean ± SEM. A power calculation indicated that 13 participants were needed to provide sufficient power (80%) to detect a 50% compensation in energy intake with alpha set at 5%.
3. Results
3.1. Exercise Responses and Resting Oxygen Consumption
During the 42 min of swimming (6 × 7 min intervals) the mean distance completed was 1875 ± 156 m. The mean swimming speed performed was 0.74 ± 0.1 m·s−1, and this elicited an estimated net energy expenditure (exercise minus resting) of 1921 ± 83 kJ. The corresponding mean heart rate and rating of perceived exertion values during the sessions were 155 ± 5 beats·min−1 and 14 ± 0. To complete the swimming session four participants swam breaststroke for all of the intervals whilst three participants used only front crawl and two participants used only backstroke. Three participants used a combination of front crawl and breast stroke whilst two participants alternated between breaststroke and backstroke. Participants' mean oxygen consumption at rest during the second hour of the control trial (i.e., the time when they were swimming during the exercise trial) was 0.32 ± 0.01 L·min−1 (6.5 ± 0.3 kJ·min−1).
3.2. Baseline Parameters
No between-trial differences existed at baseline for any of the ratings of appetite assessed or in plasma concentrations of acylated ghrelin (student's t-test, P > .05 for each).
3.3. Appetite, Energy and Macronutrient Intake
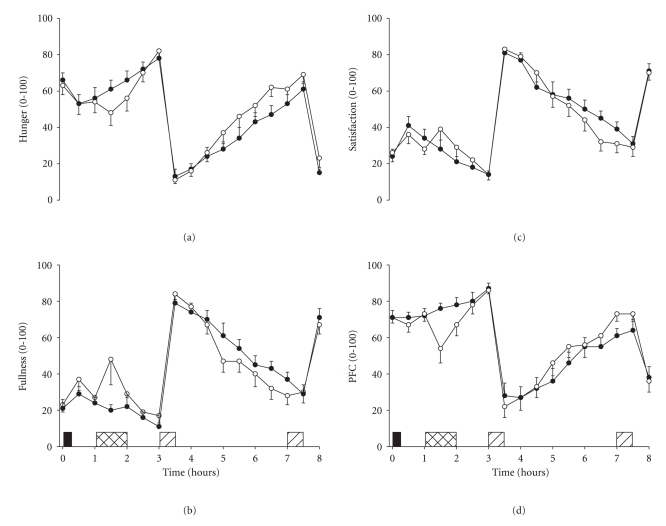
Perceived ratings of hunger and prospective food consumption were suppressed during and immediately after swimming before increasing above values exhibited during the control trial in the hours after exercise (two-factor ANOVA, trial × time interaction; P < .05 for each). Conversely, perceived ratings of fullness and satisfaction were increased transiently during swimming before decreasing below control values in the hours thereafter (two-factor ANOVA, trial × time interaction; P < .05 for each) (Figure 1). Analysis of the appetite area under the curve (AUC) data confirmed these results. After the morning meal the hunger AUC (3.5–8 h) was significantly higher in the swimming trial as compared with control (swimming 178 ± 20, control 152 ± 19; student's t-test, P = .028) whilst fullness tended to be reduced (swimming 227 ± 21, control 243 ± 17; student's t-test, P = .052). Moreover, from baseline to consumption of the morning buffet meal the fullness AUC (0–3 h) was significantly higher in the swimming trial as compared with control (swimming 74 ± 12, control 54 ± 8; student's t-test, P = .025) whilst prospective food consumption was suppressed (swimming 221 ± 12, control 231 ± 11; P = .049).
Figure 1.
Ratings of hunger (a), fullness (b), satisfaction (c), and prospective food consumption (d) in the swimming (°) and control (∙) trials. Values are mean ± SEM (n = 14). Black rectangle indicates breakfast snack, hatched rectangle indicates swimming, and diagonal rectangles indicate buffet meals. Two-factor ANOVA revealed a trial × time interaction effect for each (P < .05).
Energy intake was significantly higher at the morning buffet meals compared with the afternoon meals (two-factor ANOVA, main effect of time; P = .003); however, there were no between-trial differences in energy intake at either feeding opportunity (two-factor ANOVA, trial and interaction main effects; P > .05 for each). Relative energy intake (energy intake—net energy cost of exercise) was therefore significantly lower on the swimming trial as compared with control (swimming 7828 ± 774 kJ, control 9163 ± 720). Table 1 presents the energy intake data for the control and swimming trials.
Table 1.
Energy intake (kJ) in the control and swimming trials (n = 14). There were no significant differences between the swimming and control trials (P > .05).
| Control | Swimming | |
|---|---|---|
| Morning meal | 5517 ± 434 | 5856 ± 403 |
| (3–3.5 h) | ||
| Afternoon meal | 3644 ± 459 | 3893 ± 577 |
| (7.5–8 h) | ||
| Total trial | 9161 ± 719 | 9749 ± 809 |
Two-factor ANOVA showed no trial or interaction (trial × time) main effects for macronutrient intake (absolute amount or percentage intake) indicating that no significant differences existed between trials for the intake of fat, carbohydrate or protein (Table 2).
Table 2.
Macronutrient intake in the control and swimming trials. Values are gram and (%) (n = 14). There were no significant differences between the swimming and control trials (P > .05).
| Control trial | Fat | Carbohydrate | Protein |
|---|---|---|---|
| Morning meal (3–3.5 h) | 54 ± 5 (34.1) | 156 ± 11 (49.1) | 59 ± 9 (16.8) |
| Afternoon meal (7.5–8 h) | 33 ± 5 (33.8) | 107 ± 15 (49.9) | 38 ± 8 (16.3) |
| Total Trial | 87 ± 8 (34.9) | 263 ± 21 (49.1) | 97 ± 9 (16.0) |
| Swimming trial | Fat | Carbohydrate | Protein |
| Morning meal (3–3.5 h) | 55 ± 5 (34.0) | 164 ± 12 (49.3) | 60 ± 8 (16.7) |
| Afternoon meal (7.5–8 h) | 35 ± 5 (33.1) | 117 ± 20 (50.2) | 38 ± 7 (16.7) |
| Total Trial | 90 ± 9 (34.2) | 281 ± 26 (49.4) | 98 ± 9 (16.4) |
3.4. Acylated Ghrelin
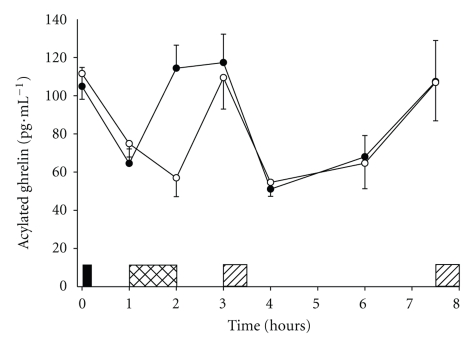
Data for ten participants showed that plasma concentrations of acylated ghrelin were suppressed during swimming and after consumption of the morning buffet meal (two-factor ANOVA, main effect of trial; P = .038). On closer inspection of the data one participant was a clear outlier exhibiting fasting values on both trials which were approximately nine times (26 standard deviations) higher than the mean fasting values of the other nine participants (949 pg·mL−1 for the outlier versus 108 ± 10 pg·mL−1 for the mean (±SEM) of the other nine participants). Upon removal of this outlier the suppression of acylated ghrelin at the end of the swimming bout and after the first meal on each trial is displayed with greater clarity (two-factor ANOVA, trial × time interaction; P < .001) (Figure 2). Examination of the acylated ghrelin AUC (outlier excluded, n = 9) confirmed suppressed concentrations of acylated ghrelin prior to the first buffet meal (0–3 h) on the swimming trial (swimming 473 ± 232, control 505 ± 217 pg·mL −1·3 h) (student's t-test, P < .001).
Figure 2.
Plasma concentrations of acylated ghrelin in the swimming (°) and control (∙) trials. Values are mean ± SEM (n = 9). Black rectangle indicates breakfast snack, hatched rectangle indicates swimming, diagonal rectangles indicate buffet meals. Two-factor ANOVA revealed a significant trial × time interaction effect (P < .001).
To examine the relationship between acylated ghrelin and energy intake, at both the morning and afternoon buffet meals correlations were performed between acylated ghrelin values immediately prior to each meal and subsequent energy intake. Moreover, correlations were also performed using the acylated ghrelin AUC leading up to the morning (0–3 h AUC) and afternoon (3–8 h AUC) buffet meals. In all instances no significant relationships were found between acylated ghrelin and energy intake.
3.5. Body Mass, Fluid Intake, and Environmental Conditions
There were no significant differences between the control and swimming trials (all P > .05) in body weight (control 76.7 ± 2.1, swimming 76.5 ± 2.2 kg), water intake (control 1402 ± 219, swimming 1302 ± 226 mL) laboratory atmospheric temperature (control 21.6 ± 0.3, swimming 21.4 ± 0.3°C), and relative humidity (control 37.8 ± 4.1, swimming 37.8 ± 4.1%). The atmospheric temperature and relative humidity at the swimming pool were 26.4 ± 0.8°C and 50.9 ± 1.7%, respectively. The temperature of the swimming pool water was 28.1 ± 0.1°C.
4. Discussion
The main findings arising from this investigation are threefold. Firstly, moderate intensity swimming exhibited a biphasic influence on appetite with an inhibition existent during exercise and a later stimulation in the hours thereafter. Secondly, swimming did not influence ad libitum energy or macronutrient intake. Finally, our exploratory analyses showed that swimming transiently suppressed circulating concentrations of the orexigenic peptide, acylated ghrelin; however, no effects were apparent after exercise. This outcome indicates that acylated ghrelin does not mediate the reported stimulation of appetite after swimming.
The suppression of appetite (decreased hunger and prospective food consumption/elevated satisfaction and fullness) observed during swimming is a novel finding yet is consistent with previous research showing a transient inhibition of appetite resulting from land-based exercise modalities such as running and cycling [20, 21]. This phenomenon has been termed exercise-induced anorexia [22] and has been consistently observed during land-based activities performed at moderate intensities or higher (>60% VO2 max). Broom et al. [12] reported suppressed hunger and plasma acylated ghrelin during treadmill running and suggested a potential role of acylated ghrelin in determining suppressed appetite during exercise. The findings from the present study confirm that acylated ghrelin and appetite are concomitantly suppressed during swimming; however, the absence of any significant correlations between acylated ghrelin and any of the appetite markers assessed, during exercise or immediately after, suggests that there may not be a strong association between these variables. Given the diversity of the role of ghrelin in human physiology [23] it is possible that the transient suppression of circulating acylated ghrelin observed during exercise is entirely unrelated to appetite regulation. At present though, the physiological relevance of this response is not known.
In the hours after consumption of the morning buffet meal, ratings of hunger and prospective food consumption were higher in the swimming trial than the control trial whilst ratings of fullness were reduced. These findings indicate that swimming stimulated a delayed increase in appetite. This response is contrary to research which has examined appetite responses to land-based activities which have typically shown no acute compensation in appetite after performing exercise, even when significant amounts of energy are expended [20–22, 24]. The mechanism responsible for these discrepant findings is not immediately clear. It has been suggested that changes in body temperature may be important [5, 6]; however, this is unlikely in the present study as appetite was not stimulated until more than two hours after swimming. By this time core temperature would almost certainly have normalised. White et al. [5] speculate that the cooling and then subsequent reheating of the body may be associated with the release of “certain hormones” which stimulate the appetite. In the present study we measured circulating concentrations of acylated ghrelin, a peptide responsible for stimulating appetite and food intake [25, 26]. The present findings suggest that acylated ghrelin is not responsible for the augmented appetite response after swimming as circulating concentrations were not different from control after the morning buffet meal. It remains possible that compensatory changes in fasting or meal-related acylated ghrelin profiles may occur over a longer duration; however, further work is needed to examine this. It must also be considered that appetite is regulated on an acute basis by many circulating peptides in addition to acylated ghrelin, including peptide YY, pancreatic polypeptide, and glucagon-like peptide-1 [10]. It is therefore feasible that changes in these peptides may have influenced the acylated ghrelin and appetite responses observed.
Research indicates that swimming may be less effective than land-based activities for inducing weight loss or reductions in body fat [3, 4]. Consistent with this, it has been observed that levels of adiposity are typically higher in swimmers than equal calibre runners [27, 28]. It has been suggested that an unparalleled stimulation of appetite and energy intake after swimming may explain these findings [6]. Despite the changes in appetite observed, the present investigation did not find any significant differences in energy or macronutrient intake between the swimming and control trials, either during the morning or afternoon meals. These findings are difficult to reconcile. It is known that food intake is influenced by a host of physiological, environmental, psychological, and social factors, some of which are learned over time and are resistant to change [29]. In this study it seems that the factors influencing appetite were insufficient to overcome other competing forces governing food intake. Nonetheless, as a consequence of the lack of change in energy intake, participants therefore failed to compensate for the energy expended during exercise and relative energy intake (energy intake—net energy cost of exercise) was subsequently lower on the swimming trial as compared with control (swimming 7828 ± 774 kJ, control 9163 ± 720 kJ; student's t-test, P = .008). This outcome contradicts the suggestion that energy intake is augmented by swimming and therefore does not support the notion that swimming is an ineffective exercise modality for successful body weight control.
When comparing the present findings to previous data water temperature emerges as an important variable influencing food intake responses to exercise performed in water. White et al. [5] examined energy intake responses in healthy participants who performed cycling exercise while immersed in either cold water (20°C) or neutral water (33°C) and compared these responses to control responses (i.e., while resting in a dry environment). Energy intake was significantly higher after exercise in cold water (3653 kJ) as compared with the neutral water (2544 kJ) and the resting trials (2586 kJ). These results indicate that exercise in cold water stimulates energy intake. In similar fashion, Dressendorfer [7] submitted six trained males to 30 min of modified cycling in cold water (22°C), warm water (34°C), cycling on land, and a resting control trial. Participants consumed significantly more energy in the cold water trial than all other trials at a buffet meal provided immediately after exercise. Furthermore, energy intake in the warm water trial was significantly less than all other trials. Collectively, these findings suggest that water temperature and possibly subsequent core body temperature are important determinants of feeding responses after exercise in water. Despite these established findings, no study has previously examined the specific effects of swimming (rather than modified cycling) on appetite and food intake. Our findings appear to support the notion that exercise only in cold water stimulates food intake because in the present study the water temperature was moderate (28–28.5°C) and no change in energy intake was observed. The idea that exercise only in cold water stimulates food intake is also supported by the finding that metabolic rate (and hence energy expenditure) is not increased by immersion in water at a temperature of 32°C (not that dissimilar from the temperature of the water in the present study) whereas metabolic rate is increased by immersion in cold water (either 14°C or 20°C) [30] and by cold air, possibly due to activation of brown adipose tissue [31]. It might be anticipated that immersion in water will only increase appetite and food intake if the water temperature lowers core temperature, eliciting an increase in metabolic rate either by shivering or nonshivering thermogenesis although this is speculation. Unfortunately core temperature was not assessed in the present study, therefore the exact relationship between this variable and energy intake cannot be explored. Further work is needed to examine this issue.
This investigation has some notable limitations. Firstly, an immersed, resting control trial was not included therefore making it difficult to determine whether the reported increase in appetite was due to immersion in water or the physical work completed. Despite this, the majority of previous investigations which have examined appetite responses to exercise have not observed increases in appetite afterwards [9], thus we believe that our findings still offer novel, interesting data. Secondly, although we have examined energy/macronutrient intake responses over an extended period, it remains possible that changes may occur over a longer duration of time, for example, on the day after exercise. An even longer period of observation would be necessary in future studies to test this hypothesis. Thirdly, this study did not directly compare the effects of swimming with those of other modes of exercise and this limits the extent to which conclusions can be drawn in this regard. Finally, participants were young, healthy males and we do not know if these findings would generalise to other populations such as females, the elderly or overweight and obese individuals. Additional work is required to examine these issues, particularly in overweight individuals as it is within this population that findings hold the most clinical importance.
In conclusion, this investigation has shown that an acute bout of moderate intensity swimming suppresses appetite during exercise before leading to an increase later on in the day. Despite this, energy intake and macronutrient selection appear resistant to change over the duration of time examined. Circulating concentrations of acylated ghrelin were suppressed during swimming and this may possibly have contributed to the reduction in appetite observed. Nonetheless, acylated ghrelin does not appear to mediate the reported increase in appetite in the hours after exercise. These findings provide novel information regarding the influence of swimming on the acute regulation of energy homeostasis.
Acknowledgments
The authors would like to thank all of the participants for dedicating their time to take part in this study. They would like to thank Mr. James Carson and Mr. Michael Sawrey for their assistance with participant recruitment and data collection. They would also like to thank Miss Karen Shilton for organising access to the university swimming pool.
References
- 1.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Medicine and Science in Sports and Exercise. 2009;41(2):459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 2.Seagle HM, Strain GW, Makris A, Reeves RS. Position of the American Dietetic Association: weight management. Journal of the American Dietetic Association. 2009;109(2):330–346. doi: 10.1016/j.jada.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 3.Gwinup G. Weight loss without dietary restriction: efficacy of different forms of aerobic exercise. American Journal of Sports Medicine. 1987;15(3):275–279. doi: 10.1177/036354658701500317. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka H, Bassett DR, Jr., Howley ET. Effects of swim training on body weight, carbohydrate metabolism, lipid and lipoprotein profile. Clinical Physiology. 1997;17(4):347–359. doi: 10.1046/j.1365-2281.1997.03939.x. [DOI] [PubMed] [Google Scholar]
- 5.White LJ, Dressendorfer RH, Holland E, McCoy SC, Ferguson MA. Increased caloric intake soon after exercise in cold water. International Journal of Sport Nutrition and Exercise Metabolism. 2005;15(1):38–47. doi: 10.1123/ijsnem.15.1.38. [DOI] [PubMed] [Google Scholar]
- 6.Burke LB. Practical Sports Nutrition. Champaign, Ill, USA: Human Kinetics; 2007. [Google Scholar]
- 7.Dressendorfer R. Effect of internal body temperature on energy intake soon after aerobic exercise. Medicine & Science in Sports & Exercise. 1993;25(supplement 5):p. S42. [Google Scholar]
- 8.Martins C, Morgan L, Truby H. A review of the effects of exercise on appetite regulation: an obesity perspective. International Journal of Obesity. 2008;32(9):1337–1347. doi: 10.1038/ijo.2008.98. [DOI] [PubMed] [Google Scholar]
- 9.Bilski J, Teległów A, Zahradnik-Bilska J, Dembiński A, Warzecha Z. Effects of exercise on appetite and food intake regulation. Medicina Sportiva. 2009;13(2):82–94. [Google Scholar]
- 10.Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444(7121):854–859. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- 11.Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiology and Behavior. 2006;89(1):71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Broom DR, Stensel DJ, Bishop NC, Burns SF, Miyashita M. Exercise-induced suppression of acylated ghrelin in humans. Journal of Applied Physiology. 2007;102(6):2165–2171. doi: 10.1152/japplphysiol.00759.2006. [DOI] [PubMed] [Google Scholar]
- 13.Marzullo P, Salvadori A, Brunani A, et al. Acylated ghrelin decreases during acute exercise in the lean and obese state. Clinical Endocrinology. 2008;69(6):970–971. doi: 10.1111/j.1365-2265.2008.03275.x. [DOI] [PubMed] [Google Scholar]
- 14.Broom DR, Batterham RL, King JA, Stensel DJ. Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males. American Journal of Physiology. 2009;296(1):R29–R35. doi: 10.1152/ajpregu.90706.2008. [DOI] [PubMed] [Google Scholar]
- 15.Durnin JVGA, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. British Journal of Nutrition. 1974;32(1):79–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- 16.Siri WE. The gross composition of the body. Advances in Biological and Medical Physics. 1956;4:239–280. doi: 10.1016/b978-1-4832-3110-5.50011-x. [DOI] [PubMed] [Google Scholar]
- 17.Borg GAV. Perceived exertion: a note on ’history’ and methods. Medicine and Science in Sports and Exercise. 1973;5(2):90–93. [PubMed] [Google Scholar]
- 18.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. International Journal of Obesity. 2000;24(1):38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 19.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Medicine and Science in Sports and Exercise. 2000;32(9):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 20.King NA, Blundell JE. High-fat foods overcome the energy expenditure induced by high-intensity cycling or running. European Journal of Clinical Nutrition. 1995;49(2):114–123. [PubMed] [Google Scholar]
- 21.Blundell JE, King NA. Exercise, appetite control, and energy balance. Nutrition. 2000;16(7-8):519–522. doi: 10.1016/s0899-9007(00)00250-1. [DOI] [PubMed] [Google Scholar]
- 22.King NA, Burley VJ, Blundell JE. Exercise-induced suppression of appetite: effects on food intake and implications for energy balance. European Journal of Clinical Nutrition. 1994;48(10):715–724. [PubMed] [Google Scholar]
- 23.van der Lely AJ, Tschöp M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocrine Reviews. 2004;25(3):426–457. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- 24.King NA, Lluch A, Stubbs RJ, Blundell JE. High dose exercise does not increase hunger or energy intake in free living males. European Journal of Clinical Nutrition. 1997;51(7):478–483. doi: 10.1038/sj.ejcn.1600432. [DOI] [PubMed] [Google Scholar]
- 25.Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. Journal of Clinical Endocrinology and Metabolism. 2001;86(12):5992–5995. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 26.Kojima M, Kangawa K. Ghrelin: structure and function. Physiological Reviews. 2005;85(2):495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 27.Novak LP, Woodward WA, Bestit C, Mellerowicz H. Working capacity, body composition, and anthropometry of Olympic female athletes. Journal of Sports Medicine and Physical Fitness. 1977;17(3):275–283. [PubMed] [Google Scholar]
- 28.Jang KT, Flynn MG, Costill DL, et al. Energy balance in competitive swimmers and runners. Journal of Swimming Research. 1987;3:19–23. [Google Scholar]
- 29.Bellisle F. Food choice, appetite and physical activity. Public Health Nutrition. 1999;2(3):357–361. doi: 10.1017/s1368980099000488. [DOI] [PubMed] [Google Scholar]
- 30.Šrámek P, Šimečková M, Janský L, Šavlíková J, Vybíral S. Human physiological responses to immersion into water of different temperatures. European Journal of Applied Physiology. 2000;81(5):436–442. doi: 10.1007/s004210050065. [DOI] [PubMed] [Google Scholar]
- 31.Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. New England Journal of Medicine. 2009;360(15):1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]