Abstract
Objectives
The study was conducted to examine sex differences in the initial level and rate of change in physical function and grip strength.
Method
The baseline survey included 2,262 Danes born in 1905 and alive in 1998 and followed-up in 2000, 2003, and 2005. Hence, the authors fully used the power of having a cohort with multiple assessments in late life and virtually complete follow-up of lifespan (through December 2008). Latent growth curve modeling was used.
Results
Men had higher initial levels and rates of decline in strength score and grip strength. Lifespan was positively correlated with intercepts and slopes.
Discussion
The Danish data suggested that the longest-living individuals have higher initial levels of strength score and grip strength and smaller rate of change. The data further suggested that the initial level of strength score and grip strength was more predictive of mortality than the rate of change was, and the predictive effects were similar in men and women.
Keywords: sex, activities of daily living, grip strength, oldest old, mortality, growth-curve analysis, Denmark
Empirical evidence accumulated over recent decades revealed an apparent contradiction between health and survival of men and women (Case & Paxson, 2005; Nathanson, 1975; Waldron, 1985). Women enjoy a longer life than men in almost all countries of the world (Barford, Dorling, Smith, & Shaw, 2006), but women tend to report poorer self-rated health (Bambra et al., 2008; Olsen & Dahl, 2007), have higher disability levels at all ages (Arber & Cooper, 1999; Leveille, Penninx, Melzer, Izmirlian, & Guralnik, 2000), and perform more poorly on physical tests than men (Frederiksen et al., 2006; Jeune et al., 2006).
It has been suggested that handgrip strength is better than chronological age in predicting the frailty of people (Syddall, Cooper, Martin, Briggs, & Sayer, 2003). Several studies have found that grip strength is predictive of disability among middle-aged men (Rantanen et al., 1999), as well as older persons (Giampaoli et al., 1999). Others have demonstrated that grip strength predicts all-cause, cardiovascular (CVD), and cancer mortality among men, but it ceased to predict mortality among elderly women in multivariate analyses (Fujita et al., 1995; Gale, Martyn, Cooper, & Sayer, 2007).
Previous research proposed that many physiological functions begin to decline around 30 years (Sehl & Yates, 2001) and that the course of the decline accelerates with increasing age (Bassey, 1998; Forrest, Zmuda, & Cauley, 2005; Forrest, Zmuda, & Cauley, 2007; Rantanen et al., 1998). The median loss for the bone-mineral density in lumbar spine from age 30 to 70 years has been estimated to be 0.3% per year, and the mean annual decline in muscle strength was 0.7% in men and 0.6% in women (Sehl & Yates, 2001). It has been indicated that the decline is steeper in individuals with greater levels of muscle strength at baseline (Forrest et al., 2005, 2007) and among men (Proctor et al., 2006).
Previous studies suggest that the rate of change in physical and cognitive functioning is associated with longevity (Deeg, Hofman, & van Zonneveld, 1990). However, little information is available on rate of change among the oldest-old population due to logistical challenges (high mortality and nonresponse). Available data suggest that although the level of functioning is predictive of survival, the rate of decline is also important. Data from the Baltimore Longitudinal Study of Aging suggest that the rates of change in muscle power and isometric strength predict mortality independently from the power and strength levels (Metter, Talbot, Schrager, & Conwit, 2004). However, little is known about sex differences in the rate of change in physical function and grip strength in the oldest population. Most previous studies were restricted to small or specific samples, such as one ethnic or sex group, were limited in the number of measurement occasions, and did not specifically focus on sex differences in the decline or on the sex-specific effect of rate of change on survival. It is also unclear to what extent the male–female health–survival paradox is due to sex differences in level and/or rate of change in physical functioning.
The present study focuses on determining whether there are sex differences in the initial level and rate of change in reported physical function and grip strength, whether it is the level or rate of change that is more predictive of mortality, and, finally, whether sex moderates the associations of the level and rate of change with lifespan. To address these research questions we used previously collected longitudinal data on the entire national Danish cohort born in 1905 and followed from ages 92 to 93 to 100 years with follow-up of survival status through December 2008. Hereby, we are using the full power of having a cohort with multiple assessments in late life and virtually complete follow-up of lifespan.
Method
Study Population
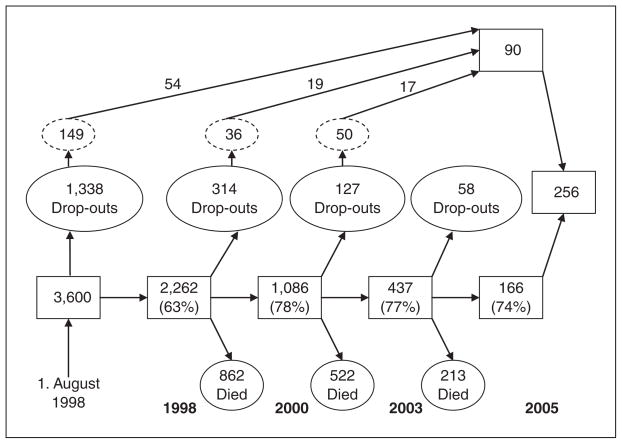
The study uses the data collected in the Danish 1905-Cohort Study described in detail elsewhere (Christensen, Bathum, & Christiansen, 2008; Nybo, Gaist, Jeune, Bathum et al., 2001). In brief, the individuals eligible to participate in the survey were identified in the Danish Civil Registration System (Pedersen, Gøtzsche, Moller, & Mortensen, 2006) and included all Danes born in 1905 and alive on August 1, 1998 (Figure 1). A total of 3,600 persons were approached irrespective of their residency, physical health, and cognitive status. If a person refused or was unable to participate in the face-to-face interview, a proxy respondent, usually a close relative, was sought. A total of 2,262 people (62.8% of the 3,600 persons eligible to participate in the baseline survey) participated in the intake survey in 1998. The consecutive waves in 2000, 2003, and 2005 were follow-up assessments of survivors from previous waves, with participation rates among survivors between 69% and 78%. In addition, for the 2005 survey all nonrespondents from previous waves were contacted, and 90 persons agreed to participate.
Figure 1.
Flow-chart of the Danish 1905-Cohort Study
Note: The square boxes give the number of participants and participation rates. The dashed circles give the initial number of dropouts who were recontacted for participation in 2005. Source: Christensen, Bathum, and Christiansen (2008).
Participants and nonparticipants were compared using the extensive registration of the Danish population. No differences were found in housing and marital status, but men and persons living in rural areas were more likely to participate than women and urban dwellers. An analysis of hospitalization patterns from 1973 to 1998 indicated that participants were not healthier than nonparticipants. Nevertheless, in the 6-month period immediately following the start of the survey, nonparticipants had higher mortality, suggesting that terminal illness was one of the reasons for nonparticipation (Nybo, Gaist, Jeune, Bathum et al., 2001; Nybo, Gaist, Jeune, McGue et al., 2001; Nybo et al., 2003).
The questionnaire included items asking respondents about their sociodemographic characteristics, family and lifecycle characteristics, health, and lifestyle behavior. Physical and cognitive functions and depression symptomatology were also assessed, and the participants were asked to give a biological sample, either blood or a cheek swab. Interviewers from the Danish National Institute of Social Research were not medically or para-medically trained but do, however, have substantial experience in interviewing the elderly (Christensen, Holm, McGue, Corder, & Vaupel, 1999). Data in each wave were collected within approximately three months.
Reported Physical Function
The assessment of physical function was based on self-reports of the activities the participant was able to do on the day of the interview. The Avlund instrument was previously validated in Denmark and was shown to discriminate between levels of functional ability among community-dwelling elderly persons (Avlund, Davidsen, & Schultz-Larsen, 1995; Schultz-Larsen, Avlund, & Kreiner, 1992). The Avlund instrument was extended to include assessment of the need for medical equipment or aids in relation to functional abilities, on the basis of results showing that equipment and aids can improve functional abilities among the elderly (Manton, Corder, & Stallard, 1993). All of the items from the Katz index of Activities of Daily Living (Katz, Ford, Moskowitz, Jackson, & Jaffe, 1963) were included, as well as questions about the ability to see and hear and the ability to engage in more demanding activities (Christensen et al., 2000). A 11-item self-reported measure of physical function was administered at each wave and ranged broadly, from relatively simple physical tasks to more demanding activities: walk around in the house, walk up and down stairs one floor, walk up and down stairs to the second floor, get outdoors, walk 400 m without resting, do light and hard exercise, walk in nice and bad weather for 30 minutes to 1 hour, run 100 meters, and carry 5 kg. Each item was answered on a scale ranging from 1 to 4 points (1 = can do without fatigue, 2 = can do with fatigue or minor difficulties, 3 = can do with aid or major difficulties, 4 = cannot do). The scale score also ranges from 1 to 4, being an average of the 11 items, and it was reversed to make higher scores correspond to higher levels of physical function. Consistent with our previous publications, this scale is called the strength score. If an item was missing or skipped, the mean for that item was substituted. If more than one item was skipped, the scale was coded as missing. This scale has been shown to provide a sensitive quantitative measure of physical ability, to be moderately heritable, and to have high internal consistency (.93) for both in-person and proxy interviews (Christensen, Frederiksen, Vaupel, & McGue, 2003; Christensen, Gaist, Vaupel, & McGue, 2002; Christensen et al., 2000). If a person refused or was unable to participate in the face-to-face interview, a proxy respondent was asked to rate the physical function of the person. Among 2,295 subjects (1,709 women and 586 men) with at least 1 measurement occasion of physical function, 462 (20.1%) were proxy respondents. In total, there were 1,084 individuals (832 women and 252 men) with at least 2 measurements of physical function, 441 persons (356 women and 85 men) with at least 3 measurements, and 155 people (124 women and 31 men) had valid measurement of physical function at all 4 waves.
Grip Strength
Grip strength in kilograms was measured using a Smedley dynamometer (TTM; Tokyo, Japan) for three trials with the stronger hand and with brief pauses between each performance (Frederiksen et al., 2006; Nybo et al., 2003). The best performance of these three was used for the analysis. To measure maximal strength, the width of the handle was adjusted to fit the hand size; the second phalanx should rest against the inner stirrup; the elbow had to be in a 90-degree position and the upper arm to be tight against the trunk (Oxford, 2000). Participants who made fewer than 3 attempts or had a difference of 20 kg or more between two measures were excluded. By definition, proxy respondents had no measurement of grip strength. In total, there were 1,617 participants (450 men and 1,167 women) with at least 1 measurement of grip strength, 689 (182 men and 507 women) with at least 2 measurements, 240 (61 men and 179 women) had their grip strength assessed at least 3 occasions, and 64 (15 men and 46 women) at all 4 waves.
Lifespan
Survival status was available through the end of December 2008. The mean observed lifespan among individuals was 95.9 years (SE = 0.09) for men and 96.6 years (SE = 0.06) for women. Half of the male population died between May 1998 and October 2000, whereas 50% of women died between February 1998 and July 2001. The lifespan of 53 individuals (9 men and 44 women) who were still alive at the end of December 2008 was estimated by adding remaining life expectancy to their last known age using male and female life tables for Denmark from the Human Mortality Database (HMD; 2009).
Statistical Analysis
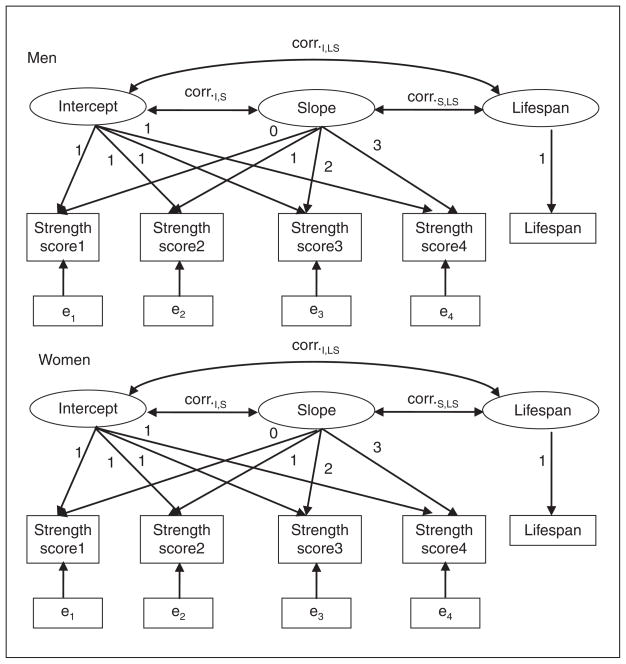
Latent growth-curve models (LGM) were used to investigate sex differences in age-related changes in strength score and grip strength by the method of maximum likelihood using the Mx software (Neale, Boker, Xie, & Maes, 2003). The LGM for change in strength score is presented in Figure 2.
Figure 2.
Graphical presentation of latent growth-curve model for change in strength score in the 1905-Cohort Study
Note: corr. = correlation coefficient; I = intercept; S = slope; LS = lifespan.
The availability of multiple measurements of strength score and grip strength permits estimation of rate of change rather than the amount of change, which is commonly examined in the analysis of the traditional difference score. LGM is a special case of multilevel or mixed model analysis in which the first, or within-subject level, is modeled as follows:
where yit is the observed score for the ith person at time t, ait is the age of the person at time t, π0i is the intercept or level random effect for the ith individual, π1i is the slope score for the ith individual, π2i is the nonlinear change score for the ith individual, and eit is the residual term. The second-, or between-subject, level models random effects in π0i, π1i, and π2i.
where β00, β10, and β20 are, respectively, the fixed (i.e., group level) intercept, slope and quadratic effects; μ0i, μ1i, and μ2i are residual terms; xij are observations on covariates; and β0j, β1j, and β2j are the effects of these covariates on individual differences in intercepts and slopes.
As the reliable estimation of random effects on the quadratic component of change requires large number of observations on each individual (Bryk & Raudenbush, 1992), we estimated the fixed quadratic effect only. In addition, the analysis focused on how sex differences in the intercept and slope are associated with lifespan and how these associations differ in men and women. The mean intercepts and slopes of strength score and grip strength were estimated freely in sex-specific groups. To reveal sex differences in the initial level and rate of change, the intercept and slope were consecutively constrained to be equal in men and women. The log likelihood ratio test was used to evaluate the model fit. A p value of .05 is considered to indicate statistical significance, and the estimates are presented with 95% confidence intervals (CI), unless otherwise stated.
In line with previous research, we expected to find sex differences in both the initial level and rate of change with men having an initial advantage over women and also a more rapid age-related decline. Sex differences in the correlations between intercept and slope and lifespan were evaluated by testing the equality between two correlation coefficients. A sex difference in the correlations between slope and lifespan would indicate that the rate-of-change in strength score and grip strength is differentially predictive of mortality in men and women. Consistent with the “terminal decline” hypothesis (Wilson, Beck, Bienias, & Bennett, 2007), we also expected the rate of change to be a stronger predictor of survival than the initial level among the oldest old.
Results
Change in Strength Score
In total, there were 2,295 (586 men [25.5%] and 1,709 women [74.5%]) elderly with at least one reported measure of physical function. In Table 1, Model 1 assumes linear change in strength score, whereas Model 2 additionally includes the fixed quadratic effect. As the fixed quadratic effect of change in strength score was very small and failed to reach statistical significance in both samples, the reduced linear model was considered.
Table 1.
Change in Strength Score Between 1998 and 2005 in the Danish 1905-Cohort Study
| Model 1 (linear) |
Model 2 (quadratic) |
|||
|---|---|---|---|---|
| Men (n = 586) | Women (n = 1,709) | Men (n = 586) | Women (n = 1709) | |
| Fixed effects | ||||
| Intercept | 2.07 (2.01, 2.14) | 1.74 (1.71, 1.77) | 2.08 (2.01, 2.15) | 1.74 (1.71, 1.78) |
| Slope | −0.20 (−0.23, −0.17) | −0.12 (−0.14, −0.11) | −0.23 (−0.27, −0.18) | −0.13 (−0.15, −0.11) |
| Lifespan | 96.0 (95.8, 96.2) | 96.8 (96.7, 96.9) | 96.0 (95.8, 96.2) | 96.8 (96.7, 96.9) |
| Quadratic effect | 0.008 (−0.002, 0.017) | 0.001 (−0.002, 0.004) | ||
| Variance components | ||||
| Variance (I) | 0.48 (0.39, 0.58) | 0.34 (0.31, 0.38) | 0.49 (0.40, 0.58) | 0.34 (0.31, 0.38) |
| Variance (S) | 0.006 (0.002, 0.011) | 0.004 (0.002, 0.005) | 0.006 (0.002, 0.006) | 0.004 (0.003, 0.004) |
| Covariance (I, S) | −0.03 (−0.05, −0.01) | −0.02 (−0.03, −0.01) | −0.03 (−0.05, −0.01) | −0.02 (−0.03,−0.01) |
| Covariance (I, LS) | 0.80 (0.63, 0.99) | 0.77 (0.68, 0.88) | 0.82 (0.65, 1.01) | 0.78 (0.68, 0.88) |
| Covariance (S, LS) | 0.03 (−0.02, 0.07) | 0.03 (0.003, 0.05) | 0.003 (−0.05, 0.06) | 0.024 (−0.002, 0.052) |
| 2 LnL | 17,631 | — | 17,628 | — |
| AIC | 5,143 | — | 5,144 | — |
| df | 6,244 | — | 6,242 | — |
Note: Total number of participants studied, n = 2,295. I = intercept; S = slope; LS = lifespan; LnL - loglikelihood; AIC - Akaike’s Information Criterion.
Initially, men aged 93 years had a higher strength score compared with women, 2.07 (2.01, 2.14) versus 1.74 (1.71, 1.77), respectively. However, the linear rate of change in strength score among women of −.12 units (−0.14, −0.11) per year was lower than the rate of change among men, −.20 (−0.23, −0.17).
As compared to the fully saturated model (−2 LnL = 17,631 with 6,244 df), models in which the intercepts (−2 LnL = 17,705 with 6,245 df, difference χ2 = 74 with 1 df, p < .00001) and the slopes (−2 LnL = 17,648 with 6,245 df, difference χ2 = 17 with 1 df, p < .00001) were constrained to be equal in men and women had significantly worse fit. This indicates that the mean initial level of strength score was significantly higher in men than in women and that the strength declined at a significantly greater rate in men than in women.
The significant variance component for the intercept suggests some remaining explainable residual variation in the initial status of strength score in both sexes. All correlation coefficients were in the expected direction (Table 2). Negative correlation coefficients between intercept and slope in both men, −.50 (−0.69, −0.23), and women, −.53 (−0.63, −0.41) indicates that elderly individuals with higher initial levels of strength score decline in their functioning at a faster pace (as smaller negative values correspond to larger absolute decline). Lifespan and intercept were positively correlated, both in men and women. Likewise, there were also positive correlations between lifespan and slope in both sex-specific samples, though it was not significant in men. These findings suggest that the persons with higher initial levels and slower rates of decline in physical function had longer lives.
Table 2.
Correlations Among Intercepts, Slopes, and Lifespan for Strength Score and Grip Strength in the Danish 1905-Cohort Study
| Men |
Women |
|||
|---|---|---|---|---|
| Intercept | Slope | Intercept | Slope | |
| Strength score | n = 586 | — | n =1,709 | — |
| Intercept | 1 | — | 1 | — |
| Slope | −0.50 (−0.69, −0.23) | 1 | −0.53 (−0.63, −0.41) | 1 |
| Lifespan | 0.46b (0.37, 0.53) | 0.13 (−0.09, 0.37) | 0.47b (0.42, 0.51) | 0.15 (0.02, 0.29) |
| Grip strengtha | n = 450 | — | n = 1,167 | — |
| Intercept | 1 | — | 1 | — |
| Slope | −0.10 (−0.75, 0.94) | 1 | −0.28 (−0.50, 0.05) | 1 |
| Lifespan | 0.29 (0.19, 0.38) | 0.33 (−0.03, 1.00) | 0.31 (0.24, 0.38) | 0.23 (−0.03, 0.59) |
Correlations for grip strength are from the linear model in men and the quadratic model – in women.
Correlation between intercept and lifespan is significantly different from the correlation between slope and lifespan in sex-specific samples.
The test of equality revealed that the correlation coefficient between lifespan and intercept was greater than that between lifespan and slope in both men and women. In addition, there was no statistically significant sex difference in the correlation coefficients of lifespan with intercept and slope. These suggest that the initial level of strength score is more important for mortality prediction than the rate of decline and that the predictive effect of the initial level is similar in men and women.
Change in Grip Strength
In total, there were 1,617 (450 men and 1,167 women) individuals with at least 1 occasion of grip strength measurement. The mean initial level of grip strength was higher in men than in women, 22.6 kg (22.0, 23.3) and 13.4 kg (13.2, 13.7), respectively. Men declined in grip strength by −1.17 kg (−1.49, −0.87) per year, whereas the decline was −0.29 kg (−0.49, −0.08) in women at the baseline, but it increased with age due to the quadratic effect in women. The fixed quadratic effect of change in grip strength was statistically significant in the women only (Table 3), so that in the final model the quadratic effect in men was fixed to zero.
Table 3.
Change in Grip Strength Between 1998 and 2005 in the Danish 1905-Cohort Study
| Model 1 (linear) |
Model 2 (quadratic) |
|||
|---|---|---|---|---|
| Men |
Women |
Men |
Women |
|
| n = 450 | n = 1,167 | n = 450 | n = 1,167 | |
| Fixed effects | ||||
| Intercept | 22.6 (22.0, 23.3) | 13.5 (13.2, 13.8) | 22.7 (22.1, 23.3) | 13.4 (13.2, 13.7) |
| Slope | −1.17 (−1.49, − 0.87) | −0.48 (−0.60,−0.36) | −1.39 (−1.80, −0.98) | −0.29 (−0.49, −0.08) |
| Lifespan | 96.4 (96.2, 96.7) | 97.4 (97.2, 97.6) | 96.4 (96.2, 96.7) | 97.4 (97.2, 97.6) |
| Quadratic effect | — | — | 0.07 (−0.02, 0.15) | −0.05 (−0.09, −0.01) |
| Variance components | ||||
| Variance (I) | 32.8 (26.6, 39.9) | 13.8 (11.8, 15.9) | 32.9 (26.7, 39.9) | 13.5 (11.5, 15.7) |
| Variance (S) | 0.22 (0.001, 0.61) | 0.13 (0.02, 0.25) | 0.15 (0.00, 0.52) | 0.14 (0.07, 0.26) |
| Covariance (I, S) | −0.28 (−1.88, 1.06) | −0.50 (−0.97, −0.06) | −0.29 (−1.83, 1.00) | −0.39 (−0.86, 0.05) |
| Covariance (I, LS) | 4.25 (2.70, 5.94) | 3.44 (2.66, 4.25) | 4.48 (2.90, 6.21) | 3.28 (2.50, 4.09) |
| Covariance (S, LS) | 0.39 (−0.04, 0.84) | 0.07 (−0.17, 0.31) | 0.14 (−0.38, 0.68) | 0.25 (−0.03, 0.53) |
| 2 LnL | 22,857 | — | 22,849 | — |
| AIC | 14,454 | — | 14,451 | — |
| df | 4,201 | — | 4,199 | — |
Note: I = intercept; S = slope; LS = lifespan. Total number of participants studied, n = 1,617; LnL-loglikelihood; AIC-Akaike’s Information Criterion.
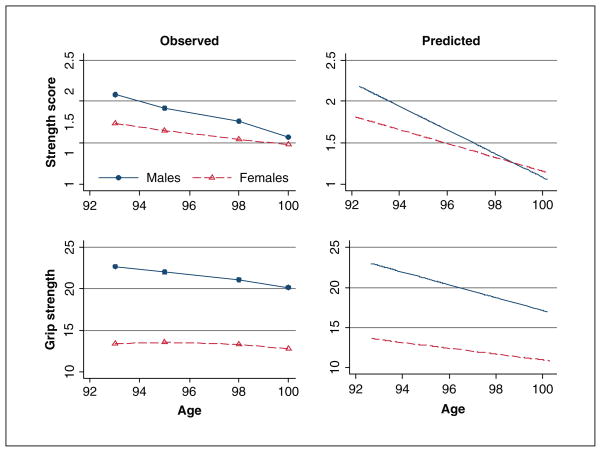
The negative fixed quadratic effect of −.05 (−0.09, −0.01) indicates that in women the rate of decline increases with advanced age. Because the pace at which women decline in grip strength changes with age, the overall annual drop is difficult to summarize by a single number. The left panel in Figure 3 illustrates observed (cross-sectional means) and right panel predicted (based on the final models) trajectories of strength score and grip strength in the Danish 1905-Cohort study from 1998 to 2005. Both the observed and predicted trajectories point toward more rapid decline in strength score and grip strength in men than in women.
Figure 3.
Observed and predicted trajectories of change in strength score and grip strength in the Danish 1905-Cohort Study (1998–2005)
a. Predicted trajectories of change in strength score are based on the linear model. Predicted trajectories of change in grip strength are based on the linear model for men and quadratic model for women.
According to the log-likelihood ratio test, the saturated model (−2 LnL = 22,851 with 4,200 df) fit the data better than the models with either the intercepts (−2 LnL = 23,310 with 4,201 df, difference χ2 = 449 with 1 df, p < .00001) or the slopes (−2 LnL = 22,873 with 4,201 df, difference χ2 = 22 with 1 df, p < .00001) constrained to be equal in the two groups. This indicates that the initial level and rate of change in grip strength are significantly higher in men than in women.
Variance components of intercepts and slopes were significant in both groups, suggesting that potentially explainable residual variations in the initial level and rate of change still remain. The correlation between the intercept and slope in men, −.10 (−0.75, 0.94), and women, −.28 (−0.50, 0.05), were negative, but they failed to reach a statistically significant level (Table 2). It may imply that among nonagenarian Danes grip strength declines at a similar pace regardless of its initial level.
Lifespan was significantly and positively correlated with intercept in men, .29 (0.19, 0.38), and women, .31 (0.24, 0.38), indicating that within both sexes individuals with higher grip strength have longer lifespan (Table 2). The correlation coefficient between lifespan and slope failed to reach statistically significant level in both men, .33 (−0.03, 1.00), and women, .23 (−0.03, 0.59). These findings suggest that among Danish nonagenarians the initial levels of grip strength are more predictive of survival than the rate of change. However, insignificant correlation coefficients between slope and lifespan may also be due to small number of individuals with at least 3 measurements of grip strength necessary to reliably estimate linear slope. The test of equality revealed no statistically significant sex difference in the correlation coefficients of lifespan with either intercept or slope.
Discussion
Consistent with previous research, the present study revealed that in the Danish 1905-cohort men had a higher strength score and grip strength, compared with women (Beckett et al., 1996; Frederiksen et al., 2006; Fujita et al., 1995; Gale et al., 2007; Taylor & Lynch, 2004). Although nonagenarian men had the initial advantage, they also experienced steeper declines in strength score and grip strength, compared with the women. The data analysis showed that the strength score declined linearly in both sexes, whereas the decline in grip strength was nonlinear in women. It appeared that among women the decline in grip strength accelerates with increasing age. The nonsignificant quadratic effect among men was possibly due to the small number of male participants (n = 15), with valid measurements at all four surveys necessary to estimate a nonlinear quadratic effect. The present study suggests that, in absolute terms, elderly men showed a more rapid decline in the reported physical function and grip strength than did women.
Our results are in agreement with a longitudinal study among Swedish twins that revealed significantly greater change for grip strength in men compared with women (Proctor et al., 2006). Hughes et al. found that although men had higher isokinetic strength in all muscle groups, the absolute 10-year decline in strength of each muscle group was greater in men than in women (Hughes et al., 2001). Forrest et al. showed that over a 10-year follow-up period women declined in grip strength by 2.4% per year, whereas men declined by 2.8% annually (Forrest et al., 2005, 2007). Other studies also reported steeper rates of decline in handgrip strength in men than in women (Proctor et al., 2006; Vianna, Oliveira, & Araujo, 2007). Several studies, however, found that longitudinal changes in handgrip strength were similar in men and women (Bassey, 1998) or even greater in women than in men (Rantanen, Era, & Heikkinen, 1997; Rantanen & Heikkinen, 1998). A greater rate of change in physical function was found among women 65 years and older than among men of the same age (Beckett et al., 1996; Liang et al., 2008; Taylor & Lynch, 2004).
Age-related decline in concentrations of testosterone and estrogen (Lamberts, van den Beld, & van der Lely, 1997) have been suggested to underlie decline in muscle strength among elderly persons. However, the evidence for causal relationship between decline in muscle strength and decrease in free testosterone levels, as well as the risks and benefits of testosterone replacement therapy are still controversial (Bassil, Alkaade, & Morley, 2009). Some studies indicated that total and bioavailable testosterone and estrogen levels were unrelated to the maximum handgrip strength, to the 3-year decline in performance of chair stand and walk tests, or to the 3-year decline in muscle strength among men and women aged 55 years and older (Schaap et al., 2008; van Geel, Geusens, Winkens, Sels, & Dinant, 2009). Other researchers, however, found that total and bioavailable testosterone were positively related with muscle strength in elderly men (van den Beld, de Jong, Grobbee, Pols, & Lamberts, 2000) and that, beyond certain concentration, no additional benefits of increased testosterone level on the performance of functional tasks was indicated (O’Donnell, Travison, Harris, Tenover, & McKinlay, 2006).
Our analysis of change in strength score revealed significant negative correlation between intercept and slope, suggesting that the oldest-old individuals with higher initial levels of strength score have higher rate of decline. However, we could not detect a similar pattern in the analysis of change in grip strength, which may suggest that a decline in grip strength among oldest-old Danes occurs at a similar pace, regardless of the initial level of muscular strength.
Consistent with previous research in mixed and sex-specific samples, our study suggests that the decline in physical performance increases with advancing age (Bassey, 1998; Forrest et al., 2005, 2007; Rantanen et al., 1998). Onder et al. reported that women aged 65 to 79 years showed a decline in grip strength by 0.50 kg per year, with those aged 80 years or older showing an annual decline of 0.60 kg (Onder et al., 2002). The 59-year longitudinal Terman study pointed out that the decline in self-rated health accelerated after age 50 for both men and women, though the mean rate of change in men was significantly higher than that found in women (McCullough & Laurenceau, 2004).
The analysis of change in strength score revealed a consistently greater correlation between lifespan and intercept than the correlation between lifespan and slope. In the analysis of change in grip strength, the correlation between lifespan and slope failed to reach statistically significant level. In both cases, no sex differences were indicated in correlation of lifespan with intercepts and slopes. These findings suggest that the initial level of strength score and grip strength are more predictive of mortality than the rate of change, but the predictive effects of the initial levels are similar in men and women. A larger correlation coefficient between slope and lifespan compared with that between intercept and lifespan in the sample of men is likely a result of the sample being small. Only 60 men and 179 women had ≥3 measurements of grip strength that are necessary to reliably fit the linear growth model. It is also possible that the initial selection of individuals, sufficiently strong at the baseline to have several follow-up measurements, may account for the more rapid drop in grip strength.
At present, knowledge about decline–mortality association based on measures of physical health is limited. Information is especially sparse for oldest-old individuals due to high mortality and loss to follow-up. Metter et al. found that changes in muscle power and muscle strength predicted mortality risk, independent of the strength and power levels (Metter et al., 2004). A study among Medicare recipients revealed that the individuals with rapid age-related decline in physical abilities had a substantially higher risk of death, compared to those with slower rates of decline or preserved functions; however, the decline–mortality association was stronger in individuals aged ≤75 years (Schupf et al., 2005).
The literature regarding “terminal decline” based on cognitive health is also contradictory (Bosworth & Siegler, 2002). Several studies demonstrated that cognitive change predicts subsequent survival (Bosworth, Schaie, & Willis, 1999; Deeg et al., 1990), whereas others have found that the level but not the decline is predictive of mortality (Ghisletta, 2008; Laukka, MacDonald, & Backman, 2006). The Nun Study has indicated that the linguistic abilities from early life were significantly inversely related with Alzheimer’s disease lesions in the brain neocortex that underscore the importance of the initial level (Snowdon, Greiner, & Markesbery, 2000). To add complexity, another study found decline–mortality association for some specific cognitive tasks and age groups only (Lavery, Dodge, Snitz, & Ganguli, 2009; Kliegel et al., 2004). It has been proposed that the effect of cognitive (Lavery et al., 2009; Kliegel, Moor, & Rott, 2004) or functional decline (Schupf et al., 2005) on mortality levels off at very old age. A diminishing decline–mortality association at very advanced age may partially explain the fact that the rate of change in our study failed to be more predictive of mortality than the initial levels of strength score and grip strength.
Some issues in our study deserve consideration. First, our analysis highlights the difficulties of estimating the rate of change in the oldest-old individuals, due to high mortality and loss to follow-up. Despite an initially large sample, with almost complete follow-up of lifespan, we still had limited power to estimate more precisely the rate of change in those health measures for which proxy interviews cannot be used. A possible decline–mortality association based on grip strength requires further investigation with a larger number of male participants.
Second, because censoring cannot be easily accommodated in growth-curve analysis, lifespan of individuals still alive at the end of December 2008 was estimated by adding the remaining life expectancy to their last known age, using the life tables for Denmark. It may restrict the variance of the lifespan variable, and, consequently, correlations of lifespan with intercept and slope may be biased towards zero. However, this bias is likely to be small and insignificant because only 53 individuals were affected.
Third, the analysis was based exclusively on the observed data, without accounting for selective loss to follow-up due to nonresponse or mortality. Recent studies in Denmark showed that correcting disability score for missing data because of nonparticipation using inverse probability weights (Dufouil, Brayne, & Clayton, 2004) gave results very similar to the unadjusted score (Christensen, McGue, Petersen, Jeune, & Vaupel, 2008). Even though the curves of the age-related decline in grip strength were shifted downwards when selective dropout due to nonparticipation was taken into consideration (Frederiksen et al., 2006), this refinement, probably, would not affect male–female differences in the initial levels and rates of change in strength score and grip strength or the sex difference in the correlations of lifespan with intercepts and slopes.
The present study had a single geographic focus, Denmark, and may not be representative of other population settings. A cross-country comparison study of grip strength among nonagenarians and centenarians in three different European regions found a significant North–South gradient in handgrip strength, with substantially lower values in Southern countries, which may be due to genetic variation, differences in early life conditions, and sociocultural differences (Jeune et al., 2006).
Finally, grip-strength measurements were performed by different lay interviewers, which could have introduced a measurement bias attributable to interviewers. However, a previous study in Denmark reported that differences attributable to interviewer effects accounted for only 1% to 2% of the population variation in grip strength (Frederiksen et al., 2006).
In conclusion, this study revealed that despite their initial advantage, nonagenarian Danish men have more rapid age-related decline in physical function and grip strength than women do. Our findings suggest that the initial levels are more important in predicting mortality than the rate of change and that the predictive effect is similar in women and men. Although this study indicated steeper decline in physical function and grip strength in men than in women, there was no indication for sex-specific effect of the initial level or rate of change on survival. Therefore, this study contributed little to the explanations for the male–female health–survival paradox.
Acknowledgments
Funding
This study was supported by research grants from the National Institute on Aging (NIA-PO1-AG08761, NIA-P01-AG031719) and the VELUX Foundation.
Footnotes
Declaration of Conflicting Interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
References
- Arber S, Cooper H. Gender differences in health in later life: The new paradox? Social Science and Medicine. 1999;48(1):61–76. doi: 10.1016/s0277-9536(98)00289-5. [DOI] [PubMed] [Google Scholar]
- Avlund K, Davidsen M, Schultz-Larsen K. Changes in functional ability from ages 70 to 75: A Danish longitudinal study. Journal of Aging and Health. 1995;7:254–282. doi: 10.1177/089826439500700205. [DOI] [PubMed] [Google Scholar]
- Bambra C, Pope DP, Swami V, Stanistreet DL, Roskam AJ, Kunst AE, et al. Gender, health inequalities and welfare state regimes: A cross-national study of thirteen European countries. Journal of Epidemiology and Community Health. 2008;63:38–44. doi: 10.1136/jech.2007.070292. [DOI] [PubMed] [Google Scholar]
- Barford A, Dorling D, Smith GD, Shaw M. Life expectancy: Women now on top everywhere. British Medical Journal. 2006;332(7545):808. doi: 10.1136/bmj.332.7545.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassey EJ. Longitudinal changes in selected physical capabilities: Muscle strength, flexibility and body size. Age and Ageing. 1998;27(Suppl 3):12–16. doi: 10.1093/ageing/27.suppl_3.12. [DOI] [PubMed] [Google Scholar]
- Bassil N, Alkaade S, Morley J. The benefits and risks of testosterone replacement therapy: A review. Journal of Therapy & Clinical Risk Management. 2009;5:427–448. doi: 10.2147/tcrm.s3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett LA, Brock DB, Lemke JH, de Leon CFM, Guralnik JM, Fillenbaum GG, et al. Analysis of change in self-reported physical function among older persons in four population studies. American Journal of Epidemiology. 1996;143:766–778. doi: 10.1093/oxfordjournals.aje.a008814. [DOI] [PubMed] [Google Scholar]
- Bosworth HB, Schaie KW, Willis SL. Cognitive and sociodemographic risk factors for mortality in the Seattle Longitudinal Study. Journals of Gerontology: Series B, Psychological Sciences and Social Sciences. 1999;54:273–282. doi: 10.1093/geronb/54b.5.p273. [DOI] [PubMed] [Google Scholar]
- Bosworth HB, Siegler IC. Terminal change in cognitive function: An updated review of longitudinal studies. Experimental Aging Research. 2002;28:299–315. doi: 10.1080/03610730290080344. [DOI] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical linear models: Applications and data analysis methods. Vol. 16. Thousand Oaks, CA: Sage; 1992. [Google Scholar]
- Case A, Paxson C. Sex differences in morbidity and mortality. Demography. 2005;42:189–214. doi: 10.1353/dem.2005.0011. [DOI] [PubMed] [Google Scholar]
- Christensen K, Bathum L, Christiansen L. Biological indicators and genetic information in Danish twin and oldest-old surveys. In: Weinstein M, Vaupel J, Wachter K, editors. Biosocial surveys. Washington, DC: National Academy Press; 2008. pp. 15–41. [Google Scholar]
- Christensen K, Frederiksen H, Vaupel JW, McGue M. Age trajectories of genetic variance in physical functioning: A longitudinal study of Danish twins aged 70 years and older. Behavioral Genetics. 2003;33(2):125–136. doi: 10.1023/a:1022501817781. [DOI] [PubMed] [Google Scholar]
- Christensen K, Gaist D, Vaupel JW, McGue M. Genetic contribution to rate of change in functional abilities among Danish twins aged 75 years or more. American Journal of Epidemiology. 2002;155(2):132–139. doi: 10.1093/aje/155.2.132. [DOI] [PubMed] [Google Scholar]
- Christensen K, Holm NV, McGue M, Corder L, Vaupel JW. A Danish population-based twin study on general health in the elderly. Journal of Aging and Health. 1999;11:49–64. doi: 10.1177/089826439901100103. [DOI] [PubMed] [Google Scholar]
- Christensen K, McGue M, Petersen I, Jeune B, Vaupel JW. Exceptional longevity does not result in excessive levels of disability. Proceedings of National Academy of Science. 2008;105:13274–13279. doi: 10.1073/pnas.0804931105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K, McGue M, Yashin A, Iachine I, Holm NV, Vaupel JW. Genetic and environmental influences on functional abilities in Danish twins aged 75 years and older. Journals of Gerontology: Series A, Biological Sciences and Medical Sciences. 2000;55:446–452. doi: 10.1093/gerona/55.8.m446. [DOI] [PubMed] [Google Scholar]
- Deeg DJH, Hofman A, van Zonneveld RJ. The association between change in cognitive function and longevity in Dutch elderly. American Journal of Epidemiology. 1990;132:973–982. doi: 10.1093/oxfordjournals.aje.a115740. [DOI] [PubMed] [Google Scholar]
- Dufouil C, Brayne C, Clayton D. Analysis of longitudinal studies with death and drop-out: A case study. Statistics in Medicine. 2004;23:2215–2226. doi: 10.1002/sim.1821. [DOI] [PubMed] [Google Scholar]
- Forrest KYZ, Zmuda JM, Cauley JA. Patterns and determinants of muscle strength change with aging in older men. Aging Male. 2005;8(3–4):151–156. doi: 10.1080/13685530500137840. [DOI] [PubMed] [Google Scholar]
- Forrest KYZ, Zmuda JM, Cauley JA. Patterns and correlates of muscle strength loss in older women. Gerontology. 2007;53(3):140–147. doi: 10.1159/000097979. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Hjelmborg J, Mortensen J, McGue M, Vaupel JW, Christensen K. Age trajectories of grip strength: Cross-sectional and longitudinal data among 8,342 Danes aged 46 to 102. Annals of Epidemiology. 2006;16:554–562. doi: 10.1016/j.annepidem.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Nakamura Y, Hiraoka J, Kobayashi K, Sakata K, Nagai M, et al. Physical-strength tests and mortality among visitors to health-promotion centers in Japan. Journal of Clinical Epidemiology. 1995;48:1349–1359. doi: 10.1016/0895-4356(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Gale CR, Martyn CN, Cooper C, Sayer AA. Grip strength, body composition, and mortality. International Journal of Epidemiology. 2007;36:228–235. doi: 10.1093/ije/dyl224. [DOI] [PubMed] [Google Scholar]
- Ghisletta P. Application of a joint multivariate longitudinal-survival analysis to examine the terminal decline hypothesis in the Swiss Interdisciplinary Longitudinal Study on the Oldest Old. Journals of Gerontology: Series B, Psychological Sciences and Social Sciences. 2008;63:185–192. doi: 10.1093/geronb/63.3.p185. [DOI] [PubMed] [Google Scholar]
- Giampaoli S, Ferrucci L, Cecchi F, Lo Noce C, Poce A, Dima F, et al. Hand-grip strength predicts incident disability in non-disabled older men. Age and Ageing. 1999;28:283–288. doi: 10.1093/ageing/28.3.283. [DOI] [PubMed] [Google Scholar]
- Human Mortality Database. 2009 Retrieved August 27, 2009, from www.mortality.org.
- Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, et al. Longitudinal muscle strength changes in older adults: Influence of muscle mass, physical activity, and health. Journals of Gerontology: Series A, Biological Sciences and Medical Sciences. 2001;56:209–217. doi: 10.1093/gerona/56.5.b209. [DOI] [PubMed] [Google Scholar]
- Jeune B, Skytthe A, Cournil A, Greco V, Gampe J, Berardelli M, et al. Handgrip strength among nonagenarians and centenarians in three European regions. Journals of Gerontology: Series A, Biological Sciences and Medical Sciences. 2006;61:707–712. doi: 10.1093/gerona/61.7.707. [DOI] [PubMed] [Google Scholar]
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged: The Index of ADL: A standardized measure of biological and psychosocial function. Journal of American Medical Association. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- Kliegel M, Moor C, Rott C. Cognitive status and development in the oldest old: A longitudinal analysis from the Heidelberg Centenarian Study. Archives of Gerontology and Geriatrics. 2004;39:143–156. doi: 10.1016/j.archger.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Lamberts SWJ, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science. 1997;278:419–424. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- Laukka EJ, MacDonald SWS, Backman L. Contrasting cognitive trajectories of impending death and preclinical dementia in the very old. Neurology. 2006;66:833–838. doi: 10.1212/01.wnl.0000203112.12554.f4. [DOI] [PubMed] [Google Scholar]
- Lavery LL, Dodge HH, Snitz B, Ganguli M. Cognitive decline and mortality in a community-based cohort: The Monongahela Valley Independent Elders Survey. Journal of the American Geriatrics Society. 2009;57(1):94–100. doi: 10.1111/j.1532-5415.2008.02052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveille SG, Penninx BW, Melzer D, Izmirlian G, Guralnik JM. Sex differences in the prevalence of mobility disability in old age: The dynamics of incidence, recovery, and mortality. Journals of Gerontology: Series B, Psychological Sciences and Social Sciences. 2000;55:41–50. doi: 10.1093/geronb/55.1.s41. [DOI] [PubMed] [Google Scholar]
- Liang J, Bennett JM, Shaw BA, Quinones AR, Ye W, Xu X, et al. Gender differences in functional status in middle and older age: Are there any age variations? Journals of Gerontology: Series B, Psychological Sciences and Social Sciences. 2008;63:282–292. doi: 10.1093/geronb/63.5.s282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manton KG, Corder L, Stallard E. Changes in the use of personal assistance and special equipment from 1982 to 1989: Results from the 1982 and 1989 NLTCS. Gerontologist. 1993;33:168–176. doi: 10.1093/geront/33.2.168. [DOI] [PubMed] [Google Scholar]
- McCullough ME, Laurenceau JP. Gender and the natural history of self-rated health: A 59-year longitudinal study. Health Psychology. 2004;23:651–655. doi: 10.1037/0278-6133.23.6.651. [DOI] [PubMed] [Google Scholar]
- Metter EJ, Talbot LA, Schrager M, Conwit RA. Arm-cranking muscle power and arm isometric muscle strength are independent predictors of all-cause mortality in men. Journal of Applied Physiology. 2004;96:814–821. doi: 10.1152/japplphysiol.00370.2003. [DOI] [PubMed] [Google Scholar]
- Nathanson CA. Illness and the feminine role: A theoretical review. Social Science and Medicine. 1975;9(2):57–62. doi: 10.1016/0037-7856(75)90094-3. [DOI] [PubMed] [Google Scholar]
- Neale M, Boker S, Xie G, Maes H. Mx: Statistical modeling. 6. Richmond, VA: Department of Psychiatry; 2003. [Google Scholar]
- Nybo H, Gaist D, Jeune B, Bathum L, McGue M, Vaupel JW, et al. The Danish 1905 Cohort: A genetic-epidemiological nationwide survey. Journal of Aging and Health. 2001;13:32–46. doi: 10.1177/089826430101300102. [DOI] [PubMed] [Google Scholar]
- Nybo H, Gaist D, Jeune B, McGue M, Vaupel JW, Christensen K. Functional status and self-rated health in 2,262 nonagenarians: The Danish 1905 Cohort Survey. Journal of the American Geriatrics Society. 2001;49:601–609. doi: 10.1046/j.1532-5415.2001.49121.x. [DOI] [PubMed] [Google Scholar]
- Nybo H, Petersen HC, Gaist D, Jeune B, Andersen K, McGue M, et al. Predictors of mortality in 2,249 nonagenarians: The Danish 1905-Cohort Survey. Journal of the American Geriatrics Society. 2003;51:1365–1373. doi: 10.1046/j.1532-5415.2003.51453.x. [DOI] [PubMed] [Google Scholar]
- O’Donnell AB, Travison TG, Harris SS, Tenover JL, McKinlay JB. Testosterone, dehydroepiandrosterone, and physical performance in older men: Results from the Massachusetts Male Aging Study. Journal of Clinical Endocrinology and Metabolism. 2006;91:425–431. doi: 10.1210/jc.2005-1227. [DOI] [PubMed] [Google Scholar]
- Olsen KM, Dahl SA. Health differences between European countries. Social Science and Medicine. 2007;64:1665–1678. doi: 10.1016/j.socscimed.2006.11.031. [DOI] [PubMed] [Google Scholar]
- Onder G, Penninx BWJH, Lapuerta P, Fried LP, Ostir GV, Guralnik JM, et al. Change in physical performance over time in older women: The Women’s Health and Aging Study. Journals of Gerontology: Series A, Biological Sciences and Medical Sciences. 2002;57:289–293. doi: 10.1093/gerona/57.5.m289. [DOI] [PubMed] [Google Scholar]
- Oxford KL. Elbow positioning for maximum grip performance. Journal of Hand Therapy. 2000;13(1):33–36. doi: 10.1016/s0894-1130(00)80050-2. [DOI] [PubMed] [Google Scholar]
- Pedersen CB, Gøtzsche H, Moller JO, Mortensen PB. The Danish Civil Registration System. A cohort of eight million persons. Danish Medical Bulletin. 2006;53:441–449. [PubMed] [Google Scholar]
- Proctor DN, Fauth EB, Hoffman L, Hofer SM, McClearn GE, Berg S, et al. Longitudinal changes in physical functional performance among the oldest old: Insight from a study of Swedish twins. Aging Clinical & Experimental Research. 2006;18:517–530. doi: 10.1007/BF03324853. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Era P, Heikkinen E. Physical activity and the changes in maximal isometric strength in men and women from the age of 75 to 80 years. Journal of the American Geriatrics Society. 1997;45:1439–1445. doi: 10.1111/j.1532-5415.1997.tb03193.x. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, et al. Midlife hand grip strength as a predictor of old age disability. Journal of the American Medical Association. 1999;281:558–560. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Heikkinen E. The role of habitual physical activity in preserving muscle strength from age 80 to age 85 years. Journal of Aging and Physical Activity. 1998;6:121–132. [Google Scholar]
- Rantanen T, Masaki K, Foley D, Izmirlian G, White L, Guralnik JM. Grip strength changes over 27 yr in Japanese-American men. Journal of Applied Physiology. 1998;85:2047–2053. doi: 10.1152/jappl.1998.85.6.2047. [DOI] [PubMed] [Google Scholar]
- Schaap LA, Pluijm SMF, Deeg DJH, Penninx BW, Nicklas BJ, Lips P, et al. Low testosterone levels and decline in physical performance and muscle strength in older men: Findings from two prospective cohort studies. Clinical Endocrinology. 2008;68(1):42–50. doi: 10.1111/j.1365-2265.2007.02997.x. [DOI] [PubMed] [Google Scholar]
- Schultz-Larsen K, Avlund K, Kreiner S. Functional ability of community dwelling elderly. Criterion-related validity of a new measure of functional ability. Journal of Clinical Epidemiology. 1992;45:1315–1326. doi: 10.1016/0895-4356(92)90172-j. [DOI] [PubMed] [Google Scholar]
- Schupf N, Tang MX, Albert SM, Costa R, Andrews H, Lee JH, et al. Decline in cognitive and functional skills increases mortality risk in non-demented elderly. Neurology. 2005;65:1218–1226. doi: 10.1212/01.wnl.0000180970.07386.cb. [DOI] [PubMed] [Google Scholar]
- Sehl ME, Yates FE. Kinetics of human aging: I. Rates of senescence between ages 30 and 70 years in healthy people. Journals of Gerontology: Series A, Biological Sciences and Medical Sciences. 2001;56(5):198–208. doi: 10.1093/gerona/56.5.b198. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Markesbery WR. Linguistic ability in early life and the neuropathology of Alzheimer’s Disease and cerebrovascular disease: Findings from the Nun Study. Annals of the New York Academy of Sciences. 2000;903:34–38. doi: 10.1111/j.1749-6632.2000.tb06347.x. [DOI] [PubMed] [Google Scholar]
- Syddall H, Cooper C, Martin F, Briggs R, Sayer AA. Is grip strength a useful single marker of frailty? Age and Ageing. 2003;32:650–656. doi: 10.1093/ageing/afg111. [DOI] [PubMed] [Google Scholar]
- Taylor MG, Lynch SM. Trajectories of impairment, social support, and depressive symptoms in later life. Journals of Gerontology: Series B, Psychological Sciences and Social Sciences. 2004;59:238–246. doi: 10.1093/geronb/59.4.s238. [DOI] [PubMed] [Google Scholar]
- van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. Journal of Clinical Endocrinology and Metabolism. 2000;85:3276–3282. doi: 10.1210/jcem.85.9.6825. [DOI] [PubMed] [Google Scholar]
- van Geel TACM, Geusens PP, Winkens B, Sels JPJE, Dinant GJ. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle mass, muscle strength and bone mineral density in post-menopausal women: A cross-sectional study. European Journal of Endocrinology. 2009;160:681–687. doi: 10.1530/EJE-08-0702. [DOI] [PubMed] [Google Scholar]
- Vianna LC, Oliveira RB, Araujo CG. Age-related decline in handgrip strength differs according to gender. Journal of Strength & Conditioning Research. 2007;21:1310–1314. doi: 10.1519/R-23156.1. [DOI] [PubMed] [Google Scholar]
- Waldron I. What do we know about causes of sex differences in mortality? A review of the literature. Population Bulletin UN. 1985;18:59–76. [PubMed] [Google Scholar]
- Wilson RS, Beck TL, Bienias JL, Bennett DA. Terminal cognitive decline: Accelerated loss of cognition in the last years of life. Psychosomatic Medicine. 2007;69(2):131–137. doi: 10.1097/PSY.0b013e31803130ae. [DOI] [PubMed] [Google Scholar]